Data from the National Comprehensive Cancer Network's Breast Cancer Outcomes Database were used to characterize the use of trastuzumab beyond disease progression in National Comprehensive Cancer Network centers prior to the 2009 publication supporting it use.
Abstract
Background.
The role of continued trastuzumab after progression in women with human epidermal growth factor receptor (HER)-2+ metastatic breast cancer is controversial. Controlled clinical trials that establish a benefit from continued trastuzumab have been difficult to complete.
Methods.
In the National Comprehensive Cancer Center Network (NCCN) Breast Cancer Outcomes Database, we identified women treated with trastuzumab for metastatic or relapsed HER-2+ breast cancer at eight NCCN centers who subsequently progressed. Patients were eligible for this analysis if they initiated treatment at an NCCN institution between July 1997 and December 2004, received trastuzumab-containing treatment, and progressed while on therapy. We calculated the proportion of patients who received trastuzumab after progression, and in a multivariate analysis assessed the association of patient and provider characteristics with continued trastuzumab therapy.
Results.
Our final cohort consisted of 218 women who experienced disease progression while on trastuzumab-containing therapy. Of these, 168 (77%) continued trastuzumab. Of these, 36 patients (17%) received therapy as part of a clinical trial. The only factors significantly associated with continuation of trastuzumab beyond progression were the presence of bone metastases and more recent year of development of progressive disease.
Conclusions.
Prior to the availability of any high-quality evidence supporting this practice, over three quarters of patients treated with trastuzumab for HER-2+ metastatic breast cancer at eight NCCN centers continued therapy beyond progression. Further work is needed to understand how physicians adopt new treatments when there is ambiguity surrounding their benefit.
Background
Trastuzumab was first approved in 1998 for the treatment of patients with metastatic breast cancer whose tumors overexpress human epidermal growth factor receptor (HER)-2. The landmark trial found a longer time to disease progression, a higher rate of objective response, and a longer overall survival time in patients who received trastuzumab in combination with chemotherapy than in those who received chemotherapy alone in the frontline setting [1]. Based on these findings, treatment with trastuzumab-based therapy is recommended for women with HER-2/neu+ breast cancer.
In the decade that followed its approval, many oncologists adopted the practice of continuing trastuzumab after the cancer progressed. Most often, the oncologist changed the chemotherapy “partner” given in combination with trastuzumab. However, prior to the 2009 publication of a clinical trial showing a higher response rate and longer progression-free survival interval, there were no randomized data to support this practice. There were only data from uncontrolled studies [2, 3] and case series [4–7] suggesting that the practice was safe and not associated with excess toxicity. In addition, responses and longer survival were seen with subsequent lines of trastuzumab-based therapy; however, given the lack of randomization, several articles called for randomized trials to clarify the true benefit [8, 9]. Given the paucity of information to support this practice, starting in 2005, guidelines from the National Comprehensive Cancer Network (NCCN) stated “the value of continued trastuzumab following progression on first line-trastuzumab containing chemotherapy for metastatic breast cancer is unknown. The optimal duration of trastuzumab in patients with long-term control of disease is unknown” [10–14]. Despite this ambiguity, a physician survey published in 2005 suggested that >80% of respondents would recommend continued trastuzumab beyond disease progression [15]. However, we are unaware of any published data on the actual rate of trastuzumab use after progression in clinical practice.
In this study, we used data from the NCCN Breast Cancer Outcomes Database to characterize the use of trastuzumab beyond disease progression in NCCN centers prior to the 2009 publication supporting it use [16]. Given the high cost of monoclonal antibodies such as trastuzumab, we believe it is important to characterize their use in settings where high-quality evidence from clinical trials is not available.
Methods
Data Source
Since July 1997, the NCCN Breast Cancer Outcomes Database Project has collected prospective data on patient and tumor characteristics, treatments, and outcomes for women with newly diagnosed localized and metastatic breast cancer treated at participating member institutions. All new patients presenting to NCCN participating centers are screened for eligibility. Patients are eligible if they receive part of their first-line therapy (surgery, chemotherapy, or hormonal therapy) at the center. Patients who receive a second opinion or radiation therapy only are not eligible.
