CMIS is increasingly diagnosed and often overtreated, although it does not affect the life expectancy of its carriers. Patients with CMIS have an increased risk of developing invasive melanoma (which warrants their enrollment in screening programs) but also a reduced risk of some epithelial cancers, which raises the intriguing hypothesis that genetic/environmental risk factors for some tumors may oppose the pathogenesis of others.
Keywords: Cutaneous melanoma, In situ, Treatment, Incidence, Risk, Population study, Relative survival analysis
Abstract
Background.
Cutaneous melanoma in situ (CMIS) is a nosologic entity surrounded by health concerns and unsolved debates. We aimed to shed some light on CMIS by means of a large population-based study.
Methods.
Patients with histologic diagnosis of CMIS were identified from the Surveillance Epidemiology End Results (SEER) database.
Results.
The records of 93,863 cases of CMIS were available for analysis. CMIS incidence has been steadily increasing over the past 3 decades at a rate higher than any other in situ or invasive tumor, including invasive skin melanoma (annual percentage change [APC]: 9.5% versus 3.6%, respectively). Despite its noninvasive nature, CMIS is treated with excision margins wider than 1 cm in more than one third of cases. CMIS is associated with an increased risk of invasive melanoma (standardized incidence ratio [SIR]: 8.08; 95% confidence interval [CI]: 7.66–8.57), with an estimated 3:5 invasive/in situ ratio; surprisingly, it is also associated with a reduced risk of gastrointestinal (SIR: 0.78, CI: 0.72–0.84) and lung (SIR: 0.65, CI: 0.59–0.71) cancers. Relative survival analysis shows that persons with CMIS have a life expectancy equal to that of the general population.
Conclusions.
CMIS is increasingly diagnosed and is often overtreated, although it does not affect the life expectancy of its carriers. Patients with CMIS have an increased risk of developing invasive melanoma (which warrants their enrollment in screening programs) but also a reduced risk of some epithelial cancers, which raises the intriguing hypothesis that genetic/environmental risk factors for some tumors may oppose the pathogenesis of others.
Introduction
Cutaneous melanoma is a serious threat for the public health because of its incidence rates—which have risen faster than those for any other malignancy in Caucasian populations over the last 30 years [1–3]—and because of its intrinsic chemoresistance, which makes this disease one of the few malignancies for which no standard systemic therapy is recommended [4–7]. Therefore, early diagnosis currently represents the “best therapy” for this type of skin cancer.
As for epithelial tumors, the invasive form of melanoma must be differentiated from the “in situ” tumor where malignant melanocytes remain confined within the skin epithelium (without trespassing the basal membrane): this nosologic entity is called cutaneous melanoma in situ (CMIS) [8, 9] and corresponds to the first level of Clark's classification system [10, 11] and to stage 0 of the AJCC TNM system [12].
The scientific importance of CMIS lies in the fact that in situ tumors are generally believed to be precursors of invasive tumors: in particular, it has been postulated that such cancers develop through the sequence dysplasia > in situ tumor > invasive tumor. This theory, first formulated for cervical cancer [13], has been rapidly applied to most epithelial tumors, and more recently a similar hypothesis has been made also for cutaneous melanoma [14–19]. The pathologic definition of in situ tumors is tightly associated with a key clinical feature, which is that these cancer precursors are by definition unable to grow and metastasize and thus their surgical removal is curative in virtually 100% of cases. This fact should confine the practical relevance of CMIS to the issue of early diagnosis and screening programs.
In contrast, despite the above considerations, CMIS is linked to some health concerns and unsolved debates regarding its clinicopathologic diagnosis [19–24], therapeutic/follow-up management [20, 25–29], and prognostic relevance [24, 30–32].
Patients diagnosed with CMIS have a higher risk of developing invasive melanoma [33], which prompts clinicians to enroll patients with CMIS into screening programs [29, 33, 34]. Moreover, CMIS has been associated with the risk of other cancers [31]. The growing incidence of CMIS is making it difficult to understand whether or not invasive skin melanoma incidence is actually stable or increasing [35] and whether or not the prognosis of patients with a melanocytic tumor is changing [36]. Finally, despite its noninvasive nature, international guidelines—which recommend excision margins ranging from 10 to 20 mm for invasive melanoma—suggest excision margins of 5 mm as the standard treatment for CMIS [4–6], although some investigators suggest the use of Mohs' surgery [37–39] or even topical therapeutics [40–42].
Because the currently available evidence regarding CMIS is based on a scattered small series, which makes it impossible to draw any reliable conclusion, we intended to perform a dedicated large-scale epidemiologic study to shed some light on the clinical relevance of this nosologic entity: to this aim we used the information obtained from the Surveillance & Epidemiology End Results (SEER) database, the largest freely available population-based databank on cancer [43].
Materials and Methods
Data were obtained from all 17 U.S. cancer registries participating in the SEER program [44] using the Seer*Stat software (version 6.5.2) [45].
We identified patients with histologically confirmed diagnosis of CMIS (1973–2006). For these cases, we searched for the variables of interest listed in Table 1, as well as those necessary for survival analyses.
