Expression of the receptor tyrosine kinase c-MET in many cancers, and its participation in multiple signal transduction pathways involved in malignant tumor growth, suggest a wide therapeutic potential for MET inhibition in human cancer. Here we describe the discovery and early clinical development of ARQ 197, a novel, selective, non–ATP-competitive inhibitor of MET. Data from ARQ 197 clinical trials in hepatocellular, germ-cell, pancreatic (in combination with gemcitabine), and colorectal (in combination with cetuximab and irinotecan) cancers further highlight the potential role of ARQ 197 in existing and emerging anticancer therapeutic regimens.
Keywords: c-MET, EGFR, Epithelial growth factor inhibitor, Kinase receptor inhibitor, Hepatocyte growth factor
Abstract
Expression of the receptor tyrosine kinase c-MET (MET, mesenchymal-epithelial transition factor) in many cancers, and its participation in multiple signal transduction pathways involved in malignant tumor growth, suggest a wide therapeutic potential for MET inhibition in human cancer. Here we describe the discovery and early clinical development of ARQ 197, a novel, selective, non–ATP-competitive inhibitor of MET. Phase I studies demonstrate that ARQ 197 has a predictable pharmacokinetics and favorable safety profile, making it a potentially ideal partner for combination with cytotoxic chemotherapies and targeted anticancer agents. Results from phase I and phase II trials demonstrate preliminary evidence of anticancer activity. New data from a global phase II randomized trial comparing a combination of ARQ 197 plus erlotinib with erlotinib/placebo, in endothelial growth factor receptor inhibitor-naïve patients with locally advanced/metastatic non–small cell lung cancer, demonstrate improvement in progression-free and overall survival with combined therapy. Results were especially pronounced for patients with non–squamous lung cancer histologies, and in particular molecularly defined subgroups including KRAS mutations. These and other data from ARQ 197 clinical trials in hepatocellular, germ-cell, pancreatic (in combination with gemcitabine), and colorectal (in combination with cetuximab and irinotecan) cancers further highlight the potential role of ARQ 197 in existing and emerging anticancer therapeutic regimens.
Introduction
A growing body of evidence establishes the role of c-MET (mesenchymal-epithelial transition factor; MET), a receptor tyrosine kinase encoded by the proto-oncogene, c-MET, in a wide variety of cancers, including colon, gastric, bladder, breast, ovarian, pancreatic, lung, and hematologic malignancies [1–4]. Once MET is bound by its high affinity ligand, hepatocyte growth factor (HGF; also known as scatter factor), the MET signaling pathway is activated and involved in a variety of physiologic processes with direct or indirect involvement in oncogenesis (Figure 1) [3]. These include angiogenesis, tumor cell proliferation, survival, migration, resistance to apoptosis, aggressive cellular invasion, and metastasis [1–3].
Figure 1.
Schematic representation of HCG/MET signaling pathway [3]. Activation of MET results in the recruitment of scaffolding proteins such as Gab1 and Grb2, which activate Shp2, Ras, and ERK/MAPK. Abbreviations: ERK/MAPK, extracellular signal–regulated kinase/mitogen-activated protein kinase; Gab1, growth factor receptor–bound protein 2 (Grb2)-associated binder 1; HGF/SF, hepatocyte growth factor/scatter factor; MET, mesenchymal-epithelial transition factor; Pak, p21-activated protein kinase; PI3K, phosphatidylinositol 3-kinase; PKB, protein kinase B; Shp2, SH2 domain–containing protein tyrosine phosphatase 2; SH2, Src-homology-2; Sos, son-of-sevenless; α and β, subunits of the receptor that are present after proteolytic cleavage. Adapted by permission from Macmillan Publishers Ltd. Birchmeier C, Birchmeier W, Gherardi E et al. Metastasis, motility and more. Nat Rev Mol Cell Biol 2003;4:915–925.
MET expression may be dysregulated in a number of human cancers, resulting in an augmented response to HGF [5]. Furthermore, genetic aberrations can lead to aberrant c-MET signaling, with germline and sporadic mutations, gene amplification, and overexpression described across a wide spectrum of tumor histologies [6].
MET overexpression and mutated c-MET appear correlated with poor clinical prognosis [3, 5, 7]. Tumors that depend on MET signaling for growth, differentiation, and/or maintenance are described as being “addicted” to MET [8]. Relevant tumors dependent on the HGF/MET axis are thought to include the majority of hereditary and sporadic papillary renal cell carcinomas (RCCs) [9], gastric cancer [10, 11], multiple myeloma [12], and glioblastoma multiforme [13]. A subset of lung, colon, ovary, pancreas, and head and neck cancers also harbor dysregulated MET (including its overexpression, constitutive activation, gene amplification, ligand-dependent activation, or mutation) [14–16].
Recent evidence suggests that acquired resistance to epithelial growth factor receptor (EGFR) inhibitors in certain cancers may be achieved through c-MET gene amplification, in turn leading to MET hyperactivation and MET-dependent phosphorylation of HER3 [8, 17]. Phosphorylated HER3 recruits phosphoinositide 3-kinase (PI3K) and stimulates PI3K-based survival pathways, causing resistance to EGFR inhibitors. Conversely, inhibition of MET signaling in these resistant cells may potentially restore sensitivity to EGFR inhibitors. It is further hypothesized that simultaneous blockade of MET and EGFR may impair growth in these tumor cells [8, 16, 17].
