The effect of postoperative radiotherapy on overall survival and tumor control in patients with resected pathological stage IIIA–N2 non-small cell lung cancer was evaluated.
Keywords: Non-small cell lung cancer, Postoperative radiotherapy, IIIA-N2, Surgery, Survival
Learning Objectives
After completing this course, the reader will be able to:
Describe the present clinical practice and controversies regarding the use of PORT in resected pIIIA-pN2 NSCLC.
Evaluate the effect of PORT on overall survival and on tumor control in this subgroup of patients.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
For patients with resected pathological stage IIIA–N2 non-small cell lung cancer (NSCLC), the role of postoperative radiotherapy (PORT) is not well defined. In this single-institutional study, we re-evaluated the effect of PORT on overall survival (OS) as well as tumor control in this subgroup of patients.
Methods.
In 2003–2005, 221 consecutive patients with resected pathological stage IIIA–N2 NSCLC at our institution were retrospectively analyzed in an institutional review board–approved study. The effect of PORT on OS, cancer-specific survival (CSS), and disease-free survival (DFS) was evaluated using the Kaplan–Meier method and log-rank tests. The impact of PORT on locoregional control and distant metastasis was also analyzed.
Results.
Compared with the control, patients treated with PORT had a significantly longer OS time (χ2, 3.966; p = .046) and DFS interval (χ2, 6.891; p = .009), as well as a trend toward a longer CSS duration (χ2, 3.486; p = .062). Patients treated with PORT also had a significantly higher locoregional recurrence-free survival rate (χ2, 5.048; p = .025) as well as distant metastasis-free survival rate (χ2, 11.248; p = .001). Multivariate analyses showed that PORT was significantly associated with a longer OS duration (p = .000).
Conclusions.
PORT can significantly improve the survival of patients with resected pathological stage IIIA–N2 NSCLC. A prospective randomized multicenter clinical trial is ongoing.
Introduction
Completely resected pathological stage IIIA–N2 non-small cell lung cancer (NSCLC) is a heterogeneous group of diseases, with 5-year overall survival rates in the range of 7%–34% [1–6]. Postoperative chemotherapy was confirmed to improve the survival of patients with pathologic stage IIIA NSCLC in several recent randomized clinical trials. However, even after complete resection followed by adjuvant chemotherapy, the locoregional failure rate of patients can still be as high as 40% [7–9]. In view of the high locoregional recurrence rate, postoperative radiotherapy (PORT) has been incorporated into multidisciplinary management to improve locoregional control, which may be further translated into an overall survival (OS) benefit.
A meta-analysis study on PORT published in 1998 concluded that PORT had a significant detrimental effect on the survival of patients with resected NSCLC [10]. Though patients with stage III and pN2 had slightly better survival with PORT, the difference was not statistically significant. Because of the lack of evidence for a survival benefit, the use of PORT for patients with resected NSCLC declined worldwide after the publication of that meta-analysis [11–13]. Improvements in conformal radiotherapy techniques have led to a resurgence of interest in studying the effect of PORT on resected pathological stage IIIA–N2 NSCLC in the past decade [13, 14]. However, because of a lack of evidence from new randomized clinical trials, the definitive role of PORT in pIIIA–N2 NSCLC patients is unknown. In this retrospective study, based on data from 221 recently treated patients in our institution, we re-evaluated the effect of PORT on OS as well as tumor control in patients with resected pathological stage IIIA–N2 NSCLC.
Materials and Methods
Patients
Between January 2003 and December 2005, 221 consecutive patients with pathologically confirmed stage IIIA–N2 NSCLC (according to the American Joint Committee on Cancer [AJCC] 1997 staging system) who survived ≥4 months after radical resection in our institution were included in this study. The medical records and follow-up data of the patients were retrospectively analyzed, which mainly included: age, gender, performance status (PS), site of primary tumor, histology, clinical and pathological tumor–node–metastasis (TNM) stage, details of tumor resection, number of positive nodes, chemotherapy, radiotherapy, pattern of treatment failure, salvage treatment, and survival status and time.
Surgery
All patients underwent lobectomy or ipsilateral pneumonectomy. Complete mediastinal lymph node dissection or systematic mediastinal lymph node sampling was performed during surgery.
Chemotherapy
Adjuvant chemotherapy was administered with a cisplatin- or paclitaxel-based regimen, with a median of four cycles. For some patients who did not receive adjuvant chemotherapy, the reasons were mainly asthenia, refusal by the patient, and choice of the physician.
