This review discusses the role of the mammalian target of rapamycin pathway in leukemia, lymphoma, multiple myeloma, and Waldenström's macroglobulinemia and presents results from recent studies on the use of mammalian target of rapamycin–targeted agents in these diseases.
Keywords: Hematologic malignancy, Leukemia, Lymphoma, Mammalian target of rapamycin, Multiple myeloma, Waldenström macroglobulinemia
Abstract
The mammalian target of rapamycin (mTOR) is an intracellular serine/threonine kinase that exists as a downstream component of numerous signaling pathways. The activation of mTOR results in the production of proteins involved in cell metabolism, growth, proliferation, and angiogenesis. Aberrant activation of mTOR signaling has been identified in a number of cancers, and targeted inhibition of mTOR has been successful in achieving tumor responses, prolonging progression-free survival, and increasing overall survival in various oncologic patient populations. In particular, persistent activation of mTOR signaling has been identified in cell lines and patient samples with leukemias, Hodgkin's lymphoma (HL), non-Hodgkin's lymphoma (NHL), multiple myeloma (MM), and Waldenström's macroglobulinemia (WM). In vitro and preclinical studies using agents that inhibit mTOR signaling have demonstrated cytostatic and cytotoxic effects in these hematologic malignancies, suggesting that mTOR is a rational target for therapy in these disease states. In addition, the combination of mTOR inhibitors with traditional therapies may help to overcome the development of resistance and may improve response rates over those seen with established regimens through synergistic or additive effects. Inhibitors of mTOR signaling currently are being investigated in clinical trials of hematologic malignancies as single agents and as components of combination regimens. Thus far, promising results have been seen with the application of mTOR inhibitors as single agents in patients with relapsed or refractory leukemia, HL, NHL, MM, and WM.
Introduction
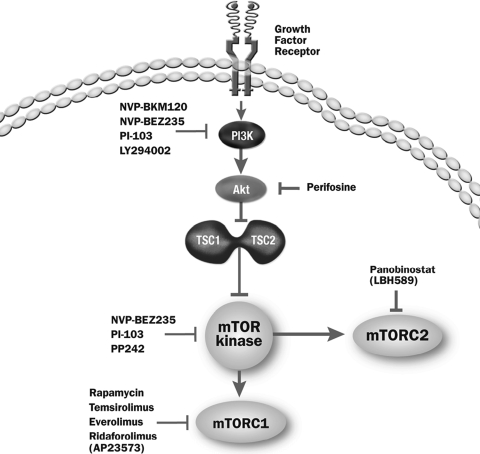
The deregulation of signaling proteins both upstream and downstream of the mammalian target of rapamycin (mTOR) has been implicated in many types of cancer, including hematologic malignancies [1, 2]. As shown in Figure 1, mTOR is an intracellular serine/threonine protein kinase that exists as a component of two distinct multiprotein complexes, mTORC1, which has well-characterized signaling pathways based on studies with pharmacologic agents such as rapamycin, and mTORC2, which is currently less well understood [3, 4]. The mTOR complex is an integration point for multiple extracellular and intracellular signals arising from growth factors, cellular energy status, and nutrient availability.
Figure 1.
Pathways involved in mTOR signaling.
Abbreviations: mTOR, mammalian target of rapamycin; PI3K, phosphoinositide 3-kinase; TSC, tuberous sclerosis.
Adapted from: Kopelovich L, Fay JR, Sigman CC, Crowell JA. The mammalian target of rapamycin pathway as a potential target for cancer chemoprevention. Cancer Epidemiol Biomarkers Prev 2007;16:1330–1340.
The stimulation of transmembrane receptors via growth factors leads to activation of the membrane-bound protein phosphoinositide 3-kinase (PI3K). PI3K in turn activates a second messenger, phosphatidylinositol-3,4,5 triphosphate (PIP3) [5, 6]. PIP3 recruits several proteins to the plasma membrane, including the serine/threonine kinase Akt, thus activating it. Akt-directed phosphorylation of the tuberous sclerosis (TSC)1–TSC2 protein complex causes its disassociation from Ras homolog enriched in brain (Rheb), allowing TSC to be activated and thus interact with the mTOR kinase [6, 7].
