Adherence to established clinical guidelines in regard to hepatitis B screening, the use of antiviral prophylaxis prior to rituximab treatment, and clinical outcomes in patients receiving rituximab since its introduction in 2001 in a large health care network were investigated. A critical need to identify at-risk patients and provide timely prophylactic antiviral therapy to prevent serious morbidity and mortality was exposed.
Keywords: Rituximab, Hepatitis B reactivation, Hepatitis B screening, Lymphoma, R-CHOP chemotherapy
Abstract
Reactivation of hepatitis B virus (HBV) replication in patients receiving rituximab is well described. Current international guidelines recommend HBV screening prior to the commencement of immunosuppressive therapy. However, adherence to such protocols has not previously been studied. We therefore audited screening practices and clinical outcomes in patients prescribed rituximab since its introduction in a large metropolitan health service. All patients receiving rituximab over an 88-month period were identified via pharmacy records. Medical records and laboratory results were reviewed to determine the timing and type of hepatitis screening. HBV flares were identified and correlated with clinical outcomes and any screening or prophylaxis given. Rituximab was given to 355 patients over 88 months (average age, 61 years; 51% male, 48% born overseas); 83% received rituximab for treatment of a hematological malignancy. HBV screening occurred in 31% of patients and, of these, 66% had pre-emptive screening. Five patients given cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab experienced HBV flares. Four died from viral reactivation. None received antiviral prophylaxis. Hepatitis screening rates in patients receiving rituximab in this study were lower than recommended in clinical guidelines. The identification of five patients with clinically important flares and four deaths in this group highlight the critical need to identify at-risk patients and provide timely prophylactic antiviral therapy to prevent serious morbidity and mortality. Even those with evidence of HBV seroconversion are at risk for fatal flares without active prophylactic antiviral therapy.
Introduction
One third of the world's population has evidence of previous exposure to the hepatitis B virus (HBV) and are hepatitis B core antibody (anti-HBc) positive [1, 2]. Chronic hepatitis B infection—hepatitis B surface antigen (HBsAg) positivity—now affects >400 million people worldwide [3]. Reactivation flares of hepatitis B [4] have been well described in patients receiving chemotherapy or other immunosuppressive therapy, including rituximab, a monoclonal antibody directed at CD20+ B cells. Currently, rituximab is commonly used to treat indolent non-Hodgkin's lymphoma; other indications include refractory rheumatoid arthritis and lupus nephritis. With the recent approval for chronic lymphocytic leukemia [5], the most common form of adult leukemia, the number of patients receiving rituximab therapy will likely increase. Current international guidelines recommend screening patients for HBV infection prior to chemotherapy or immunosuppression and commencing prophylactic antiviral therapy in those with detectable HBsAg [6, 7]. The aim of this study was to investigate adherence to established clinical guidelines in regard to hepatitis B screening, the use of antiviral prophylaxis prior to rituximab treatment, and clinical outcomes in patients receiving rituximab since its introduction in 2001 in a large health care network.
Materials and Methods
All patients receiving rituximab in a metropolitan health service from January 1, 2001 to April 30, 2008 were identified from pharmacy dispensing records that included clinical trial patients, ambulatory care patients, and hospital inpatients. Medical records and pathology results were reviewed to determine whether hepatitis B screening had been performed, and if so, the method and timing of screening was noted, particularly, whether patients had undergone intentional pre-emptive screening. Compliance with the American Association for the Study of Liver Disease (AASLD) 2007 clinical practice guidelines on hepatitis B [8] was noted and, in particular, the timing and type of antiviral prophylaxis were recorded. As recommended by these guidelines, hepatitis serology (HBsAg, anti-HBc) was assessed as the trigger to institute antiviral prophylaxis rather than abnormal liver function tests given that the latter could result from other causes, such as drugs and lymphoma. Evidence of reactivation of hepatitis B replication and clinical outcomes were identified. A hepatitis flare was defined as an increase in serum alanine aminotransferase (ALT) to >3× the upper limit of normal [9].
Hepatitis serology was measured by the hospital pathology laboratory using a commercially available assay (Architect® HBsAg; Abbott Diagnostics Abbott Park, IL). Hepatitis B replication was determined by viral load measurement using two different assays. Prior to 2007, the Digene Hybrid Capture II HBV DNA assay (Digene Diagnostic Corporation, Gaithersburg, MD) was used (quantification range, 0.5–6,000 pg/ml). From 2007, the Abbott RealTime HBV assay (Abbott Laboratories, Abbott Park, IL) was used (quantification range, 10–109 IU/ml). Liver function tests were measured by standard autoanalyzer methodology.