Radiotherapy
The administration of PORT for a patient with N2 disease was based on the attending radiation oncologist's decision and, partially, the referring surgeon's suggestion. Basically, widespread mediastinal lymph node involvement was the main reason for recommending PORT. Radiotherapy techniques included three-dimensional conformal radiotherapy (3DCRT) and conventional two-dimensional radiotherapy (2DRT). For 3DCRT, the clinical target volume (CTV) included the subcarinal nodes, ipsilateral mediastinum, and ipsilateral hilum. The planning target volume was defined as the CTV plus 0.8-cm margins. For 2DRT, the irradiation fields covered the bronchial stump, subcarinal nodes, ipsilateral mediastinum, and ipsilateral hilum. 2DRT was performed with two parallel-opposed anterior–posterior fields to 40 Gy, followed by irradiation with two opposed oblique fields in order to avoid the spinal cord. Both 3DCRT and 2DRT were administered with a linear accelerator using 6–8 MV x-rays at 2 Gy per fraction, 5 days per week, to a total dose of 60 Gy.
Follow-Up
Patients were followed up 1 month after radiotherapy, then every 3 months for the first year, then every 3–6 months thereafter. The last follow-up date was March 2009. All patients were evaluated with a physical examination, CBC, serum biochemistry, thoracic computed tomography scans, abdomen B-ultrasound examination, and other necessary imaging examinations based on the patient's symptoms.
Data Analysis
SPSS statistical software (version 13.0; SPSS Inc., Chicago, IL) was used for the statistical analyses. OS was defined from the day of surgery to the time of death from any cause or last follow-up. Cancer-specific survival (CSS) was defined from the day of surgery to the time of cancer death or last follow-up. Disease-free survival (DFS) was defined from the day of surgery to the time of death, tumor recurrence, or last follow-up. The Kaplan–Meier method was used to calculate survival rates, and the log-rank test was used to analyze differences between the groups. Univariate and multivariate analyses were used to evaluate potential prognostic factors for OS. The Pearson χ2 test was used to compare the constituent ratios in different groups. A statistically significant difference was set as p < .05.
Results
Patients and Tumor Characteristics
Characteristics of the 221 patients are presented in Table 1. The median follow-up time was 35.1 months (range, 4.2–80.7 months). The median age of the patients was 60 years (range, 27–79 years). According to AJCC 1997 definitions, the primary tumor stage was pT1 in 17 patients (7.7%), pT2 in 166 patients (75.1%), and pT3 in 38 patients (17.2%). Adenocarcinoma (51.6%; n = 114) and squamous cell carcinoma (40.3%; n = 89) were the predominant pathological types, followed by adenosquamous carcinoma (5.4%; n = 12) and large cell carcinoma (2.7%; n = 6). The median number of dissected nodes was 22 (range, 1–60).
Table 1.
Patient characteristics
aSmoking index is the number of cigarettes smoked per day × the number of cigarette-years.
Abbreviations: KPS, Karnofsky performance status; PORT, postoperative radiotherapy.
Of all 221 patients, 161 (72.9%) received adjuvant chemotherapy. Ninety-six patients (43.4%) received PORT, including 41 treated with 3DCRT and 55 treated with conventional 2DRT. The median interval between surgery and PORT was 2.1 months. In the PORT group, 61 patients (63.5%) also received adjuvant chemotherapy. Table 1 also compares the baseline characteristics of patients between the PORT and non-PORT groups. Male patients and patients with preoperative clinical stage N2 and squamous cell carcinoma were more prevalent in the PORT group than in the non-PORT group. There were fewer patients receiving adjuvant chemotherapy in the PORT group. The remaining listed characteristics were comparable between the two groups.
Survival
For all patients, the median survival time (MST) was 37.9 months. The 1-, 3-, and 5-year OS rates were 85.1%, 51.3%, and 32.7%, respectively, and the corresponding CSS rates were 86.4%, 55.5%, and 36.7%.