Once activated, the mTOR protein associates with regulatory associated protein of TOR and G protein β-subunit-like protein (mLST8/GßL) to form mTORC1. mTORC1 initiates a downstream cascade that triggers cellular translational machinery to produce proteins required for cell metabolism, growth, proliferation, and angiogenesis. mTOR-mediated phosphorylation of eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) allows its dissociation from eIF4E to promote cap-dependent mRNA translation [8]. In addition, phosphorylation of p70 ribosomal S6 kinase (p70S6K) by mTOR promotes translation of 5′-polypyrimidine tract mRNA transcripts for the synthesis of components of the translation machinery [9]. On the other hand, mTOR kinase also binds with rapamycin-insensitive companion of TOR, mLST8/GßL, and mSin1 to form mTORC2 [10]. The function of mTORC2 is still under investigation. From what is known, it has the ability to activate Akt and lead to the full activation of this protein [11]. Fully activated Akt phosphorylates several proteins (including TSC2, as described above), which drives cell growth, proliferation, migration, and metabolism [12]. This diverse pathway is referred to more simply as the PI3K/Akt/mTOR pathway.
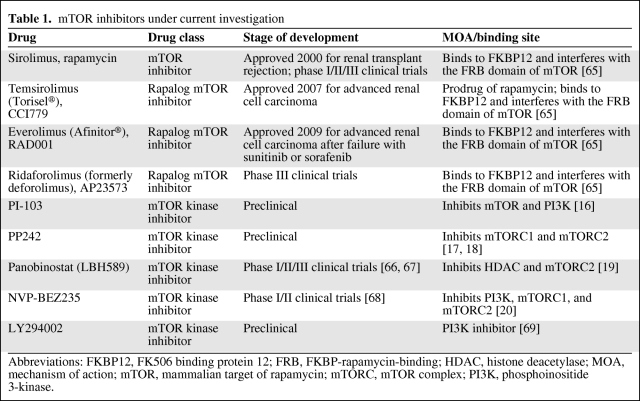
Therapeutic approaches that target the inhibition of the PI3K/Akt/mTOR pathway are currently under active investigation (Table 1). Rapamycin was the first agent found to interfere with this pathway, leading to the partial inhibition of mTORC1 [13]. Temsirolimus and everolimus, two analogs of rapamycin (i.e., rapalogs) have shown clinical efficacy in a variety of tumors and are approved by the U.S. Food and Drug Administration (FDA) as single-agent therapy for the treatment of patients with advanced renal cell carcinoma (RCC). Temsirolimus is indicated as first-line therapy for patients with poor-prognosis RCC, and everolimus is the standard of care after failure of vascular endothelial growth factor receptor tyrosine kinase inhibitor (TKI) therapies. Temsirolimus and everolimus are under investigation as single agents or components of combination regimens in several hematologic malignancies.
Table 1.
mTOR inhibitors under current investigation
Abbreviations: FKBP12, FK506 binding protein 12; FRB, FKBP-rapamycin-binding; HDAC, histone deacetylase; MOA, mechanism of action; mTOR, mammalian target of rapamycin; mTORC, mTOR complex; PI3K, phosphoinositide 3-kinase.
Second-generation mTOR inhibitors (known as mTOR kinase inhibitors) currently are in development and differ from rapalogs by binding to the ATP-binding pocket directly on mTOR. These compounds may have more dramatic effects on cell growth and proliferation with their ability to inhibit both mTORC1 and mTORC2 [14]. Another class of agents capable of interfering with the PI3K/Akt/mTOR pathway at two points via the dual inhibition of mTOR kinase and PI3K also is under investigation. These agents include PI-103, which inhibits mTOR and PI3K [15, 16]; PP242, which inhibits mTORC1 and mTORC2 [17, 18]; panobinostat, which inhibits histone deacetylase (HDAC) and mTORC2 [19]; and NVP-BEZ235, which inhibits PI3K, mTORC1, and mTORC2 [20]. Many of these agents are being applied to hematologic malignancies and are in various phases of clinical study (Table 1).
