Subjective and objective assessments were used to describe the natural history of oxaliplatin-induced neuropathy. Findings establish the persistence of subjective and objective deficits in oxaliplatin-treated patients post-oxaliplatin, suggesting that sensory neuropathy is a long-term outcome, thereby challenging the literature on the reversibility of oxaliplatin-induced neuropathy.
Keywords: Oxaliplatin, Neuropathy, Long-term follow-up, Reversibility, Persistence
Learning Objectives
After completing this course, the reader will be able to:
Define the symptoms of sensory neurotoxicity in oxaliplatin-treated patients and identify the long-term natural history of nerve dysfunction as a long-lasting complication of treatment that does not necessarily resolve within 6 months.
Use sensory excitability techniques to predict long-standing changes in sensory nerve function produced by oxaliplatin.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Objectives.
Oxaliplatin-induced neuropathy is a significant and dose-limiting toxicity that adversely affects quality of life. However, the long-term neurological sequelae have not been adequately described. The present study aimed to describe the natural history of oxaliplatin-induced neuropathy, using subjective and objective assessments.
Methods.
From a population of 108 oxaliplatin-treated patients referred for neurological assessment in 2002–2008, 52.2% of the surviving patient cohort (n = 24) was available for follow-up at a median of 25 months post-oxaliplatin. Patients underwent a protocol that incorporated clinical assessment scales, patient questionnaires, standard electrodiagnostic assessments, and novel nerve excitability studies to precisely assess nerve function.
Results.
At follow-up, 79.2% of patients reported residual neuropathic symptoms, with distal loss of pin-prick sensibility in 58.3% of patients and loss of vibration sensibility in 83.3% of patients. Symptom severity scores were significantly correlated with cumulative dose. There was no recovery of sensory action potential amplitudes in upper and lower limbs, consistent with persistent axonal sensory neuropathy. Sensory excitability parameters had not returned to baseline levels, suggesting persisting abnormalities in nerve function. The extent of excitability abnormalities during treatment was significantly correlated with clinical outcomes at follow-up.
Conclusions.
These findings establish the persistence of subjective and objective deficits in oxaliplatin-treated patients post-oxaliplatin, suggesting that sensory neuropathy is a long-term outcome, thereby challenging the literature on the reversibility of oxaliplatin-induced neuropathy.
Introduction
Oxaliplatin, a third-generation platinum-based chemotherapy, is now a central component of the chemotherapeutic treatment approach to colorectal cancer [1–3]. However, dose-limiting neurotoxicity resulting from oxaliplatin treatment is a prominent feature, with both acute and chronic manifestations. Whereas acute neuropathic symptoms typically resolve within a week [4–8], with increasing cumulative dose, severe chronic sensory neuropathy develops in 20%–50% of patients [1, 3], characterized by distal paresthesia and numbness, leading to functional disability. Because colorectal cancer survivors constitute the third largest group of cancer survivors [9], the implications of persistent long-term nerve damage imposes unacceptable burdens on quality of life in survivorship.
There is limited information available about the natural history of neuropathy in oxaliplatin-treated patients. Although it has been reported that oxaliplatin-induced neurotoxicity is reversible upon treatment cessation [1, 3, 5], other reports suggest that oxaliplatin-induced neurotoxicity may be long lasting [6, 10, 11]. Recently, axonal excitability studies [12, 13] provided evidence for striking progressive changes in peripheral nerve function that developed across oxaliplatin treatment, predicting the development of neuropathy [6, 8, 14, 15]. In the present study, prospective and longitudinal assessments of oxaliplatin-treated patients were undertaken to determine the extent to which oxaliplatin treatment induces long-standing alterations in peripheral nerve function.
Materials and Methods
Study Design
Clinical assessment, grading scales, nerve conduction studies, and axonal excitability studies were prospectively undertaken in a series of oxaliplatin-treated patients consecutively referred from the Department of Medical Oncology, Prince of Wales Hospital. The study was approved by the South Eastern Sydney Area Health Service (Eastern Section) Human Research Ethics Committee and University of New South Wales Human Research Ethics Committee. Participants provided written informed consent in accordance with the Declaration of Helsinki. Patients were excluded from the study if they received other neurotoxic chemotherapy, had an Eastern Cooperative Oncology Group performance status score >2, or had pre-existing neuropathic symptoms or baseline abnormalities in nerve function.
Patients received standard oxaliplatin-containing treatment regimens. Oxaliplatin was administered i.v. over 2 hours at an initial dose of 85 mg/m2 (with the 5-fluorouracil, leucovorin, and oxaliplatin [FOLFOX]4 regimen) [3], 100 mg/m2 (with the FOLFOX6 regimen) [16], or 130 mg/m2 (with the capecitabine plus oxaliplatin [XELOX] regimen) [17]. Nerve conduction studies were completed by a neurologist who was blinded to the clinical status of patients. Clinical assessment was undertaken by oncologists using the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI CTCAE) scale (version 3) and who were blinded to the results of the electrophysiological investigations. Nerve excitability and questionnaire assessments were completed by trained investigators.