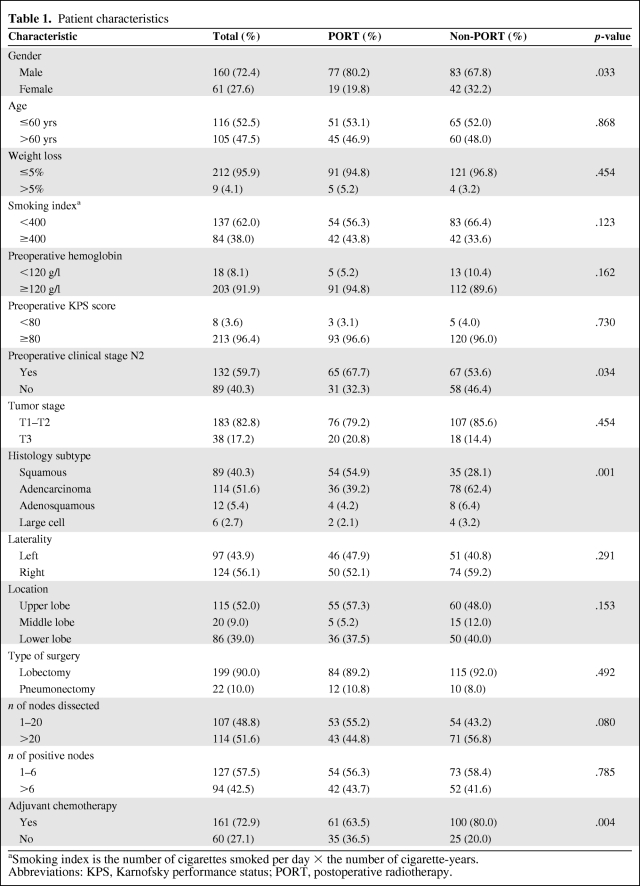
For patients in the PORT and non-PORT groups, the MSTs were 43.9 months and 31.8 months, respectively. The 1-, 3-, and 5-year OS rates were 94.8%, 59.1%, and 36.6%, respectively, in the PORT group, which were statistically significantly higher than the 77.6%, 45.4%, and 30.6% respective rates in the non-PORT group (χ2, 3.966; p = .046) (Fig. 1A). The 1-, 3-, and 5-year CSS rates were 94.5%, 61.8%, and 40.6% in the PORT group and 80.1%, 50.1%, and 33.2% in the non-PORT group, respectively. The difference in CSS rates between the two groups trended toward significance (χ2, 3.486; p = .062) (Fig. 1B). The 1-, 3-, and 5-year DFS rates were 76.1%, 39.8%, and 32.1% in the PORT group and 56.4%, 28.2%, and 16.5% in the non-PORT group, respectively. The difference in the DFS rates between the two groups was significant (χ2, 6.891; p = .009) (Fig. 1C).
Figure 1.
Comparison of overall survival (A), cancer-specific survival (CSS) (B), and disease-free survival (DFS) (C) between the postoperative radiotherapy (PORT) and non-PORT groups.
Results of the univariate and multivariate analyses of different potential prognostic factors for OS are shown in Table 2. On univariate analysis, the use of PORT (p = .046), not having preoperative clinical stage N2 disease (p = .000), a lower T stage (p = .001), having a lower number of positive nodes (p = .000), having a lower percentage of positive nodes (p = .000), and having a single N2 station (p = .002) were significant positive prognostic factors for OS. The use of lobectomy (p = .080) was marginally associated with a higher OS rate, whereas gender, age, weight loss, smoking index (number of cigarettes smoked per day × number of cigarette-years), preoperative hemoglobin level, histology subtype, laterality and lobe location of the primary tumor, the number of dissected nodes, and treatment with adjuvant chemotherapy were not prognostic factors. On multivariate analysis, treatment with PORT (p = .000), having preoperative clinical stage N2 disease (p = .000), the number of positive nodes (p = .000), the percentage of positive nodes (p = .000), and treatment with adjuvant chemotherapy (p = .002) were independent prognostic factors for OS. T stage (p = .067) was a potential prognostic factor. The remaining factors were not significantly associated with OS.
Table 2.
Results of univariate and multivariate analyses of prognostic factors for OS
Table 2a.
(continued)
aSmoking index is the number of cigarettes smoked per day × the number of cigarette-years.
Abbreviations: CI, confidence interval; HR, hazard ratio; MST, median survival time; OS, overall survival; PORT, postoperative radiotherapy.
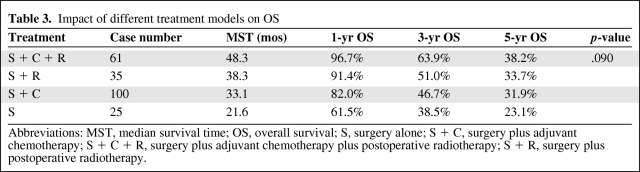
In a subset analysis, patients were divided into four groups: surgery plus adjuvant chemotherapy plus PORT (S + C + R), surgery plus PORT (S + R), surgery plus adjuvant chemotherapy (S + C), and surgery alone (S). The impact of different treatment modalities on OS is presented in Table 3 and Figure 2. The S + C + R group had a longer MST and higher OS rate than the other three groups, with a significant difference when compared with the S group (χ2, 6.311; p = .012) and difference approaching significance when compared with the S + C group (χ2, 3.642; p = .057). Although the S + R group had consistently higher 1-, 3-, and 5-year OS rates (91.4%, 51.0%, and 33.7%, respectively) than the S group (61.5%, 38.5%, and 23.1%, respectively), the difference was not statistically significant.
Table 3.