The eligibility criteria and data collection procedures for the database were described previously [17–19]. Briefly, clinical, treatment, and recurrence information is abstracted from medical records by dedicated and trained clinical research associates at each site. Whenever a chemotherapy regimen is discontinued or changed, reasons for discontinuation are coded using a standardized list based on documentation in the record. Information regarding menopausal status, educational status, and employment status are collected by patient survey at initial presentation to the NCCN center. Rigorous data quality assurance processes were in place to validate the accuracy of the data used in this study. These included initial and follow-up data management training, online edit checking during Web-based data entry, programmed logic checks against the pooled data repository, routine quality assurance reports to the centers for rectification by the data managers, and audits of a random sample of source documents against the submitted data, conducted within the first few months of data collection and repeated annually at each institution.
Eight institutions contributed data to this analysis: City of Hope Comprehensive Cancer Center, Duarte, CA; Dana-Farber Cancer Institute, Boston, MA; Fox Chase Cancer Center, Philadelphia, PA; The University of Texas MD Anderson Cancer Center, Houston, TX; Roswell Park Cancer Institute, Buffalo, NY; University of Michigan Comprehensive Cancer Center, Ann Arbor, MI; Arthur G. James Cancer Hospital and Richard Solove Research Institute at Ohio State University, Columbus, OH; and H. Lee Moffitt Cancer Center and Research Institute at the University of South Florida, Tampa, FL. Each institution is an academic center where the physicians who treat breast cancer generally devote most or all of their clinical effort to breast cancer care. The institutional review boards (IRB) at each center approved the data collection, transmission, and storage protocols. When institutional IRBs required signed informed consent for data collection, only patients who provided consent were included in the database. The Data Coordinating Center is located at City of Hope National Medical Center.
Patients
We included women with HER-2/neu+ breast cancer who initiated treatment at one of the eight NCCN institutions between July 1997 and December 2004 and who progressed on trastuzumab-containing treatment for recurrent or metastatic disease.
Definition of Disease Progression on Therapy
Patients were considered to have progressed on therapy if: (a) treatment was discontinued or changed and the reason for discontinuation was coded as progression, (b) treatment was discontinued or changed and no reason was provided in the record, or (c) a second agent was added >60 days after the initiation of single-agent trastuzumab therapy.
Analysis
Trastuzumab-containing regimens before and after progression were categorized based on whether the regimen also consisted of (a) an anthracycline, (b) a taxane, (c) vinorelbine, (d) other single-agent chemotherapy, (e) combination chemotherapy, or (f) trastuzumab alone. These categories included patients who were on hormonal therapy in conjunction with trastuzumab with or without chemotherapy. All regimens were also classified based on whether they were given as a component of an IRB-approved clinical trial.
We used multivariate logistic regression analysis to assess the association of the following clinical and patient factors with receipt of continued trastuzumab after cancer progression: year that progression occurred while on trastuzumab, patient age at cancer progression, tumor hormone receptor status at diagnosis of metastases, number and sites of metastatic disease (classified as bone, central nervous system [CNS], visceral, or other), and NCCN institution. Age at progression was classified as <50, 50 to <70, or ≥70 years, and year of progression was classified as 1999–2000, 2001–2002, 2003–2004, or 2005 onward. Only those explanatory variables with a p-value ≤.2 based on the univariate analysis were included in the logistic model. All p-values were two-sided, and analyses were conducted using SAS software (version 9.1.3; SAS Institute, Inc., Cary, NC).
Results
Patients
We identified 547 patients with HER-2/neu+ metastatic breast cancer who began a component of their initial cancer treatment at one of the eight NCCN institutions in July 1997 to December 2004. Of this group, 369 patients (67.5%) received trastuzumab at some point for treatment of metastatic disease. Of the 369 patients who received trastuzumab, 218 (59%) progressed while receiving a trastuzumab-containing regimen; this group comprised the analytic cohort for this study.