Study Time Course
Patients were assessed at the following time points: baseline prior to the initial oxaliplatin administration (pre-oxaliplatin, n = 24), within 28 days after the final oxaliplatin treatment (post-oxaliplatin; n = 21), and at a long-term follow-up (median, 25 months; n = 24). A subset of patients (n = 8) was assessed during oxaliplatin treatment at a median of treatment cycle 5. Patients were included in the follow-up study if their final oxaliplatin treatment occurred >10 months before the study census date. Follow-up assessment was completed 10–67 months following patients' final oxaliplatin treatment. In order to avoid assessment of the acute neurotoxic effects of oxaliplatin treatment, all assessments were completed at least 14 days following acute oxaliplatin infusion.
Clinical Assessment: Grading of Neurotoxicity
A clinical examination was performed that included a neurological history incorporating sensory, motor, and autonomic symptoms, evaluation of deep tendon reflexes, evaluation of muscle strength, and evaluation of sensory function, including pin-prick (Neurotip™ disposable needle; Owen Mumford Ltd., Oxford, U.K.) and vibration (128-Hz tuning fork) sensibility in the upper and lower limbs. Clinical data were classified according to the Total Neuropathy Scale (TNS) [18, 19] using the clinical version (TNSc) [20] and the reduced version (TNSr), incorporating neurophysiological measures [21] with severity rankings of 0 (none) to 4 (very severe), as previously validated in patients with chemotherapy-induced neuropathy [21, 22].
Patients' descriptions of symptoms were staged using the Neuropathy Symptom Score (NSS) into negative (subset IIA) and positive (subset IIB) sensory symptoms summed to give a composite score out of 5 [23]. The Neuropathy Sensory Subscale of the NCI CTCAE scale (version 3) was used to classify patients into neurotoxicity grades [24]: grade 1, mild; grade 2, moderate; and grade 3, severe. The European Organization for Research and Treatment of Cancer Chemotherapy Induced Peripheral Neuropathy module was used to assess significance of patient symptoms [25], incorporating questions regarding neuropathic symptoms and functional deficits, with results presented as the percentage of patients reporting the presence of a particular symptom.
Neurophysiological assessment of nerve function was undertaken using conventional nerve conduction studies [14, 26] and axonal excitability studies [12, 27]. A Medelec Synergy system (Oxford Instruments, Oxfordshire, U.K.) was used for nerve conduction studies of upper and lower limb nerves. Nerve amplitudes were normalized to baseline values or expressed as a percentage of normal age-matched values [26, 28]. Sensory axonal excitability studies were undertaken using specialized software (Qtrac©; Institute of Neurology, Queen Square, U.K.). The median nerve was stimulated at the wrist with a reference electrode placed 10 cm distal over bone and compound sensory action potentials (CSAPs) were recorded from the second digit. Using established protocols [12, 27], multiple excitability parameters were recorded, as in prior studies of oxaliplatin-induced neurotoxicity [8, 15], as indirect measures of resting axonal membrane potential. The recovery of excitability following impulse conduction, marking the function of voltage-gated Na+ channels, was assessed with the parameters refractoriness and superexcitability, calculated as the percent change in threshold 2.5 msec and 7 msec after supramaximal stimulation [12, 29, 30].
Statistical Analysis
To ensure comparability of the examined patient cohort, patient demographics and characteristics were compared with those of a group of oxaliplatin-treated patients who were not available for long-term follow-up (n = 22) using Mann-Whitney U tests (two-tailed). Symptom severity scores and clinical and nerve function parameters were correlated within individual patients with Spearman rank correlation coefficients. To determine the relationship between excitability measures during oxaliplatin treatment and follow-up measures, a sum score of composite excitability was determined by combining the measures refractoriness, superexcitability, and threshold electrotonus [8, 15] and was correlated within individual patients' clinical neurotoxicity scores (TNSc and TNSr). To compare assessments across multiple time points (pre-oxaliplatin, post-oxaliplatin, and follow-up), assessments were paired and compared within patients using Wilcoxon signed rank tests (two-tailed). Results are expressed as the mean ± standard error of the mean (SEM) or, alternatively, as the median and interquartile range (IQR) as a measure of variability [15]. Significance was defined as p ≤ .05. All statistics were performed in SPSS (version 18; SPSS, Inc., Chicago, IL).