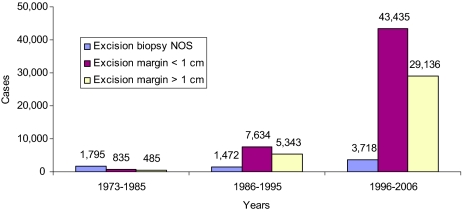
Table 1.
Clinical and pathologic characteristics of 93,853 cases of CMIS retrieved from the SEER database (1973–2006)
Abbreviations: ALM, acro-lengiginous; CMIS, cutaneous melanoma in situ; IQR, interquartile range, LMM, Lentigo maligna; NOS, not otherwise specified; SD, standard deviation; SSM, superficial spreading.
CMIS incidence and survival rates were compared with those of invasive skin melanoma (n = 183,175), which were also retrieved from the SEER database.
Both overall and disease-specific survival rates were considered, survival estimates being calculated according to the actuarial method (time intervals: 12 months).
Relative survival rates for patients with CMIS were also computed using the Seer*Stat software. Relative survival is a net survival measure representing cancer survival in the absence of other causes of death: it is defined as the ratio of the proportion of observed survivors in a cohort of cancer patients to the proportion of expected survivors in a comparable set of cancer-free individuals, the formulation being based on the assumption of independent competing causes of death. The relative survival rate adjusts for the survival rate of the general population (in this case, the United States population) for that race, sex, age, and date at which the age was coded (if this information is missing, that individual is excluded from the analysis).
The risk of developing other tumors was measured using the standardized incidence ratio (SIR), which is the ratio between the observed and the expected cases of a given event (according to the event incidence in the general population).
Ninety-five percent confidence interval (CI) was used as the measure of estimate variation, as appropriate. Probability values (p values) lower than 5% were considered significant.
Results
The search retrieved 93,853 cases of CMIS in 82,127 patients (Table 1), the great majority being white people (93.1%); 7,597 patients (9.3%) had two or more CMIS. The median age was 63 years (interquartile range [IQR]: 50–74), the disease frequency being higher in males (55.7%); interestingly, also invasive melanoma shows a higher incidence in men (55.5%), whereas the median age is lower (57 years).
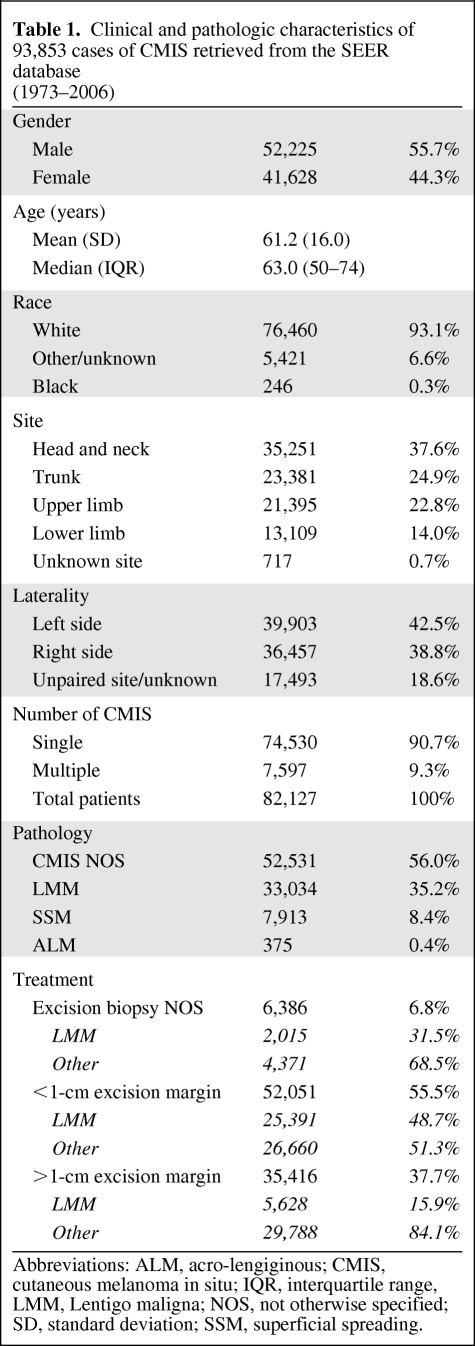
Considering the whole time span (1973–2006), CMIS incidence increased from less than 1 case to 14 cases per 100,000 per year, with an annual percentage change (APC) equal to 9.5% (CI: 8.6%–10.5%); this increasing trend outperforms that of invasive skin melanoma (APC: 3.6%, CI: 3.4%–3.8%), as shown in Figure 1. Remarkably, this steep rise of CMIS diagnosis outperforms also that of lung cancer in women (APC: 2.6%, CI: 2.1%–3.0%; SEER data from 293,083 patients) and is slightly higher than the increase in breast in situ carcinoma (APC: 7.1%, CI: 6.1%–8.1%; SEER data from 135,015 patients).
