The role of trastuzumab for the treatment of patients with human epidermal growth factor receptor 2–positive metastatic breast cancer is reviewed.
Keywords: Monoclonal antibody, Trastuzumab, Cardiotoxicity, Safety monitoring
Abstract
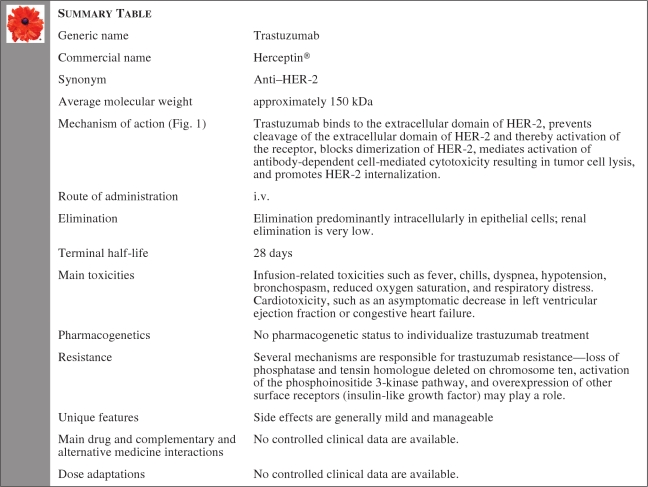
Trastuzumab is standard of care in the treatment of human epidermal growth factor receptor (HER)-2+ early and advanced breast cancer. Recently, it has been approved for the treatment of HER-2+ advanced gastric cancer. Trastuzumab is an IgG1 humanized monoclonal antibody administered by intravenous infusion on a weekly or three weekly schedule. In all registered indications, trastuzumab is almost always given in combination with chemotherapy. In hormonal receptor-positive breast cancer in postmenopausal women, trastuzumab can be combined with an aromatase inhibitor. Main toxicity is reduction in the left ventricular ejection fraction, which in a minority of patients can become symptomatic, but in many patients is at least partly reversible. Long-term safety needs to be further determined.
Introduction
Trastuzumab (Herceptin®; F. Hoffmann-La Roche, Basel, Switzerland) is registered for the treatment of human epidermal growth factor receptor (HER)-2+ metastatic breast cancer, for adjuvant treatment of localized HER-2+ breast cancer, and for HER-2+ metastatic adenocarcinoma of the stomach or gastroesophageal junction. In the U.S. and European Union (EU), trastuzumab is indicated for breast cancer patients with a proven amplification of the HER-2 oncogene or overexpression of the HER-2 protein in the tumor. Overexpression of HER-2 or amplification of HER-2 is associated with adverse disease prognosis and shorter overall and disease-free survival times [1, 2]. Trastuzumab is indicated in metastatic HER-2+ breast cancer patients: (a) as monotherapy after at least one or more chemotherapy regimens, (b) in combination with paclitaxel (U.S., EU), (c) in combination with docetaxel, and (d) in combination with an aromatase inhibitor in postmenopausal women with endocrine-responsive breast cancer not previously treated with trastuzumab (EU). Patients with endocrine-responsive breast cancer must have failed hormonal therapy before trastuzumab is indicated (EU) [3–6]. Trastuzumab is indicated in HER-2+ early breast cancer: (a) as adjuvant treatment (U.S., EU) and (b) as neoadjuvant treatment (EU). Trastuzumab is also indicated in HER-2+ metastatic adenocarcinoma of the stomach or gastroesophageal junction: (a) in combination with capecitabine or 5-fluorouracil and cisplatin in patients who have not received prior anticancer therapy for their metastatic disease (U.S., EU).
Side effects of trastuzumab treatment are often mild and mostly manageable. The major side effect of trastuzumab treatment is a reduction in left ventricular ejection fraction (LVEF), in a small proportion of patients even leading to advanced congestive heart failure (CHF), which appears to be at least partly reversible [7].
Clinical Benefit of Trastuzumab Treatment
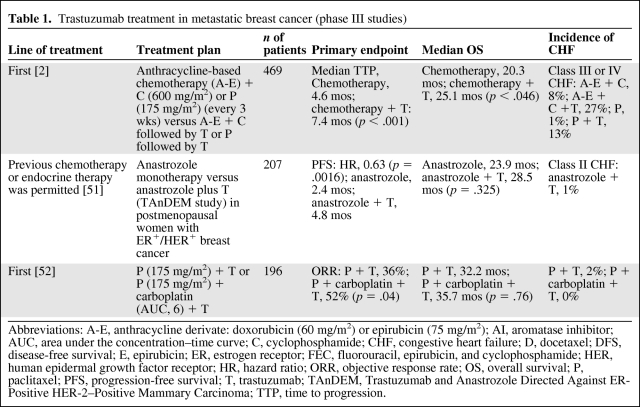
Trastuzumab has been shown to benefit patients with HER-2+ metastatic breast cancer when applied as monotherapy or used in combination with chemotherapy. In phase II studies, trastuzumab treatment was effective and well tolerated. An overview of the phase II studies in which trastuzumab was tested in advanced breast cancer was presented in a recent review [8]. In phase III studies, the addition of trastuzumab to standard chemotherapy was associated with a longer time to disease progression (7.4 months versus 4.6 months), longer duration of response (9.1 months versus 6.1 months), and longer overall survival time (25.1 month versus 20.3 months) (Table 1).