The objectives of this review are to discuss the role of the mTOR pathway in leukemia, lymphoma, multiple myeloma (MM), and Waldenström's macroglobulinemia (WM) and to present results from recent studies on the use of mTOR-targeted agents in these diseases.
The PI3K/Akt/mTOR Pathway in Leukemia
Aberrant regulation of the mTOR signaling pathway has been identified in several types of leukemia, including acute myeloid leukemia (AML), T-cell acute lymphoblastic leukemia (T-ALL), Philadelphia chromosome positive B precursor acute lymphoblastic leukemia (Ph+ B-ALL), B-cell chronic lymphocytic leukemia (B-CLL), chronic myelogenous leukemia (CML), and high-risk myelodysplastic syndromes (MDS) [1, 2]. High levels of phosphorylated Akt have been found in patient-derived AML cells in patients with newly diagnosed AML [21]. To study the significance of this finding, a myristoylated and thus constitutively active form of Akt was transplanted into a mouse model, which demonstrated that increased Akt signaling induced the development of myeloproliferative disease (MPD), AML, and T-cell lymphoma [22].
The lipid phosphatase and tensin homolog gene (PTEN) leading to PTEN protein is a negative regulator of Akt, and loss of PTEN expression via mutation has been implicated in many cancers. In one study, constitutive hyperactivation of the PI3K/Akt pathway was detected in 87.5% of patient-derived T-ALL specimens [23]. Surprisingly, both PTEN− and PTEN+ samples displayed hyperactive PI3K/Akt pathways, suggesting that PTEN gene alterations are not the only means of PTEN loss of function in leukemia. Despite normal levels of PTEN expression in T-ALL specimens, the protein was found to be inactivated via phosphorylation secondary to upregulation of casein kinase 2 (CK2) activity [23]. The pharmacologic inhibition of CK2 in these cell lines resulted in significant cell death, suggesting the importance of CK2-mediated activation of the PI3K/Akt pathway via the downregulation of PTEN.
In Vitro Data with mTOR Inhibitors in Leukemia
Theoretically, inhibition of the PI3K/Akt/mTOR pathway should inhibit cell growth and proliferation and induce apoptosis. Preclinical studies have confirmed that inhibition of this pathway impairs the clonogenic properties of leukemic cells [24–27]. A 2005 study showed that mTOR inhibition by rapamycin decreased the growth of AML cell lines [24]. Subsequently, everolimus and temsirolimus blocked mTORC1 and Akt activation via mTORC2 in AML cells [25]. Kojima et al. [15] found that PI-103 enhances downstream p53 signaling, suggesting that a combination strategy directed toward PI3K/Akt/mTOR signaling and activating p53 signaling might be effective in AML. Dual inhibition of mTORC1 and the insulin-like growth factor 1 pathway induced additive antiproliferative effects in AML cells [27]. To document the clinical significance of Akt upregulation in AML cell lines, investigators examined the effects of Akt inhibition via the PI3K inhibitor LY294002 [28]. Patient-derived AML cells incubated in LY294002 exhibited lower levels of phosphorylated Akt, p70S6K, and 4E-BP1, which resulted in apoptosis. Interestingly, the level of PTEN expression in these cells did not correlate with the amount of activated Akt.
In one study, T-ALL cell lines containing constitutively active PI3K/Akt/mTOR signaling were treated with different concentrations of PI-103, a small-molecule inhibitor of both PI3K and mTOR [26]. When compared with pharmacologic agents that inhibit either PI3K or mTOR alone, PI-103 exerted a stronger effect on cell growth retardation and displayed both cytostatic and cytotoxic properties. PI-103 also was capable of dephosphorylating Akt and downstream mTOR targets such as p70S6K and 4E-BP1 [26]. In addition, bone marrow and peripheral blood cells from pediatric T-ALL patients demonstrated higher levels of phosphorylated Akt and 4E-BP1 than peripheral blood lymphocytes of normal controls, and after 96 hours of treatment with increasing concentrations of PI-103, cell viability was significantly lower than in untreated cells [26].