Figure 1.
Incidence of cutaneous melanoma (invasive melanoma, CMIS, and globally considered) over the past 3 decades (1973–2006) according to the Surveillance Epidemiology End Results (SEER) data.
Abbreviation: CMIS, cutaneous melanoma in situ.
With regard to CMIS location, “head and neck” was the body site with the highest incidence (37.6%), followed by the extremities (36.8%), with a modest prevalence of left-sided lesions (52.2% of lateralized lesions). Noticeably, also invasive melanoma shows a slightly higher incidence in the left side of the body (51.9% of lateralized lesions), whereas the trunk is the most frequently affected site (34.7%). At pathology evaluation, CMIS not otherwise specified (NOS) was the most common diagnosis (56.0%). Lentigo maligna CMIS (n = 33,034; 35.2%) occurred in older patients (median age: 70 years) and was mainly (but not exclusively) localized in the head and neck region (66.2%).
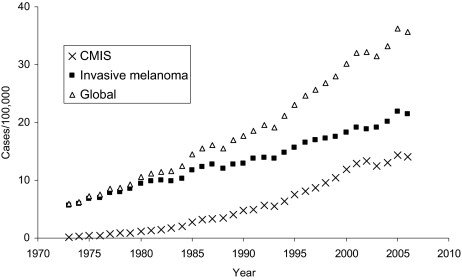
Treatment consisted of surgical removal of the lesion with an excision margin <1 cm in most cases (55.5%); however, 37.7% of cases were treated with wider excision (margin >1 cm), with no significant change of this percentage over the past 2 decades (Figure 2). Different extent of surgical excision margins (i.e., <1 cm versus >1 cm) did not appear to affect melanoma-specific survival (log-rank test p value = 0.52).
Figure 2.
Treatment of cutaneous melanoma in situ over time (1973–2006) according to the Surveillance Epidemiology End Results (SEER) data.
Abbreviation: NOS, not otherwise specified.
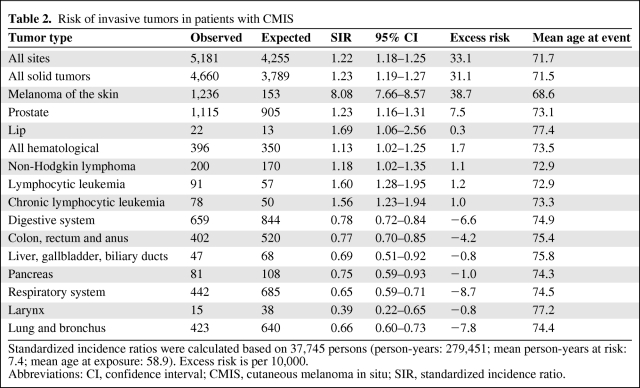
CMIS associated with a significantly increased risk of cancer in general and invasive melanoma in particular (standardized incidence ratio [SIR]: 8.08, 95% CI = 7.66–8.57), the mean onset time (mean age at event − mean age at exposure) being 13.8 years. Unexpectedly, CMIS was also associated with a reduced risk of malignancies arising from the respiratory (SIR: 0.65, 95% CI = 0.59–0.71) and digestive (SIR: 0.78, 95% CI = 0.72–0.84) system (Table 2), a feature shared by invasive melanoma (SIR: 0.77 [95% CI = 0.73–0.82] and 0.89 [95% CI = 0.85–0.93], respectively).
Table 2.
Risk of invasive tumors in patients with CMIS
Standardized incidence ratios were calculated based on 37,745 persons (person-years: 279,451; mean person-years at risk: 7.4; mean age at exposure: 58.9). Excess risk is per 10,000.
Abbreviations: CI, confidence interval; CMIS, cutaneous melanoma in situ; SIR, standardized incidence ratio.