Early Development
Pharmacologic Profile
In Vitro Studies
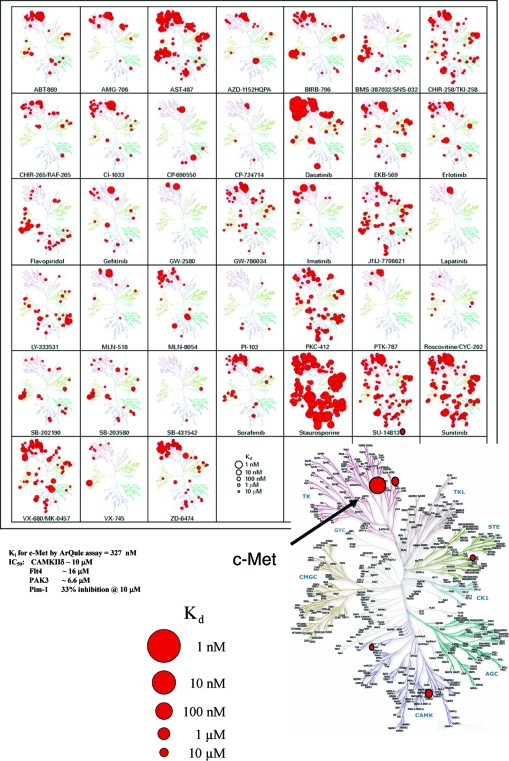
ARQ 197 (chemical formula (−)-(3R,4R)-3-(5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)pyrrolidine-2,5-dione) (Figure 2) is the most advanced agent in a new class of trans-3,4-bisubstituted pyrrolidine-2,5-diones [18]. Among >230 human protein kinases tested, ARQ 197 concentrations of 5–10 μM selectively inhibit only MET to any appreciable extent (Figure 3) [18, 19]. ARQ 197 binds to an inactive, or nonphosphorylated, conformation of MET and locks it in this inactive state [20]. Kinetic analyses of ARQ 197 demonstrate high in vitro potency (inhibitory constant [Ki] ≈ 35.5 nM) and a non–ATP-competitive mechanism of action, which may explain a high degree of kinase selectivity that distinguishes the compound from other MET inhibitors [18, 21, 22]. ARQ 197 inhibits both constitutive and ligand-mediated MET autophosphorylation in different human cancer cell lines, with a 50% inhibitory concentration (IC50) of 100–300 nM (Table 1), in turn inhibiting downstream MET effectors Akt, Erk-1/2, and STAT-3 [18]. Maximal MET inhibition is achieved by 24 hours, and it can be sustained for up to 8–12 hours following withdrawal of ARQ 197, demonstrating prolonged durability of MET kinase receptor inhibition [23]. ARQ 197 also inhibits HGF-induced phosphorylation of MET (IC50 ≈ 0.1 μM) and HGF-induced downstream targets, such as ERK1/2, MEK1/2, and FAK [18].
Figure 2.
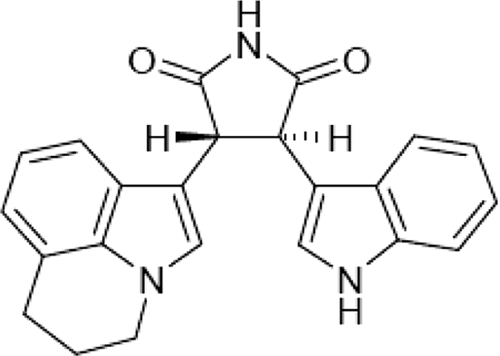
Chemical structure of ARQ 197 [18].
Figure 3.
Kinase specificity of ARQ 197 [18]. The inhibitory effect of ARQ 197 was profiled against 230 human kinases. The size of the red circles on the kinome tree are proportional to the potency determined by repeat dose-response follow-up inhibition studies against the top five kinases inhibited by ARQ 197. Abbreviations: IC50, 50% inhibitory concentration; Ki, inhibitory constant; MET, mesenchymal-epithelial transition factor. Illustration reproduced courtesy of Cell Signaling Technology, Inc. (http://www.cellsignal.com).
Table 1.
MET status and ARQ 197 IC50 values for 12 evaluated human cancer cell lines
Abbreviations: IC50, 50% inhibitory concentration; MET, mesenchymal-epithelial transition factor; NSCLC, non–small-cell lung cancer; SCLC, small-cell lung cancer.
Adapted with permission from the American Association for Cancer Research: Munshi N, Jeay S, Li Y et al. ARQ 197, a novel and selective inhibitor of the human c-Met receptor tyrosin kinase with anti-tumor activity. Mol Cancer Ther 2010;9:1544–1553.
In Vivo Studies
Xenograft mouse models using several human cancer cell lines demonstrate marked antitumor activity with orally administered ARQ 197 200 mg/kg, as indicated by significant tumor growth reductions ranging from 45% to 79% (all P values <0.05) in colon, gastric, breast, prostate, and pancreatic cancer models [18, 24, 25]. Compared with control animals, the level of phospho-MET was dramatically reduced in immunosuppressed mice with established HT-29 human colon cancer 24 hours after administration of a single oral dose of ARQ 197 (200 mg/kg) [18]. Moreover, tumor xenografts were exposed to sustained ARQ 197 plasma levels following a single oral dose of 200 mg/kg in mice, consistent with concentrations shown to inhibit MET enzymatic activity and proliferation of MET-harboring cancer cell lines in vitro. ARQ 197 plasma levels 10 hours after dosing were 1.3 μM—greater than threefold above the ARQ 197 Ki for MET [18]. ARQ 197 also demonstrated the ability to prevent bone metastases in a humanized mouse model of metastatic breast cancer [24], as well as significant inhibition of liver metastases in murine xenograft models of human cancer [25].
Preclinical Pharmacokinetics and Metabolism
Studies of individual human cytochrome (CY) P450 isozymes demonstrate that ARQ 197 is rapidly metabolized by CYP2C19 (half-life [t1/2] = 2.8 minutes) and moderately metabolized by CYP3A4 (t1/2 = 16.3 minutes) [26]. ARQ 197 does not appear to be a strong inhibitor of any of the major CYP450 enzymes tested [26]. Metabolic studies in rat, dog, mouse, and human hepatocytes indicate that oxidative biotransformation is the primary metabolic pathway [26]. On the basis of pharmacokinetic (PK) data, oral bioavailability was ≥20% in the species investigated: mouse, rat, and dog [26].
