This review focuses on the rationale for targeting angiogenesis in oncology and the current and possible future applications of antiangiogenic agents in esophagogastric adenocarcinoma.
Keywords: Angiogenesis, Bevacizumab, Biomarkers, Gastric cancer, Esophageal cancer, VEGF
Learning Objectives
After completing this course, the reader will be able to:
Describe the receptors and ligands with identified roles in tumor angiogenesis and the mechanism of action of established and investigational antiangiogenic agents.
Describe aspects of antiangiogenic agents that are incompletely understood and need further investigation to define their role in esophagogastric cancer.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
The possibility of targeting tumor angiogenesis was postulated almost 40 years ago. The vascular endothelial growth factor (VEGF) family and its receptors have since been characterized and extensively studied. VEGF overexpression is a common finding in solid tumors, including esophagogastric cancer, and frequently correlates with poor prognosis. Monoclonal antibodies, soluble receptors, and small-molecule tyrosine kinase inhibitors have been developed to inhibit tumor angiogenesis, and antiangiogenic therapy is now a component of standard treatment for advanced renal cell, hepatocellular, colorectal, breast, and non-small cell lung carcinomas. The small-molecule tyrosine kinase inhibitors sunitinib and sorafenib have been evaluated in phase II studies in esophagogastric cancer but appear to have only modest activity. Similarly, despite promising efficacy signals from phase II studies, the addition of the anti-VEGF-A monoclonal antibody bevacizumab to cisplatin plus capecitabine failed to result in a longer overall survival duration than with the chemotherapy doublet plus placebo. The response rate and progression-free survival interval were significantly greater with bevacizumab, confirming some efficacy in advanced gastric cancer, but with inadequate benefit to justify the high cost of treatment. Evaluation of bevacizumab in the neoadjuvant and perioperative settings continues, hypothesizing that a higher response rate will translate into longer survival in patients with operable disease. Despite extensive research, the discovery of a reliable predictive biomarker for antiangiogenic therapy continues to elude the scientific and oncology communities, and mechanisms of primary and acquired resistance are incompletely understood. We are therefore currently unable to personalize antiangiogenic therapy for established indications, or use molecular selection for clinical trials evaluating novel indications.
Introduction
Gastric and esophageal cancers are the fourth and eighth most common cancers worldwide, with a combined annual incidence of almost 1.5 million cases and resulting in >1 million deaths per year [1]. For patients with operable disease, multimodality therapy is an internationally accepted standard, because surgery alone results in relatively poor long-term survival. Perioperative chemotherapy [2], adjuvant chemotherapy [3], and chemoradiation [4] produce longer overall survival (OS) times for gastric cancer patients. Similarly, perioperative chemotherapy [2], neoadjuvant chemotherapy [5], and chemoradiation [6] lead to longer OS times for patients with operable esophageal adenocarcinomas.
The majority of patients presenting with esophagogastric carcinoma have advanced disease, with a median survival time of ∼3 months with supportive care alone [7]. With combination chemotherapy, median survival times of 9 and 14 months have been reported for patients with metastatic and locally advanced inoperable disease, respectively [8]. There is no international consensus regarding the optimal first-line chemotherapy regimen; however, treatment with a platinum and fluoropyrimidine doublet [9] or a triplet regimen with the addition of epirubicin [10] or docetaxel [11] is most frequently used. Following successful results in other solid tumors, targeted agents are now being evaluated in esophagogastric cancer. The recent positive results from the randomized phase III ToGA (Trastuzumab for Gastric Cancer) study have changed the treatment paradigm for patients with human epidermal growth factor receptor 2–positive disease, for whom treatment with a platinum and fluoropyrimidine doublet plus trastuzumab is now the standard of care [12].
The second targeted agent to undergo phase III evaluation in advanced esophagogastric cancer was an antiangiogenic agent, bevacizumab. Angiogenesis is an essential event for small, established tumors to grow beyond a critical size of a few millimeters. It is thought that without the necessary microenvironment for neovascularization, tumor growth is arrested. This proposed dependency on angiogenesis, in addition to the lack of angiogenesis in normal tissues under physiological conditions other than embryogenesis, the female menstrual cycle, wound healing, and muscle growth, made angiogenesis a logical therapeutic target, with minimal toxicity to normal tissues expected [13].
Bevacizumab, a monoclonal antibody directed against vascular endothelial growth factor (VEGF)-A, was the first antiangiogenic drug to be clinically evaluated and was licensed for the first-line treatment of patients with metastatic colorectal cancer (mCRC) following the report of an almost 5-month survival benefit when added to irinotecan and 5-fluorouracil (5-FU) [14]. Small-molecule inhibitors of VEGF receptor (VEGFR) tyrosine kinase activity, sunitinib and sorafenib, have since been established as standard first- and second-line therapies, respectively, for patients with metastatic renal cell carcinoma (RCC) [15, 16]. Sorafenib also leads to longer survival in patients with metastatic hepatocellular carcinoma (HCC) and is a standard first-line therapy [17]. Both drugs exert at least part of their therapeutic activity via inhibition of VEGFRs.
This review focuses on the rationale for targeting angiogenesis in oncology and the current and possible future applications of antiangiogenic agents in esophagogastric adenocarcinoma.
The VEGF Family
VEGF-A
VEGF-A is secreted by several human and rodent tumor cell lines [18] and was shown to stimulate endothelial cell growth and angiogenesis [19]. Serum VEGF-A levels in patients with cancer are often higher than normal physiological levels [20]. There are at least 12 isoforms of VEGF-A, although the soluble VEGF-A121 and VEGF-A165 isoforms have been most studied [21]. VEGF-A mediates its effects by binding to two endothelial cell surface tyrosine kinase receptors, VEGFR-1 and VEGFR-2, but activation of VEGFR-2 is considered to be more critical to angiogenesis [22]. Proof of concept for the therapeutic activity of VEGF inhibition was reported in 1993, when a monoclonal antibody directed at VEGF-A was reported to inhibit angiogenesis and tumor growth in human tumor xenografts. That group successfully humanized the antibody, which became known as bevacizumab [23].
Regulation of VEGF-A Expression
Transcription of the gene encoding VEGF-A is mediated by hypoxia inducible factor (HIF)-1, a heterodimeric protein composed of α and β subunits. Under normoxic conditions, prolyl hydroxylase domain proteins hydroxylate the oxygen-dependent degradation domain of HIF-1α, precipitating interaction with the von Hippel Lindau (VHL) protein and subsequent degradation of HIF-1α. A second regulator of HIF-1α, known as factor inhibiting HIF-1, also prevents activation of the HIF pathway in well-oxygenated cells, via hydroxylation of the transcriptional activation domain. However, under hypoxic conditions, neither enzyme is able to hydroxylate its target on HIF-1α, allowing transcription of hypoxic response genes, including VEGF-A [24]. Targeted inhibition of HIF-1α inhibits tumor growth and angiogenesis in animal models, providing a rationale for therapeutic targeting of HIF-1α in oncology [25].
The efficacy of antiangiogenic agents in RCC patients is likely to relate to the frequent inactivation of the VHL gene, accumulation of HIF-1α, and subsequent overexpression of VEGF-A and other proangiogenic factors [26].