Results
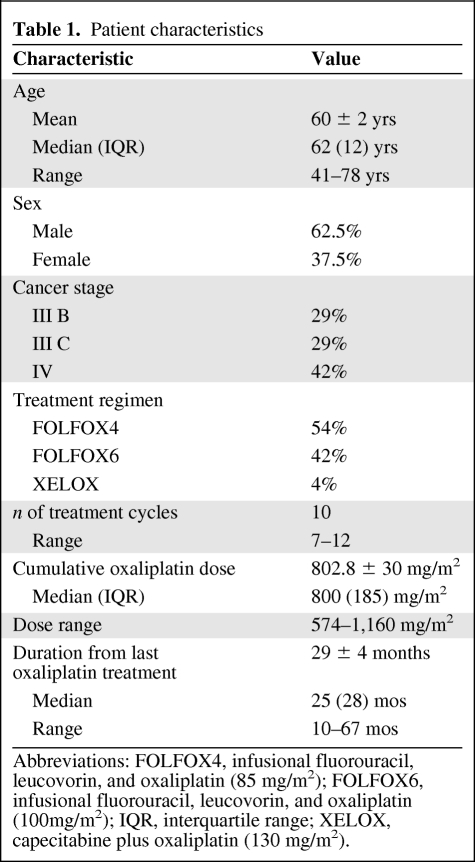
Patient Characteristics
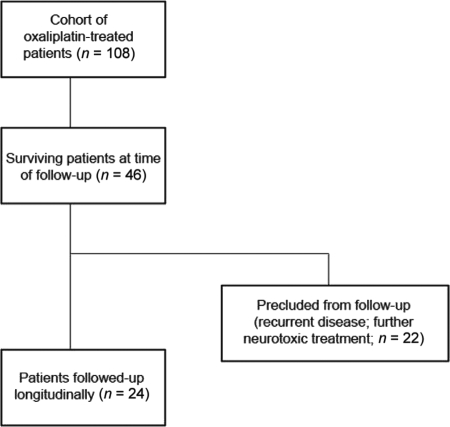
Between September 2002 and December 2008, in total, 108 oxaliplatin-treated patients were consecutively referred from the Department of Medical Oncology, Prince of Wales Hospital for nerve studies and underwent a total of 1,647 neurophysiological assessments (Fig. 1). Of this cohort, 7% declined full participation in the study. At the time of follow-up, 46 of 108 patients were alive (42.6%). Of this cohort, 24 patients (52.2%) were assessed after 2 years of follow-up: median, 25 months; IQR, 28 months. Patient characteristics are shown in Table 1. These patients underwent at total of 560 neurophysiological assessments. The remaining patients were precluded from follow-up because of reasons that included recurrent disease or additional neurotoxic treatment.
Figure 1.
Flow diagram of patient inclusion in the study. From a total cohort of 108 oxaliplatin-treated patients referred for nerve studies, 46 patients were alive at the time of follow-up. Of these, 24 patients were available for follow-up and assessed at a mean of 2.5 years after oxaliplatin treatment.
Table 1.
Patient characteristics
Abbreviations: FOLFOX4, infusional fluorouracil, leucovorin, and oxaliplatin (85 mg/m2); FOLFOX6, infusional fluorouracil, leucovorin, and oxaliplatin (100mg/m2); IQR, interquartile range; XELOX, capecitabine plus oxaliplatin (130 mg/m2).
To ensure comparability of the examined patient cohort, individuals in the remaining patient cohort who were treated at the same center and were precluded from follow-up (n = 22) were compared with those recruited for the present study. There were no significant differences between groups, expressed in terms of the median (IQR) as follows—age: recruited cohort, 62 (12) years; nonrecruited cohort, 58 (9) years (p = .34); oxaliplatin dose level: initial dose for recruited cohort, 85 (15) mg/m2; initial dose for nonrecruited cohort, 100 (15) mg/m2 (p = .15); cumulative dose for recruited cohort, 800 (185) mg/m2; cumulative dose for nonrecruited cohort, 850 (224) mg/m2 (p = .86); time since final oxaliplatin treatment: recruited cohort, 25 (28) months; nonrecruited cohort, 22 (14) months (p = .99); and maximal neurotoxicity: maximum NCI grade in recruited cohort, 2 (2); maximum NCI grade in nonrecruited cohort: 2 (1) (p = .13)—suggesting that the examined patient cohort was representative of the oxaliplatin-treated population at this center.
Development of Neuropathy
While undergoing oxaliplatin treatment, the majority of patients (95.8%) reported acute neuropathic symptoms, including cold-triggered paresthesia and dysesthesia, muscle fasciculations, and cramps. Significantly, 30.4% of patients experienced an oxaliplatin dose reduction because of the severity of persisting neuropathic symptoms and 33.3% of patients ceased oxaliplatin treatment prematurely because of neurotoxicity (on average, 2.4 ± 0.4 treatment cycles were stopped prematurely).
Similar to other platinum-based chemotherapies, patients treated with oxaliplatin may develop worsening neuropathic symptoms after treatment has been ceased, a process termed the “coasting” phenomenon. Overall, 25% of patients reported worsening of neuropathic symptoms following completion of oxaliplatin treatment in the present cohort. During treatment, 29.2% of patients experienced a maximum rank of mild neurotoxicity (grade 1), 41.6% of patients experienced moderate neurotoxicity (grade 2), and 29.2% experienced severe neurotoxicity (grade 3).