The Ph chromosome generated by the t(9;22)(q34;q11) translocation results in the production of a fusion gene encoding a constitutively active Bcr-Abl tyrosine kinase, which leads to the development of CML and some cases of ALL. One downstream target of Bcr-Abl phosphorylation is mTOR kinase. In an experimental mouse model of Ph+ B-ALL and Ph+ CML cell lines, the efficacy of three types of mTOR inhibition was tested using rapamycin, PI-103, and PP242, a compound that binds to the ATP-catalytic binding site on mTOR kinase, thus inhibiting both mTORC1 and mTORC2 [17, 18]. Cell cycle analysis confirmed that, whereas rapamycin primarily caused cell cycle arrest, both PI-103 and PP242 caused cell cycle arrest and apoptosis.
Combination therapy with mTOR inhibitors and cytotoxic chemotherapy with other targeted therapies are under investigation in numerous in vitro and preclinical studies. In vitro AML cells incubated with rapamycin display greater sensitivity to the apoptotic effects of cytarabine, an S-phase–specific drug commonly used to treat AML [29]. Because rapamycin can increase levels of activated Akt, the authors combined rapamycin with a PI3K inhibitor (LY294002) and demonstrated a much stronger apoptotic effect in these cells than with rapamycin alone. The subsequent addition of cytarabine to these cells further enhanced this effect [29]. In T-ALL cell lines, PI-103 demonstrated strong synergism with vincristine, an agent used in the standard treatment of T-ALL. Earlier in vitro data using cells with myristoylated Akt demonstrated that more Akt may confer resistance to microtubule inhibitors such as vincristine [30]. Cytotoxicity induced by this combination was higher than with either of the two agents alone [26]. Experiments with precursor B-ALL cell lines and patient-derived samples showed that administration of everolimus in combination with bortezomib (a proteosome inhibitor) significantly enhanced cell death [31].
TKIs are the mainstay of treatment for Ph+ CML and may be used in combination with chemotherapy for Ph+ B-ALL. The TKI imatinib is indicated by the U.S. FDA for the treatment of newly diagnosed Ph+ CML and for relapsed or refractory Ph+ ALL. Dasatinib is a multikinase inhibitor indicated by the U.S. FDA for the treatment of Ph+ ALL and CML with resistance or intolerance to prior therapy, including imatinib in the latter indication. Upregulation of mTOR in these diseases may lead to propagation of a leukemic phenotype with resistance to these agents, providing sound rationale for studying the effects of TKIs with mTOR inhibitors.
In a xenograft model of human Ph+ B-ALL cells transplanted into interleukin-2 receptor-γ knockout nonobese diabetic severe combined immunodeficient (NOD-SCID) interleukin 2 receptor γ null mice, the combined administration of PP242 and dasatinib caused regression of leukemic disease and prevented the spread of disease to the central nervous system [17]. When PP242 was combined with higher doses of dasatinib, the decrease in leukemic burden was significantly higher than with dasatinib monotherapy [17]. The combination of everolimus and imatinib enhanced the cytotoxicity induced by imatinib alone in Bcr-Abl–expressing cells via persistent inhibition of cell proliferation and apoptosis [32, 33]. Greater nuclear translocation of the normal p145 c-Abl protein occurred in response to the combined presence of everolimus and imatinib, leading to apoptosis [33].
Everolimus also may have a role in the blockade of imatinib resistance in leukemic cells. One mechanism of imatinib resistance involves activation of the PI3K/Akt/mTOR pathway; however, treatment with everolimus antagonized the late reactivation of mTOR triggered by imatinib treatment in Bcr-Abl–expressing cells [32]. The T315I mutation in the Abl kinase domain coding region alters the imatinib binding site on tyrosine kinase and confers resistance to imatinib. One study showed that, in imatinib-resistant Ph+ leukemia cell lines containing Bcr-Abl with the T315I mutation, treatment with everolimus was able to overcome resistance to imatinib and induce cell death [34].