Clinical Development
Pharmacokinetic Data
Evaluation of ARQ 197 PK parameters was performed for studies ARQ 197-101, ARQ 197-103, ARQ 197-111 (with erlotinib), ARQ 197-114, ARQ 197-204, ARQ 197-116 (with sorafenib), and ARQ 197-117 (with gemcitabine) [27–34]. Across these studies, ARQ 197 was administered at doses ranging from 10 to 480 mg twice daily (bid). Additionally, in most studies, several active pharmaceutical ingredient (API) forms were used, including amorphous, crystalline A, and crystalline B. The different API forms were encountered as the API manufacturing process was scaled to larger batch size. Ultimately, crystalline B was determined to be the most stable and is currently being dosed in patients at 360 mg bid (the proposed phase II dose at the time of this publication). In general, across studies, exposure (area under the plasma concentration time curve [AUC] and maximum plasma concentration [Cmax]) was highly variable but generally increased as the dose was increased. In most cases, however, the resulting increase in exposure was less than dose proportional. In a recent study in which patients were dosed 360 mg bid with crystalline B ARQ 197, on day 1, mean Cmax (n = 8) was 1,766 ± 1,452 ng/ml and mean AUC(0–12) was 14,053 ± 13,736 h*ng/ml. On day 29, mean Cmax (n = 6) was 1,986 ± 1,487 ng/ml, and mean AUC(0–12) was 15,003 ± 13,428 h*ng/ml [35]. In general, mean values for t1/2 and apparent clearance remained relatively constant up to the maximum tolerated dose (MTD). In combination therapy (with erlotinib, sorafenib, or gemcitabine), ARQ 197 exposure (and adverse event [AE] profile) appears similar to that reported for monotherapy studies and indicates the absence of drug-drug interactions [26–33].
Phase I and II Studies
Monotherapy ARQ 197-101: Phase I Dose-Escalation Study in Metastatic Solid Tumors (USA)
Initiated in 2006, ARQ 197-101 was a phase I dose-escalation study of ARQ 197 in 74 patients with metastatic solid tumors [30–32]. The two dosing schedules evaluated, one intermittent and one continuous, demonstrated favorable safety profiles, with no dose-limiting toxicities (DLTs) observed and no MTD identified [30, 32]. The most common (≥5%) drug-related AEs included fatigue (16.2%), nausea (13.5%), vomiting (6.8%), and diarrhea (5.6%).
A total of 61 patients were evaluable for response by Response Evaluation Criteria In Solid Tumors (RECIST) 1.0 criteria [31]. Among these, three patients (5%; one each with neuroendocrine, prostate, and testicular cancer) achieved a partial response (PR), 38 patients (62%) demonstrated stable disease (SD), and 20 (33%) patients experienced progressive disease (PD). Disease control (complete response + PR + SD) was achieved in 41 of the evaluable patients (67%) [31].
ARQ 197–103: Phase I Dose-Escalation Study in Advanced Solid Tumors (U.K.)
Because no MTD was identified in the earlier phase I trial, an additional phase I trial, ARQ 197-103, was initiated in 2007 [27, 33]. Fifty-one patients were assigned to one of five continuous 28-day cycle dosing cohorts: 100 (n = 3), 200 (n = 6), 300 (n = 23), 360 (n = 15), and 400 (n = 4) mg bid.
In the 200 mg bid cohort, one DLT of grade 3 fatigue was observed, which resolved <24 hours after drug cessation. In the 400 mg bid cohort, a DLT of grade 3 febrile neutropenia was observed in each of two patients; in one of these patients, two other grade 3 DLTs were observed (mucosal inflammation and palmar-plantar erythrodysesthesia). All DLTs resolved within 2 weeks of ARQ 197 discontinuation [33]. ARQ 197 300 mg bid was initially identified as the MTD but was subsequently adjusted to 360 mg bid following introduction of a modified commercial-grade formulation and PK studies demonstrating a 5:6 conversion factor [33]. Safety of the 360 mg bid dose using the modified formulation was confirmed in an expanded cohort of 20 patients [29, 36]. In total, 51 patients experienced 73 drug-related AEs, with gastrointestinal AEs (n = 18; 25%) and fatigue (n = 10; 14%) reported most frequently [33].
Regarding efficacy, SD by RECIST 1.0 was the best observed response for 14 patients (27%), demonstrating evidence of tumor regression [33]. Tumor response was also examined using dynamic contrast enhanced-magnetic resonance imaging (DCE-MRI) and diffusion weighted-MRI imaging of lesions of interest. Preliminary DCE-MRI data showed nonstatistically significant changes in mean and median transfer constant after 7 days of ARQ 197 treatment, suggesting only a possible antiangiogenic effect of the drug.
ARQ 197-114: Phase Ib Study in Cirrhotic Patients with Hepatocellular Carcinoma
ARQ 197-114 is a recently conducted multicenter, single-cohort, Phase Ib study evaluating safety/toxicity of ARQ 197 in Child-Pugh A or B cirrhotic patients with hepatocellular carcinoma (HCC) who received two or fewer prior systemic chemotherapy regimens (last treatment completed at least 4 weeks before the first dose of ARQ 197) [28, 37, 38]. As of March 19, 2010, a total of 21 patients (19 male, 2 female; mean age 70 years) were treated with ARQ 197 at the recommended phase II dose of 360 mg bid [28, 37, 38].
Drug-related AEs were reported in 20 patients (95.2%), with the most commonly reported drug-related AEs of any grade being anemia (43%), asthenia (43%), neutropenia (38%), leukopenia (33%), diarrhea (29%), anorexia (29%), and fatigue (24%). Study drug-related serious adverse events (SAEs) were observed in four patients (19%), including grade 3 anemia (n = 2), grade 4 neutropenia (n = 2), grade 4 leukopenia (n = 1), grade 5 pneumonia (n = 1), and sepsis (n = 1). No drug-related worsening of liver function was observed [38].