Table 1.
Trastuzumab treatment in metastatic breast cancer (phase III studies)
Abbreviations: A-E, anthracycline derivate: doxorubicin (60 mg/m2) or epirubicin (75 mg/m2); AI, aromatase inhibitor; AUC, area under the concentration–time curve; C, cyclophosphamide; CHF, congestive heart failure; D, docetaxel; DFS, disease-free survival; E, epirubicin; ER, estrogen receptor; FEC, fluorouracil, epirubicin, and cyclophosphamide; HER, human epidermal growth factor receptor; HR, hazard ratio; ORR, objective response rate; OS, overall survival; P, paclitaxel; PFS, progression-free survival; T, trastuzumab; TAnDEM, Trastuzumab and Anastrozole Directed Against ER-Positive HER-2–Positive Mammary Carcinoma; TTP, time to progression.
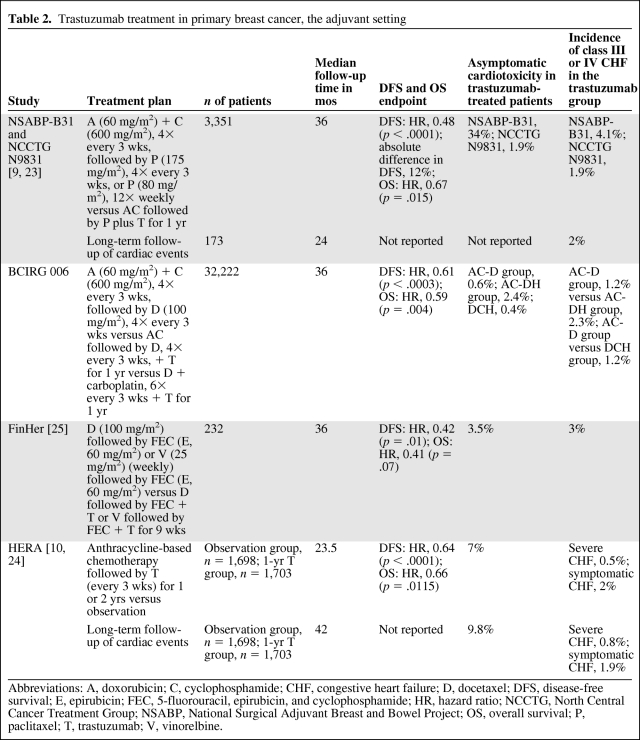
The addition of trastuzumab in the (neo)adjuvant setting resulted in an lower risk for recurrence or death. Three large, randomized trials evaluating the use of trastuzumab after adjuvant standard chemotherapy showed beneficial effects of the addition of trastuzumab to standard adjuvant treatment. A combined analysis of the North Central Cancer Treatment Group (NCCTG) N9831 trial and the National Surgical Adjuvant Breast and Bowel Project (NSABP)-B31 trial (n = 3,351) showed beneficial effects in terms of the disease-free survival rate (87% versus 75%) and overall survival rate (94% versus 92%) after a median follow-up of 3 years [9]. A large European study, Herceptin® Adjuvant (HERA), showed that patients treated with trastuzumab had an absolute disease-free survival benefit of 6.3% (80.6% versus 74.3%) at 3 years [10]. A fourth adjuvant trastuzumab trial, the Breast Cancer International Research Group 006 study, also showed a disease-free survival benefit for patients treated with trastuzumab when combined with standard chemotherapy. Table 2 presents an overview of the published adjuvant trastuzumab trials.
Table 2.
Trastuzumab treatment in primary breast cancer, the adjuvant setting
Abbreviations: A, doxorubicin; C, cyclophosphamide; CHF, congestive heart failure; D, docetaxel; DFS, disease-free survival; E, epirubicin; FEC, 5-fluorouracil, epirubicin, and cyclophosphamide; HR, hazard ratio; NCCTG, North Central Cancer Treatment Group; NSABP, National Surgical Adjuvant Breast and Bowel Project; OS, overall survival; P, paclitaxel; T, trastuzumab; V, vinorelbine.
Recently, clinical benefit was demonstrated in other malignancies with HER-2 overexpression, in particular, gastric cancer. The international phase III Trastuzumab for Gastric Cancer trial showed an overall survival duration of 13.5 months in the treatment group, compared with 11 months in the control group (hazard ratio, 0.74; 95% confidence interval, 0.60–091; p = .0048) [11]. This clinical improvement was considered convincing enough to halt the trial and to obtain U.S. Food and Drug Administration and European Medicines Agency registration for the first-line treatment of HER-2+ gastric cancer.
Clinical Use
Trastuzumab is administrated by i.v. infusion and is applied in a weekly or 3-weekly schedule. The weekly schedule is initiated for monotherapy or in combination with chemotherapy. The weekly dose of trastuzumab is 2 mg/kg starting 1 week after a loading dose of trastuzumab of 4 mg/kg. The 3-weekly schedule of trastuzumab starts with a loading dose of trastuzumab of 8 mg/kg, followed by 6 mg/kg trastuzumab every 21 days. Trastuzumab in doses of 2 mg/kg can be administered as a 30-minute infusion, but higher doses, of 4 mg/kg or 6 mg/kg, require approximately 90 minutes.