Impact of different treatment models on OS
Abbreviations: MST, median survival time; OS, overall survival; S, surgery alone; S + C, surgery plus adjuvant chemotherapy; S + C + R, surgery plus adjuvant chemotherapy plus postoperative radiotherapy; S + R, surgery plus postoperative radiotherapy.
Figure 2.
Overall survival according to treatment pattern: S + C + R = surgery followed by chemotherapy and radiotherapy; S + R= surgery followed by radiotherapy; S + C = surgery followed by chemotherapy; S = surgery alone.
Impact of PORT on Locoregional Recurrence
Up to the last follow-up, treatment failures were observed in 161 patients, including 41 with locoregional recurrence alone, 83 with distant metastases, and 37 with both.
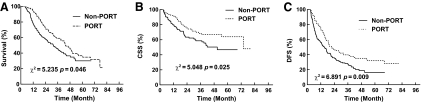
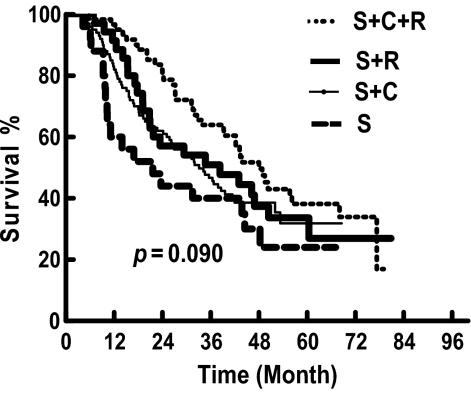
Among the 78 patients (35.3%) with locoregional recurrence, there were 29 in the PORT group (30.2%) and 49 in the non-PORT group (39.2%). The 1-, 3-, and 5-year locoregional recurrence-free survival (LRFS) rates were 92.4%, 68.4%, and 63.9% in the PORT group, respectively, which were significantly higher than the 79.8%, 58.6%, and 46.7% respective rates in the non-PORT group (χ2, 5.048; p = .025) (Fig. 3).
Figure 3.
Comparison of locoregional recurrence-free survival (LRFS) between the postoperative radiotherapy (PORT) and non-PORT groups.
Impact of PORT on Distant Metastasis
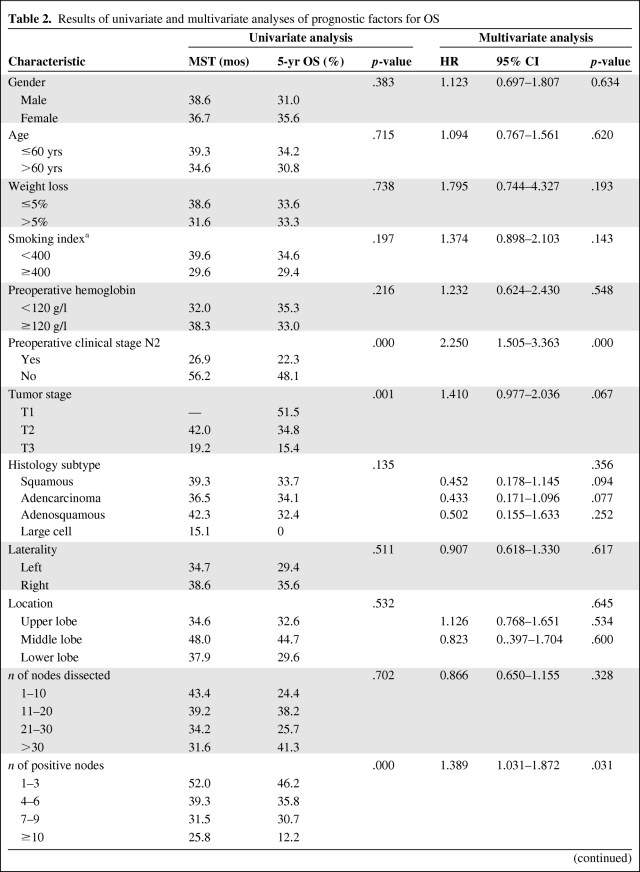
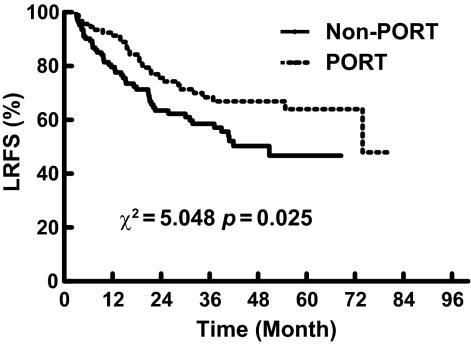
There were 120 patients who developed distant metastases (54.3%), including 47 in the PORT group (49.0%) and 83 in the non-PORT group (66.4%). As shown in Figure 4, the 1-, 3-, and 5-year distant metastasis-free survival (DMFS) rates were, respectively, 80.1%, 54.3%, and 43.8% in the PORT group and 64.8%, 34.4%, and 23.6% in the non-PORT group. The difference between the two groups was statistically significant (χ2, 11.248; p = .001).