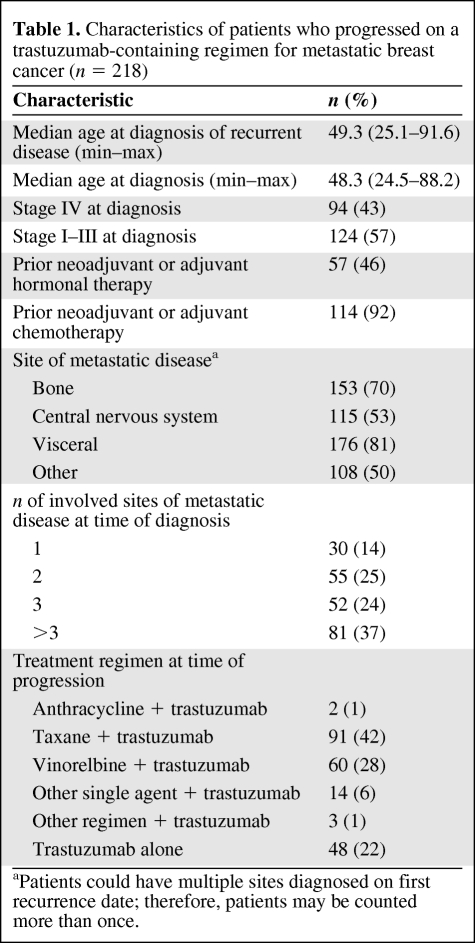
The characteristics of the 218 patients included in this analysis are shown in Table 1. The median age at the time of diagnosis of metastatic breast cancer was 49.1 years (range, 24.5–91.6 years). Slightly less than half of the patients (43%) had stage IV disease at presentation. Of the 57% with a metastatic recurrence, almost all (92%) had received prior neoadjuvant or adjuvant chemotherapy without trastuzumab. The majority of patients had more than one site of metastatic disease, with visceral sites in 81%, bone in 70%, and CNS in 53% of patients. At the time of disease progression on trastuzumab, the majority of patients were receiving a single chemotherapy agent in conjunction with trastuzumab, most commonly a taxane; 22% were receiving trastuzumab alone.
Table 1.
Characteristics of patients who progressed on a trastuzumab-containing regimen for metastatic breast cancer (n = 218)
aPatients could have multiple sites diagnosed on first recurrence date; therefore, patients may be counted more than once.
Use of Trastuzumab After Progression
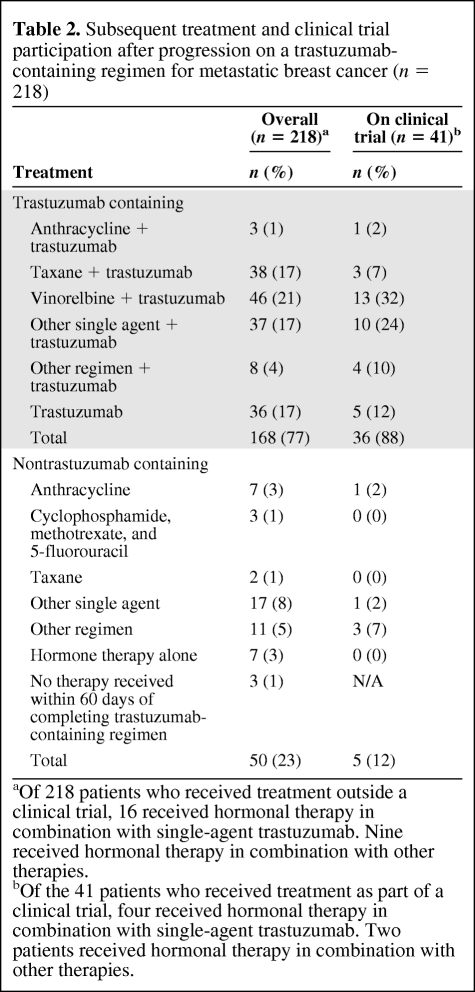
Characteristics of treatments received beyond disease progression on trastuzumab are shown in Table 2. Seventy-seven percent (168 of 218) of the patients received further trastuzumab. After disease progression, single-agent vinorelbine was the most commonly used concomitant chemotherapy (21%), followed by single-agent taxane (17%). Seventeen percent of patients were treated with trastuzumab alone after disease progression. Of the patients who continued to receive trastuzumab, only 21% (36 of 168) received their treatment as part of a clinical trial.
Table 2.
Subsequent treatment and clinical trial participation after progression on a trastuzumab-containing regimen for metastatic breast cancer (n = 218)
aOf 218 patients who received treatment outside a clinical trial, 16 received hormonal therapy in combination with single-agent trastuzumab. Nine received hormonal therapy in combination with other therapies.
bOf the 41 patients who received treatment as part of a clinical trial, four received hormonal therapy in combination with single-agent trastuzumab. Two patients received hormonal therapy in combination with other therapies.