Placental Growth Factor, VEGF-B, VEGF-C, VEGF-D, and VEGF-E
Placental growth factor (PlGF) mediates the angiogenic response to VEGF by activating VEGFR-1 [27] and regulating crosstalk between VEGFR-1 and VEGFR-2 [28]. Direct anti-PlGF targeting is of particular interest because of the lack of effect on normal vessels coupled with the activity of an anti-PlGF antibody, 5D11D4, reported in VEGF-resistant tumors [29]. However, these data were recently challenged by a report of impaired wound healing but no inhibition of angiogenesis or growth in tumors by four novel anti-PlGF antibodies [30]. Further preclinical studies of 5D11D4 have confirmed the antitumor effect of this antibody in HCC [31], but the reason for the inconsistent efficacy in preclinical models remains unclear.
VEGF-C is normally expressed in multiple human tissues and preferentially binds to VEGFR-3, although it also binds to and activates VEGFR-2, albeit with lower affinity [32]. VEGF-C expression in animal studies is associated with the frequent development of lymph node metastases [33]. Similarly, detection of VEGF-C in a study of 139 resected gastric cancers with submucosal invasion was significantly associated with the presence of lymph node metastases on multivariate analysis (odds ratio, 4.18; 95% confidence interval [CI], 1.38–12.7; p = .0116) [34].
VEGF-B activates VEGFR-1 but has little angiogenic activity outside the myocardium, where loss of VEGF-B impairs angiogenesis in the ischemic heart [35]. VEGF-D activates VEGFR-2 and VEGFR-3 and stimulates the growth of endothelial cells in vitro, but is approximately five times less potent than VEGF-A and therefore may be a less important therapeutic target [36] VEGF-E appears to bind only to VEGFR-2 and has similar proangiogenic activity to that of VEGF-A [37], but the gene encoding VEGF-E is not present in the human genome and it is therefore unlikely to have a role in cancer treatment.
VEGF Receptors
VEGFR-1, VEGFR-2, and VEGFR-3
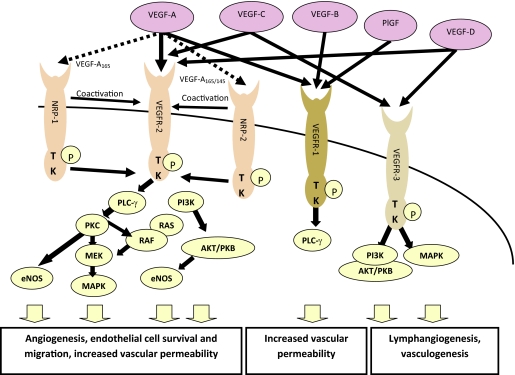
VEGFR-1 through VEGFR-3 are receptor tyrosine kinases that are expressed by vascular and lymphatic endothelial cells, and their expression has also been identified on several normal embryological and adult tissues as well as tumor cells [22]. Figure 1 depicts VEGFRs and downstream signaling pathways.
Figure 1.
The three VEGF receptors, two coreceptors, and downstream signaling pathways. VEGF-A binds to VEGFR-1 and VEGFR-2, with additional isoform-specific binding to the NRP receptors, which coactivate VEGFR-2. VEGF-B and PlGF bind to VEGFR-1, and VEGF-C and VEGF-D both bind to VEGFR-3 and VEGFR-2. Activation of these receptors stimulates a signaling cascade resulting in angiogenesis, increased vascular permeability, and lymphangiogenesis.
Abbreviations: eNOS, endothelial nitric oxide synthase; MAPK, mitogen-activated protein kinase; MEK, MAPK/extracellular signal–related kinase kinase; NRP, neuropilin; PI3K, phospatidylinositol-3-kinase; PKB, protein kinase B; PKC, protein kinase C; PLCγ, phospholipase Cγ; PlGF, placental growth factor; TK, tyrosine kinase; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
VEGFR-2 is considered to be the principal receptor by which VEGF-A induces angiogenesis. The downstream effects of VEGFR-2 activation are mediated by several signaling pathways, including the phospholipase C (PLC)-γ, protein kinase C (PKC), extracellular signal–related kinase (ERK), phospatidylinositol 3-kinase (PI3K), and endothelial nitric oxide synthase (eNOS) pathways [22]. Inhibition of VEGFR-2 was shown to suppress angiogenesis and tumor growth in numerous preclinical models, validating it as a potential target [38, 39].
Despite high-affinity binding to VEGF-A, the level of VEGFR-1 kinase activity is low. Downstream signaling pathways are ill defined, but VEGF induces phosphorylation of PLC-γ, PI3K, PKC, and ERK/mitogen-activated protein kinase (MAPK) [22]. It is thought that VEGFR-1 may act as a decoy receptor, thereby regulating the VEGF-A available to bind VEGFR-2 [22], or act to refine VEGF signaling by heterodimerization with VEGFR-2 [28].
VEGFR-3 is widely expressed in benign and malignant vascular tumors, but not in solid tumors, including undifferentiated carcinomas, in which only the capillaries at the site of neovascularization stain for VEGFR-3 [40]. Downstream signaling via PKC-dependent MAPK activation has been reported in lymphatic endothelial cells [41] and in the Ras–MAPK pathway in human hematopoietic cells [42], but these pathways have not been fully defined. Blockade of VEGFR-3 using a soluble fusion protein, VEGFR-3 immunoglobulin, in a human lung cancer cell line xenograft suppressed tumor lymphangiogenesis and lymph node metastasis but not visceral metastasis [43], suggesting that dual targeting of VEGFR-3 and VEGFR-2 may be valuable.
Several small-molecule inhibitors of VEGFR tyrosine kinase activity have also been developed, including sunitinib, a multi–tyrosine kinase inhibitor (TKI) that potently inhibits VEGFR-1, VEGFR-2, VEGFR-3, platelet-derived growth factor receptors (PDGFRs), and the Kit receptor. Several other TKIs have been evaluated, with those reaching clinical testing including sorafenib, pazopanib, cediranib (AZD2171), and axitinib (AG-013736) [44].
Neuropilin 1 and Neuropilin 2
Neuropilin (NRP)-1 is a molecule that may play multiple roles in angiogenesis. It is perhaps best known as an isoform-specific coreceptor for VEGF-A165 and may promote signaling through VEGFR-2 when the two receptors are coexpressed [45]. However, NRP-1 also mediates signaling of the semaphorins, which may be involved in inhibition of vessel formation [46], and can act as a cell adhesion receptor [47]. NRP-1 can also be expressed in tumor cells. Overexpression of NRP-1 in pancreatic cancer cell lines induces MAPK signaling and is associated with resistance to gemcitabine and 5-FU chemotherapy [48]. Inhibition of NRP-1 suppresses neovascularization in animal models [49], demonstrating the potential of the receptor as a therapeutic target. A small-molecule inhibitor of VEGF-A binding to NRP-1, EG00229, has in vitro activity in lung cancer cell lines, and an apparent synergistic effect with paclitaxel and 5-FU has been reported [50].
NRP-2 is also isoform specific for VEGF-A, binding only the VEGF165 and VEGF145 isoforms, but it also binds PlGF [51]. VEGF-A and VEGF-C can induce interaction of NRP-2 with VEGFR-2, enhancing VEGFR-2 signaling and consequent endothelial cell survival [52]. VEGF-C also weakly induces NRP-2 interaction with VEGFR-3 [52]. Inhibition of NRP-2 in colorectal cancer cell lines leads to impaired tumor growth both in vitro and in vivo [53]. NRP-2 is upregulated in gastric cancer endothelial cells, enhancing the proproliferation and migration effects of VEGF [54].