Figure 4.
Comparison of distant metastasis-free survival (DMFS) between the postoperative radiotherapy (PORT) and non-PORT groups.
Causes of Death
Up to the last follow-up, 140 deaths in total were recorded and 123 deaths were cancer related, including 53 in the PORT group and 70 in the non-PORT group. Among 17 noncancer-related deaths, seven were in the PORT group (cerebrovascular accident, pulmonary infection, tracheoesophageal fistula, pulmonary embolism, and cachexia in one case each, unknown cause in two cases). Ten were in the non-PORT group (congestive heart failure, myocardial infarction, cerebellar atrophy, acute pancreatitis, thoracic empyema, and cerebrovascular accident in one case each, pulmonary infection in two cases, unknown cause in two cases). The difference in the noncancer-related mortality rate between the two groups was not significant (5.3% versus 6.4%; χ2, 0.470; p = .493).
Discussion
The main purpose of this retrospective study was to evaluate the impact of PORT on survival in patients with resected stage IIIA–N2 NSCLC. For this subgroup of patients, the rate of locoregional failure is still unacceptably high after radical resection, even followed by adjuvant chemotherapy [7–9]. PORT has been administered to improve locoregional tumor control for a long time. However, a clear benefit in terms of survival has not been described so far. An early published PORT meta-analysis indicated that PORT had a significant detrimental effect on survival for all resected patients, especially those patients with pN0 and pN1 disease [10].That meta-analysis was criticized for the comparatively high morbidity and mortality rates resulting from suboptimal radiotherapy techniques [15]. However, a potentially favorable effect of PORT on OS in pN2 patients was observed. This is in agreement with more recent studies [13, 14]. Based on the Surveillance, Epidemiology, and End Results (SEER) database, an analysis for patients with resected NSCLC in 1998–2002 also showed that, although PORT had a detrimental effect on survival for patients with pN0 or pN1 disease, it significantly prolonged survival for patients with pN2 disease (hazard ratio [HR], 0.855; p = .008) [13]. Another subgroup analysis of the Adjuvant Navelbine International Trialist Association (ANITA) trial, which compared adjuvant cisplatin plus vinorelbine with observation in patients with completely resected stage IB–IIIA NSCLC in 1994–2000, showed that PORT had a beneficial effect on survival, compared with the control (MST, 50.2 months versus 25.9 months; 5-year OS rate, 42.6% versus 31.4%) [14]. Our study showed that patients treated with PORT had a longer MST (43.9 months versus 31.8 months) and significantly higher 5-year OS rate (36.6% versus 30.6%; p = .046) than those not treated with PORT (Fig. 1A). A multivariate analysis also showed that PORT was a positive independent prognostic factor. The result is consistent with the SEER study and ANITA trial, as well as two other more recently published retrospective clinical studies [16, 17]. A further randomized clinical trial is also warranted.
Several recent large trials have confirmed the benefit of adjuvant chemotherapy for patients with completely resected stage IIIA–N2 NSCLC, and postoperative chemotherapy has been the standard of care in the treatment of this subgroup [7–9]. However, there are still certain patients who will not receive adjuvant chemotherapy because of advanced age, asthenia, patient refusal, or some other reason. It is essential to separately evaluate the effect of PORT in resected pN2 patients treated with adjuvant chemotherapy and those not treated with chemotherapy. In the ANITA study [14], patients with pN2 disease who received PORT had a strikingly longer MST and higher 5-year OS rate, both in the chemotherapy group (47.4 months versus 23.8 months, 47.4% versus 34.0%) and in the observation group (22.7 months versus 12.7 months, 21.3% versus 16.6%). Similar results were also observed in our study, in which 72.9% of patients received adjuvant chemotherapy. In patients treated with chemotherapy, the MSTs were 48.3 months and 38.1 months and the 5-year OS rates were 38.2% and 31.9% in the PORT and non-PORT groups, respectively. For those not treated with adjuvant chemotherapy, the corresponding MSTs and 5-year OS rates were 38.3 months and 21.6 months and 33.7% and 23.1%. These consistent results indicate that both patients treated with and without adjuvant chemotherapy may benefit from PORT for pN2 disease. On the other hand, our data showed that, for resected pN2 disease, patients treated with PORT combined with adjuvant chemotherapy achieved the highest OS rate, followed by those treated with PORT only and those treated with adjuvant chemotherapy only (33.7% versus 31.9%). Based on the similar results in the ANITA trial, as well as Radiation Therapy Oncology Group 9705 study, which confirmed the efficacy of the combination of PORT and chemotherapy [18], PORT plus adjuvant chemotherapy seems to be the most optimal approach in the treatment of patients with resected stage IIIA–pN2 NSCLC.