Summary Table
Mechanism of Action
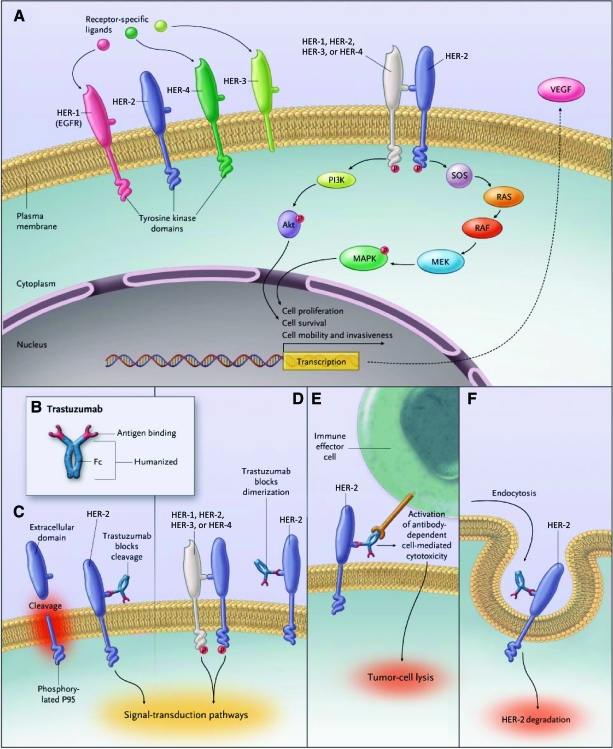
Trastuzumab is a recombinant humanized IgG1 monoclonal antibody against the extracellular domain of the HER-2 receptor (ErbB-2). The HER-2 receptor consists of an extracellular ligand-binding domain, a transmembrane region, and an intracellular or cytoplasmic tyrosine kinase domain. Trastuzumab binds to the extracellular domain of HER-2 and prevents cleavage of the extracellular domain of HER-2 and thereby activation of the receptor; blocks the dimerization of HER-2; mediates activation of antibody-dependent cell-mediated cytotoxicity, resulting in tumor cell lysis; and promotes HER-2 internalization (Fig. 1). Trastuzumab treatment is effective only in patients with amplified HER-2 or overexpression of HER-2 [12].
Figure 1.
Signal transduction by the HER family and potential mechanisms of action of trastuzumab.
Abbreviations: EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; MAPK, mitogen-activated protein kinase; MEK, MAPK/extracellular signal–related kinase kinase; PI3K, phosphoinositide 3-kinase; SOS, son of sevenless; VEGF, vascular endothelial growth factor.
Reprinted with kind permission from Hudis CA. Trastuzumab—Mechanism of action and use in clinical practice. N Engl J Med 2007;357:39–51. ©2007 Massachusetts Medical Society.
Molecular Pathologic Diagnosis
The accuracy of HER-2 assays is essential for testing HER-2 status in breast and gastric cancer patients; however, the outcome of assays to determine HER-2 status varies substantially among laboratories. Validated methods for HER-2 testing need to be used for resolving discrepancies in HER-2 testing. HER-2 status is mostly tested by immunohistochemistry (IHC, HercepTest™; Dako, Glostrup, Denmark), and in some cases by fluorescent in situ hybridization (FISH) or by chromogenic in situ hybridization (CISH) [12]. In current practice, the use of HercepTest™ is considered insufficient in patients with, for example, a HER-2 2+ outcome. Equivocal IHC samples (2+) must be retested for HER-2 amplification by FISH or CISH [13]. Equivocal FISH or CISH results must be confirmed by counting additional cells or repeating the test with a different method. The pathologist scores the test as 0, 1+, 2+, or 3+, and only 3+ and/or showing HER-2 amplification by FISH or CISH identifies patients for trastuzumab treatment. In the case of gastric cancer a 3+ IHC score combined with a positive FISH result is considered necessary for trastuzumab treatment [13].
Bioanalysis of Trastuzumab
Trastuzumab can be quantified in human serum by an enzyme-linked immunosorbent assay (ELISA). A precipitate-enhanced immunoassay (PEIA) has also been developed and results demonstrate good correlation between the ELISA and PEIA methods [14]. It is presently not clear whether variations in plasma concentrations are predictive of toxicity or treatment failure.
Pharmacokinetics
The relevance of trastuzumab pharmacokinetics is unclear in relation to response or toxicity.
Elimination
Trastuzumab is metabolized to peptides and amino acids. The elimination is a complex of processes in humans but is specifically mediated by epithelial cells. Trastuzumab binds to HER-2 and is metabolized intracellularly. The consequence of intracellular binding explains a dose-dependent (nonlinear) elimination. The elimination of antibodies from the plasma is complex and dependent on factors such as genetics and the clinical status of the patient. The half-life of trastuzumab is approximately 28 days. The washout period is up to 24 weeks after cessation of trastuzumab treatment. The renal excretion of trastuzumab is very low.
Contraindications for Trastuzumab Treatment
Contraindications for trastuzumab treatment include a history of hypersensitivity to trastuzumab treatment or murine proteins, severe dyspnea at rest resulting from complications of advanced malignancy, and requiring supplementary oxygen therapy.