Clinical Data in Esophagogastric Cancer
Monoclonal Antibodies: Bevacizumab
Bevacizumab first underwent phase III evaluation in mCRC patients, with a significant OS benefit reported [14], precipitating licensing worldwide for this indication. The optimal duration of bevacizumab treatment has not yet been established in this setting, with data from an observational study suggesting that treatment beyond disease progression is associated with longer survival [55], but there are no confirmatory data from a randomized study. Combination with chemotherapy appears necessary for mCRC patients, with minimal monotherapy activity reported in a second-line study [56]. In the curative-intent setting, the addition of bevacizumab to adjuvant 5-FU, leucovorin, and oxaliplatin (FOLFOX) did not result in a longer disease-free survival interval in patients with resected stage II–III colon cancer [57]. Moreover, in the AVANT (Avastin as Chemotherapy for Adjuvant Colon Carcinoma) study of bevacizumab added to adjuvant FOLFOX or capecitabine plus oxaliplatin, both the disease-free and preliminary OS results favored the control arm. Although greater use of bevacizumab after disease progression in the control arm may account, in part, for the longer survival time in that group, it cannot explain the apparent detrimental effect on disease-free survival [58]. This lack of benefit in early disease, on the background of proven efficacy in advanced disease, was previously reported with both the chemotherapeutic agent irinotecan [59, 60] and the targeted agent cetuximab [61]. This suggests significant differences between early and advanced stage disease in terms of drug sensitivity and molecular alterations. Correlative translational work from these adjuvant studies may yet define subgroups of patients who benefit from such agents. Bevacizumab is additionally licensed in combination with interferon for the treatment of patients with advanced RCC, and with chemotherapy for the treatment of patients with advanced breast and non-small cell lung cancers.
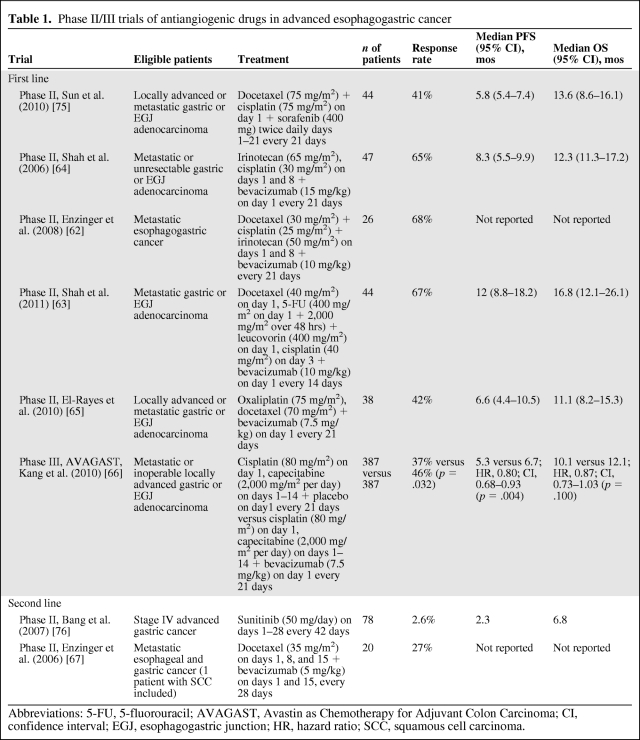
In esophagogastric cancer, response rates (RRs) of 65%–68% were reported in three phase II studies of bevacizumab with combination chemotherapy [62–64], with encouraging median progression-free survival (PFS) and OS times reported in combination with irinotecan and cisplatin [64]. However, a further phase II study reported more modest efficacy in combination with oxaliplatin and docetaxel [65], comparable with the results with chemotherapy triplet regimens in phase III studies [10, 11]. Unfortunately, these results reflect those reported in the international, randomized phase III AVAGAST (Avastin for Advanced Gastric Cancer) study, in which 774 patients with advanced gastric or esophagogastric junction (EGJ) adenocarcinoma were randomized to a cisplatin–fluoropyrimidine doublet with bevacizumab or placebo in just 14 months. Although bevacizumab showed efficacy in this disease in terms of a higher RR and longer median PFS interval, the study failed to meet the primary endpoint of a statistically significant longer OS duration. Possible regional variation in efficacy was reported in a subgroup analysis, with an apparent benefit noted in patients treated in pan-America but no benefit in those treated in Asia [66]. One possible explanation for the apparent geographical variation is the wide variation in the use of second-line chemotherapy, whereby the highest rates were reported in Asian patients (66%) and the lowest rates were reported in pan-American patients (21%). In view of the hypothesis-generating data from mCRC [55], it may be valuable to evaluate bevacizumab beyond disease progression in combination with second-line chemotherapy in a randomized study in advanced gastric cancer.
Bevacizumab was also evaluated in a small phase II study in the second-line treatment of esophagogastric cancer. An encouraging RR of 27% was reported in combination with weekly docetaxel in the 20 evaluable patients, and the final results of the study are awaited [67]. These results are summarized in Table 1.
Table 1.
Phase II/III trials of antiangiogenic drugs in advanced esophagogastric cancer
Abbreviations: 5-FU, 5-fluorouracil; AVAGAST, Avastin as Chemotherapy for Adjuvant Colon Carcinoma; CI, confidence interval; EGJ, esophagogastric junction; HR, hazard ratio; SCC, squamous cell carcinoma.
Initial phase I studies of bevacizumab as monotherapy [68] and in combination with three chemotherapy regimens [69] showed no dose-limiting toxicities, and it was not until phase II and phase III evaluation in mCRC patients that the characteristic toxicities of bevacizumab, including hypertension, proteinuria, arterial and venous thromboembolism, and gastrointestinal perforation, were recognized [14, 70]. In a phase II study combining bevacizumab (15 mg/kg) with irinotecan and cisplatin every 3 weeks in patients with advanced gastric cancer, grade 3–4 venous thromboembolic events were reported in 25.5% of patients, myocardial infarction was reported in one patient, and gastric perforation was reported in two of 47 patients [64]. These unexpectedly high rates of thromboembolic events were inconsistent with published phase III studies of bevacizumab for other solid tumors and may relate, in part, to better detection of asymptomatic pulmonary emboli on computed tomography CT scans. However, additionally, the underlying disease, the irinotecan-based chemotherapy regimen, and possibly the bevacizumab dose may also have contributed. In support of this, in the AVAGAST study, in which bevacizumab (7.5 mg/kg every 3 weeks) was delivered with a cisplatin–fluoropyrimidine doublet, there was not a higher incidence of venous or arterial thromboembolic events than in patients treated with chemotherapy plus placebo. The rates of other expected bevacizumab-related toxicities, including hypertension, bleeding, wound-healing events, and gastrointestinal perforations, were higher in the bevacizumab arm, but the rates of serious complications were low. The recognized rare toxicities of fistula or abscess formation and reversible posterior leukoencephalopathy syndrome were each reported in two of 386 patients randomized to receive bevacizumab [66].