In spite of the debated role of PORT in terms of OS, there was more convincing evidence showing that PORT could improve locoregional control in stage IIIA–pN2 disease [10, 13, 14]. Among some early published randomized studies, the Lung Cancer Study Group 773 study assigned 230 patients with resected stage II or stage III squamous cell lung cancer to receive either adjuvant radiotherapy or no adjuvant treatment. The overall recurrence rate was significantly lower with radiotherapy in patients with N2 disease (29% versus 57%; p < .05) [19]. Another study by the Medical Research Council Lung Cancer Working Party [20] showed a trend toward a lower locoregional recurrence rate with PORT, 29% versus 41%, in patients with stage N2 disease (HR, 1.81; confidence interval, 0.95–3.46; p = .07). This lower rate of locoregional recurrence with PORT was also observed in the ANITA study, in which PORT led to a lower locoregional recurrence rate both in patients treated with adjuvant chemotherapy (18.6% versus 6.3%) and in those not treated with chemotherapy (28.9% versus 14.7%) for resected pN2 disease [14]. Our previous randomized study, including 366 patients with resected pN1–N2 squamous cell lung cancer, also showed that PORT resulted in a significantly lower locoregional recurrence rate, 13% versus 33% (p < .01) [21]. Those results are consistent with our present study: the locoregional recurrence rates were 30.2% and 39.2% in the PORT and non-PORT groups, respectively, which resulted in a significantly longer LRFS duration in favor of PORT (χ2, 5.048; p = .025) (Fig. 3). Similar results were also confirmed in several more recently published retrospective studies [16, 17, 22]. All these findings support the use of PORT, with a locoregional control benefit for patients with resected pN2 NSCLC, but no consistent improvement in OS.
For most studies with positive results, the effect of PORT in decreasing the locoregional recurrence rate is believed to be the mechanism for the longer OS duration in patients with pN2 disease [13, 16]. Unlike most other studies, a significantly lower rate of distant metastasis with PORT was observed in our present study. This could be a result of the effect of PORT in eradicating microresidual tumor after surgery, which could further lessen the opportunity for tumor metastasis from these sites. Our data demonstrated that locoregional recurrence was significantly associated with distant metastasis (p = .04, data not shown). We believe that success in reducing distant metastasis is as important as decreasing locoregional recurrence in improving OS with PORT. In our previous randomized study focusing on patients with pN1 and pN2 squamous cell carcinoma, PORT did not result in a significantly lower distant failure rate (48.5% versus 51.2%) and also failed to lead to a higher OS rate (43.4% versus 40.5%), although significantly better locoregional control was observed [21]. This result also validates the importance of the lower distant failure rate seen with PORT.
In older studies, radiotherapy-related morbidity and mortality, such as pneumonitis, cardiac toxicity, and severe esophagitis, were believed to be the most important factors impacting outcome with PORT. In some randomized studies and meta-analyses on PORT in resected NSCLC patients, patients receiving PORT had a significantly higher radiation-related mortality rate than those not treated with PORT [10, 23]. This even resulted in a markedly lower OS rate with PORT, especially with suboptimal, outdated radiation equipment and techniques. Today, there has been significant improvement in radiotherapy delivery techniques. The radiotherapy-related toxicity rate has not been higher in various studies using modern PORT techniques [24–27]. In our study, all patients were treated with linear accelerators, and the dose delivered to important adjacent organs was kept below their tolerance levels. Furthermore, 42.7% of patients received 3DCRT to minimize toxicity to surrounding normal tissues. As a result, no severe radiation toxicities were observed (data not shown). There was no significant difference in terms of the noncancer-related death rate between the PORT and non-PORT groups (5.3% versus 6.4%; p = .493). A similar result was also confirmed by another recently published study from China, in which PORT was delivered via a linear accelerator and 72% of patients received treatment with three-dimensional computerized dosimetry planning and a standard fraction size. That study showed a comparable death rate from intercurrent disease in the PORT plus chemotherapy and chemotherapy alone groups (28.8% versus 21.5%; p = .282) [16].