Preliminary antitumor activity of ARQ 197 was observed among 16 patients evaluable for tumor response. Progression-free rates at 2 and 4 months were 59.7% and 39.8%, respectively. Median time on study was 13 weeks, and median time to progression was 15.3 weeks. One patient remained with SD for >13 months, with a decrease in tumor density observed by computed tomography scan [38].
Tumor biomarker analyses revealed that all patient biopsies were positive for total MET and at least weakly positive for HGF. Of particular note is that plasma biomarker analyses suggest that neutropenia may have correlated with reductions in plasma HGF (and to a lesser extent with reductions in MET levels) and, in turn, tumor response. Conversely, plasma vascular endothelial growth factor levels did not appear to correlate with ARQ 197 activity [38].
ARQ 197-204: Phase II Monotherapy Study in Patients with Microphthalmia Transcription Factor–Associated Tumors
ARQ 197-204 is a recently completed phase II trial evaluating ARQ 197 as monotherapy in patients with a rare set of microphthalmia transcription factor–associated tumors, including translocation-associated RCC, alveolar soft-part sarcoma (ASPS), and clear cell sarcoma (CCS) [34]. Patients aged 13 years or older were initially administered oral ARQ 197 120 mg bid, and the protocol was subsequently amended to increase the dose to 360 mg bid following identification of the phase I MTD [34].
As of June 1, 2009, 36 patients (mean age, 26.5 years; 8 with CCS, 19 with ASPS, and 9 with RCC) were evaluable for efficacy analysis [39]. A PR was observed in 1 patient with CCS, whereas SD was observed in 21 total patients (15 with ASPS, 3 with CCS, and 3 with RCC). The disease control rate was 79% in patients with ASPS versus 50% and 33% in those with CCS and RCC, respectively. Median progression-free survival (PFS) was 37 and 8 weeks in patients with ASPS and CCS, respectively. These data are difficult to interpret given the paucity of existing historical benchmarks for efficacy but are intriguing given the extremely poor prognosis of these tumor types. Further development opportunities are being explored.
Regarding safety, the most common drug-related AEs observed here were fatigue (46%), nausea (41%), and vomiting (34%). Two drug-related SAEs of grade 3 febrile neutropenia were observed in a patient treated with ARQ 197 360 mg bid.
ARQ 197-215: Phase II Monotherapy Study in Patients with Unresectable HCC (ClinicalTrials.gov Identifier: NCT00802555)
On the basis of results of the Phase Ib ARQ 197-114 study, a phase II clinical trial evaluating ARQ 197 monotherapy in HCC is currently underway and enrolling patients [40]. ARQ 195-215 is a global, randomized, double-blind, placebo-controlled, phase II clinical trial in patients who experienced disease progression following—or who were unable to tolerate—one prior line of systemic chemotherapy. Approximately 99 patients with Child-Pugh A status will be enrolled from multiple study sites. The primary study endpoint is median time to progression; secondary endpoints include overall survival (OS), disease control rate, and biomarker analyses (including circulating MET and HGF levels).
ARQ 197-A-U251: Phase II Study in Patients with Relapsed/Refractory Germ Cell Tumors (ClinicalTrials.gov Identifier: NCT01055067)
ARQ 197 is currently being investigated in a multicenter phase II study in patients with relapsed/refractory germ cell tumors. No proven therapies currently exist in this extremely difficult-to-treat patient population. The primary objective of this monotherapy trial is to determine the objective response and progression-free rates following four cycles of ARQ 197 360 mg bid [41].
Combination Therapy
ARQ 197-111: Phase I Dose-Escalation Study in Combination with Erlotinib in Advanced Solid Tumors
This phase I dose-escalation trial evaluated the combination of ARQ 197 (administered at 120 [n = 8], 240 [n = 4], and 360 mg bid [n = 20]) and the EGFR inhibitor erlotinib (150 mg once daily [qd]) in patients with advanced solid tumors [29, 36]. Intrapatient dose escalation was allowed in the absence of DLTs through one cycle of therapy (21 days). The combination was well tolerated, with fatigue (28.1%), nausea (18.8%), and rash (18.8%) being the most commonly observed AEs, and primarily grade 1–2 in severity [36]. Two patients experienced drug-related SAEs: neutropenia at 360 mg bid and sinus bradycardia at 240 mg bid [29]. DLTs were observed in two patients at 360 mg bid (grade 4 neutropenia in one patient, and grade 4 neutropenia and leukopenia and grade 3 thrombocytopenia in the other); all events resolved after treatment discontinuation. In the absence of a formally identified MTD, 360 mg bid was selected as the ARQ 197 recommended phase II dose (RP2D) for subsequent phase II combination studies with erlotinib at its full approved dose of 150 mg daily [36].
ARQ 197-116: Phase I Dose-Escalation Study in Combination with Sorafenib in Advanced Solid Tumors (ClinicalTrials.gov Identifier: NCT00827177)
This ongoing phase I dose-escalation trial is evaluating the safety and tolerability of ARQ 197 administered in combination with sorafenib [35, 42]. An initial cohort was treated with ARQ 197 360 mg bid + sorafenib 200 mg bid (dose level 1 [DL1]). Because no DLTs were observed, dosing was increased to the full single-agent doses of both drugs: ARQ 197 360 mg bid + sorafenib 400 mg bid (dose level 2 [DL2]). Intrapatient dose escalation was allowed, and an extension cohort was opened following determination of the RP2D, with planned enrollment of up to 50 patients with RCC, HCC, breast cancer, non–small-cell lung cancer (NSCLC), and melanoma [35].