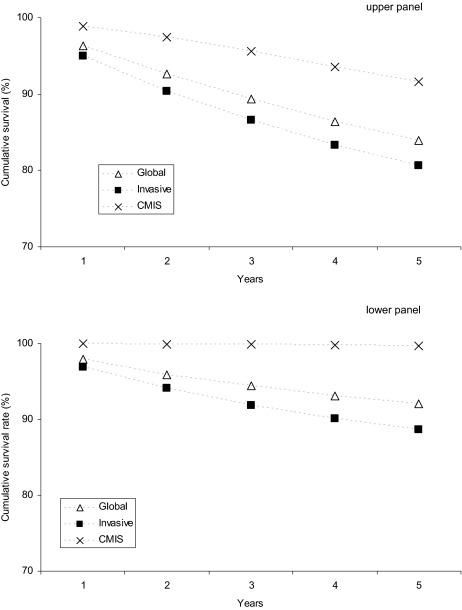
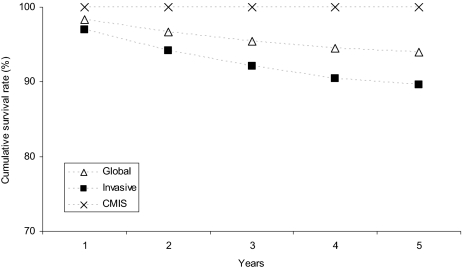
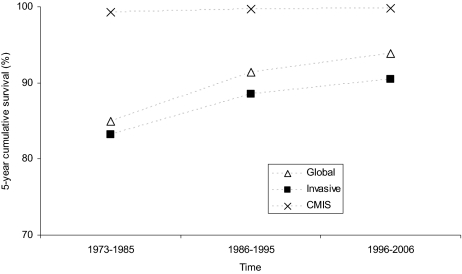
After a mean follow-up of 68.2 months, 12,649 patients (15.4%) have died, 532 (0.6%) of melanoma: overall (upper panel) and melanoma-specific (lower panel) survival rates of patients with CMIS and with invasive melanoma are shown in Figure 3. Importantly, life expectancy of patients diagnosed with CMIS does not differ from that of the general population, which is clearly not the case for patients with invasive melanoma (relative survival rates for both populations as well as their combination are shown in Figure 4). Considering the 5-year survival rates, the prognosis of patients diagnosed with cutaneous melanoma (invasive + CMIS) has been improving over the past 3 decades (the melanoma-specific survival rate increased from 85.0% to 93.9%); though slightly reduced, this favorable trend is maintained also after removing the potentially “diluting” effect of CMIS (Figure 5).
Figure 3.
Overall (upper panel) and melanoma-specific (lower panel) survival rates at 1, 2, 3, 4, and 5 years for patients with CMIS, invasive melanoma, and globally considered (Surveillance Epidemiology End Results [SEER] data 1973–2006).
Abbreviation: CMIS, cutaneous melanoma in situ.
Figure 4.
Relative survival rates at 1, 2, 3, 4, and 5 years for patients with CMIS, invasive melanoma, and globally considered (Surveillance Epidemiology End Results [SEER] data 1973–2006).
Abbreviation: CMIS, cutaneous melanoma in situ.
Figure 5.
Trends of melanoma-specific 5-year survival rates for patients with cutaneous melanoma in situ (CMIS), invasive melanoma and globally considered over the past 3 decades (Surveillance Epidemiology End Results [SEER] data 1973–2006).
Abbreviation: CMIS, cutaneous melanoma in situ.
Discussion
Using the largest series ever reported on, we have described some key epidemiologic features of cutaneous melanoma in situ (CMIS) that could shed some light on the clinical relevance of this nosologic entity. While presenting the results, we are aware of the fact that although the SEER database provides investigators with a unique opportunity of generating and testing medical hypotheses on an unprecedented large series of patients, under-reporting is a potential limit of this databank, especially when it comes to conditions like CMIS that are routinely and increasingly treated in the outpatient setting.
Although most investigators agree that the incidence of skin melanoma is rising worldwide [1–3], some have questioned this statement, sustaining either that benign lesions are erroneously included in the calculations [46] or that the increased incidence regards mainly invasive [47] or in situ lesions [48], or even that the incidence is declining [49]. As shown in Figure 1, according to the SEER data, the incidence of CMIS is remarkably increasing over the past 3 decades, which might be related to an actual “epidemic” of CMIS (as postulated for invasive melanoma [36]) but also to the increased attention paid by physicians and the general population to the early diagnosis of skin melanoma (so called “skin awareness”) [50–52]. As reported in the Results section, this rise of incidence is steeper than that of any other invasive and in situ malignancy. Importantly, the increase in CMIS incidence does not account for the whole increment in skin melanoma (in situ + invasive) incidence, as invasive melanoma rates are also growing, although at a slower pace (Figure 1).
The parallel increase of both invasive and in situ melanoma supports the hypothesis that the former tumor passes through the phase of CMIS before becoming capable of infiltrating surrounding tissues and metastasizing. The small but consistent prevalence of left-sided lesions and male gender in both invasive and in situ melanoma might be seen as addition epidemiologic evidence of the relationship between the two diseases. On the other hand, the higher rate of CMIS in the head and neck region supports the hypothesis that lentigo maligna CMIS (the most frequent type of CMIS in this body area) has a lower tendency to progress to invasive melanoma.
Considering the last decade, the ratio between invasive and in situ melanoma is about 1.5:1 (Figure 1); assuming that each invasive melanoma passes through the phase of CMIS (although no direct proof of this hypothesis exists), this ratio might imply that approximately 3 out of 5 cases of CMIS might progress to invasive melanoma. Lending support to this hypothesis, the risk of developing invasive melanoma is approximately eight times higher in people diagnosed with CMIS as compared with the general population (Table 2). The increased risk of invasive melanoma in patients with a previous diagnosis of CMIS was already known, although in smaller series its extent was probably overestimated: for instance, in a series of 3,766 Swedish patients diagnosed with CMIS from 1958 to 1992, Wassberg and colleagues reported a SIR of 22.2 [31], which is almost three times higher than that observed in the SEER series. Moreover, the same investigators described a higher risk of cancer in general (SIR: 2.2, 95% CI: 2.0–2.4) and some tumors in particular, such as breast cancer (in females), gastrointestinal cancers, and hematologic malignancies [31]. In this regard, the SEER data allowed us to confirm the higher risk of some hematologic tumors (Table 2), whereas no increased risk of breast carcinoma could be found (SIR: 1.01, 95% CI: 0.91–1.11). Surprisingly, we found that the risk of gastrointestinal tumors is even diminished in patients with CMIS (along with the risk of lung cancer; see Table 2), which highlights the importance of having at disposal large databases to draw reliable conclusions, especially when the event rates are relatively low.