Assessment of Peripheral Neuropathy at Long-Term Follow-Up
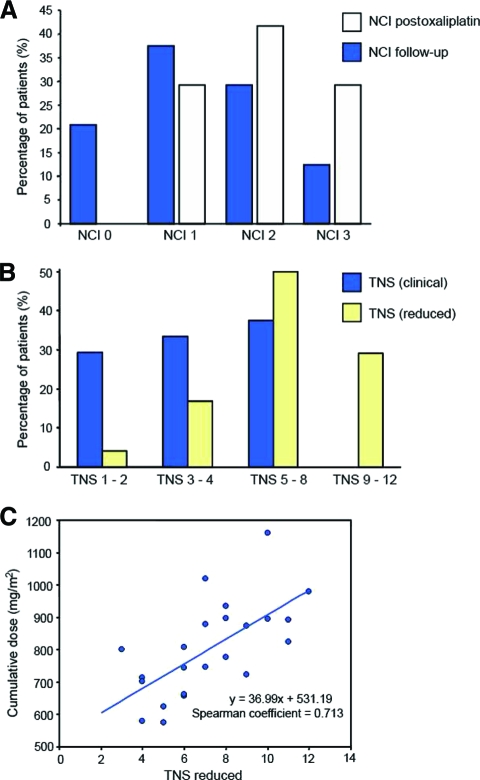
At the time of follow-up (29 ± 4 months post-oxaliplatin), the majority of patients reported persistent neuropathic symptoms (79.2%). Residual sensory neuropathic symptoms in the upper limbs were reported by 45.8% of patients and 79.2% of patients reported residual symptoms in the lower limbs, primarily numbness in the extremities. All symptomatic patients reported numbness as the primary symptom. At follow-up, 20.8% of patients were classified with no neurotoxicity (grade 0), 37.5% were classified with mild neurotoxicity (grade 1), 29.2% were classified with moderate neurotoxicity (grade 2), and 12.5% were classified with severe neurotoxicity (grade 3) (Fig. 2A). The NSS was assessed in all patients at follow-up, with the most patients demonstrating a score of 1, reflecting the primary symptom of numbness (NSS 0, 20.8%; NSS 1, 41.7%; NSS 2, 33.3%; and NSS 3, 4.2%).
Figure 2.
Persistence of neuropathy at long-term follow-up. (A): NCI neuropathy severity grade displayed post-oxaliplatin and at the time of follow-up, depicting the percentage of patients characterized with NCI grade 0 (post-oxaliplatin, 0%; follow-up, 20.8%), NCI grade 1 (post-oxaliplatin, 29.2%; follow-up, 37.5%), NCI grade 2 (post-oxaliplatin, 41.6%; follow-up, 29.2%), and NCI grade 3 (post-oxaliplatin, 29.2%: follow-up, 12.5%). (B): TNS at the time of follow-up (TNS clinical version, blue; TNS reduced version, yellow). (C): Plot of the association of oxaliplatin cumulative dose (mg/m2) with neuropathic severity as assessed by the TNS (reduced) (correlation coefficient, 0.704; p = .0005).
Abbreviations: NCI, National Cancer Institute; TNS, Total Neuropathy Scale.
Examination revealed distal loss of pin-prick sensibility in 58.3% of patients and loss of vibration sensibility in 83.3% of patients, with reduced or absent ankle reflexes in 79.2% of patients. Clinical features were summed into the TNSc, and 37.5% patients had a TNSc ≥5 (Fig. 2B). When neurophysiological recordings were added, 29.2% patients had a TNSr score of 9–12, indicating the presence of significant objective and subjective signs of neuropathy (Fig. 2B). Cumulative dose was an important predictor of the development of neuropathy, and symptom severity scores were significantly correlated with cumulative oxaliplatin dose at follow-up—correlation coefficients: TNSc, 0.658 (p = .001); TNSr, 0.704; (p = .0005); NCI grade, 0.603 (p = .002); and NSS, 0.453 (p = .03)—illustrating the dose dependence of oxaliplatin-induced neuropathy (Fig. 2C).
The majority of patients (66.7%) experienced minor, incomplete improvement in neuropathic symptoms during the follow-up period, mostly by one severity grade. However, 33.3% of patients did not experience any improvement and a further 18.8% experienced improvement only in the upper limbs while the lower limb symptoms remained unabated. Of note, at the time of follow-up, 41.7% of patients reported persistent functional difficulties with fine motor skills or walking balance. Importantly, >40% of patients were still experiencing significant functional difficulties with daily tasks, including walking problems and fine motor deficits, which will inevitably impinge on the lifestyle of cancer survivors.