As of April 2, 2010, 22 patients were enrolled and treated at the two dose levels (5 at DL1, 9 at DL2, and 8 at the RP2D). A total of 81 AEs considered related to either or both drugs were reported in 20 of 22 patients (90.9%), with the most commonly reported drug-related AEs of any grade being fatigue (36.4%), diarrhea (27.3%), anorexia (22.7%), and rash (22.7%). No DLTs were reported at DL1, and 1 of 9 patients (11.1%) at DL2 experienced two DLTs (grade 3 fatigue and grade 3 dyspnea) [35, 42].
As of May 5, 2010, 14 of 18 patients (77.8%) evaluable for efficacy by RECIST 1.1 demonstrated a best response of SD for 7+ to 32+ weeks (median 12+ weeks) [35]. All 7 evaluable patients with RCC experienced SD for 7+ to 31+ weeks (median 15+ weeks); 4 of 5 patients with HCC experienced SD for 8+ to 24+ weeks (median 15+ weeks); and 3 of 6 evaluable patients with other tumors experienced SD for 8–32 weeks (median 8 weeks). These results suggest that combined inhibition of MET and angiogenic signaling may have therapeutic potential [35]. Further development plans are being discussed.
ARQ 197-117: Phase I Dose-Escalation Study in Combination with Gemcitabine in Advanced Solid Tumors (ClinicalTrials.gov identifier: NCT00874042)
This ongoing multicenter, dose-escalation phase Ib study conducted in patients with advanced solid tumors is examining the safety and tolerability of competitive doses (120–360 mg bid) and schedules (either continuous or interrupted) of ARQ 197 given in combination with gemcitabine (1000 mg/m2 ¾ weekly) [43]. To date, no DLTs have been observed with intermittent ARQ 197 dosing, and all 21 patients initially enrolled are now being entered into the continuous dosing cohorts. AEs considered to be at least possibly drug-related were reported in 52% of patients, with the most commonly observed AEs including neutropenia (33%), thrombocytopenia (24%), anemia (19%), fatigue (14%), leukopenia (10%), and anorexia (10%). To date, one patient experienced a drug-related SAE (anemia), and one non–drug-related death was reported [43]. On the basis of the favorable safety profile, phase II combination studies are being considered in several indications.
ARQ 197-209: Phase II Combination Study with Erlotinib Versus Erlotinib/Placebo in Metastatic NSCLC
ARQ 197-209 is a recently concluded global, randomized, placebo-controlled, double-blind phase II clinical trial that evaluated erlotinib + ARQ 197 compared with erlotinib + placebo in second-/third-line chemotherapy-experienced, EGFR-inhibitor–naïve patients (N = 167) with inoperable, locally advanced/metastatic NSCLC [36, 44]. Eligible patients were randomly assigned to receive erlotinib 150 mg qd + ARQ 197 360 mg bid (n = 84), or erlotinib 150 mg qd + placebo (n = 83; 28-day cycles in both groups) [36, 44]. The primary study endpoint was PFS.
Results presented at the 2010 Annual Meeting of the American Society of Clinical Oncology demonstrated that median time on therapy was 101 days in the combination arm versus 65 days in the erlotinib/placebo arm. Treatment discontinuation occurred in 71 (85%) and 74 (89%) patients, respectively, primarily due to PD (50 [60%] vs 58 [70%] patients, respectively). In the intention-to-treat population (N = 167), PFS was prolonged with the ARQ 197/erlotinib combination versus erlotinib/placebo (16.1 vs 9.7 weeks). The hazard ratio (HR) for progression was statistically significant when adjusting for imbalances in the treatment arms using a prespecified Cox regression model (HR = 0.68; 95% confidence interval [CI]: 0.47, 0.98; P < 0.05). This improvement in PFS was paralleled by a similar improvement in median OS (36.6 vs 29.4 weeks). PFS and OS benefits were most pronounced in patients with non–squamous-cell histology (n = 117), with a 9.2-week improvement in median PFS (18.9 vs 9.7 weeks) and a 13.7-week improvement in median OS (43.1 vs 29.4 weeks). These hazard ratios were statistically significant when adjusting for key prognostic factors: 0.61 (95% CI: 0.47, 0.98; P < 0.05) for PFS and 0.58 (95% CI: 0.34, 0.99; P < 0.05) for OS. Analyses of specific biologic subgroups showed benefits of the ARQ 197/erlotinib combination in patients with MET FISH gene copy number >4, EGFR wild-type status, and KRAS mutation status.
Of intriguing interest, furthermore, was evidence demonstrated in this clinical trial of ARQ 197's potential antimetastatic effect. Among intention-to-treat patients, median time to new metastatic lesions was increased from 3.6 months in the erlotinib–placebo arm to 7.3 months in the combination arm (HR 0.49; 95% CI: 0.31, 0.78). This effect was even more pronounced in non–squamous-cell patients, among whom median time to metastatic disease was increased from 3.6 to 11.0 months (HR 0.46; 95% CI: 0.26, 0.82) [45].
RECIST PRs were observed in 7/73 evaluable patients (10%) in the ARQ 197/erlotinib arm compared with 5/72 evaluable patients (7%) in the erlotinib/placebo arm. SD was observed in 41 (56%) and 34 (47%) patients, yielding disease control rates of 66% and 54%, respectively (Table 2) [44].
Table 2.
Evaluation of erlotinib/ARQ 197 versus erlotinib/placebo in patients with advanced NSCLC
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; PR, partial response; SD, stable disease.
Thirty-four patients in the erlotinib/placebo arm were offered crossover to the ARQ 197/erlotinib arm at the time of progression, and 23 of these patients were evaluable for a postprogression response. Two patients (8.7%) demonstrated a PR, 9 (39.1%) demonstrated SD, and 12 (52.2%) had PD as their best response per RECIST 1.0.