Factors Associated with the Use of Trastuzumab After Disease Progression
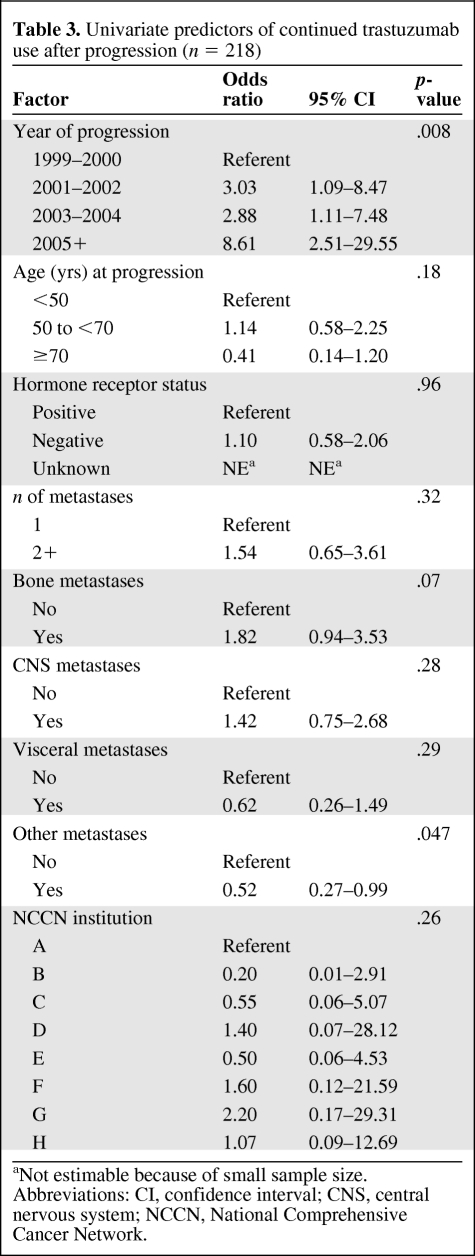
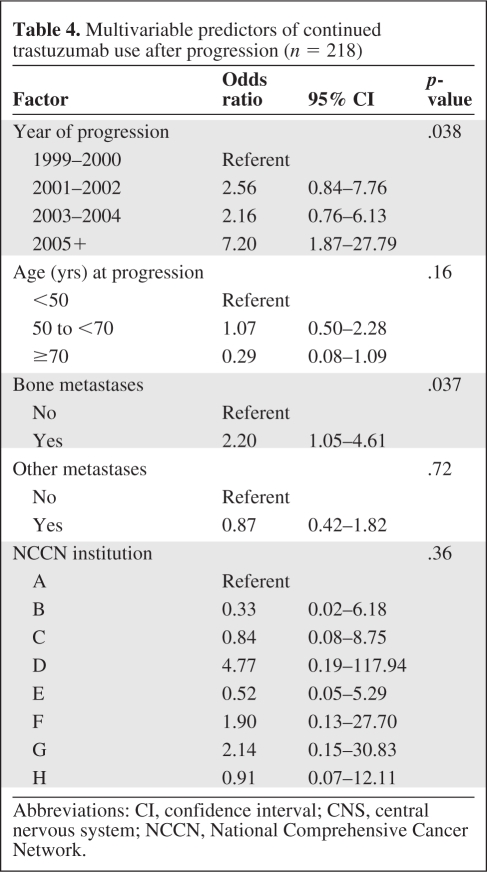
As shown in Tables 3 and 4, for both the univariate and multivariate analyses, the only factors significantly associated with use of trastuzumab after progression were more recent year of progression (p = .038) and the presence of bone metastases (p = .037). None of the other prespecified covariates (age, hormone receptor status, presence of visceral or other metastatic sites, number of metastatic sites, or NCCN center) were found to be significantly associated with continued trastuzumab.
Table 3.
Univariate predictors of continued trastuzumab use after progression (n = 218)
aNot estimable because of small sample size.
Abbreviations: CI, confidence interval; CNS, central nervous system; NCCN, National Comprehensive Cancer Network.
Table 4.
Multivariable predictors of continued trastuzumab use after progression (n = 218)
Abbreviations: CI, confidence interval; CNS, central nervous system; NCCN, National Comprehensive Cancer Network.