Results
Over the 88-month period, 355 patients were identified; the mean age was 61 years (range, 12–92); 51% were male. Most (52%) were born in Australia, with the remainder from Greece (13%), the U.K. (12%), Italy (11%), India (5%), and Scotland (5%). Thirty-eight other countries were represented in small numbers. Rituximab was administered mainly for treatment of lymphoma (83%), and all patients with lymphoma received cyclophosphamide, doxorubicin, vincristine, and prednisone with rituximab (R-CHOP). Other indications included renal transplantation (7%), lupus nephritis (5%), rheumatoid arthritis/scleroderma (3%), thrombotic thrombocytopenic purpura (1%), and others (1%). The number of patients receiving rituximab in each year is shown in Figure 1.
Figure 1.
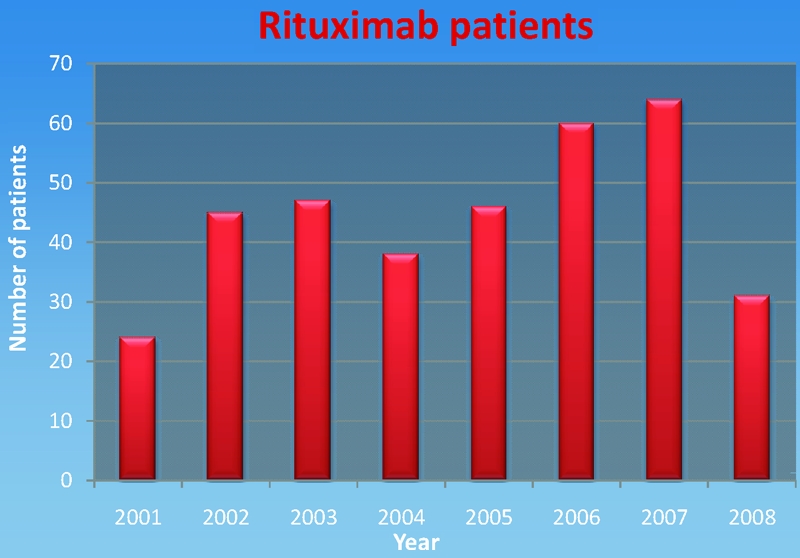
Rituximab use by year (only the first 6 months of 2008 were surveyed).
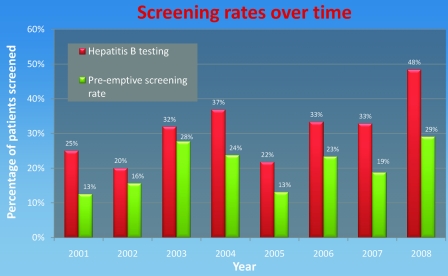
Of the 355 patients, 110 (31%) underwent hepatitis screening. Of these, 55% had hepatitis screening performed prior to rituximab therapy, 16% were tested during therapy, and 17% were tested after therapy. The annual proportion of rituximab-treated patients who underwent any form of hepatitis B screening and the percentage screened pre-emptively, consistent with clinical guidelines, are shown in Figure 2. The screening rate for hepatitis B in patients who received rituximab for nononcological reasons was higher than for the lymphoma group (51%); however, none of the screened patients in that group had evidence of prior hepatitis B exposure.
Figure 2.
Hepatitis B screening rates over time.
Of the 110 screened patients, 92% were tested for HBsAg and 18% were tested for antibody to hepatitis B surface antigen (anti-HBs). Of the latter, 75% were also tested for HBsAg. Only 25% of those who had any hepatitis serology performed were tested for anti-HBc. Of all patients screened, six (5.4%) had serological evidence of hepatitis B exposure (four with detectable HBsAg and two were anti-HBc+ but with undetectable HBsAg). Of these, five patients experienced reactivation of HBV replication initially detected by raised serum ALT activity and confirmed by HBV DNA testing. One patient who was HBsAg− but anti-HBC+ and anti-HBs+ did not have an ALT flare or evidence of active HBV replication.