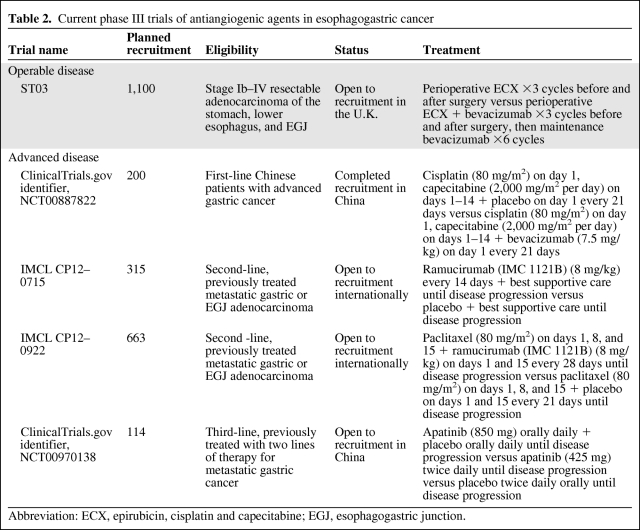
The evaluation of bevacizumab in localized esophagogastric cancer is ongoing in phase II and phase III studies, and only safety data are available. Of 14 evaluable patients treated with bevacizumab (7.5 mg/kg every 3 weeks) in combination with weekly irinotecan and cisplatin chemoradiation, 10 underwent surgery, in whom there were no unexpected surgical or wound-healing problems, but anastomotic leaks were reported in two patients (20%) [71]. In contrast, in the phase II/III ST03 study of perioperative epirubicin, cisplatin, and capecitabine with or without bevacizumab (7.5 mg/kg every 3 weeks), preliminary safety data from the first 104 patients randomized showed no difference in the incidence of wound-healing complications or anastomotic leaks and similar rates of thromboembolic events [72]. Current phase III studies evaluating antiangiogenic agents in esophagogastric cancer patients are listed in Table 2.
Table 2.
Current phase III trials of antiangiogenic agents in esophagogastric cancer
Abbreviation: ECX, epirubicin, cisplatin and capecitabine; EGJ, esophagogastric junction.
VEGFR Antibodies: Ramucirumab (IMC1121B)
Ramucirumab is a fully human IgG1 monoclonal antibody to VEGFR-2. A phase I study of 37 patients with previously treated advanced solid tumors identified a safety profile similar to that of bevacizumab, with serious adverse events including dose-related hypertension, venous thromboembolism, and proteinuria reported. The maximum tolerated weekly dose was 13 mg/kg, although pharmacokinetic studies demonstrated that clearance of the drug was saturated at 8 mg/kg. Partial responses were observed in four patients, including one with previously treated gastric cancer [73]. Phase III evaluation of ramucirumab as monotherapy and in combination with weekly paclitaxel in previously treated advanced gastric cancer patients is under way.
TKIs
Sorafenib is an oral multitargeted TKI that inhibits VEGFR-1, VEGFR-2, and VEGFR-3, PDGFRs, B-Raf, Raf-1, and c-Kit. Sorafenib monotherapy led to a longer OS time in metastatic HCC patients [17] and a longer PFS interval as second-line therapy in metastatic RCC patients [15]. Common toxicities included diarrhea, fatigue, hypertension, hand–foot syndrome, rash, alopecia, anorexia, and nausea. Serious cardiotoxicity, such as myocardial ischemia or infarction, is rare [15]. In gastric cancer, a phase I evaluation of sorafenib plus capecitabine and cisplatin defined diarrhea and neutropenia as dose-limiting toxicities, with an encouraging RR (62.5%), median PFS duration (10 months; 95% CI, 7.4–13.8 months), and median OS duration (14.7 months; 95% CI, 12.0–20.0 months) reported in the 21 patients enrolled [74]. A subsequent phase II study of sorafenib with 3-weekly docetaxel and cisplatin reported possible additive efficacy, with a median OS time of 13.6 months (90% CI, 8.6–16.1 months). However, the median PFS time of 5.8 months (90% CI, 5.4–7.4 months) is less than that reported in a phase III study of chemotherapy alone [10] and could suggest that the longer OS duration reflects the use of second-line chemotherapy [75].
Sunitinib also targets VEGFRs among other intracellular targets and led to a higher RR and longer PFS interval as monotherapy for the first-line treatment of advanced RCC patients [16]. Frequently reported adverse events include those of sorafenib, but, additionally, neutropenia and biochemical abnormalities such as elevated serum lipase are common [16]. A single-arm phase II study of sunitinib monotherapy in 78 patients with previously treated gastric or EGJ adenocarcinoma reported a disappointing radiological RR of 2.6%, median PFS time of 2.3 months (95% CI, 1.6–2.6 months), and median OS time of 6.8 months (95% CI, 4.4–9.7 months), demonstrating modest efficacy in this disease setting [76]. Phase III evaluation compared with supportive care alone is anticipated.
Other VEGFR TKIs—axitinib, vatalinib, cediranib, and pazopanib—have not yet been evaluated in patients with esophagogastric cancer.
Other Potential Antiangiogenic Therapies for Esophagogastric Cancer
Aflibercept (VEGF Trap) binds to and inactivates circulating VEGF-A and PlGF, with higher affinity for VEGF-A than bevacizumab, and has undergone phase I and phase II evaluation in several solid tumors. Of interest, in previously treated mCRC patients, monotherapy activity was apparent in patients who had received prior bevacizumab [77]. The fully human anti–NRP-1 antibody MNRP1685A is currently undergoing phase Ib evaluation in combination with bevacizumab and with weekly paclitaxel [78]. An oral inhibitor of HIF-1α, PX-478, has undergone phase I testing in patients with advanced solid tumors [79]. However, none of these agents have yet been evaluated in esophagogastric cancer.
Evidence for Tumor Rebound Effects and Greater Tumor Aggressiveness in Response to VEGF-Targeted Therapies
A study of s.c. Lewis lung carcinoma cell lines in mice treated with an anti-VEGF TKI showed rapid regrowth of the tumor vasculature after withdrawal of the TKI [80]. These data suggest that continuous administration of antiangiogenic therapy may be necessary for maximum efficacy and to avoid rebound growth of tumors. There have been several preclinical reports of a paradoxical increase in local tumor invasion and development of distant metastases apparently induced by antiangiogenic therapy. A study using metastatic breast cancer and melanoma xenografts treated with sunitinib reported the worrying observation that treatment with TKIs resulted in a higher incidence of metastasis and shorter survival time [81]. This effect is not limited to small-molecule TKIs, because in a murine model of pancreatic neuroendocrine tumors, antibody-mediated blockade of VEGFR-2 (DC101) increased the invasiveness of the tumors and there were more involved lymph nodes on histological examination after 1 or 4 weeks of treatment, despite a smaller tumor volume and longer OS time in treated mice than in controls [82]. However, this is in contrast to a study using an anti-VEGF antibody, in which slower regrowth after anti-VEGF antibody monotherapy was reported, and suppression of a rebound growth effect was noted after discontinuation of chemotherapy when the antibody was delivered concurrently [83].
In clinical practice, there are very limited data to support a “rebound phenomenon” after cessation of antiangiogenic monotherapy. A retrospective study of 12 patients with RCC treated with sunitinib or sorafenib (with or without surgery) to complete response showed disease relapse in five patients within 8 months of discontinuation of the drug, all of whom responded to reintroduction of the TKI [84]. A case series of 53 patients with high-grade gliomas reported rapid regrowth in 11 of the 40 patients with disease progression after cessation of bevacizumab, with an apparent survival advantage in four of the 11 patients retreated with bevacizumab [85]. However, a recent meta-analysis of 4,205 patients treated in five randomized studies assessed time to disease progression or death after cessation of bevacizumab or placebo prior to disease progression and demonstrated no detrimental effect in patients who received bevacizumab [86]. At present, there is no clinical evidence that rebound growth is a consequence of antiangiogenic therapy or of any negative effect of antiangiogenic therapy on survival.