Discussion
We found that three quarters of patients with metastatic breast cancer treated with a trastuzumab-containing regimen in these eight NCCN centers were continued on trastuzumab after disease progression. This high rate is notable because, during the time period we studied, there were no randomized controlled clinical trial data supporting the continuation of trastuzumab after progression, and the NCCN breast cancer practice guidelines during that time stated that “the value of continued trastuzumab following progression on first line-trastuzumab containing chemotherapy for metastatic breast cancer is unknown” [10–14]. In addition, more recent year of disease progression was associated with a higher likelihood of receiving continued trastuzumab, suggesting that this practice became substantially more common over time, and predated the publication of the first randomized trial results.
During the period studied, all experience supporting the use of continued trastuzumab beyond disease progression came from uncontrolled retrospective case series and single-arm clinical trials. The results of these studies were inconsistent. A study of 40 patients who had previously received trastuzumab-based therapy for metastatic breast cancer evaluated the role of capecitabine and trastuzumab. This was associated with a clinical benefit rate of 70%. In addition, the time to progression (TTP) of 8 months and overall survival duration of 24 months were much higher than those seen in historical controls of patients treated with capecitabine monotherapy [3]. A retrospective analysis of 136 patients with HER-2/neu+ breast cancer found that 66 and 47 patients received trastuzumab in the first and second line, respectively. Of these, 23 patients received trastuzumab beyond progression. Patients who received at least two lines of trastuzumab had a longer TTP than those who received only one (64.2 months versus 38.5 months), leading the authors to conclude that continued trastuzumab beyond disease progression may improve survival [20]. However, a larger retrospective study found that, among patients with metastatic HER-2/neu+ breast cancer whose disease progressed on trastuzumab-based therapy, the progression-free and overall survival times were similar between patients who did (n = 83) and did not (n = 112) continue on trastuzumab [4]. Another analysis published in 2010 performed as part of the Hermine prospective cohort study of patients with metastatic breast cancer treated with trastuzumab found a benefit in terms of TTP and overall survival in patients who received trastuzumab beyond disease progression (n = 107), compared with those who did not (n = 70). The authors identified continuation of trastuzumab as an independent prognostic factor for survival, but also acknowledged that an imbalance in prognostic factors between the groups could have influenced the results [21].
Despite the high prevalence of breast cancer, randomized clinical trials to address this important question have suffered from very poor accrual. A Southwest Oncology Group study closed after accruing only 17 patients from 30 centers over 18 months [22]. The only published randomized trial (German Breast Group 26/Breast International Group 03–05) found benefits in terms of both the response rate and TTP in favor of trastuzumab plus capecitabine, compared with capecitabine alone, among women who had progressed on a prior trastuzumab-containing regimen. In that study, the median TTP was 8.2 months in the combination arm and 5.6 months in the capecitabine alone arm (hazard ratio, 0.69; 95% confidence interval, 0.48–0.97; two-sided log-rank p = .0338). The response rate was also higher in the combination arm than in the single-agent arm (48% versus 27%; odds ratio, 2.50; p = .0115). However, there was no statistically significant difference in terms of overall survival. That study, published in early 2009, also closed early because of poor accrual after recruiting only 156 of 482 planned patients, perhaps as a result of diminished interest in the question following the release of data showing the benefit of lapatinib among patients who had progressed on trastuzumab [16]. Reflecting the somewhat equivocal findings from the German study, the NCCN guidelines were modified in 2010 to state that “continued trastuzumab following progression on first line-trastuzumab containing chemotherapy for metastatic breast cancer is an option” [23].
There is little question that the practice patterns we observed were not based on high-quality evidence during the period we studied. There was some debate in the literature at the time about whether or not the strategy of continuing trastuzumab in the absence of supporting randomized trial data represented an appropriate weighing of the risks and benefits for patients or a rational use of societal resources [22]. Now that the results from the first randomized trial addressing this question are available [16], it appears that, in hindsight, in this case, patients may have benefited from their physicians' decision to “practice ahead of the evidence.” Unfortunately, such hindsight may hide the fact that such early adoption practices have real potential to cause harm.
The larger question for contemporary practice is whether or not this pattern will be adopted for other disease sites in the absence of clinical trial data. Physicians may be tempted to continue other targeted agents beyond disease progression in other malignancies, extrapolating from their experience with trastuzumab in breast cancer patients. However, given the very high costs and potential toxicities associated with these agents, it is important that physicians support clinical trials to address these questions, rather that treating patients “off study” outside clinical trials with these potentially toxic and expensive agents. These important questions will not be answered without the widespread agreement of both academic and community oncologists to enroll their patients in ongoing studies.