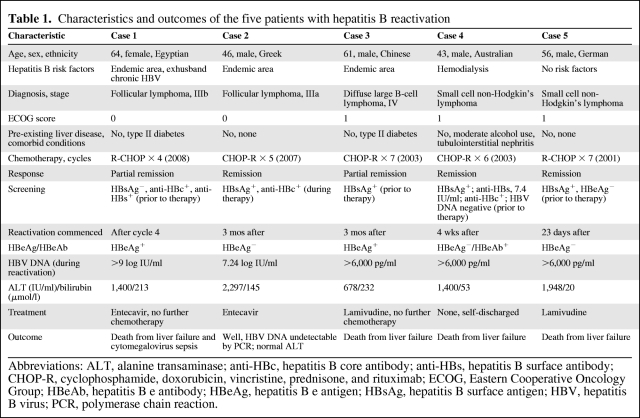
The characteristics of the five patients with HBV reactivation are summarized in Table 1. Four were male, one was female, and their ages were in the range of 43–61 years. Ethnic backgrounds varied, with only one born in Australia. All five had non-Hodgkin's lymphoma of varying stages with low Eastern Cooperative Oncology Group scores of 0–1 (all were still employed at the time of diagnosis). The patients received four to seven cycles of R-CHOP chemotherapy, with three patients attaining complete remission. Of the patients with hepatitis flares, hepatitis B serology testing occurred prior to rituximab therapy in four and during therapy in one. Testing for HBsAg was performed in all five cases; three were also tested for anti-HBc. This is relevant given that patient 1 was HBsAg− and anti-HBs+ on screening (Table 1); thus, evidence of previous hepatitis B exposure in this patient was detected only by testing for anti-HBc. One patient was tested for HBV DNA prior to treatment. None of the HBsAg+ patients was given antiviral prophylaxis prior to the commencement of therapy as recommended by clinical guidelines. All had high HBV DNA levels and significant ALT flares that occurred mainly after therapy (23 days to 3 months) with only one flare occurring during therapy.
Table 1.
Characteristics and outcomes of the five patients with hepatitis B reactivation
Abbreviations: ALT, alanine transaminase; anti-HBc, hepatitis B core antibody; anti-HBs, hepatitis B surface antibody; CHOP-R, cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab; ECOG, Eastern Cooperative Oncology Group; HBeAb, hepatitis B e antibody; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; PCR, polymerase chain reaction.
Antiviral therapy was offered to all five patients following recognition of acute severe HBV reactivation. Patients 1 and 2 received entecavir therapy, one survived and one died. Patients 3 and 5 received lamivudine and both died. Patient 4 refused treatment and died. Of the four patients who died, three deaths occurred despite institution of antiviral treatment. In regard to the cause of death, three were directly attributable to fulminant hepatic failure with no associated neutropenia or sepsis. The fourth patient had both fulminant hepatic failure and cytomegalovirus sepsis. Two of the four patients who died were in remission from their lymphoma and the other two were in partial remission.
Discussion
Guidelines published by the AASLD in 2007 and revised in 2009 recommend hepatitis B screening in persons at high risk prior to the initiation of chemotherapy or other immunosuppressive therapy [8]. Recently published guidelines from the European Association for the Study of the Liver (EASL) recommend screening in all patients prior to chemotherapy or immunosuppressive treatment because serious viral reactivations have been described with other chemotherapy regimens, for example, chronic lymphocytic leukemia patients treated with purine analogs alone or in combination [7]. These guidelines are also echoed by the National Comprehensive Cancer Network guidelines [10, 11].
The background prevalence of hepatitis B in our population was difficult to ascertain given the low screening rates. Previous work on the epidemiology of hepatitis B infection in Australia by Tawk et al. [12] shows that patients born in Asia or the Pacific Islands have an adjusted odds ratio of 12.4 for hepatitis B infection whereas those from North Africa, the Middle East, and the Mediterranean have an adjusted odds ratio of 6 compared with those born in Australia. The patients who received rituximab in our study were from 44 different countries. Patient 4, who was born in Australia, illustrates that other risk factors should also be considered, in this case, hemodialysis. Notably, this particular risk factor was not considered in the study by Tawk et al. [12] and so the hepatitis B status of patient 4 would have been missed if a purely risk factor–based protocol for screening was used. Patient 5, from Germany, also had no identifiable risk factors. Although evidence from formal cost-efficacy studies is not currently available, we suggest that, given the low cost of hepatitis B serology ($AU47.25) [13], compared with the potential risks of delayed chemotherapy and mortality, universal screening should be strongly considered prior to rituximab therapy.