Despite the fact that CMIS is associated with a significantly increased risk of invasive tumors, it is interesting to note that the life expectancy overlaps with that of the general population (5-year relative survival rate = 100%; Figure 4). This might be linked (at least in part) by two factors: first, the melanoma-specific mortality rates are quite low (0.3% at 5 years), which suggests that invasive melanomas arising in people with CMIS are mainly represented by thin lesions with a high curability rate; this phenomenon might in turn be related to the enrollment of these patients into screening programs. Second—as mentioned above—persons with CMIS are at lower risk of cancers that are considered “big killers,” such as lung and colorectal carcinomas, an observation never reported before.
CMIS association with higher incidence of invasive melanoma could well be due to the similar genetic background and/or environmental risk factors characterizing the two nosologic entities; in contrast, it appears much more difficult to explain the finding that people with CMIS carry a lower risk of epithelial tumors generally featuring worse prognosis (in terms of mortality rates intended as ratio of disease-specific deaths to incidence) as compared with invasive melanoma. Considering that invasive melanoma shares this epidemiologic feature with CMIS, an intriguing hypothesis might be that the genetic background and/or the environmental risk factors underlying skin melanoma development can oppose the carcinogenesis of some epithelial tumors. Besides representing a scientific challenge, such findings could represent a hint (or a warning) while analyzing and/or comparing molecular profiles of different tumors, as they are (at least in past) conflicting with the theory of common cancer pathways [53–55].
The extent of CMIS-free margins obtained with surgical excision does not appear to have a significant impact on melanoma-specific survival, as suggested by the similar rates of melanoma-related deaths in patients with <1 cm as compared with those with >1 cm excision margins. As a corollary, CMIS is likely best managed simply with a pathologically margin-free excision: wider margin excisions—which continue to represent a significant proportion of the surgical treatments (more than one third; see Figure 2) over the past 2 decades—should be discouraged.
Finally, the SEER data allowed us to verify whether the prognosis of invasive melanoma has been changing over the past 30 years independently of the impact of the increasing CMIS incidence. Interestingly, as shown in Figure 5, the prognosis of patients diagnosed with cutaneous melanoma (invasive + CMIS) has been improving over the past 3 decades (the melanoma-specific survival rate increased from 85.0% to 93.9%); although slightly reduced, this favorable trend—which has been questioned by some investigators [56]—is maintained also after removing the potentially “diluting” effect of CMIS advocated by some authors [57, 58]. Our data do not allow discernment on whether this positive finding is due to earlier diagnosis (which is notoriously accompanied by lower risk of melanoma-related death) or ameliorated therapeutic strategies (e.g., adjuvant interferon-alpha, sentinel node biopsy), which is a matter of continuous debate [57–61]; however, within the fame of this debate, it appears important to have clarified—by means of such a large series—the separate impact of invasive and in situ melanoma in the epidemiology of the deadliest skin disease.
Taken together, the above analysis of the SEER data can be regarded both as the basis of a practical guideline for the management of CMIS and as a source of some hints regarding melanoma epidemiology and cancer predisposition. In particular, we conclude that CMIS is a condition more and more frequently diagnosed with no significant impact on life expectancy: accordingly, its relevance is mainly linked to the clinical [22, 62] and pathologic [21, 63, 64] difficulty in making a differential diagnosis with invasive melanoma. Although their enrollment in screening programs for the early detection of skin melanoma is warranted because of the risk of developing invasive melanoma, patients diagnosed with CMIS should be reassured on the benign nature of their condition that is associated with a normal life expectancy. Finally, the fact that CMIS increasing incidence does not nullify the positive trend regarding the prognosis of invasive melanoma and the fact that an in situ tumor is associated with a lower risk of some invasive cancers deserves further attention and investigation.
Acknowledgments
We are grateful to our data manager Dr. Marta Briarava for setting up the dedicated database utilized for this work.
Author Contributions
Conception/Design: Simone Mocellin, Donato Nitti
Provision of study material or patients: Simone Mocellin, Donato Nitti
Collection and/or assembly of data: Simone Mocellin, Donato Nitti
Data analysis and interpretation: Simone Mocellin
Manuscript writing: Simone Mocellin, Donato Nitti
Final approval of manuscript: Simone Mocellin, Donato Nitti
References
- 1.MacKie RM, Hauschild A, Eggermont AM. Epidemiology of invasive cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):vi1–vi7. doi: 10.1093/annonc/mdp252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol. 2009;27:3–9. doi: 10.1016/j.clindermatol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 3.StatBite: Distribution of melanoma incidence and mortality by age, 2001–2005. J Natl Cancer Inst. 2008;100:1498. doi: 10.1093/jnci/djn393. [DOI] [PubMed] [Google Scholar]
- 4.Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365:687–701. doi: 10.1016/S0140-6736(05)17951-3. [DOI] [PubMed] [Google Scholar]
- 5.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 6.NCCN. Melanoma. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology, 2009. Available at http://www.nccn.org/index.asp.