Our study has several strengths. Although the NCCN Breast Cancer Outcomes Database Project is not a population-based sample, it does provide insight into the use of trastuzumab at centers across the country. Our results demonstrated that continued trastuzumab use beyond progression was not limited to one particular region of the country or center. In addition, this dataset provides information about disease recurrence, reasons for treatment discontinuation, and clinical trial enrollment, data that are not readily available from other sources, such as linked claims from registry and claims data (e.g., the Surveillance Epidemiology, and End Results database or Medicare) or commercial insurance data.
Our study also has several limitations. Reasons for discontinuation of therapy were not always documented in the medical record. Therefore, we had to rely on surrogate measures of progression in some cases. For example, we assumed that patients who were started on chemotherapy or endocrine therapy >60 days after starting single-agent trastuzumab were considered to have progressive disease. However, it is possible that chemotherapy or endocrine therapy could have been started without evidence of disease progression. However, we believe that bias caused by both these limitations should be nondifferential and should not significantly affect our results. In addition, we restricted our sample to patients who continued to receive their care at the NCCN center beyond the end of first-line trastuzumab-containing therapy for advanced disease. Some patients whose treatment was initiated at an NCCN center may have opted to transfer their care to oncologists at other centers or in community practice while they were still receiving active treatment. However, it seems unlikely that this would have biased our results because there is no a priori reason to think that patients who chose to transfer their care would have received systematically different recommendations at the time of progression than those who remained at the NCCN center.
Conclusion
Over three quarters of patients at NCCN institutions received trastuzumab-based therapy beyond disease progression prior to the publication of randomized controlled clinical trial data supporting its use. Understanding how physicians adopt new treatments when there are limited trial data is especially important given the recent introduction of promising but costly and potentially toxic treatments. Patients should be encouraged to enroll in clinical trials.
Acknowledgment
This work was supported in part by grant P50 CA089393 from the National Cancer Institute to Dana-Farber Cancer Institute. Dr. Wong was supported by in part by P30 CA006927.
Author Contributions
Conception/Design: Yu-Ning Wong, Melissa Hughes, Jane C. Weeks, Joyce Niland
Provision of study material or patients: Yu-Ning Wong, Jane C. Weeks, Stephen Edge, Richard L. Theriault, Douglas Blayney
Collection and/or assembly of data: Yu-Ning Wong, Melissa Hughes, Jane C. Weeks, Rebbeca Ottesen, Joyce Niland
Data analysis and interpretation: Yu-Ning Wong, Melissa Hughes, Jane C. Weeks, Rebbeca Ottesen, Joyce Niland
Manuscript writing: Yu-Ning Wong, Melissa Hughes, Jane C. Weeks, Rebbeca Ottesen, Joyce Niland, Stephen Edge, Richard L. Theriault, Douglas Blayney
Final approval of manuscript: Yu-Ning Wong, Melissa Hughes, Jane C. Weeks, Rebbeca Ottesen, Joyce Niland, Stephen Edge, Richard L. Theriault, Douglas Blayney
References
- 1.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 2.Tripathy D, Slamon DJ, Cobleigh M, et al. Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol. 2004;22:1063–1070. doi: 10.1200/JCO.2004.06.557. [DOI] [PubMed] [Google Scholar]
- 3.Bartsch R, Wenzel C, Altorjai G, et al. Capecitabine and trastuzumab in heavily pretreated metastatic breast cancer. J Clin Oncol. 2007;25:3853–3858. doi: 10.1200/JCO.2007.11.9776. [DOI] [PubMed] [Google Scholar]
- 4.Montemurro F, Redana S, Viale G, et al. Retrospective evaluation of clinical outcomes in patients with HER2-positive advanced breast cancer progressing on trastuzumab-based therapy in the pre-lapatinib era. Clin Breast Cancer. 2008;8:436–442. doi: 10.3816/CBC.2008.n.053. [DOI] [PubMed] [Google Scholar]