The type of serology ordered in screening is important [14]. Comprehensive hepatitis screening should include anti-HBc testing [15] in addition to the usual measurements of HBsAg and anti-HBs. The death of patient 1, who was HBsAg− and anti-HBs+, highlights the utility of anti-HBc testing and the dangers in labeling patients as “resolved” hepatitis B or assuming that anti-HBs is present because of prior immunization. In fact, flares [16, 17] and deaths [18] associated with prior HBsAg negativity and anti-HBc and anti-HBs positivity have been well described [14]. Of concern, only 7.6% (27 of 355) of the patients in our series were tested for anti-HBc.
Although both AASLD and EASL guidelines recommend empiric antiviral therapy with oncologic treatment for any patient with detectable HBsAg, debate continues regarding antiviral prophylaxis in patients who are HBsAg− and anti-HBc+ [15], with recommendations for monitoring of HBV DNA rather than instituting antiviral prophylaxis. The structure, compliance, and outcome of a monitoring strategy for HBV DNA during rituximab therapy in such patients have not been evaluated. A recent prospective analysis of HBV reactivation in patients with diffuse large B-cell lymphoma after rituximab combination chemotherapy suggests that entecavir prophylaxis could be started when serum HBV DNA is detected during monitoring rather than at the start of therapy [19]. In that study, patients who were anti-HBs− and anti-HBc+ underwent monitoring for HBV DNA every month for 2 years. For anti-HBc+ patients with detectable anti-HBs, weekly monitoring of anti-HBs titers occurred, and if levels decreased below a predetermined level then HBV DNA was monitored monthly. Whether such an intensive monitoring program is feasible in terms of availability of laboratory testing, cost, and compliance by patients and clinicians has not been confirmed. If rituximab is given as maintenance therapy, then monitoring may be even longer term. We identified a significant mortality risk, even in patients who were thought to have resolved hepatitis B, and others have shown that the presence of anti-HBs may not be protective [16, 17], although this could be addressed in the future by measuring anti-HBs titers. Thus, there is accumulating evidence that a strategy of active prophylaxis rather than monitoring should be considered for patients with any serological evidence of hepatitis B infection for whom rituximab therapy is indicated.
The low rate of pre-emptive screening that we encountered likely reflects insufficient awareness of the potential mortality associated with hepatitis B reactivation, especially before international guidelines were widely promulgated [20], although over time pre-emptive screening rates have not increased significantly (Fig. 2). Pre-emptive screening can be effective only if the outcomes are recognized and acted upon appropriately. In some patients, the screening outcome will lead to the institution of prophylactic antiviral therapy [21]. A recent randomized, controlled trial demonstrated that prophylactic lamivudine use is superior to therapeutic use, with a lower rate of severe hepatitis (serum ALT >10× the upper limit of normal), 0% versus 36% (p < .001), in that study [9]. More importantly, a recent meta-analysis showed that lamivudine therapy reduces HBV reactivation–related mortality [22].
The prophylactic agent of choice is unclear. Most of the published evidence has involved lamivudine [23], although antiviral resistance is a likely outcome in patients requiring long-term therapy to reach standard treatment endpoints. In the case of the oncological use of rituximab, resistance is less likely with 6–12 months of antiviral therapy. Entecavir has been used with clinical reactivation [24]. A recent retrospective study showed that patients given prophylactic entecavir during chemotherapy and for 6 months after the completion of chemotherapy had significantly lower rates of HBV reactivation and chemotherapy disruption than patients given lamivudine [25]. In our study, only one of the two patients given entecavir survived, which reflects the severity of acute hepatitis in this patient group. Interestingly, the patient who survived recommenced prednisolone, which may have inhibited the immunological mechanisms that lead to hepatocyte death [26, 27], but we do not recommend this as routine practice. Adefovir prophylaxis has been unsuccessful previously [28] and would not be favored because of a relatively slow onset of its antiviral effect. EASL guidelines recommend using entecavir or tenofovir for prophylaxis [7], given their high potency and low resistance rates.