Pregnancy and Lactation
Data on the effect of trastuzumab treatment on the development of the human fetus are limited [15]. Some cases of oligohydramnios during the second and third trimesters and reversible fatal renal failure have been reported in pregnant women receiving trastuzumab treatment [16]. Trastuzumab treatment is indicated if the potential benefit for the mother outweighs the potential risk to the fetus, but the fetus must be strictly monitored. Lactation should be avoided during trastuzumab treatment and for 6 months after the last administration of trastuzumab.
Drug Interaction and Complementary and Alternative Medicine Interaction
No controlled clinical data are available on drug interactions or complementary and alternative medicine interactions.
Alterations in Patient Characteristics
Data suggest that the disposition of trastuzumab is not influenced by age or renal function.
Pharmacogenetics
The aim of pharmacogenetics and trastuzumab treatment is to determine whether there is a correlation between genetic polymorphism, such as in HER-2, and response to trastuzumab treatment or the development of trastuzumab-associated toxicity, such as cardiotoxicity. Most reported polymorphisms affecting the efficacy of anticancer treatment are single nucleotide polymorphisms (SNPs) [17]. Several SNPs in the extracellular, transmembrane, and intracellular regions of HER-2 have been studied; however, their reported influence on trastuzumab efficacy remains controversial [18, 19]. Currently, there are no arguments to determine the pharmacogenetic status of HER-2 to individualize trastuzumab treatment.
Pharmacodynamics
Recommended trastuzumab dosages are for monotherapy and for trastuzumab in combination with chemotherapy, used in the (neo-)adjuvant or metastatic setting. Higher doses and longer dosing intervals show no significant benefit over standard dose schedules. There are no algorithms for dose reductions of trastuzumab if significant toxicity develops.
Special Precautions
Caution should be exercised in patients who are experiencing dyspnea at rest as a result of complications of advanced malignancy and comorbidities; in patients with chronic heart failure, hypertension, and coronary artery disease; and in patients treated with prior anthracycline-based chemotherapy.
Cardiac Dysfunction
Short-term side effects of trastuzumab are generally mild and manageable. However, cardiac dysfunction is an important side effect of trastuzumab treatment. Cardiac dysfunction has been reported in patients who received trastuzumab as a single agent or in combination with chemotherapy for metastatic disease and in primary breast cancer [20, 21]. An indirect comparison of cardiac events among clinical studies is hampered by differences in the applied treatments, in inclusion and exclusion criteria, in the time interval between anthracycline-based chemotherapy and trastuzumab treatment, and in the definition of cardiotoxicity. A meta-analysis of randomized clinical trials in patients treated with sequential anthracycline-based chemotherapy and trastuzumab in the adjuvant setting reported significantly higher risks of 1.4% and 5.6% for grade III–IV CHF and asymptomatic cardiotoxicity, respectively [22].
However, the highest incidence of cardiac dysfunction was reported in metastatic breast cancer patients who were treated concurrently with anthracycline-based chemotherapy and trastuzumab [2]. Based on this observation, strict cardiac monitoring was incorporated into the adjuvant trastuzumab trials.
Recently, long-term cardiac safety data from three large randomized adjuvant trastuzumab trials (NCCTG N983, NSABP-B31, and HERA) were presented. Patients with CHF in the NSABP-B31 and NCCTG N9831 trials were reviewed by an independent Adjuvant Cardiac Review and Evaluation Committee (ACREC) [23]. CHF was defined as clinical symptoms, objective physical findings, and an LVEF drop of 10% or an LVEF drop to an absolute LVEF <50%. Based on previously documented cardiotoxicity data, 173 patients were evaluated: 40 patients treated with chemotherapy alone and 133 trastuzumab-treated patients. The ACREC confirmed CHF in eight patients (0.45%) who received chemotherapy alone and in 36 trastuzumab-treated patients (2%) after a median follow-up of 2 years. A higher rate of CHF was associated with age >50 years and a lower LVEF at the start of trastuzumab treatment.
A long-term follow-up study of the HERA trial evaluated the incidences of asymptomatic cardiotoxicity and CHF after a median follow-up of 3.6 years [24]. A significant LVEF decrease (asymptomatic cardiotoxicity) was defined as an absolute decline of at least 10 percentage points from the baseline LVEF and to <50%. Severe CHF was defined as New York Heart Association class III or class IV CHF, confirmed by a cardiologist, and a significant LVEF decrease. Symptomatic CHF was defined as symptomatic CHF confirmed by a cardiologist and a significant LVEF decrease. A total of 164 trastuzumab-treated patients (9.8%) and 49 patients (2.9%) who received chemotherapy alone experienced asymptomatic cardiotoxicity. Thirteen trastuzumab-treated patients (0.8%) developed severe CHF, versus none treated with chemotherapy alone. Thirty-two trastuzumab patients (1.9%) developed symptomatic CHF, versus two patients (0.1%) in the chemotherapy alone group.