Overall, there were no clinically relevant or statistically significant differences in AE rates between treatment and control arms. The most common AEs included rash, diarrhea, anorexia, anemia, and fatigue and were generally grade 1/2 in severity. Rates of neutropenia for the ARQ 197/erlotinib and erlotinib/placebo arms were 6% and 4%, respectively [36, 44]. On the basis of these results, the sponsors are currently planning a global phase III trial of ARQ 197 and erlotinib as second-/third-line treatment in patients with metastatic non–squamous-cell grade 3/4 NSCLC.
ARQ 197-A-U252: Phase I/II Combination Study with Irinotecan and Cetuximab in Metastatic Colorectal Cancer (ClinicalTrials.gov: NCT01075048)
This ongoing phase I/II, randomized, placebo-controlled clinical trial is evaluating ARQ 197 in combination with irinotecan/cetuximab in patients with metastatic CRC and wild-type KRAS status who have progressed on front-line systemic therapy [46]. Recently, the safety, tolerability, and RP2D of the ARQ 197/irinotecan/cetuximab combination were established in the phase I stage of this trial, and a phase II stage comparing the study treatments for PFS began enrollment.
Additional Studies
Additional phase I–III studies, evaluating safety of ARQ 197, as monotherapy or in combination with erlotinib, and efficacy of ARQ 197 in NSCLC and gastric cancer (ClinicalTrials.gov identifier: NCT01152645) are being planned or performed in Japan by Kyowa Hakko Kirin Co., Ltd.
Future and Planned Studies
Future Phase I Studies
A Children's Oncology Group–led phase I dose-escalation trial of ARQ 197 in children with advanced tumors is expected to begin accrual in 2011. On the basis of the favorable safety profile observed in the phase I combination studies of ARQ 197 with gemcitabine and sorafenib in patients with advanced solid tumors, phase II combination studies with these agents are being planned. Other ARQ 197–based combinations currently being evaluated include those containing pemetrexed, vascular endothelial growth factor inhibitors, irreversible EGFR inhibitors, and mammalian target of rapamycin inhibitors. Many of these combinations are included in the National Cancer Institute's Cancer Therapy Evaluation Program clinical development plan for ARQ 197.
Molecular-Guided Trials
A series of carefully targeted ARQ 197 trials are being planned in lung cancer and other metastatic malignancies based on a variety of disease biomarkers. These include plans to investigate ARQ 197 in NSCLC patients with KRAS-mutation–positive lung cancer. It is anticipated that these focused analyses of ARQ 197 efficacy and safety, in both monotherapy and/or combination therapy, will define those targeted patient subgroups most likely to benefit from treatment with ARQ 197.
Conclusion
ARQ 197 is a novel, selective, non–ATP-competitive inhibitor of the receptor tyrosine kinase c-MET, a key mediator of oncogenic signaling implicated in multiple stages of tumor progression, in a wide variety of human cancers. ARQ 197 demonstrates efficacy in both in vitro preclinical models and multiple human cancer xenograft models. In the clinic, ARQ 197 has been orally administered to >400 cancer patients and demonstrates favorable safety and predictable PK profiles.
Results from phase I studies additionally demonstrate favorable safety profiles for ARQ 197 in combination with erlotinib, sorafenib, and gemcitabine. Across all studies, the most commonly reported drug-related AEs appear to be fatigue and nausea. The most frequent drug-related SAEs, which are hematologic in nature, appear manageable and consistent with the involvement of MET in the maturation of bone marrow progenitor cells.
Data from both phase I and II clinical trials evaluating ARQ 197 across multiple tumor types demonstrate the promising anticancer activity of ARQ 197 achieved through selective inhibition of the MET signaling pathway. Patients with notable tumor reduction or long periods of stable disease are included in Table 3. Of particular clinical relevance are recent data from a global randomized trial in second-/third-line NSCLC, where the combination of ARQ 197 and erlotinib resulted in notable improvements in PFS and OS, as well as provocative increases in the time to new metastatic disease. Further data from ongoing and planned phase I–III clinical trials will determine whether ARQ 197 can influence current cancer treatment paradigms as a single agent or through its inclusion in multidrug anticancer regimens.
Table 3.
Table 3a.
(continued)
Abbreviations: ECOG, Eastern Collaborative Oncology Group; NOS, not otherwise specified; NSCLC, non–small cell lung cancer; PR, partial response; SD, stable disease.
Author Contributions
Conception/Design: Alex A. Adjei, Brian Schwartz, Edward Garmey
Provision of study material or patients: Alex A. Adjei, Brian Schwartz, Edward Garmey
Collection and/or assembly of data: Alex A. Adjei, Brian Schwartz, Edward Garmey
Data analysis and interpretation: Alex A. Adjei, Brian Schwartz, Edward Garmey
Manuscript writing: Alex A. Adjei, Brian Schwartz, Edward Garmey
Final approval of manuscript: Alex A. Adjei, Brian Schwartz, Edward Garmey
The authors take full responsibility for the content of the paper but thank Michael G. Pellegrino, Ph.D., of Chameleon Communications International, supported by ArQule, Inc., and Daiichi-Sankyo, Inc., for his editorial assistance in the preparation of the manuscript.