Conclusions
In conclusion, we have shown that failure to adhere to current guidelines can have serious implications for patient outcomes. Timely and appropriate screening for hepatitis B infection and development of a management plan based on those results is essential for optimal patient care. Institution of prophylactic therapy can decrease HBV reactivation rates and the severity of reactivation episodes, and has the potential to decrease mortality [22]. The mortality rate found in our study suggests that active antiviral prophylaxis rather than monitoring should be considered for patients with any serological evidence of hepatitis B infection for whom rituximab therapy is indicated, including patients who are HBsAg− but anti-HBc+ and anti-HBs+. Although our study is limited by its retrospective nature, we have reported a “real world” experience in a large number of patients that provides important information to guide future clinical practice. Ultimately, cooperation among oncologists, haematologists, hepatologists, and infectious disease physicians should drive institutional protocols to ensure that prophylactic screening and treatment is implemented universally. Given that rituximab is used across a broad range of specialties, pharmacy dispensing records could be used as a method of triggering adherence. Regular auditing to ensure adherence to such protocols would be essential in preventing potential mortality.
Acknowledgments
The authors gratefully acknowledge the support of Mr. Ian Larmour from the Southern Health Department of Pharmacy and Dr. Stephen Opat from the Southern Health Department of Haematology in this study.
Previously presented as an oral presentation during Australian Gastroenterology Week, Brisbane, Australia, October 22–25, 2008.
Author Contributions
Conception/Design: Christopher Leung, William Sievert
Provision of study material or patients: Christopher Leung, Edward Tsoi, Gareth Burns, William Sievert
Collection and/or assembly of data: Christopher Leung, Edward Tsoi, Gareth Burns
Data analysis and interpretation: Christopher Leung, Edward Tsoi, Gareth Burns, William Sievert
Manuscript writing: Christopher Leung, William Sievert
Final approval of manuscript: Christopher Leung, Edward Tsoi, Gareth Burns, William Sievert
References
- 1.World Health Organization. Hepatitis B. Fact Sheet No 204. Revised August 2008. [accessed March 19, 2011]. Available at http://www.who.int/mediacentre/factsheets/fs204.
- 2.van der Sande MA, Waight P, Mendy M, et al. Long-term protection against carriage of hepatitis B virus after infant vaccination. J Infect Dis. 2006;193:1528–1535. doi: 10.1086/503433. [DOI] [PubMed] [Google Scholar]
- 3.Maynard JE. Hepatitis B: Global importance and need for control. Vaccine. 1990;(8 suppl):S18–S20. doi: 10.1016/0264-410x(90)90209-5. discussion S21–S23. [DOI] [PubMed] [Google Scholar]
- 4.Zell JA, Yoon EJ, Ignatius Ou SH, et al. Precore mutant hepatitis B reactivation after treatment with CHOP-rituximab. Anticancer Drugs. 2005;16:83–85. doi: 10.1097/00001813-200501000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Bosch F, Abrisqueta P, Villamor N, et al. Rituximab, fludarabine, cyclophosphamide, and mitoxantrone: A new, highly active chemoimmunotherapy regimen for chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4578–4584. doi: 10.1200/JCO.2009.22.0442. [DOI] [PubMed] [Google Scholar]
- 6.Lok AS, McMahon BJ. Chronic hepatitis B: Update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 9.Hsu C, Hsiung CA, Su IJ, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin's lymphoma: A randomized trial. Hepatology. 2008;47:844–853. doi: 10.1002/hep.22106. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. New NCCN Guidelines for Primary Cutaneous B-Cell Lymphoma and Updates to NCCN Guidelines for Non-Hodgkin's Lymphomas. [accessed February 24, 2010]. Available at http://www.nccn.org.
- 11.Zelenetz AD, Abramson JS, Advani RH, et al. NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin's lymphomas. J Natl Compr Canc Netw. 2010;8:288–334. doi: 10.6004/jnccn.2010.0021. [DOI] [PubMed] [Google Scholar]
- 12.Tawk HM, Vickery K, Bisset L, et al. The current pattern of hepatitis B virus infection in Australia. J Viral Hepat. 2006;13:206–215. doi: 10.1111/j.1365-2893.2005.00664.x. [DOI] [PubMed] [Google Scholar]
- 13.Sydney South West Pathology Service (SSWPS) Results. [accessed March 18, 2011]. Available at http://www.sswahs.nsw.gov.au/sswps/default_hb.htm.