In a subset of patients, trastuzumab treatment was discontinued because of cardiac disorders. In the NSABP-B31 and NCCTG N9831 trials, 16.4% of patients discontinued trastuzumab treatment because of a confirmed asymptomatic decline in LVEF and 4.7% of patients discontinued trastuzumab treatment because of symptoms of CHF [9]. In an analysis of the HERA study, 5.1% of patients discontinued trastuzumab treatment before completion of the treatment plan because of cardiac dysfunction. Premature discontinuation of trastuzumab treatment might limit trastuzumab-associated treatment benefit in the adjuvant setting. However, the optimal duration of adjuvant trastuzumab treatment has not yet been determined. The efficacy of 9 weeks of trastuzumab in one small adjuvant trastuzumab study (the Finland Herceptin® study) was similar to that seen in the large adjuvant trials [25]. This raises the question of whether or not discontinuation of trastuzumab treatment is associated with a worse prognosis. Currently, the standard duration of adjuvant trastuzumab treatment is 1 year. However, the optimal duration of trastuzumab treatment has yet to be determined.
Results from the NCCTG N9831 trial, NSABP-B31 trial, and HERA trial suggest that trastuzumab-associated cardiac dysfunction has a high rate of reversibility. Complete or partial recovery was observed in 86.1% of the trastuzumab-treated patients with CHF in the combined analysis of the NSABP-B31 and NCCTG N9831 trials. In the HERA trial, 81% of the patients reached acute recovery of a cardiac event. An acute recovery was defined as two or more sequential LVEF assessments ≥50% after the date of the cardiac event.
In their editorial, Morris and Hudis criticized these reports on the point of data collection [26]. Data from the long-term follow-up studies were not selected prospectively but were based on retrospectively documented cardiotoxicity data. The retrospective design of these studies can lead to an underestimation of the incidence and reversibility of cardiotoxicity. Incomplete follow-up of the patient and underdiagnosis of other cardiac diseases can influence the accuracy of trastuzumab-associated cardiotoxicity.
All adjuvant trials had strict exclusion criteria concerning pre-existing cardiovascular morbidity. This makes the outcome of these trials difficult to translate to an unselected patient population. It is unclear whether or not classical cardiac risk factors are predisposing factors for trastuzumab-associated cardiotoxicity. The incidence of cardiotoxicity may well be higher in unselected patient populations than reported in clinical trials in selected patients. Pre-existing hypertension, a smoking history, and a family history of coronary artery disease were risk factors for developing CHF in a retrospective trial in a Canadian trastuzumab-treated patient population [27]. Another retrospective trial suggested no relationship between these factors and trastuzumab-associated cardiotoxicity, but there was a significant relationship between baseline LVEF and the risk for cardiotoxicity (p = .001) [28]. Therefore, more studies are needed to investigate the cardiac safety of trastuzumab treatment in a general population.
In conclusion, trastuzumab treatment is associated with cardiac dysfunction, is mostly medically manageable with CHF medication, and is in most cases reversible when trastuzumab is discontinued. Trastuzumab-associated cardiotoxicity is different from anthracycline-associated cardiotoxicity, which is dose dependent, not reversible, and results in ultrastructural abnormalities, as observed in myocardial biopsies. Based on current data, HER-2+ breast cancer patients can be safely treated with trastuzumab. However, we need longer follow-up from adjuvant studies, further research to establish the incidence of trastuzumab-induced cardiotoxicity in general patient populations, and research on screening methods and cardioprotective drugs in trastuzumab-treated patients.
It is important to decrease the morbidity and mortality of trastuzumab treatment in breast cancer patients. Currently, a prospective, randomized, double-blind, placebo-controlled trial is ongoing in The Netherlands to evaluate the efficacy of the angiotensin II receptor blocker candesartan in the prevention of trastuzumab-associated cardiotoxicity.
Mechanisms of Trastuzumab-Associated Cardiotoxicity
In embryonic wild-type mice, HER-2 is immunohistochemically present in myocardial and endocardial cells [29, 30]. Cardiomyocyte HER-2 expression is mostly restricted to the T-tubular network, indicating a nonrandom cardiac distribution pattern [31]. It is therefore likely that HER-2 regulates circumscriptive processes in cardiac physiology. Evidence of HER-2 involvement in the physiology and pathophysiology of the heart is demonstrated in conditional mutant mice with cardiac-restricted HER-2 deletion. These mice showed no abnormalities at birth, but shortly after birth they developed dilated cardiomyopathy [29, 31–33].
HER-2 appears to play an important role in compensatory cardiac hypertrophy. Hypertrophic growth can serve as a compensatory mechanism for different mechanical, hemodynamic, hormonal, and pathologic stimuli. Aortic banding in conditionally mutated mice with cardiac-restricted HER-2 deficiency did not result in a hypertrophic growth response.
The precise role of HER-2 in human cardiac physiology and disease is still unknown. Myocardial HER-2 mRNA expression was studied in left ventricle biopsies from 36 patients with severe CHF resulting from ischemic or nonischemic cardiomyopathy undergoing left ventricular assist device implantation. HER-2 was upregulated after implantation of the device, whereas HER-2 prior to implantation was comparable with that of healthy controls [34]. Recently, in six of 60 severe CHF patients, immunohistochemical expression of HER-2 (and HER-4) was shown in myocardial biopsies [35].
Clinical Monitoring
Monitoring Cardiac Function
For identification of trastuzumab-related cardiotoxicity, all trastuzumab-treated patients should undergo a complete medical history, physical examination, electrocardiogram, and measurement of LVEF at baseline of trastuzumab treatment. Furthermore, it is recommended that cardiac function be monitored by LVEF evaluation every 3 months during trastuzumab treatment.