- 7.Agarwala SS. Current systemic therapy for metastatic melanoma. Expert Rev Anticancer Ther. 2009;9:587–595. doi: 10.1586/era.09.25. [DOI] [PubMed] [Google Scholar]
- 8.Ackerman AB. Malignant melanoma in situ: the flat, curable stage of malignant melanoma. Pathology. 1985;17:298–300. doi: 10.3109/00313028509063771. [DOI] [PubMed] [Google Scholar]
- 9.Clark WH, Jr., Tucker MA. Problems with lesions related to the development of malignant melanoma: common nevi, dysplastic nevi, malignant melanoma in situ, and radial growth phase malignant melanoma. Hum Pathol. 1998;29:8–14. doi: 10.1016/s0046-8177(98)90384-7. [DOI] [PubMed] [Google Scholar]
- 10.Elder DE, Jucovy PM, Tuthill RJ, et al. The classification of malignant melanoma. Am J Dermatopathol. 1980;2:315–320. doi: 10.1097/00000372-198000240-00005. [DOI] [PubMed] [Google Scholar]
- 11.Clark WH., Jr Malignant melanoma in situ. Hum Pathol. 1990;21:1197–1198. doi: 10.1016/s0046-8177(06)80029-8. [DOI] [PubMed] [Google Scholar]
- 12.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson JH, Jr., Averette HE, Richart RM. Dysplasia, carcinoma in situ, and early invasive cervical carcinoma. CA Cancer J Clin. 1984;34:306–327. doi: 10.3322/canjclin.34.6.306. [DOI] [PubMed] [Google Scholar]
- 14.Miller AJ, Mihm MC., Jr Melanoma. N Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 15.Gutkowicz-Krusin D, Rabinovitz HS. Kinetic model of progression of cutaneous melanoma population. Melanoma Res. 2007;17:354–359. doi: 10.1097/CMR.0b013e3282f120bf. [DOI] [PubMed] [Google Scholar]
- 16.Haass NK, Herlyn M. Normal human melanocyte homeostasis as a paradigm for understanding melanoma. J Investig Dermatol Symp Proc. 2005;10:153–163. doi: 10.1111/j.1087-0024.2005.200407.x. [DOI] [PubMed] [Google Scholar]
- 17.Crowson AN, Magro CM, Sanchez-Carpintero I, et al. The precursors of malignant melanoma. Recent Results Cancer Res. 2002;160:75–84. doi: 10.1007/978-3-642-59410-6_11. [DOI] [PubMed] [Google Scholar]
- 18.Tannous ZS, Lerner LH, Duncan LM, et al. Progression to invasive melanoma from malignant melanoma in situ, lentigo maligna type. Hum Pathol. 2000;31:705–708. doi: 10.1053/hupa.2000.7640. [DOI] [PubMed] [Google Scholar]
- 19.Dubow BE, Ackerman AB. Ideas in pathology. Malignant melanoma in situ: the evolution of a concept. Mod Pathol. 1990;3:734–744. [PubMed] [Google Scholar]
- 20.Charles CA, Yee VS, Dusza SW, et al. Variation in the diagnosis, treatment, and management of melanoma in situ: a survey of US dermatologists. Arch Dermatol. 2005;141:723–729. doi: 10.1001/archderm.141.6.723. [DOI] [PubMed] [Google Scholar]
- 21.Megahed M, Schon M, Selimovic D, et al. Reliability of diagnosis of melanoma in situ. Lancet. 2002;359:1921–1922. doi: 10.1016/S0140-6736(02)08741-X. [DOI] [PubMed] [Google Scholar]
- 22.Pizzichetta MA, Argenziano G, Talamini R, et al. Dermoscopic criteria for melanoma in situ are similar to those for early invasive melanoma. Cancer. 2001;91:992–997. [PubMed] [Google Scholar]
- 23.Okamura JM, Barr RJ, Cantos KA. Benign atypical junctional melanocytic hyperplasia associated with intradermal nevi: a common finding that may be confused with melanoma in situ. Mod Pathol. 2000;13:857–860. doi: 10.1038/modpathol.3880152. [DOI] [PubMed] [Google Scholar]
- 24.Bartoli C, Bono A, Clemente C, et al. Clinical diagnosis and therapy of cutaneous melanoma in situ. Cancer. 1996;77:888–892. doi: 10.1002/(sici)1097-0142(19960301)77:5<888::aid-cncr12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Moller MG, Pappas-Politis E, Zager JS, et al. Surgical management of melanoma-in-situ using a staged marginal and central excision technique. Ann Surg Oncol. 2009;16:1526–1536. doi: 10.1245/s10434-008-0239-x. [DOI] [PubMed] [Google Scholar]
- 26.Clark GS, Pappas-Politis EC, Cherpelis BS, et al. Surgical management of melanoma in situ on chronically sun-damaged skin. Cancer Control. 2008;15:216–224. doi: 10.1177/107327480801500304. [DOI] [PubMed] [Google Scholar]