Compared with the SEER database and ANITA study, our data have more detailed information on the number, location, and extent of lymph node dissections; the number and location of positive lymph nodes; the irradiation techniques; and the chemotherapy used (some details not shown). Furthermore, to our knowledge, our report is the largest retrospective study focusing on the effect of PORT on survival in resected stage IIIA–N2 patients from a single institution, especially within a relatively short time period (2003–2005). These all can be helpful to draw a relatively convincing conclusion. On the other hand, like all other retrospective analyses, our study has some limitations. The study may have selection bias and the results should be interpreted cautiously. Although most of the baseline characteristics in our study were comparable between the PORT and non-PORT groups, there were more male patients (80.2% versus 67.8%) and more squamous cell carcinoma patients (67.7% versus 53.6%) in the PORT group, which might be influenced by the specialist's choice. Moreover, there was a significantly lower proportion of patients receiving adjuvant chemotherapy in the PORT group (63.5% versus 80.8%). This is because our postoperative treatment approach is adjuvant chemotherapy followed by radiotherapy. Some patients refused to take further PORT because of severe gastrointestinal side effects, hematological toxicities, and worsening PS caused by chemotherapy. We should also admit that a median follow-up of 35.1 months is not long enough to observe late toxicity from radiotherapy. The intercurrent death rates were similar in the PORT and non-PORT groups in our present study, but a subsequent increase in PORT related deaths might emerge with further follow-up, which could influence the OS results.
Based on our present results, as well as other related studies, patients with resected stage IIIA–N2 NSCLC are likely to benefit from PORT. In order to define a clearer role for PORT in survival using more advanced radiation technology, a randomized, multicenter clinical trial is ongoing in China, which is expected to accrue 500 patients with completely resected pN2 NSCLC. There are also some other parallel studies throughout the world [28]. By using advanced modern equipment and techniques, the findings of these phase III studies will shed more light on the definitive role of PORT in the treatment of resected IIIA–N2 NSCLC patients.
Acknowledgments
The authors Honghai Dai and Zhouguang Hui contributed equally to this manuscript. We thank professor Bin S. Teh from the Methodist Cancer Center for English editing of the manuscript.
Author Contributions
Conception/Design: Luhua Wang
Administrative support: Luhua Wang
Provision of study material or patients: Luhua Wang, Jun Liang, Jima Lu, Guangfei Ou, Zongmei Zhou, Qinfu Feng, Zefen Xiao, Dongfu Chen, Hongxing Zhang, Weibo Yin, Jie He
Collection and/or assembly of data: Honghai Dai, Zhouguang Hui, Wei Ji
Data analysis and interpretation: Luhua Wang, Honghai Dai, Zhouguang Hui
Manuscript writing: Honghai Dai, Zhouguang Hui
Final approval of manuscript: Luhua Wang, Honghai Dai, Zhouguang Hui
References
- 1.Vansteenkiste JF, De Leyn PP, Deneffe GJ, et al. Clinical prognostic factors in surgically treated stage IIIA-N2 non-small cell lung cancer: Analysis of the literature. Lung Cancer. 1998;19:3–13. doi: 10.1016/s0169-5002(97)00072-x. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki K, Nagai K, Yoshida J, et al. The prognosis of surgically resected N2 non-small cell lung cancer: The importance of clinical N status. J Thorac Cardiovasc Surg. 1999;118:145–153. doi: 10.1016/S0022-5223(99)70153-4. [DOI] [PubMed] [Google Scholar]
- 3.Kim KJ, Ahn YC, Lim do H, et al. Analyses on prognostic factors following tri-modality therapy for stage IIIa non-small cell lung cancer. Lung Cancer. 2007;55:329–336. doi: 10.1016/j.lungcan.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Martini N, Flehinger BJ. The role of surgery in N2 lung cancer. Surg Clin North Am. 1987;67:1037–1049. doi: 10.1016/s0039-6109(16)44341-0. [DOI] [PubMed] [Google Scholar]
- 5.Casali C, Stefani A, Natali P, et al. Prognostic factors in surgically resected N2 non-small cell lung cancer: The importance of patterns of mediastinal lymph nodes metastases. Eur J Cardiothorac Surg. 2005;28:33–38. doi: 10.1016/j.ejcts.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Andre F, Grunenwald D, Pignon JP, et al. Survival of patients with resected N2 non-small-cell lung cancer: Evidence for a subclassification and implications. J Clin Oncol. 2000;18:2981–2989. doi: 10.1200/JCO.2000.18.16.2981. [DOI] [PubMed] [Google Scholar]
- 7.Arriagada R, Bergman B, Dunant A, et al. International Adjuvant Lung cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 8.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 9.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 10.PORT Meta-analysis Trialists Group. Postoperative radiotherapy in non-small-cell lung cancer: Systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet. 1998;352:257–263. [PubMed] [Google Scholar]