Preliminary data suggest that plasma N-terminal pro B-type natriuretic peptide (NT-proBNP) and troponin I may be parameters to detect or predict trastuzumab-induced cardiotoxicity. In a study by Perik et al. [36], pretreatment plasma NT-proBNP levels were higher in patients with heart failure during trastuzumab treatment (n = 3) than in patients without heart failure (n = 12). A recently published trial revealed a significant relationship between troponin I, a well-established specific and sensitive marker of myocardial injury, and trastuzumab-associated cardiotoxicity. Patients with elevated troponin I levels were at risk for trastuzumab-associated cardiotoxicity, and recovery of trastuzumab-associated cardiotoxicity was unlikely [37]. These findings suggest that NT-proBNP and troponin I may be useful parameters for identifying patients at risk for trastuzumab-induced cardiotoxicity. However, more evidence is needed before these parameters can be applied as biomarkers to identify patients at risk for the development of trastuzumab-associated cardiotoxicity during trastuzumab treatment.
Infusion-Related Reactions of Trastuzumab
Trastuzumab may cause infusion-related reactions. Most trastuzumab-related reactions occur during the first infusion or within 24 hours after infusion. These are generally mild and occur infrequently with subsequent trastuzumab infusions. The overall incidence of severe infusion-related reactions is rare and is <1%. These infusion-related reactions include fever, chills, dyspnea, hypotension, bronchospasm, reduced oxygen saturation, and respiratory distress. Fatal reactions, however, have been reported. In patients with severe trastuzumab-related hypersensitivity, the safety of rechallenge is unknown. In 33 (85%) of 39 patients with a previous severe hypersensitivity reaction, rechallenge of trastuzumab treatment was safe with supportive therapy [38].
Patient Instructions and Recommendations for Supportive Care
Trastuzumab should be administrated by i.v. infusion. Patients should be observed for hypersensitivity reactions during trastuzumab administration, especially during the first infusion. It is advised to monitor cardiac function before the start of trastuzumab treatment, every 3 months during trastuzumab treatment, and at least 6 months after discontinuation of trastuzumab treatment.
Biomarkers of Trastuzumab Resistance
Not all HER-2+ breast cancer patients respond to trastuzumab treatment. Several mechanisms have been proposed that might explain intrinsic trastuzumab resistance. Deficiency of phosphatase and tensin homologue and activation of phosphoinositide 3-kinase results in greater activity of the Akt–mammalian target of rapamycin signal transduction pathway and these have been shown to be important biomarkers of trastuzumab resistance [39, 40]. Also, the overexpression of other surface receptors, such as insulin-like growth factor, provides alternative growth-factor signaling and is related to lower trastuzumab sensitivity [41]. In vitro studies have shown that greater expression of mucin 4 results in greater retention of HER-2 and HER-3 at the cell surface. As a result, growth factor receptors cannot be degraded, with greater signaling potential and lower trastuzumab sensitivity as results [42].
Trastuzumab Treatment Beyond Progression
The optimal therapeutic strategy beyond progression during trastuzumab treatment is not well known. A prospective trial (prematurely closed) described the clinical outcome of 156 patients after progressive disease during treatment with trastuzumab. Patients received capecitabine alone or in combination with trastuzumab. The addition of trastuzumab to capecitabine was associated with a longer time to disease progression (8.2 months versus 5.6 months), longer overall survival time (25.5 months versus 20.4 months), and higher overall response rate (48.1% versus 27%) [43]. Although prospective data are limited, on the basis of retrospective analysis, there is consensus that trastuzumab should be continued until tumor progression. Trastuzumab is also applied beyond progression, whereby the accompanying chemotherapy is switched. For example, trastuzumab plus paclitaxel is changed into trastuzumab plus capecitabine or trastuzumab plus vinorelbine. Phase II studies to support this strategy are lacking, as are studies to compare this strategy with replacement of trastuzumab by lapatinib. At present, we should focus on well-designed clinical trials to establish the optimal strategy in this setting for patients.
Novel Anti-ErbB-2 Therapies
Patients with HER-2+ breast cancer eventually experience relapse or progression on trastuzumab treatment. Trastuzumab binds to the extracellular domain of HER-2, but inhibition of one signal transduction pathway may not be enough because it does not control all HER-2+ breast cancer tumors. Therefore, there is a need for novel, effective anti-ErbB-2 therapies. Several studies in patients with HER-2+ metastatic breast cancer have been initiated to develop multiple lines of anti-ErbB-2 therapy. Small molecule tyrosine kinase inhibitors (TKIs) may add therapeutic benefit to established antibody-based treatment. TKIs bind to the intracellular domain of HER-2, usually the ATP-binding domain, thereby blocking the HER-2 dimerization step by kinase inhibition and interrupting downstream pathways. Lapatinib, an orally available small molecule, is the only TKI registered for the treatment of HER-2+ metastatic breast cancer (U.S., EU). The most frequently reported side effects of lapatinib therapy are diarrhea, skin rash, and asymptomatic cardiotoxicity. Lapatinib is established as effective treatment in advanced breast cancer patients, including those with cancers progressing on trastuzumab-based therapy [44]. In a phase III study, 321 HER-2+ metastatic breast cancer patients (previously progressive on trastuzumab treatment) were randomized to receive lapatinib plus capecitabine or capecitabine alone. The median time to progression was 8.4 months in the combination therapy group, compared with 4.4 months in the capecitabine monotherapy group (p < .001) [45, 46]. Lapatinib in combination with endocrine therapy provided clinical benefit in untreated estrogen receptor–positive or HER+ postmenopausal patients. Patients received lapatinib plus letrozol or letrozol plus placebo. The median progression-free survival (PFS) interval was significantly longer in the combination group of lapatinib with letrozol compared with letrozol plus placebo—8.2 months, versus 3 months. A combination of trastuzumab and lapatinib resulted in a significantly longer median PFS time—12 weeks in the lapatinib plus trastuzumab group versus 8.1 weeks in the lapatinib alone group. The overall tumor response rate was not significantly different between the two treatment arms [47].