- 27.Wolf IH, Cerroni L, Kodama K, et al. Treatment of lentigo maligna (melanoma in situ) with the immune response modifier imiquimod. Arch Dermatol. 2005;141:510–514. doi: 10.1001/archderm.141.4.510. [DOI] [PubMed] [Google Scholar]
- 28.Zalaudek I, Horn M, Richtig E, et al. Local recurrence in melanoma in situ: influence of sex, age, site of involvement and therapeutic modalities. Br J Dermatol. 2003;148:703–708. doi: 10.1046/j.1365-2133.2003.05155.x. [DOI] [PubMed] [Google Scholar]
- 29.Thorn M, Ponten F, Johansson AM, et al. Rapid increase in diagnosis of cutaneous melanoma in situ in Sweden, 1968–1992. Cancer Detect Prev. 1998;22:430–437. doi: 10.1046/j.1525-1500.1998.00052.x. [DOI] [PubMed] [Google Scholar]
- 30.Flotte TJ, Mihm MC., Jr Lentigo maligna and malignant melanoma in situ, lentigo maligna type. Hum Pathol. 1999;30:533–536. doi: 10.1016/s0046-8177(99)90197-1. [DOI] [PubMed] [Google Scholar]
- 31.Wassberg C, Thorn M, Yuen J, et al. Cancer risk in patients with earlier diagnosis of cutaneous melanoma in situ. Int J Cancer. 1999;83:314–317. doi: 10.1002/(sici)1097-0215(19991029)83:3<314::aid-ijc5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Wagner RF, Jr., Murphy CM. Risk classification of life and health insurance applicants with atypical melanocytic hyperplasia or malignant melanoma in situ. Cutis. 1992;50:352–354. [PubMed] [Google Scholar]
- 33.McCaul KA, Fritschi L, Baade P, et al. The incidence of second primary invasive melanoma in Queensland, 1982–2003. Cancer Causes Control. 2008;19:451–458. doi: 10.1007/s10552-007-9106-5. [DOI] [PubMed] [Google Scholar]
- 34.Francken AB, Shaw HM, Thompson JF. Detection of second primary cutaneous melanomas. Eur J Surg Oncol. 2008;34:587–592. doi: 10.1016/j.ejso.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Linos E, Swetter SM, Cockburn MG, et al. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666–1674. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beddingfield FC., 3rd The melanoma epidemic: res ipsa loquitur. The Oncologist. 2003;8:459–465. doi: 10.1634/theoncologist.8-5-459. [DOI] [PubMed] [Google Scholar]
- 37.Raziano RM, Clark GS, Cherpelis BS, et al. Staged margin control techniques for surgical excision of lentigo maligna. G Ital Dermatol Venereol. 2009;144:259–270. [PubMed] [Google Scholar]
- 38.Shumaker PR, Kelley B, Swann MH, et al. Modified Mohs micrographic surgery for periocular melanoma and melanoma in situ: long-term experience at Scripps Clinic. Dermatol Surg. 2009;35:1263–1270. doi: 10.1111/j.1524-4725.2009.01222.x. [DOI] [PubMed] [Google Scholar]
- 39.Dawn ME, Dawn AG, Miller SJ. Mohs surgery for the treatment of melanoma in situ: a review. Dermatol Surg. 2007;33:395–402. doi: 10.1111/j.1524-4725.2007.33085.x. [DOI] [PubMed] [Google Scholar]
- 40.Powell AM, Robson AM, Russell-Jones R, et al. Imiquimod and lentigo maligna: a search for prognostic features in a clinicopathological study with long-term follow-up. Br J Dermatol. 2009;160:994–998. doi: 10.1111/j.1365-2133.2009.09032.x. [DOI] [PubMed] [Google Scholar]
- 41.Hopson B, Richey D, Sajben FP. Treatment of lentigo maligna with imiquimod 5% cream. J Drugs Dermatol. 2007;6:1037–1040. [PubMed] [Google Scholar]
- 42.Woodmansee C, Pillow J, Skinner RB., Jr The role of topical immune response modifiers in skin cancer. Drugs. 2006;66:1657–1664. doi: 10.2165/00003495-200666130-00001. [DOI] [PubMed] [Google Scholar]
- 43.SEER. Bethesda, MD: National Cancer Institute; 2009. Surveillance Epidemiology and End Results. Available at http://seer.cancer.gov/ [Google Scholar]
- 44.SEER. Bethesda, MD: National Cancer Institute; 2009. SEER*Stat Database: Incidence - SEER 17 Regs Limited-Use + Hurricane Katrina Impacted Louisiana Cases, Nov 2008 Sub (1973–2006 varying) Available at http://seer.cancer.gov/data/index.html. [Google Scholar]
- 45.SEER. Bethesda, MD: National Cancer Institute; 2009. Surveillance Research Program, SEER*Stat software. Available at http://seer.cancer.gov/seerstat/ [Google Scholar]