Objective Measures of Nerve Function
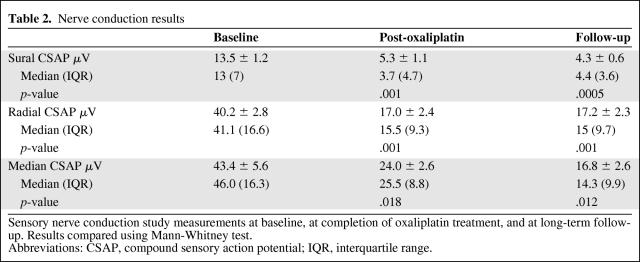
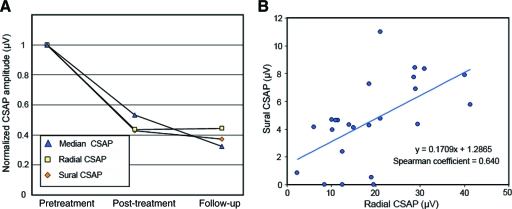
Neurophysiological assessment revealed persistent, significant reductions in peak sensory amplitudes in both the upper and lower limbs (Table 2). There was no significant improvement in sensory amplitudes from completion of oxaliplatin treatment to the time of follow-up. Sural amplitudes were reduced by 21%–100% at the time of follow-up, with a mean reduction of 65.7% ± 6% from baseline, whereas upper limb radial amplitudes were reduced by a mean of 55.7% ± 6% and median amplitudes were reduced by 67.4% ± 7.4% from baseline (Fig. 3A). Conduction velocity and motor amplitudes were relatively preserved, consistent with an axonal sensory neuropathy.
Table 2.
Nerve conduction results
Sensory nerve conduction study measurements at baseline, at completion of oxaliplatin treatment, and at long-term follow-up. Results compared using Mann-Whitney test.
Abbreviations: CSAP, compound sensory action potential; IQR, interquartile range.
Figure 3.
Nerve conduction findings across oxaliplatin treatment. (A): Change in compound sensory action potential (CSAP) peak amplitude immediately following oxaliplatin treatment and at follow-up, normalized to baseline recordings, for median (triangle), radial (square), and sural (diamond) sensory nerves. (B): Relationship of lower limb sural and upper limb radial amplitudes at follow-up, demonstrating symmetrical upper and lower limb presentation (correlation coefficient, .617; p = .001).
Sural amplitudes were significantly correlated with radial amplitudes at follow-up, confirming symmetrical upper and lower limb presentation (correlation coefficient, 0.617; p = .001) (Fig. 3B). In addition, there were no significant differences in the degree of reduction in upper and lower limb amplitudes at follow-up—sural, 0.34 ± .06; radial, 0.44 ± .06 (p = .18); median, 0.30 ± .07 (p = .86)—confirming that oxaliplatin-induced neuropathy was not lower-limb predominant.
In order to assess axonal function and membrane potential in surviving axons, sensory axonal excitability studies were followed longitudinally. By completion of oxaliplatin treatment, a suite of sensory excitability changes developed, consistent with global axonal dysfunction [8, 15]. The most sensitive measures in predicting oxaliplatin-induced neurotoxicity were assessed at follow-up. In 25% of patients, axonal excitability studies could not be undertaken at follow-up because of the severity of peripheral neuropathy and the low amplitude of CSAPs.
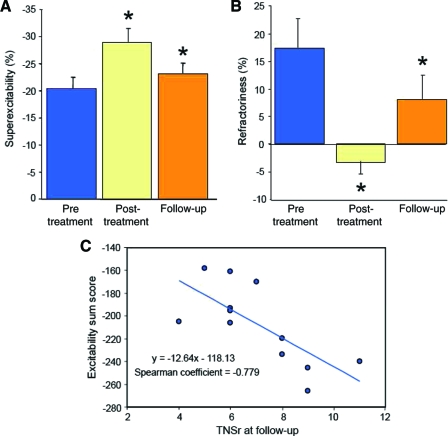
By the time of follow-up, the stimulus threshold and latency had normalized to baseline levels, suggesting that surviving axons were conducting normally (presented as median (IQR), mean ± SEM; Threshold follow-up: 4.4 (1.5) mA; 4.4 ± 0.3 mA; pre-oxaliplatin: 5.9 (3.7) mA; 6.1 ± 1.0 mA; p = .008; Latency follow-up: 3.9 (0.9) ms; 4.1 ± 0.2 ms; pre-oxaliplatin: 3.8 (0.9) ms; 3.8 ± 0.2 ms; p = .004). However, deficits persisted in the recovery cycle parameters superexcitability (Fig. 4A) and refractoriness (Fig. 4B), suggesting that residual abnormalities remained in the function of sensory nerves (presented as median (IQR), mean ± SEM; superexcitability pre-oxaliplatin: −19.6% (9.4%); −20.4% ± 2.1%; post-oxaliplatin: −31.3% (11.3%); −28.9% ± 2.6%; p = .028; follow-up: −20.9% (6.1%); −23.1 ± 2.0%; p = .05; Refractoriness pre-oxaliplatin: 17.2% (23.2%); 17.4% ± 5.3%; post-oxaliplatin: −2.4% (9.3%); −3.2 ± 2.4%; p = .036; follow-up: 8.1% (18.8%); 8.1% ± 4.4%; p = .05).