Predictive Markers of Response
Despite extensive preclinical and clinical research, there currently are no validated biomarkers to select patients for antiangiogenic therapy. However, several candidate surrogate markers of response to bevacizumab have been identified from clinical trials.
Tumor VEGF
Tumor VEGF expression was first identified as a marker of poor prognosis in gastric cancer patients when a significant correlation between VEGF expression and the presence of lymphatic and vascular invasion, lymph node and liver metastases, and OS was observed in a study of 129 patients with gastric cancer resection (p < .05 for each comparison) [87]. Tumor expression of VEGF-D and VEGFR-3 were also reported as independent prognostic markers in a study of 91 patients with gastric adenocarcinoma undergoing complete resection. The carcinoma-specific survival rate was significantly shorter in patients with VEGF-D (relative risk, 3.08; 95% CI, 1.22–7.80; p = .017) or VEGFR-3 (relative risk, 2.36; 95% CI, 1.174.74; p = .016) expression [88]. A small retrospective study also identified tumor VEGF-C expression as a marker of poor prognosis in patients with resected gastric cancer [89]. Prospective validation of these possible prognostic biomarkers is warranted.
Circulating Angiogenic Factors
Several clinical trials have reported contrasting results when evaluating circulating VEGF as a possible predictive or prognostic biomarker [90–94]. These differences may relate to the assays used, disease setting, tumor type, or treatment regimen. However, a recent analysis of phase III studies across three tumor types demonstrated that circulating VEGF is prognostic, with high levels correlating with shorter PFS and OS times irrespective of treatment with bevacizumab, rather than predictive of response to bevacizumab [95].
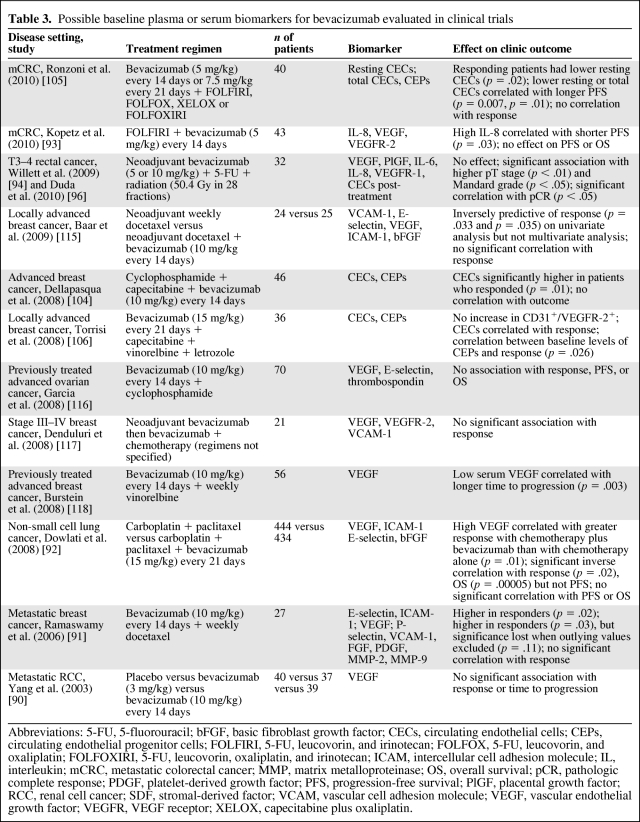
Soluble VEGFR-1 was evaluated as a possible predictive biomarker in a phase I/II study of 32 patients treated with neoadjuvant bevacizumab plus 5-FU chemoradiation for T3–4 adenocarcinoma of the rectum. A high plasma VEGFR-1 level at baseline was correlated with a higher pT-stage at surgery [94] (p < .05), higher Mandard regression score (p < .01), and lower risk for serious adverse events (p < .05), suggesting that such patients are refractory to both the therapeutic and toxic effects of bevacizumab [96]. However, this result has not been reproduced in other studies and prospective validation of this potential biomarker in a larger study is warranted. Baseline circulating biomarkers evaluated for bevacizumab are summarized in Table 3.
Table 3.
Possible baseline plasma or serum biomarkers for bevacizumab evaluated in clinical trials
Abbreviations: 5-FU, 5-fluorouracil; bFGF, basic fibroblast growth factor; CECs, circulating endothelial cells; CEPs, circulating endothelial progenitor cells; FOLFIRI, 5-FU, leucovorin, and irinotecan; FOLFOX, 5-FU, leucovorin, and oxaliplatin; FOLFOXIRI, 5-FU, leucovorin, oxaliplatin, and irinotecan; ICAM, intercellular cell adhesion molecule; IL, interleukin; mCRC, metastatic colorectal cancer; MMP, matrix metalloproteinase; OS, overall survival; pCR, pathologic complete response; PDGF, platelet-derived growth factor; PFS, progression-free survival; PlGF, placental growth factor; RCC, renal cell cancer; SDF, stromal-derived factor; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; XELOX, capecitabine plus oxaliplatin.
Genetic Polymorphisms
VEGF genotyping was investigated in the phase III E1200 study in advanced breast cancer patients, showing a longer OS duration in patients with the VEGF-2578-AA or VEGF-1154-A alleles treated with bevacizumab, but not in those treated with chemotherapy alone [97].
A recent study of angiogenesis-related genetic polymorphisms in resected esophageal cancer patients reported no correlation between VEGF or VEGFR-2 polymorphisms and relapse or survival. A predictive effect could not be evaluated because no patient received antiangiogenic agents. Two independent markers of poor prognosis were identified, however. Polymorphisms in the gene encoding a receptor involved in VEGF regulation, proteinase-activated-receptor 1 (PAR-1 −506 any insertion allele), and the epidermal growth factor (EGF +61 A>G (A/A)) were correlated with a higher risk for disease recurrence [98].
CEPs and CECs
Candidate predictive biomarkers include the quantification of circulating endothelial progenitor cells (CEPs) and circulating endothelial cells (CECs). Bone marrow-derived CEPs, or “angioblasts,” were first reported to be incorporated into sites of active angiogenesis, where they differentiate into endothelial cells [99]. These CEPs may regulate the angiogenic switch, promoting angiogenesis-mediated progression of micrometastases. Blockade of CEP mobilization blocked angiogenesis and tumor growth [100], inhibited progression of metastatic disease, and prolonged survival in animal models [101]. CEPs have been reported to be mobilized during neoadjuvant chemotherapy [102], an effect not seen using metronomic dosing [103]. Conflicting results exist regarding the association between high baseline levels of CEPs and response to chemotherapy plus bevacizumab [104–106]. CEPs were recently described in patients with advanced gastric cancer undergoing chemotherapy [107]; therefore, prospective evaluation of CEPs as a possible marker of response to bevacizumab in this population may be feasible.
CECs were identified in the plasma of cancer patients, with resting and activated cells reported at five times higher levels than in healthy controls (p < .008) [108]. These cells are derived from vessel walls [109] and were shown to be suppressed by antiangiogenic agents but not by chemotherapy in preclinical studies, suggesting a role for monitoring response to antiangiogenic drugs [110]. However, results from clinical studies are currently inconsistent [104–106]. We are not aware of any reports of CEC quantification in esophagogastric cancer. Difficulties associated with understanding the origins and roles of these cells, as well as the technical complexity of isolating and identifying these cells, may make the use of CECs as a biomarker challenging.