References
- 1.Furge KA, Zhang YW, Vande Woude GF. Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene. 2000;19:5582–5589. doi: 10.1038/sj.onc.1203859. [DOI] [PubMed] [Google Scholar]
- 2.Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8:404–410. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- 3.Birchmeier C, Birchmeier W, Gherardi E, et al. Metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YW, Vande Woude GF. HGF/SF-met signaling in the control of branching morphogenesis and invasion. J Cell Biochem. 2003;88:408–417. doi: 10.1002/jcb.10358. [DOI] [PubMed] [Google Scholar]
- 5.Jiang W, Hiscox S, Matsumoto K, et al. Hepatocyte growth factor/scatter factor, its molecular, cellular and clinical implications in cancer. Crit Rev Oncol Hematol. 1999;29:209–248. doi: 10.1016/s1040-8428(98)00019-5. [DOI] [PubMed] [Google Scholar]
- 6.Toschi L, Janne PA. Single-agent and combination therapeutic strategies to inhibit hepatocyte growth factor/MET signaling in cancer. Clin Cancer Res. 2008;14:5941–5946. doi: 10.1158/1078-0432.CCR-08-0071. [DOI] [PubMed] [Google Scholar]
- 7.Jiang WG, Hiscox SE, Parr C, et al. Antagonistic effect of NK4, a novel hepatocyte growth factor variant, on in vitro angiogenesis of human vascular endothelial cells. Clin Cancer Res. 1999;5:3695–3703. [PubMed] [Google Scholar]
- 8.Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7:504–516. doi: 10.1038/nrd2530. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt L, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Han SU, Cho H, et al. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene. 2000;19:4947–4953. doi: 10.1038/sj.onc.1203874. [DOI] [PubMed] [Google Scholar]
- 11.Heideman DA, Snijders PJ, Bloemena E, et al. Absence of tpr-met and expression of c-met in human gastric mucosa and carcinoma. J Pathol. 2001;194:428–435. doi: 10.1002/path.934. [DOI] [PubMed] [Google Scholar]
- 12.Derksen PW, de Gorter D. J, Meijer HP, et al. The hepatocyte growth factor/Met pathway controls proliferation and apoptosis in multiple myeloma. Leukemia. 2003;17:764–774. doi: 10.1038/sj.leu.2402875. [DOI] [PubMed] [Google Scholar]
- 13.Beroukhim R, Getz G, Nghiemphu L, et al. Assessing the significance of chromosomal aberrations in cancer: methodology and application to glioma. Proc Natl Acad Sci U S A. 2007;104:20007–20012. doi: 10.1073/pnas.0710052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng Z, Weiser MR, D'Alessio M, et al. Immunoblot analysis of c-Met expression in human colorectal cancer: overexpression is associated with advanced stage cancer. Clin Exp Metastasis. 2004;21:409–417. doi: 10.1007/s10585-005-1617-4. [DOI] [PubMed] [Google Scholar]
- 15.Christensen JG, Burrows J, Salgia R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005;225:1–26. doi: 10.1016/j.canlet.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 16.Cipriani NA, Abidoye OO, Vokes E, et al. MET as a target for treatment of chest tumors. Lung Cancer. 2009;63:169–179. doi: 10.1016/j.lungcan.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munshi N, Jeay S, Li Y, et al. ARQ197, a novel and selective inhibitor of the human c-Met receptor tyrosin kinase with anti-tumor activity. Mol Cancer Ther. 2010;9:1544–1553. doi: 10.1158/1535-7163.MCT-09-1173. [DOI] [PubMed] [Google Scholar]
- 19.Jeay S, Munshi N, Hill J, et al. ARQ 197, a highly selective small molecule inhibitor of c-Met, with selective antitumor properties in a broad spectrum of human cancer cells. Paper presented at: 98th AACR Annual Meeting; April 14, 2007; Los Angeles, CA. Abstract 2369. [Google Scholar]
- 20.Jarvis LM. One pill, many uses. Chem Eng News. 2007;85:15–23. [Google Scholar]
- 21.Underiner TL, Herbertz T, Miknyoczki SJ. Discovery of small molecule c-Met Inhibitors: evolution and profiles of clinical candidates. Anticancer Agents Med Chem. 2010;10:7–27. doi: 10.2174/1871520611009010007. [DOI] [PubMed] [Google Scholar]
- 22.Pan BS, Chan GK, Chenard M. MK-2461, a novel multitargeted kinase inhibitor, preferentially inhibits the activated c-Met receptor. Cancer Res. 2010;70:1524–1533. doi: 10.1158/0008-5472.CAN-09-2541. [DOI] [PubMed] [Google Scholar]
- 23.Gu X, Wang C, Yu Y, et al. Inhibition of HGF/c-Met pathway by ARQ 197: characterization of pharmacodynamic markers in vitro and in vivo. Poster presented at: 2009 AACR Annual Meeting; April 18, 2009; Denver, CO. Abstract 1748. [Google Scholar]
- 24.Anderson K, Li C, Moreau J, et al. ARQ 197, a small molecule inhibitor of c-met, prevents bone metastasis in a humanized mouse model of breast cancer. Poster presented at: AACR-NCI-EORTC International Conference B184; October 22, 2007; San Francisco, CA. Abstract B184. [Google Scholar]
- 25.Li Y, Chen D, Zhou W, et al. Broad spectrum anti-cancer activity of ARQ 197, a highly selective oral c-Met inhibitor, in multiple xenograft models. Poster presented at: 98th AACR Annual Meeting; April 14–18, 2007; Los Angeles, CA. Poster 2216. [Google Scholar]
- 26.Savage RE, Zhong C, Hall T, et al. In vitro ADME Properties of ARQ-197. Comparison to in vivo data. Poster presented at: 99th AACR Annual Meeting; April 12, 2009; Philadelphia, PA. Poster 1291. [Google Scholar]
- 27.Yap TA, Barriuso J, Frentzas S, et al. Pharmacokinetic (PK) and pharmacodynamic (PD) phase I study of an oral c-Met inhibitor ARQ197 reaches maximum tolerated dose (MTD) in a twice daily (bid) dosing schedule. Eur J Cancer. 2008;6(12 suppl) Abstract 386. [Google Scholar]