Figure 4.
Sensory excitability values pre-oxaliplatin treatment (blue), post-oxaliplatin treatment (yellow), and at follow-up (orange). (A): Superexcitability at pre-oxaliplatin, post-oxaliplatin (pre-oxaliplatin versus post-oxaliplatin, p = .028), and follow-up (pre-oxaliplatin versus follow-up, p = .05). (B): Refractoriness at pre-oxaliplatin, post-oxaliplatin (pre-oxaliplatin versus post-oxaliplatin, p =.036), and follow-up (pre-oxaliplatin versus follow-up, p = .05). (C): Summed excitability change during treatment correlated with final clinical neurological outcome (Total Neuropathy Scale reduced [TNSr]) at long-term follow-up (correlation coefficient, −0.779; p = .003).
Critically, when excitability recordings obtained from patients during oxaliplatin treatment were compared with neurological outcome at follow-up, there were significant correlations between excitability parameters during treatment and severity of neuropathy at follow-up (Fig. 4C). Specifically, the composite sum of the change in excitability parameters during oxaliplatin treatment was significantly correlated with TNSr at follow-up (correlation coefficient, −0.779; p = .003). Patients with the greatest change in excitability during treatment demonstrated the most severe neuropathy at follow-up, indicating that sensory axonal excitability techniques provide a predictive measure of clinical severity of neurotoxicity at long-term follow-up.
To determine the relationship between early changes in excitability and final outcome, the excitability parameter previously identified as the earliest marker of excitability change [15], superexcitability, as assessed in early treatment, was compared with the final neurological outcome. A subset of patients (n = 8) was assessed at a median of cycle 5 (or after 2.5 months of 6 months of oxaliplatin treatment). Importantly, the extent of superexcitability change by cycle 5 was significantly correlated with TNSr at follow-up (correlation coefficient, 0.830; p = .011), suggesting that sensory excitability techniques provide an early predictor of clinical outcome at long-term follow-up.
Discussion
The present study provides a prospective, comprehensive, neurological assessment of long-term outcomes in WD: ox-aliplatin-treated patients, indicating that oxaliplatin produces persistent and long-lasting neuropathy. Contrary to previous reports [1, 3, 5], oxaliplatin-induced neurotoxicity was not reversible within 6 months, suggesting that clinician-based grading scales underreport the level of chronic neurotoxicity. Significant neuropathic symptoms and objective signs of neuropathy remained in most patients, with severe neurotoxicity in a substantial proportion. Importantly, the extent of sensory axonal excitability dysfunction as assessed early during oxaliplatin treatment, prior to clinical detection, was predictive of the degree of neurotoxicity at long-term follow-up, suggesting that clinical excitability measures provide a sensitive measure of neurotoxicity in oxaliplatin-treated patients.
Prior to interpretation of the present findings, the limitations of the study are acknowledged. Although the study was conducted at a single center and the small sample size may limit generalization of the results to a wider clinical population, there were no significant differences identified between patients recruited for the study and those not recruited from the same center. Although quality of life was not formally assessed in the present cohort, patient questionnaires detailing functional disability in daily tasks were undertaken. In addition, significant objective neurophysiological dysfunction was identified in the present study, providing detailed assessment and insight into patient impairment.
Sensory neurotoxicity has long been recognized as the major dose-limiting toxicity of oxaliplatin treatment [3, 5, 31]. The present study confirms that persistent peripheral neuropathy is a common occurrence following oxaliplatin treatment, with 79.2% of patients with residual neuropathic symptoms at the time of follow-up. Grading of chemotherapy-induced neuropathy is problematic, with the lack of standardization and ambiguity in measurement contributing to underestimation of neuropathy incidence and severity [32, 33]. Using clinical grading scales, oxaliplatin-induced neuropathy has been characterized as completely reversible within 6 months [3, 5, 31], with the majority of patients experiencing improvement or recovery within 1 month [1, 2].
Previous studies have highlighted the discrepancy between clinician and patient grading of neuropathic symptoms, with self-report of severe neuropathic symptoms persisting in 32% of patients in the lower limbs and in 22% of patients in the upper limbs 12 months post-oxaliplatin treatment but clinician-scored NCI grades registering only 5.2% of patients as having moderate to severe neurotoxicity in the same cohort [10]. Objective measures of nerve function reveal further lasting deficits in sensory nerves [11, 14, 34], suggesting that, rather than recovery, patients undergo adaptation to chronic symptoms [35].
Importantly, sensory axonal excitability studies obtained during treatment were significantly correlated with neurological outcome on long-term follow-up, suggesting that these techniques provide early markers of the risk for developing long-term neurological sequelae following oxaliplatin treatment. Excitability studies performed during follow-up also demonstrated persistent changes in sensory nerve function, suggesting that the changes that develop during oxaliplatin treatment contribute to the final severity of neuropathy.