Hypertension
Development of hypertension was correlated with the RR and a longer PFS interval in a small study of patients with mCRC treated with chemotherapy plus bevacizumab (p < .04), suggesting a possible predictive effect [111]. Similarly, in a retrospective analysis of a phase III study of interferon-α with or without bevacizumab for patients with advanced RCC, the PFS and OS times were longer in patients treated with bevacizumab who developed grade ≥2 hypertension (p < .01), but no significant effect on the RR was observed [112]. However, this potential clinical biomarker needs to be validated in a large, prospective study.
Imaging Biomarkers
Conventional imaging with CT scans with response evaluation using conventional CT-based criteria appears not to be the optimal imaging modality for assessing response to antiangiogenic agents. Dynamic-contrast magnetic resonance imaging has been investigated as a novel method to evaluate response to antiangiogenic agents, allowing noninvasive estimation of vascular permeability and endothelial surface area. However, the available results are mostly derived from small studies, resulting in few significant results [113], and prospective evaluation within larger studies will determine the future use of this modality in clinical practice. Positron emission tomography is frequently used in the staging of esophagogastric cancers and is an effective tool for early assessment of response to neoadjuvant therapy, with metabolic response correlating with longer survival [114]. Alternative tracers to the standard 18-fluorodeoxyglucose to potentially better image tumors in patients treated with antiangiogenic agents are currently under evaluation [113]. Imaging methods may offer a promising approach for early prediction of treatment response in patients treated with antiangiogenic therapy.
Conclusions
Despite extensive international research in the field of angiogenesis, many aspects of antiangiogenic agents and how to optimally integrate them into clinical care remain poorly understood. Preclinical concerns of a rebound effect on growth after antiangiogenic agents are withdrawn have not been borne out in clinical trials, but the reason for this discrepancy is unknown. We still lack definitive evidence to determine the optimal duration of therapy and whether antiangiogenic agents are most effectively used until or beyond disease progression. Furthermore, the mechanism underlying the lack of efficacy in the adjuvant treatment of colorectal cancer is unknown.
The monotherapy activity of the TKIs sorafenib and sunitinib in RCC patients is thought to relate to the frequent inactivation of VHL, which is not a feature of esophagogastric cancer. Evaluation of the activity of TKIs in gastric cancer will need to be undertaken with correlative translational studies to define both the mechanism of activity and any subgroups of patients who may gain most benefit.
With the absence of a validated biomarker, we are currently unable to preselect patients who may benefit from antiangiogenic drugs, or predict those who will develop toxicities. Parallel prospective translational research in current trials is critical to bridge these gaps in our knowledge and, it is hoped, one day allow us to select patients most likely to benefit from these high-cost drugs that have uncommon, but potentially serious, toxicities.
Whereas antiangiogenic agents have some activity in esophagogastric cancer patients, no trial to date has reported an OS benefit. The results of the AVAGAST study demonstrated some clinical efficacy in the first-line advanced disease setting, but failure to achieve the primary endpoint of the study meant that bevacizumab will not be integrated into routine clinical care. However, ongoing studies of bevacizumab added to neoadjuvant or perioperative chemotherapy and of ramucirumab and anti-VEGFR TKIs in the second-line treatment of advanced disease may identify a future role for these agents in esophagogastric cancer.
Author Contributions
Conception/Design: Alicia F.C. Okines, David Cunningham
Collection and/or assembly of data: Alicia F.C. Okines, Andrew R. Reynolds
Data analysis and interpretation: Alicia F.C. Okines, Andrew R. Reynolds, David Cunningham
Manuscript writing: Alicia F.C. Okines, Andrew R. Reynolds
Final approval of manuscript: Alicia F.C. Okines, Andrew R. Reynolds, David Cunningham
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 3.Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 5.Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 6.Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet Oncol. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 7.Murad AM, Santiago FF, Petroianu A, et al. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37–41. doi: 10.1002/1097-0142(19930701)72:1<37::aid-cncr2820720109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2010;362:858–859. doi: 10.1056/NEJMc0911925. [DOI] [PubMed] [Google Scholar]
- 9.Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: A randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 11.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 12.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 14.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 15.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 16.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 18.Senger DR, Perruzzi CA, Feder J, et al. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. [PubMed] [Google Scholar]
- 19.Connolly DT, Heuvelman DM, Nelson R, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. The Journal of clinical investigation. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kut C, Mac Gabhann F, Popel AS. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br J Cancer. 2007;97:978–985. doi: 10.1038/sj.bjc.6603923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houck KA, Ferrara N, Winer J, et al. The vascular endothelial growth factor family: Identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- 22.Olsson AK, Dimberg A, Kreuger J, et al. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 24.Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 25.Stoeltzing O, McCarty MF, Wey JS, et al. Role of hypoxia-inducible factor 1α in gastric cancer cell growth, angiogenesis, and vessel maturation. J Natl Cancer Inst. 2004;96:946–956. doi: 10.1093/jnci/djh168. [DOI] [PubMed] [Google Scholar]
- 26.Lainakis G, Bamias A. Targeting angiogenesis in renal cell carcinoma. Curr Cancer Drug targets. 2008;8:349–358. doi: 10.2174/156800908785133132. [DOI] [PubMed] [Google Scholar]
- 27.Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 28.Autiero M, Waltenberger J, Communi D, et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 29.Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 30.Bais C, Wu X, Yao J, et al. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell. 2010;141:166–177. doi: 10.1016/j.cell.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 31.Van de Veire S, Stalmans I, Heindryckx F, et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell. 2010;141:178–190. doi: 10.1016/j.cell.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 32.Kukk E, Lymboussaki A, Taira S, et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 33.Mandriota SJ, Jussila L, Jeltsch M, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amioka T, Kitadai Y, Tanaka S, et al. Vascular endothelial growth factor-C expression predicts lymph node metastasis of human gastric carcinomas invading the submucosa. Eur J Cancer. 2002;38:1413–1419. doi: 10.1016/s0959-8049(02)00106-5. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Tjwa M, Van Hove I, et al. Reevaluation of the role of VEGF-B suggests a restricted role in the revascularization of the ischemic myocardium. Arterioscler Thromb Vasc Biol. 2008;28:1614–1620. doi: 10.1161/ATVBAHA.107.158725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Achen MG, Jeltsch M, Kukk E, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci U S A. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogawa S, Oku A, Sawano A, et al. A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J Biol Chem. 1998;273:31273–31282. doi: 10.1074/jbc.273.47.31273. [DOI] [PubMed] [Google Scholar]