- 5.Montemurro F, Donadio M, Clavarezza M, et al. Outcome of patients with HER2-positive advanced breast cancer progressing during trastuzumab-based therapy. The Oncologist. 2006;11:318–324. doi: 10.1634/theoncologist.11-4-318. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Sàenz JA, Martin M, Puente J, et al. Trastuzumab associated with successive cytotoxic therapies beyond disease progression in metastatic breast cancer. Clin Breast Cancer. 2005;6:325–329. doi: 10.3816/CBC.2005.n.035. [DOI] [PubMed] [Google Scholar]
- 7.Bartsch R, Wenzel C, Hussian D, et al. Analysis of trastuzumab and chemotherapy in advanced breast cancer after the failure of at least one earlier combination: An observational study. BMC Cancer. 2006;6:63. doi: 10.1186/1471-2407-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelmon KA, Mackey J, Verma S, et al. Use of trastuzumab beyond disease progression: Observations from a retrospective review of case histories. Clin Breast Cancer. 2004;5:52–58. doi: 10.3816/cbc.2004.n.010. discussion 59–62. [DOI] [PubMed] [Google Scholar]
- 9.Fountzilas G, Razis E, Tsavdaridis D, et al. Continuation of trastuzumab beyond disease progression is feasible and safe in patients with metastatic breast cancer: A retrospective analysis of 80 cases by the Hellenic Cooperative Oncology Group. Clin Breast Cancer. 2003;4:120–125. doi: 10.3816/cbc.2003.n.017. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network (NCCN) Fort Washington, PA: National Comprehensive Cancer Network, Inc.; 2005. The NCCN Breast Cancer Clinical Practice Guidelines in Oncology (Version 1.2005) [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network (NCCN) Fort Washington, PA: National Comprehensive Cancer Network, Inc.; 2006. The NCCN Breast Cancer Clinical Practice Guidelines in Oncology (Version 2.2006) [Google Scholar]
- 12.National Comprehensive Cancer Network (NCCN) Fort Washington, PA: National Comprehensive Cancer Network, Inc.; 2007. The NCCN Breast Cancer Clinical Practice Guidelines in Oncology (Version 1.2007) [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network (NCCN) Fort Washington, PA: National Comprehensive Cancer Network, Inc.; 2008. The NCCN Breast Cancer Clinical Practice Guidelines in Oncology (Version 1.2008) [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network (NCCN) Fort Washington, PA: National Comprehensive Cancer Network, Inc.; 2009. The NCCN Breast Cancer Clinical Practice Guidelines in Oncology (Version 1.2009) [PubMed] [Google Scholar]
- 15.Love N, editor. HER2-Positive Disease. Patterns of Care in Medical Oncology. [accessed March 28, 2011];Volume 2 Available at http://www.patternsofcare.com/2005/1/her-2-positive-disease.htm. [Google Scholar]
- 16.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: A German Breast Group 26/Breast International Group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 17.Christian CK, Niland J, Edge SB, et al. A multi-institutional analysis of the socioeconomic determinants of breast reconstruction: A study of the National Comprehensive Cancer Network. Ann Surg. 2006;243:241–249. doi: 10.1097/01.sla.0000197738.63512.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weeks J. Outcomes assessment in the NCCN: 1998 update. National Comprehensive Cancer Network. Oncology (Williston Park) 1999;13:69–71. [PubMed] [Google Scholar]
- 19.Weeks JC. Outcomes assessment in the NCCN. Oncology (Williston Park) 1997;11:137–140. [PubMed] [Google Scholar]
- 20.Stemmler HJ, Kahlert S, Siekiera W, et al. Prolonged survival of patients receiving trastuzumab beyond disease progression for HER2 overexpressing metastatic breast cancer (MBC) Onkologie. 2005;28:582–586. doi: 10.1159/000088296. [DOI] [PubMed] [Google Scholar]
- 21.Extra JM, Antoine EC, Vincent-Salomon A, et al. Efficacy of trastuzumab in routine clinical practice and after progression for metastatic breast cancer patients: The observational Hermine study. The Oncologist. 2010;15:799–809. doi: 10.1634/theoncologist.2009-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hortobagyi GN. In reply. J Clin Oncol. 2005;23:2868–2869. [Google Scholar]
- 23.National Comprehensive Cancer Network (NCCN) Fort Washington, PA: National Comprehensive Cancer Network, Inc.; 2010. The NCCN Breast Cancer Clinical Practice Guidelines in Oncology (Version 1.2010) [PubMed] [Google Scholar]