Neratinib, a pan-ErbB receptor TKI, was shown to be clinically active and well tolerated in patients previously treated with trastuzumab [48]. Patients with advanced HER-2+ breast cancer received neratinib at a dose of 240 mg once daily. Sixty-six patients had received prior trastuzumab treatment and 70 patients were trastuzumab naïve. The median PFS times were 22.3 weeks for patients with prior trastuzumab exposure and 39.6 weeks for patients with no prior trastuzumab treatment. Diarrhea was the most frequently reported side-effect [48]. Phase I and II studies of trastuzumab-DM1 (T-DM1), an antibody-drug conjugate, have shown activity and was well tolerated in patients with prior trastuzumab treatment [49]. The combination of trastuzumab and pertuzumab, a recombinant humanized monoclonal antibody preventing HER-2 dimerization with HER-1, HER-3, and HER-4, was evaluated in a phase II study. The combination of trastuzumab and pertuzumab showed activity, with a median PFS interval of 5.5 months, and was well tolerated in patients who had progressed during prior trastuzumab treatment [50]. Ongoing trials are investigating the efficacy of various new TKIs of HER-2. Moreover, other studies are aiming to define subsets of patients with specific characteristics of the ERB gene who will most likely benefit from these new strategies.
Author Contributions
Conception/Design: A.H. Boekhout, J.H. Beijnen, Jan H.M. Schellens
Provision of study material or patients: A.H. Boekhout, J.H. Beijnen, Jan H.M. Schellens
Collection and/or assembly of data: A.H. Boekhout
Data analysis and interpretation: A.H. Boekhout, J.H. Beijnen, Jan H.M. Schellens
Manuscript writing: A.H. Boekhout, J.H. Beijnen, Jan H.M. Schellens
Final approval of manuscript: A.H. Boekhout, J.H. Beijnen, Jan H.M. Schellens
References
- 1.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Burstein HJ, Keshaviah A, Baron AD, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: The trastuzumab and vinorelbine or taxane study. Cancer. 2007;110:965–972. doi: 10.1002/cncr.22885. [DOI] [PubMed] [Google Scholar]
- 4.Infante JR, Yardley DA, Burris HA, 3rd, et al. Phase II trial of weekly docetaxel, vinorelbine, and trastuzumab in the first-line treatment of patients with HER2-positive metastatic breast cancer. Clin Breast Cancer. 2009;9:23–28. doi: 10.3816/CBC.2009.n.004. [DOI] [PubMed] [Google Scholar]
- 5.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 6.Schaller G, Fuchs I, Gonsch T, et al. Phase II study of capecitabine plus trastuzumab in human epidermal growth factor receptor 2 overexpressing metastatic breast cancer pretreated with anthracyclines or taxanes. J Clin Oncol. 2007;25:3246–3250. doi: 10.1200/JCO.2006.09.6826. [DOI] [PubMed] [Google Scholar]
- 7.Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 8.Brufsky A. Trastuzumab-based therapy for patients with HER2-positive breast cancer: From early scientific development to foundation of care. Am J Clin Oncol. 2010;33:186–195. doi: 10.1097/COC.0b013e318191bfb0. [DOI] [PubMed] [Google Scholar]
- 9.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 10.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 11.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 12.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 13.Vocaturo A, Novelli F, Benevolo M, et al. Chromogenic in situ hybridization to detect HER-2/neu gene amplification in histological and ThinPrep-processed breast cancer fine-needle aspirates: A sensitive and practical method in the trastuzumab era. The Oncologist. 2006;11:878–886. doi: 10.1634/theoncologist.11-8-878. [DOI] [PubMed] [Google Scholar]
- 14.Damen CW, Speijer H, Hermens WT, et al. The bioanalysis of trastuzumab in human serum using precipitate-enhanced ellipsometry. Anal Biochem. 2009;393:73–79. doi: 10.1016/j.ab.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Pant S, Landon MB, Blumenfeld M, et al. Treatment of breast cancer with trastuzumab during pregnancy. J Clin Oncol. 2008;26:1567–1569. doi: 10.1200/JCO.2008.16.0309. [DOI] [PubMed] [Google Scholar]
- 16.Bader AA, Schlembach D, Tamussino KF, et al. Anhydramnios associated with administration of trastuzumab and paclitaxel for metastatic breast cancer during pregnancy. Lancet Oncol. 2007;8:79–81. doi: 10.1016/S1470-2045(06)71014-2. [DOI] [PubMed] [Google Scholar]
- 17.Candelaria M, Taja-Chayeb L, Arce-Salinas C, et al. Genetic determinants of cancer drug efficacy and toxicity: Practical considerations and perspectives. Anticancer Drugs. 2005;16:923–933. doi: 10.1097/01.cad.0000180120.39278.c9. [DOI] [PubMed] [Google Scholar]
- 18.Beauclair S, Formento P, Fischel JL, et al. Role of the HER2 [Ile655Val] genetic polymorphism in tumorogenesis and in the risk of trastuzumab-related cardiotoxicity. Ann Oncol. 2007;18:1335–1341. doi: 10.1093/annonc/mdm181. [DOI] [PubMed] [Google Scholar]
- 19.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 20.Suter TM, Procter M, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac adverse effects in the Herceptin Adjuvant trial. J Clin Oncol. 2007;25:3859–3865. doi: 10.1200/JCO.2006.09.1611. [DOI] [PubMed] [Google Scholar]