- 28.Santoro A, Simonelli M, Zucali P. A Phase Ib safety trial of ARQ 197 in cirrhotic patients with hepatocellular carcinoma (HCC). Poster presented at: American Society of Clinical Oncology Gastrology; 2010; Poster 1961. [Google Scholar]
- 29.Laux I, Goldman J, Goldman J, et al. Phase I dose escalation trial (ARQ 197–111) evaluating combination of selective c-Met inhibitor ARQ 197 and erlotinib. American Society of Clinical Oncology 2010. J Clin Oncol. 2009;27(15):3549. [Google Scholar]
- 30.Rosen L, Senzer N, Nemunaitis J, et al. Phase 1 dose escalation study and signs of anti-metastatic activity of ARQ 197, a selective c-Met inhibitor. Paper presented at: AACR-NCI-EORTC International Conference; October 22, 2007; San Francisco, CA. Abstract B91. [Google Scholar]
- 31.Mekhail T, Rich T, Rosen L, et al. Final results: a dose escalation phase I study of ARQ 197, a selective c-Met inhibitor, in patients with metastatic solid tumors. J Clin Oncol. 2009;27(15s):3548. [Google Scholar]
- 32.Garcia A, Rosen L, Cunningham C, et al. Phase 1 study of ARQ 197, a selective inhibitor of the c-Met RTK in patients with metastatic solid tumors reaches recommended phase 2 dose. J Clin Oncol. 2007;25(suppl 18):3525. [Google Scholar]
- 33.Yap TA, Frentzas S, Tunuriu N, et al. Final results of a pharmacokinetic (PK) and pharmacodynamic (PD) phase I trial of ARQ 197 incorporating dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) studies investigating the antiangiogenic activity of selective c-Met inhibition. J Clin Oncol. 2009;27(15):3523. [Google Scholar]
- 34.Goldberg J, Demetri GD, Choy E, et al. Preliminary results from a phase II study of ARQ 197 in patients with microphthalmia transcription factor family (MiT)-associated tumors. J Clin Oncol. 2009;27(15):10502. [Google Scholar]
- 35.Adjei AA, Sosman J, Dy GK, et al. A Phase 1 dose-escalation trial of the c-MET inhibitor ARQ 197 administered in combination with sorafenib in adult patients with advanced solid tumors. Poster presented at: American Society of Clinical Oncology meeting; June 4–8, 2010; Chicago, IL. Poster 3024. [Google Scholar]
- 36.Goldman J, Rosen L, Laux I, et al. Phase I dose escalation trial (ARQ 197–111) evaluating combination of selective c-Met inhibitor ARQ 197 and erlotinib. Oral presentation at: IASLC 13th World Conference on Lung Cancer; August 1, 2009; San Francisco, CA, USA. Presentation A6.5. [Google Scholar]
- 37.Santoro A, Simonelli M, Zucali P, et al. Preliminary results from ARQ 197–144: a phase 1b safety trial evaluating ARQ 197 in cirrhotic patients with hepatocellular carcinoma (HCC). Poster presented at: American Society of Clinical Oncology meeting; June 4–8, 2010; Chicago, IL. Poster 1961. [Google Scholar]
- 38.Zucali P, Santoro A, Rodriguez-Lope C, et al. Final results from ARQ 197–114: a Phase 1b safety trial evaluating the c-MET inhibitor ARQ-197 in cirrhotic patients with hepatocellular carcinoma. Poster presented at: American Society of Clinical Oncology; June 4–8, 2010; Chicago, IL. Poster 4137. [Google Scholar]
- 39.Wagner A, Demetri GD, Choy E, et al. Preliminary results from a Phase 2 study of ARQ 197 in patients with microphthalmia transcription factor family (MiT) associated tumors. Oral presentation to: Connective Tissue Oncology Society; November 26, 2009; Miami, FL. Oral presentation 39313. [Google Scholar]
- 40.Borbath I, Santoro A, Van Laethem J, et al. ARQ 197–215: a randomized, placebo-controlled phase 2 clinical trial evaluating the c-Met inhibitor, ARQ 197, in patients (pts) with hepatocellular carcinoma (HCC). Paper presented at: American Society of Clinical Oncology meeting; June 4–8, 2010; Chicago, IL. Abstract TPS215. [Google Scholar]
- 41.ClinicalTrials.gov. ARQ 197 for subjects with relapsed or refractory germ cell tumors. [Accessed 2010]. Avaiable at: http://www.clinicaltrials.gov/ct2/show/NCT01055067?term=nct01055067&rank=1.
- 42.Adjei AA, Puzanov I, Chai F, et al. A phase 1 dose-escalation trial evaluating ARQ 197 administered in combination with sorafenib in adult patients with advanced solid tumors. Paper presented at: American Society of Clinical Oncology meeting; June 4–8, 2010; Chicago, IL. Abstract 3024. [Google Scholar]
- 43.Camacho L, Chen CL, Kazakin J, et al. A phase 1b dose-escalation trial evaluating ARQ 197 administered in combination with gemcitabine to patients with advanced solid tumors. Paper presented at: American Society of Clinical Oncology meeting; June 4–8, 2010; Chicago, IL. Abstract e13008. [Google Scholar]
- 44.Schiller JH, Akerley WL, Brugger W, et al. Results from ARQ 197–209: a global randomized placebo-controlled Phase 2 clinical trial comparing erlotinib plus ARQ 197 to erlotinib plus placebo in previously treated EGFR-inhibitor naive patients with locally advanced or metastatic non-small cell lung cancer. Paper presented at: American Society of Clinical Oncology meeting oral presentation; June 4–8, 2010; Chicago, IL. Abstract LBA7502. [Google Scholar]
- 45.Sequist LV, Akerley WL, Brugger W, et al. Final results from ARQ 197–209: a global randomized placebo-controlled phase 2 clinical trial of erlotinib plus ARQ 197 versus erlotinb plus placebo in previously treated EGFR-inhibitor naïve patients with advanced non-small cell lung cancer (NSCLC). Paper presented at: European Society for Medical Oncology meeting; October 8–12, 2010; Milan, Italy. Abstract LBA7502. [Google Scholar]
- 46.ClinicalTrials.gov. ARQ 197 in combination with chemotherapy in patients with metastatic colorectal cancer. [Accessed 2010]. Available at: http://www.clinicaltrials.gov/ct2/show/NCT01075048?term=nct01075048&rank=1.