With the increasing use of oxaliplatin in the adjuvant treatment of colorectal cancer patients, it is important to determine the long-term prognosis of oxaliplatin-induced neuropathy to provide patients and clinicians with accurate information regarding the natural history of oxaliplatin-induced neuropathy. The present study demonstrates that oxaliplatin-induced nerve damage does not resolve at long-term follow-up, with significant clinical and neurophysiological deficits persisting in the majority of patients at a follow-up of 2 years. Importantly, the lack of recovery following oxaliplatin treatment indicates that dose-limiting strategies are needed unless preventative neuroprotection becomes a successful strategy, making the search for a neuroprotective agent critical. The development of a prognostic marker of long-term neurotoxicity would enable patients at risk for severe neurological sequelae to be identified early, potentially enabling patient-specific dosing strategies and facilitating the assessment of future neuroprotective strategies.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (Project grant 400938; 570233), the Sydney Foundation for Medical Research, and an Australian Postgraduate Award (S.B.P.).
Author Contributions
Conception/Design: Susanna B. Park, Cindy S.Y. Lin, Arun V. Krishnan, David Goldstein, Michael L. Friedlander, Matthew C. Kiernan
Provision of study material or patients: David Goldstein, Michael L. Friedlander, Matthew C. Kiernan
Collection and/or assembly of data: Susanna B. Park, Cindy S.Y. Lin, Arun V. Krishnan
Data analysis and interpretation: Susanna B. Park, Cindy S.Y. Lin, Arun V. Krishnan, Matthew C. Kiernan
Manuscript writing: Susanna B. Park, Cindy S.Y. Lin, Arun V. Krishnan, David Goldstein, Michael L. Friedlander, Matthew C. Kiernan
Final approval of manuscript: Susanna B. Park, Cindy S.Y. Lin, Arun V. Krishnan, David Goldstein, Michael L. Friedlander, Matthew C. Kiernan
References
- 1.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 2.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 3.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 4.Gamelin E, Gamelin L, Bossi L, et al. Clinical aspects and molecular basis of oxaliplatin neurotoxicity: Current management and development of preventive measures. Semin Oncol. 2002;29(5 suppl 15):21–33. doi: 10.1053/sonc.2002.35525. [DOI] [PubMed] [Google Scholar]
- 5.Grothey A. Oxaliplatin-safety profile: Neurotoxicity. Semin Oncol. 2003;30(4 suppl 15):5–13. doi: 10.1016/s0093-7754(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan AV, Goldstein D, Friedlander M, et al. Oxaliplatin and axonal Na+ channel function in vivo. Clin Cancer Res. 2006;12:4481–4484. doi: 10.1158/1078-0432.CCR-06-0694. [DOI] [PubMed] [Google Scholar]
- 7.Lehky TJ, Leonard GD, Wilson RH, et al. Oxaliplatin-induced neurotoxicity: Acute hyperexcitability and chronic neuropathy. Muscle Nerve. 2004;29:387–392. doi: 10.1002/mus.10559. [DOI] [PubMed] [Google Scholar]
- 8.Park SB, Goldstein D, Lin CSY, et al. Acute abnormalities of sensory nerve function associated with oxaliplatin-induced neurotoxicity. J Clin Oncol. 2009;27:1243–1249. doi: 10.1200/JCO.2008.19.3425. [DOI] [PubMed] [Google Scholar]
- 9.Ganz PA. Why and how to study the fate of cancer survivors: Observations from the clinic and the research laboratory. Eur J Cancer. 2003;39:2136–2141. doi: 10.1016/s0959-8049(03)00489-1. [DOI] [PubMed] [Google Scholar]
- 10.Land SR, Kopec JA, Cecchini RS, et al. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol. 2007;25:2205–2211. doi: 10.1200/JCO.2006.08.6652. [DOI] [PubMed] [Google Scholar]
- 11.Pietrangeli A, Leandri M, Terzoli E, et al. Persistence of high-dose oxaliplatin-induced neuropathy at long-term follow-up. Eur Neurol. 2006;56:13–16. doi: 10.1159/000094376. [DOI] [PubMed] [Google Scholar]
- 12.Kiernan MC, Burke D, Andersen KV, et al. Multiple measures of axonal excitability: A new approach in clinical testing. Muscle Nerve. 2000;23:399–409. doi: 10.1002/(sici)1097-4598(200003)23:3<399::aid-mus12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan AV, Lin CSY, Park SB, et al. Axonal ion channels from bench to bedside: A translational neuroscience perspective. Prog Neurobiol. 2009;89:288–313. doi: 10.1016/j.pneurobio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan AV, Goldstein D, Friedlander M, et al. Oxaliplatin-induced neurotoxicity and the development of neuropathy. Muscle Nerve. 2005;32:51–60. doi: 10.1002/mus.20340. [DOI] [PubMed] [Google Scholar]