- 21.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 22.Bria E, Cuppone F, Fornier M, et al. Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: The dark side of the moon? A meta-analysis of the randomized trials. Breast Cancer Res Treat. 2008;109:231–239. doi: 10.1007/s10549-007-9663-z. [DOI] [PubMed] [Google Scholar]
- 23.Russell SD, Blackwell KL, Lawrence J, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: A combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28:3416–3421. doi: 10.1200/JCO.2009.23.6950. [DOI] [PubMed] [Google Scholar]
- 24.Procter M, Suter TM, de Azambuja E, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010;28:3422–3428. doi: 10.1200/JCO.2009.26.0463. [DOI] [PubMed] [Google Scholar]
- 25.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 26.Morris PG, Hudis CA. Trastuzumab-related cardiotoxicity following anthracycline-based adjuvant chemotherapy: How worried should we be? J Clin Oncol. 2010;28:3407–3410. doi: 10.1200/JCO.2009.26.0125. [DOI] [PubMed] [Google Scholar]
- 27.Wadhwa D, Fallah-Rad N, Grenier D, et al. Trastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: A retrospective study. Breast Cancer Res Treat. 2009;117:357–364. doi: 10.1007/s10549-008-0260-6. [DOI] [PubMed] [Google Scholar]
- 28.Guarneri V, Lenihan DJ, Valero V, et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: The M.D. Anderson Cancer Center experience. J Clin Oncol. 2006;24:4107–4115. doi: 10.1200/JCO.2005.04.9551. [DOI] [PubMed] [Google Scholar]
- 29.Camenisch TD, Schroeder JA, Bradley J, et al. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med. 2002;8:850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- 30.Lee KF, Simon H, Chen H, et al. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 31.Ozcelik C, Erdmann B, Pilz B, et al. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci U S A. 2002;99:8880–8885. doi: 10.1073/pnas.122249299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crone SA, Zhao YY, Fan L, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 33.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 34.Uray IP, Connelly JH, Thomàzy V, et al. Left ventricular unloading alters receptor tyrosine kinase expression in the failing human heart. J Heart Lung Transplant. 2002;21:771–782. doi: 10.1016/s1053-2498(02)00390-x. [DOI] [PubMed] [Google Scholar]
- 35.Fuchs IB, Landt S, Bueler H, et al. Analysis of HER2 and HER4 in human myocardium to clarify the cardiotoxicity of trastuzumab (Herceptin) Breast Cancer Res Treat. 2003;82:23–28. doi: 10.1023/b:brea.0000003916.39959.73. [DOI] [PubMed] [Google Scholar]
- 36.Perik PJ, Lub-De Hooge MN, Gietema JA, et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2006;24:2276–2282. doi: 10.1200/JCO.2005.03.8448. [DOI] [PubMed] [Google Scholar]
- 37.Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: Clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–3916. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 38.Cook-Bruns N. Retrospective analysis of the safety of Herceptin immunotherapy in metastatic breast cancer. Oncology. 2001;61(suppl 2):58–66. doi: 10.1159/000055403. [DOI] [PubMed] [Google Scholar]
- 39.Pandolfi PP. Breast cancer–loss of PTEN predicts resistance to treatment. N Engl J Med. 2004;351:2337–2338. doi: 10.1056/NEJMcibr043143. [DOI] [PubMed] [Google Scholar]
- 40.Park BH, Davidson NE. PI3 kinase activation and response to trastuzumab therapy: What's neu with Herceptin resistance? Cancer Cell. 2007;12:297–299. doi: 10.1016/j.ccr.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Harris LN, You F, Schnitt SJ, et al. Predictors of resistance to preoperative trastuzumab and vinorelbine for HER2-positive early breast cancer. Clin Cancer Res. 2007;13:1198–1207. doi: 10.1158/1078-0432.CCR-06-1304. [DOI] [PubMed] [Google Scholar]
- 42.Moasser MM. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: A German Breast Group 26/Breast International Group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 44.Blackwell KL, Pegram MD, Tan-Chiu E, et al. Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol. 2009;20:1026–1031. doi: 10.1093/annonc/mdn759. [DOI] [PubMed] [Google Scholar]
- 45.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 46.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 47.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 48.Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301–1307. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 49.Krop IE, Beeram M, Modi S, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28:2698–2704. doi: 10.1200/JCO.2009.26.2071. [DOI] [PubMed] [Google Scholar]
- 50.Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: Results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 52.Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006;24:2786–2792. doi: 10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]