- 46.Levell NJ, Beattie CC, Shuster S, et al. Melanoma epidemic: a midsummer night's dream? Br J Dermatol. 2009;161:630–634. doi: 10.1111/j.1365-2133.2009.09299.x. [DOI] [PubMed] [Google Scholar]
- 47.Downing A, Newton-Bishop JA, Forman D. Recent trends in cutaneous malignant melanoma in the Yorkshire region of England; incidence, mortality and survival in relation to stage of disease, 1993–2003. Br J Cancer. 2006;95:91–95. doi: 10.1038/sj.bjc.6603216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nevins T, Su Y, Doucette S, et al. Incidence of cutaneous malignant melanoma in the Ottawa region: 1996 to 2006. J Cutan Med Surg. 2008;12:276–281. doi: 10.2310/7750.2008.07075. [DOI] [PubMed] [Google Scholar]
- 49.Chuang TY, Charles J, Reizner GT, et al. Melanoma in Kauai, Hawaii, 1981–1990: the significance of in situ melanoma and the incidence trend. Int J Dermatol. 1999;38:101–107. doi: 10.1046/j.1365-4362.1999.00511.x. [DOI] [PubMed] [Google Scholar]
- 50.Stratigos AJ, Katsambas AD. The value of screening in melanoma. Clin Dermatol. 2009;27:10–25. doi: 10.1016/j.clindermatol.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Halpern AC, Lieb JA. Early melanoma diagnosis: a success story that leaves room for improvement. Curr Opin Oncol. 2007;19:109–115. doi: 10.1097/CCO.0b013e32801497b2. [DOI] [PubMed] [Google Scholar]
- 52.Janda M, Baade PD, Youl PH, et al. The skin awareness study: promoting thorough skin self-examination for skin cancer among men 50 years or older. Contemp Clin Trials. 2010;31:119–130. doi: 10.1016/j.cct.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Ajani J, Allgood V. Molecular mechanisms in cancer: what should clinicians know? Semin Oncol. 2005;32:2–4. doi: 10.1053/j.seminoncol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 54.Basil CF, Zhao Y, Zavaglia K, et al. Common cancer biomarkers. Cancer Res. 2006;66:2953–2961. doi: 10.1158/0008-5472.CAN-05-3433. [DOI] [PubMed] [Google Scholar]
- 55.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 56.Downing A, Yu XQ, Newton-Bishop J, et al. Trends in prognostic factors and survival from cutaneous melanoma in Yorkshire, UK and New South Wales, Australia between 1993 and 2003. Int J Cancer. 2008;123:861–866. doi: 10.1002/ijc.23495. [DOI] [PubMed] [Google Scholar]
- 57.Criscione VD, Weinstock MA. Melanoma thickness trends in the United States, 1988–2006. J Invest Dermatol. 2010;130:793–797. doi: 10.1038/jid.2009.328. [DOI] [PubMed] [Google Scholar]
- 58.Coory M, Baade P, Aitken J, et al. Trends for in situ and invasive melanoma in Queensland, Australia, 1982–2002. Cancer Causes Control. 2006;17:21–27. doi: 10.1007/s10552-005-3637-4. [DOI] [PubMed] [Google Scholar]
- 59.Wolff T, Tai E, Miller T. Screening for skin cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;150:194–198. doi: 10.7326/0003-4819-150-3-200902030-00009. [DOI] [PubMed] [Google Scholar]
- 60.Terushkin V, Halpern AC. Melanoma early detection. Hematol Oncol Clin North Am. 2009;23:481–500. viii. doi: 10.1016/j.hoc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Weinstock MA. Progress and prospects on melanoma: the way forward for early detection and reduced mortality. Clin Cancer Res. 2006;12:2297s–2300s. doi: 10.1158/1078-0432.CCR-05-2559. [DOI] [PubMed] [Google Scholar]
- 62.Strauss RM, Elliott F, Affleck P, et al. A retrospective study addressed to understanding what predicts severe histological dysplasia/early melanoma in excised atypical melanocytic lesions. Br J Dermatol. 2007;157:758–764. doi: 10.1111/j.1365-2133.2007.08136.x. [DOI] [PubMed] [Google Scholar]
- 63.McCalmont TH. Melanoma and melanoma in situ: build a better diagnosis through architecture. Semin Cutan Med Surg. 1997;16:97–107. doi: 10.1016/s1085-5629(97)80003-x. [DOI] [PubMed] [Google Scholar]
- 64.Ruiter DJ, van Dijk MC, Ferrier CM. Current diagnostic problems in melanoma pathology. Semin Cutan Med Surg. 2003;22:33–41. doi: 10.1053/sder.2003.50003. [DOI] [PubMed] [Google Scholar]