- 38.Millauer B, Longhi MP, Plate KH, et al. Dominant-negative inhibition of Flk-1 suppresses the growth of many tumor types in vivo. Cancer Res. 1996;56:1615–1620. [PubMed] [Google Scholar]
- 39.Prewett M, Huber J, Li Y, et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- 40.Partanen TA, Alitalo K, Miettinen M. Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor 3 in 185 vascular tumors. Cancer. 1999;86:2406–2412. [PubMed] [Google Scholar]
- 41.Mäkinen T, Veikkola T, Mustjoki S, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang JF, Ganju RK, Liu ZY, et al. Signal transduction in human hematopoietic cells by vascular endothelial growth factor related protein, a novel ligand for the FLT4 receptor. Blood. 1997;90:3507–3515. [PubMed] [Google Scholar]
- 43.He Y, Kozaki K, Karpanen T, et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 44.Jain RK, Duda DG, Clark JW, et al. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 45.Soker S, Takashima S, Miao HQ, et al. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 46.Miao HQ, Soker S, Feiner L, et al. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: Functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murga M, Fernandez-Capetillo O, Tosato G. Neuropilin-1 regulates attachment in human endothelial cells independently of vascular endothelial growth factor receptor-2. Blood. 2005;105:1992–1999. doi: 10.1182/blood-2004-07-2598. [DOI] [PubMed] [Google Scholar]
- 48.Wey JS, Gray MJ, Fan F, et al. Overexpression of neuropilin-1 promotes constitutive MAPK signalling and chemoresistance in pancreatic cancer cells. Br J Cancer. 2005;93:233–241. doi: 10.1038/sj.bjc.6602663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oh H, Takagi H, Otani A, et al. Selective induction of neuropilin-1 by vascular endothelial growth factor (VEGF): A mechanism contributing to VEGF-induced angiogenesis. Proc Natl Acad Sci U S A. 2002;99:383–388. doi: 10.1073/pnas.012074399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jarvis A, Allerston CK, Jia H, et al. Small molecule inhibitors of the neuropilin-1 vascular endothelial growth factor A (VEGF-A) interaction. J Med Chem. 2010;53:2215–2226. doi: 10.1021/jm901755g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gluzman-Poltorak Z, Cohen T, Herzog Y, et al. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165. J Biol Chem. 2000;275:29922. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- 52.Favier B, Alam A, Barron P, et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108:1243–1250. doi: 10.1182/blood-2005-11-4447. [DOI] [PubMed] [Google Scholar]
- 53.Gray MJ, Van Buren G, Dallas NA, et al. Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver. J Natl Cancer Inst. 2008;100:109–120. doi: 10.1093/jnci/djm279. [DOI] [PubMed] [Google Scholar]
- 54.Kim WH, Lee SH, Jung MH, et al. Neuropilin2 expressed in gastric cancer endothelial cells increases the proliferation and migration of endothelial cells in response to VEGF. Exp Cell Res. 2009;315:2154–2164. doi: 10.1016/j.yexcr.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 55.Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: Results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26:5326–3453. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 56.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 57.Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2010;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Gramont A, Van Cutsem E, Tabernero J, et al. AVANT: Results from a randomized, three-arm multinational phase III study to investigate bevacizumab with either XELOX or FOLFOX4 vs. FOLFOX4 alone as adjuvant treatment for colon cancer [abstract 362]. Presented at the 2011 Gastrointestinal Cancer Symposium; January 22, 2011; San Francisco, CA. [Google Scholar]
- 59.Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J Clin Oncol. 2007;25:3456–3461. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 60.Van Cutsem E, Labianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol. 2009;27:3117–3125. doi: 10.1200/JCO.2008.21.6663. [DOI] [PubMed] [Google Scholar]
- 61.Alberts SR, Sargent DJ, Smyrk TC, et al. Adjuvant mFOLFOX6 with or without cetuxiumab (Cmab) in KRAS wild-type (WT) patients (pts) with resected stage III colon cancer (CC): Results from NCCTG Intergroup Phase III Trial N0147. J Clin Oncol. 2010;28(18 suppl):3508. [Google Scholar]
- 62.Enzinger PC, Ryan DP, Regan E, et al. Phase II trial of docetaxel, cisplatin, irinotecan, and bevacizumab in metastatic esophagogastric cancer [abstract 97]. Presented at the 2008 Gastrointestinal Cancers Symposium; January 25, 2008; San Francisco, CA. [Google Scholar]
- 63.Shah MA, Jhawer M, Ilson DH, et al. Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol. 2011;29:868–874. doi: 10.1200/JCO.2010.32.0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shah MA, Ramanathan RK, Ilson DH, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201–5206. doi: 10.1200/JCO.2006.08.0887. [DOI] [PubMed] [Google Scholar]
- 65.El-Rayes BF, Zalupski M, Bekai-Saab T, et al. A phase II study of bevacizumab, oxaliplatin, and docetaxel in locally advanced and metastatic gastric and gastroesophageal junction cancers. Ann Oncol. 2010;21:1999–2004. doi: 10.1093/annonc/mdq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang Y, Ohtsu A, Van Cutsem E, et al. AVAGAST: A randomized, double-blind, placebo-controlled, phase III study of first-line capecitabine and cisplatin plus bevacizumab or placebo in patients with advanced gastric cancer (AGC) [abstract LBA4007] J Clin Oncol. 2010;28(18 suppl):950S. [Google Scholar]
- 67.Enzinger PC, Fidias P, Meyerhardt J, et al. Phase II study of bevacizumab and docetaxel in metastatic esophageal and gastric cancer [abstract 68]. Presented at the 2006 Gastrointestinal Cancers Symposium; January 26–28, 2006; San Francisco, CA. [Google Scholar]
- 68.Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- 69.Margolin K, Gordon MS, Holmgren E, et al. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: Pharmacologic and long-term safety data. J Clin Oncol. 2001;19:851–856. doi: 10.1200/JCO.2001.19.3.851. [DOI] [PubMed] [Google Scholar]
- 70.Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: Results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 71.Ilson D, Bains M, Rizk N, et al. Phase II trial of preoperative bevacizumab (Bev), irinotecan (I), cisplatin (C), and radiation (RT) in esophageal adenocarcinoma: Preliminary safety analysis. J Clin Oncol. 2009;27(15 suppl):4573. [Google Scholar]
- 72.Okines AF, Langley R, Cafferty FH, et al. Preliminary safety data from a randomized trial of perioperative epirubicin, cisplatin plus capecitabine (ECX) with or without bevacizumab (B) in patients (pts) with gastric or oesophagogastric junction (OGJ) adenocarcinoma. J Clin Oncol. 2010;28(15 suppl):4019. [Google Scholar]
- 73.Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780–787. doi: 10.1200/JCO.2009.23.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim C, Lee JL, Choi YH, et al. Phase I dose-finding study of sorafenib in combination with capecitabine and cisplatin as a first-line treatment in patients with advanced gastric cancer [abstract 4559] Invest New Drugs. 2010 doi: 10.1007/s10637-010-9531-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 75.Sun W, Powell M, O'Dwyer PJ, et al. Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. J Clin Oncol. 2010;28:2947–2951. doi: 10.1200/JCO.2009.27.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bang YJ, Kang YK, Kang WK, et al. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs. 2010 May 12; doi: 10.1007/s10637-010-9438-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang P, Cohen SJ, Bjarnason GA, et al. Phase II trial of aflibercept (VEGF Trap) in previously treated patients with metastatic colorectal cancer (MCRC): A PMH phase II consortium trial. J Clin Oncol. 2008;26(15 suppl):4027. [Google Scholar]