- 15.Park SB, Lin CSY, Krishnan AV, et al. Oxaliplatin-induced neurotoxicity: Changes in axonal excitability precede development of neuropathy. Brain. 2009;132:2712–2723. doi: 10.1093/brain/awp219. [DOI] [PubMed] [Google Scholar]
- 16.Maindrault-Goebel F, Louvet C, André T, et al. Oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX6). GERCOR. Eur J Cancer. 1999;35:1338–1342. doi: 10.1016/s0959-8049(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 17.Cassidy J, Tabernero J, Twelves C, et al. XELOX (capecitabine plus oxaliplatin): Active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol. 2004;22:2084–2091. doi: 10.1200/JCO.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhry V, Cornblath DR, Corse A, et al. Thalidomide-induced neuropathy. Neurology. 2002;59:1872–1875. doi: 10.1212/01.wnl.0000037480.59194.85. [DOI] [PubMed] [Google Scholar]
- 19.Cornblath DR, Chaudhry V, Carter K, et al. Total neuropathy score: Validation and reliability study. Neurology. 1999;53:1660–1664. doi: 10.1212/wnl.53.8.1660. [DOI] [PubMed] [Google Scholar]
- 20.Cavaletti G, Jann S, Pace A, et al. Multi-center assessment of the Total Neuropathy Score for chemotherapy-induced peripheral neurotoxicity. J Peripher Nerv Syst. 2006;11:135–141. doi: 10.1111/j.1085-9489.2006.00078.x. [DOI] [PubMed] [Google Scholar]
- 21.Cavaletti G, Bogliun G, Marzorati L, et al. Grading of chemotherapy-induced peripheral neurotoxicity using the Total Neuropathy Scale. Neurology. 2003;61:1297–1300. doi: 10.1212/01.wnl.0000092015.03923.19. [DOI] [PubMed] [Google Scholar]
- 22.Cavaletti G, Frigeni B, Lanzani F, et al. The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: Comparison with the National Cancer Institute-Common Toxicity Scale. J Peripher Nerv Syst. 2007;12:210–215. doi: 10.1111/j.1529-8027.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- 23.Dyck P, Karnes JL, O'Brien PC, et al. The Rochester Diabetic Neuropathy Study: Reassessment of tests and criteria for diagnosis and staged severity. Neurology. 1992;42:1164–1170. doi: 10.1212/wnl.42.6.1164. [DOI] [PubMed] [Google Scholar]
- 24.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 25.Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur J Cancer. 2005;41:1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Kimura J. Philadelphia: F.A. Davis; 1983. Electrodiagnosis in diseases of nerve and muscle; pp. 1–951. [Google Scholar]
- 27.Kiernan MC, Lin CSY, Andersen KV, et al. Clinical evaluation of excitability measures in sensory nerve. Muscle Nerve. 2001;24:883–892. doi: 10.1002/mus.1085. [DOI] [PubMed] [Google Scholar]
- 28.Burke D, Skuse NF, Lethlean AK. Sensory conduction of the sural nerve in polyneuropathy. J Neurol Neurosurg Psychiatry. 1974;37:647–652. doi: 10.1136/jnnp.37.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bostock H, Cikurel K, Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve. 1998;21:137–158. doi: 10.1002/(sici)1097-4598(199802)21:2<137::aid-mus1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 30.Burke D, Kiernan MC, Bostock H. Excitability of human axons. Clin Neurophysiol. 2001;112:1575–1585. doi: 10.1016/s1388-2457(01)00595-8. [DOI] [PubMed] [Google Scholar]
- 31.Raymond E, Faivre S, Woynarowski JM, et al. Oxaliplatin: Mechanism of action and antineoplastic activity. Semin Oncol. 1998;25(2 suppl 5):4–12. [PubMed] [Google Scholar]
- 32.Postma TJ, Heimans JJ, Muller MJ, et al. Pitfalls in grading severity of chemotherapy-induced peripheral neuropathy. Ann Oncol. 1998;9:739–744. doi: 10.1023/a:1008344507482. [DOI] [PubMed] [Google Scholar]
- 33.Stubblefield MD, Burstein HJ, Burton AW, et al. NCCN task force report: Management of neuropathy in cancer. J Natl Compr Canc Netw. 2009;7(suppl 5):S1–S28. doi: 10.6004/jnccn.2009.0078. [DOI] [PubMed] [Google Scholar]
- 34.Brouwers EE, Huitema AD, Boogerd W, et al. Persistent neuropathy after treatment with cisplatin and oxaliplatin. Acta Oncol. 2009;48:832–841. doi: 10.1080/02841860902806609. [DOI] [PubMed] [Google Scholar]
- 35.Bennett B, Goldstein D, Friedlander ML, et al. Oxaliplatin-induced peripheral neuropathy: A qualitative investigation. Asia-Pacific J Clin Oncol. 2009;5(suppl 2):A229. [Google Scholar]