- 11.Bekelman JE, Rosenzweig KE, Bach PB, et al. Trends in the use of postoperative radiotherapy for resected non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;66:492–499. doi: 10.1016/j.ijrobp.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Uno T, Sumi M, Kihara A, et al. Postoperative radiotherapy for non-small-cell lung cancer: Results of the 1999–2001 patterns of care study nationwide process survey in Japan. Lung Cancer. 2007;56:357–362. doi: 10.1016/j.lungcan.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Lally BE, Zelterman D, Colasanto JM, et al. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2006;24:2998–3006. doi: 10.1200/JCO.2005.04.6110. [DOI] [PubMed] [Google Scholar]
- 14.Douillard JY, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: The adjuvant Navelbine International Trialist Association (ANITA) randomized trial. Int J Radiat Oncol Biol Phys. 2008;72:695–701. doi: 10.1016/j.ijrobp.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 15.Munro AJ. What now for postoperative radiotherapy for lung cancer? Lancet. 1998;352:250–251. doi: 10.1016/S0140-6736(98)22030-7. [DOI] [PubMed] [Google Scholar]
- 16.Zou B, Xu Y, Li T, et al. A multicenter retrospective analysis of survival outcome following postoperative chemoradiotherapy in non-small-cell lung cancer patients with N2 nodal disease. Int J Radiat Oncol Biol Phys. 2010;77:321–328. doi: 10.1016/j.ijrobp.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 17.Moretti L, Yu DS, Chen H, et al. Prognostic factors for resected non-small cell lung cancer with pN2 status: Implications for use of postoperative radiotherapy. The Oncologist. 2009;14:1106–1115. doi: 10.1634/theoncologist.2009-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley JD, Paulus R, Graham MV, et al. Phase II trial of postoperative adjuvant paclitaxel/carboplatin and thoracic radiotherapy in resected stage II and IIIA non-small-cell lung cancer: Promising long-term results of the Radiation Therapy Oncology Group–RTOG 9705. J Clin Oncol. 2005;23:3480–3487. doi: 10.1200/JCO.2005.12.120. [DOI] [PubMed] [Google Scholar]
- 19.Lung Cancer Study Group. Effects of postoperative mediastinal radiation on completely resected stage II and stage III epidermoid cancer of the lung. N Engl J Med. 1986;315:1377–1381. doi: 10.1056/NEJM198611273152202. [DOI] [PubMed] [Google Scholar]
- 20.Stephens RJ, Girling DJ, Bleehen NM, et al. The role of post-operative radiotherapy in non-small-cell lung cancer: A multicentre randomised trial in patients with pathologically staged T1–2, N1–2, M0 disease. Br J Cancer. 1996;74:632–639. doi: 10.1038/bjc.1996.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng QF, Wang M, Wang LJ, et al. A study of postoperative radiotherapy in patients with non-small-cell lung cancer: A randomized trial. Int J Radiat Oncol Biol Phys. 2000;47:925–929. doi: 10.1016/s0360-3016(00)00509-5. [DOI] [PubMed] [Google Scholar]
- 22.Scotti V, Meattini I, Saieva C, et al. Post-operative radiotherapy in N2 non-small cell lung cancer: A retrospective analysis of 175 patients. Radiother Oncol. 2010;96:84–88. doi: 10.1016/j.radonc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Dautzenberg B, Arriagada R, Chammard AB, et al. A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma. Cancer. 1999;86:265–273. doi: 10.1002/(sici)1097-0142(19990715)86:2<265::aid-cncr10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: A phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:318–328. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 25.Keller SM, Adak S, Wagner H, et al. A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non-small-cell lung cancer. Eastern Cooperative Oncology Group. N Engl J Med. 2000;343:1217–1222. doi: 10.1056/NEJM200010263431703. [DOI] [PubMed] [Google Scholar]
- 26.Machtay M, Lee JH, Shrager JB, et al. Risk of death from intercurrent disease is not excessively increased by modern postoperative radiotherapy for high-risk resected non-small-cell lung carcinoma. J Clin Oncol. 2001;19:3912–3917. doi: 10.1200/JCO.2001.19.19.3912. [DOI] [PubMed] [Google Scholar]
- 27.Wakelee HA, Stephenson P, Keller SM, et al. Post-operative radiotherapy (PORT) or chemoradiotherapy (CPORT) following resection of stages II and IIIA non-small cell lung cancer (NSCLC) does not increase the expected risk of death from intercurrent disease (DID) in Eastern Cooperative Oncology Group (ECOG) trial E3590. Lung Cancer. 2005;48:389–397. doi: 10.1016/j.lungcan.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Le Péchoux C, Dunant A, Pignon JP, et al. Need for a new trial to evaluate adjuvant postoperative radiotherapy in non-small-cell lung cancer patients with N2 mediastinal involvement. J Clin Oncol. 2007;25:e10–e11. doi: 10.1200/JCO.2006.09.6263. [DOI] [PubMed] [Google Scholar]