- 78.Patnaik A, Weekes CD, Hegde P, et al. A phase Ib study to evaluate the fully human monoclonal antibody MNRP1685A (anti-NRP1) administered intravenously in combination with bevacizumab with or without weekly paclitaxel in patients with advanced solid tumors. J Clin Oncol. 2010;28(15 suppl):TPS180. [Google Scholar]
- 79.Tibes R, Falchook GS, Von Hoff D, et al. Results from a phase I, dose-escalation study of PX-478, an orally available inhibitor of HIF-1α. J Clin Oncol. 2010;28(15 suppl):3076. [Google Scholar]
- 80.Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Píez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bagri A, Berry L, Gunter B, et al. Effects of anti-VEGF treatment duration on tumor growth, tumor regrowth, and treatment efficacy. Clin Cancer Res. 2010;16:3887–3900. doi: 10.1158/1078-0432.CCR-09-3100. [DOI] [PubMed] [Google Scholar]
- 84.Johannsen M, Florcken A, Bex A, et al. Can tyrosine kinase inhibitors be discontinued in patients with metastatic renal cell carcinoma and a complete response to treatment? A multicentre, retrospective analysis. Eur Urol. 2009;55:1430–1438. doi: 10.1016/j.eururo.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 85.Zuniga RM, Torcuator R, Jain R, et al. Rebound tumour progression after the cessation of bevacizumab therapy in patients with recurrent high-grade glioma. J Neurooncol. 2010;99:237–242. doi: 10.1007/s11060-010-0121-0. [DOI] [PubMed] [Google Scholar]
- 86.Miles D, Harbeck N, Escudier B, et al. Disease course patterns after discontinuation of bevacizumab: Pooled analysis of randomized phase III trials. J Clin Oncol. 2011;29:83–88. doi: 10.1200/JCO.2010.30.2794. [DOI] [PubMed] [Google Scholar]
- 87.Maeda K, Chung YS, Takatsuka S, et al. Tumour angiogenesis and tumour cell proliferation as prognostic indicators in gastric carcinoma. Br J Cancer. 1995;72:319–323. doi: 10.1038/bjc.1995.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jüttner S, Wissmann C, Jöns T, et al. Vascular endothelial growth factor-D and its receptor VEGFR-3: Two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol. 2006;24:228–240. doi: 10.1200/JCO.2004.00.3467. [DOI] [PubMed] [Google Scholar]
- 89.Ding S, Li C, Lin S, et al. Distinct roles of VEGF-A and VEGF-C in tumour metastasis of gastric carcinoma. Oncol Rep. 2007;17:369–375. [PubMed] [Google Scholar]
- 90.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramaswamy B, Elias AD, Kelbick NT, et al. Phase II trial of bevacizumab in combination with weekly docetaxel in metastatic breast cancer patients. Clin Cancer Res. 2006;12:3124–3129. doi: 10.1158/1078-0432.CCR-05-2603. [DOI] [PubMed] [Google Scholar]
- 92.Dowlati A, Gray R, Sandler AB, et al. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab—an Eastern Cooperative Oncology Group study. Clin Cancer Res. 2008;14:1407–1412. doi: 10.1158/1078-0432.CCR-07-1154. [DOI] [PubMed] [Google Scholar]
- 93.Kopetz S, Hoff PM, Morris JS, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: Efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010;28:453–459. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: A multidisciplinary phase II study. J Clin Oncol. 2009;27:3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bernaards C, Hedge P, Chen D, et al. Circulating vascular endothelial growth factor (VEGF) as a biomarker for bevacizumab-based therapy in metastatic colorectal, non-small cell lung, and renal cell cancers: Analysis of phase III studies. J Clin Oncol. 2010;28(15 suppl):10519. [Google Scholar]
- 96.Duda DG, Willett CG, Ancukiewicz M, et al. Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer. The Oncologist. 2010;15:577–583. doi: 10.1634/theoncologist.2010-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schneider BP, Wang M, Radovich M, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lurje G, Leers JM, Pohl A, et al. Genetic variations in angiogenesis pathway genes predict tumor recurrence in localized adenocarcinoma of the esophagus. Ann Surg. 2010;251:857–864. doi: 10.1097/SLA.0b013e3181c97fcf. [DOI] [PubMed] [Google Scholar]
- 99.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 100.Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 101.Gao D, Nolan DJ, Mellick AS, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 102.Fürstenberger G, von Moos R, Lucas R, et al. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer. Br J Cancer. 2006;94:524–531. doi: 10.1038/sj.bjc.6602952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bertolini F, Paul S, Mancuso P, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–4346. [PubMed] [Google Scholar]
- 104.Dellapasqua S, Bertolini F, Bagnardi V, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol. 2008;26:4899–4905. doi: 10.1200/JCO.2008.17.4789. [DOI] [PubMed] [Google Scholar]
- 105.Ronzoni M, Manzoni M, Mariucci S, et al. Circulating endothelial cells and endothelial progenitors as predictive markers of clinical response to bevacizumab-based first-line treatment in advanced colorectal cancer patients. Ann Oncol. 2010;21:2382–2389. doi: 10.1093/annonc/mdq261. [DOI] [PubMed] [Google Scholar]
- 106.Torrisi R, Bagnardi V, Cardillo A, et al. Preoperative bevacizumab combined with letrozole and chemotherapy in locally advanced ER- and/or PgR-positive breast cancer: Clinical and biological activity. Br J Cancer. 2008;99:1564–1571. doi: 10.1038/sj.bjc.6604741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ahn JB, Rha SY, Shin SJ, et al. Circulating endothelial progenitor cells (EPC) for tumor vasculogenesis in gastric cancer patients. Cancer Lett. 2010;288:124–132. doi: 10.1016/j.canlet.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 108.Mancuso P, Burlini A, Pruneri G, et al. Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood. 2001;97:3658–3661. doi: 10.1182/blood.v97.11.3658. [DOI] [PubMed] [Google Scholar]
- 109.Lin Y, Weisdorf DJ, Solovey A, et al. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Monestiroli S, Mancuso P, Burlini A, et al. Kinetics and viability of circulating endothelial cells as surrogate angiogenesis marker in an animal model of human lymphoma. Cancer Res. 2001;61:4341–4344. [PubMed] [Google Scholar]
- 111.Scartozzi M, Galizia E, Chiorrini S, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20:227–230. doi: 10.1093/annonc/mdn637. [DOI] [PubMed] [Google Scholar]
- 112.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: Final results of CALGB 90206. J Clin Oncol. 2010;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murukesh N, Dive C, Jayson GC. Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br J Cancer. 2010;102:8–18. doi: 10.1038/sj.bjc.6605483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: The MUNICON phase II trial. Lancet Oncol. 2007;8:797–805. doi: 10.1016/S1470-2045(07)70244-9. [DOI] [PubMed] [Google Scholar]
- 115.Baar J, Silverman P, Lyons J, et al. A vasculature-targeting regimen of preoperative docetaxel with or without bevacizumab for locally advanced breast cancer: Impact on angiogenic biomarkers. Clin Cancer Res. 2009;15:3583–3590. doi: 10.1158/1078-0432.CCR-08-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garcia AA, Hirte H, Fleming G, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: A trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 117.Denduluri N, Yang SX, Berman AW, et al. Circulating biomarkers of bevacizumab activity in patients with breast cancer. Cancer Biol Ther. 2008;7:15–20. doi: 10.4161/cbt.7.1.5337. [DOI] [PubMed] [Google Scholar]
- 118.Burstein HJ, Chen YH, Parker LM, et al. VEGF as a marker for outcome among advanced breast cancer patients receiving anti-VEGF therapy with bevacizumab and vinorelbine chemotherapy. Clin Cancer Res. 2008;14:7871–7877. doi: 10.1158/1078-0432.CCR-08-0593. [DOI] [PubMed] [Google Scholar]