- 14.Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27:605–611. doi: 10.1200/JCO.2008.18.0182. [DOI] [PubMed] [Google Scholar]
- 15.Koo YX, Tan DS, Tan BH, et al. Risk of hepatitis B virus reactivation in patients who are hepatitis B surface antigen negative/antibody to hepatitis B core antigen positive and the role of routine antiviral prophylaxis. J Clin Oncol. 2009;27:2570–2571. doi: 10.1200/JCO.2009.21.9352. author reply 2571–2572. [DOI] [PubMed] [Google Scholar]
- 16.Dervite I, Hober D, Morel P. Acute hepatitis B in a patient with antibodies to hepatitis B surface antigen who was receiving rituximab. N Engl J Med. 2001;344:68–69. doi: 10.1056/NEJM200101043440120. [DOI] [PubMed] [Google Scholar]
- 17.Koo YX, Tan DS, Tan IB, et al. Hepatitis B virus reactivation in a patient with resolved hepatitis B virus infection receiving maintenance rituximab for malignant B-cell lymphoma. Ann Intern Med. 2009;150:655–656. doi: 10.7326/0003-4819-150-9-200905050-00024. [DOI] [PubMed] [Google Scholar]
- 18.Sarrecchia C, Cappelli A, Aiello P. HBV reactivation with fatal fulminating hepatitis during rituximab treatment in a subject negative for HBsAg and positive for HBsAb and HBcAb. J Infect Chemother. 2005;11:189–191. doi: 10.1007/s10156-005-0385-z. [DOI] [PubMed] [Google Scholar]
- 19.Niitsu N, Hagiwara Y, Tanae K, et al. Prospective analysis of hepatitis B virus reactivation in patients with diffuse large B-cell lymphoma after rituximab combination chemotherapy. J Clin Oncol. 2010;28:5097–5100. doi: 10.1200/JCO.2010.29.7531. [DOI] [PubMed] [Google Scholar]
- 20.Loomba R, Rowley A, Wesley R, et al. Systematic review: The effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519–528. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz LH, Fraser A, Gafter-Gvili A, et al. Lamivudine prevents reactivation of hepatitis B and reduces mortality in immunosuppressed patients: Systematic review and meta-analysis. J Viral Hepat. 2008;15:89–102. doi: 10.1111/j.1365-2893.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- 22.Martyak LA, Taqavi E, Saab S. Lamivudine prophylaxis is effective in reducing hepatitis B reactivation and reactivation-related mortality in chemotherapy patients: A meta-analysis. Liver Int. 2008;28:28–38. doi: 10.1111/j.1478-3231.2007.01618.x. [DOI] [PubMed] [Google Scholar]
- 23.Yeo W, Chan PK, Ho WM, et al. Lamivudine for the prevention of hepatitis B virus reactivation in hepatitis B s-antigen seropositive cancer patients undergoing cytotoxic chemotherapy. J Clin Oncol. 2004;22:927–934. doi: 10.1200/JCO.2004.05.161. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Rodriguez MJ, Canales MA, Hernandez-Maraver D, et al. Late reactivation of resolved hepatitis B virus infection: An increasing complication post rituximab-based regimens treatment? Am J Hematol. 2008;83:673–675. doi: 10.1002/ajh.21214. [DOI] [PubMed] [Google Scholar]
- 25.Li HR, Huang JJ, Guo HQ, et al. Comparison of entecavir and lamivudine in preventing hepatitis B reactivation in lymphoma patients during chemotherapy. J Viral Hepat. 2010 Nov 5; doi: 10.1111/j.1365-2893.2010.01386.x. doi: 10.1111/j.1365-2893.2010.01386.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Cheng AL. Steroid-free chemotherapy decreases the risk of hepatitis flare-up in hepatitis B virus carriers with non-Hodgkin's lymphoma. Blood. 1996;87:1202. [PubMed] [Google Scholar]
- 27.Kanda T, Yokosuka O, Imazeki F, et al. Corticosteroids and lamivudine combined to treat acute severe flare-up in a chronic hepatitis B and C patient. J Gastroenterol Hepatol. 2004;19:238–239. doi: 10.1111/j.1440-1746.2004.03269.x. [DOI] [PubMed] [Google Scholar]
- 28.Khaled Y, Hanbali A. Hepatitis B virus reactivation in a case of Waldenstrom's macroglobulinemia treated with chemotherapy and rituximab despite adefovir prophylaxis. Am J Hematol. 2007;82:688. doi: 10.1002/ajh.20861. [DOI] [PubMed] [Google Scholar]