The combination of rapamycin and tipifarnib, a farnesyltransferase inhibitor, achieved synergistic inhibition of the growth of both Bcr-Abl− human myeloid leukemia cell lines and Bcr-Abl+ cell lines established from patients with CML [35]. Growth inhibitory effects involved apoptosis and cell cycle blockade [35]. When patient-derived peripheral blood AML cells were treated with each agent and with both agents in combination, combination therapy again showed a synergistic effect on growth inhibition (Fig. 2) [35]. The addition of rapamycin to tipifarnib treatment of tipifarnib-resistant cells overcame tipifarnib resistance and resulted in synergistic growth inhibition [35].
Figure 2.
Synergistic effect of combination therapy with tipifarnib and rapamycin on patient-derived acute myeloid leukemia cells. The growth inhibitory effect of each agent on primary leukemia cells from the peripheral blood of a patient with acute myeloid leukemia is surpassed by that with combination therapy with both agents.
Reprinted from Nagai T, Ohmine K, Fujiwara S et al. Combination of tipifarnib and rapamycin synergistically inhibits the growth of leukemia cells and overcomes resistance to tipifarnib via alteration of cellular signaling pathways. Leuk Res 2010;34:1057–1063, with permission from Elsevier.
These preclinical results suggest that combination therapy with mTOR inhibitors and other agents may improve response rates in patients with leukemia through synergistic or additive effects.
Clinical Studies with mTOR Inhibitors in Leukemia
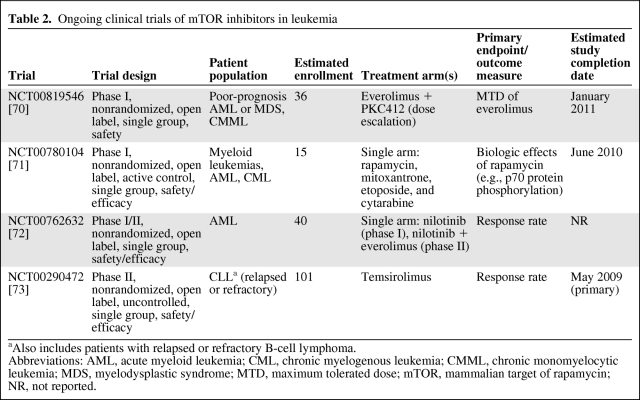
Clinically, mTOR inhibition has achieved responses in patients with relapsed or refractory leukemia. Ongoing clinical trials of mTOR inhibitors in leukemia are summarized in Table 2. In a trial of five patients with chemotherapy-refractory AML, 14 days of treatment with a low dose of rapamycin (2 mg/day) conferred a mild antileukemic effect in half of the patients [36]. The authors suggested that higher doses of rapamycin or a combination of mTOR inhibitors may increase the chances of driving these patients into remission [36]. A phase I study examined rapamycin in combination with mitoxantrone, etoposide, and cytarabine in 29 patients with AML [37]. Six of 27 patients who completed chemotherapy experienced a clinical response (complete response [CR] or partial response [PR]).
Table 2.
Ongoing clinical trials of mTOR inhibitors in leukemia
aAlso includes patients with relapsed or refractory B-cell lymphoma.
Abbreviations: AML, acute myeloid leukemia; CML, chronic myelogenous leukemia; CMML, chronic monomyelocytic leukemia; MDS, myelodysplastic syndrome; MTD, maximum tolerated dose; mTOR, mammalian target of rapamycin; NR, not reported.
The first phase I/II study of 5 mg or 10 mg everolimus in 27 patients with refractory hematologic malignancies demonstrated acceptable toxicity and possible activity in one of five patients with MDS but not in the nine patients with AML [38]. Results of a phase II clinical trial of everolimus (10 mg/day) in patients with previously treated indolent hematologic malignancies showed that, in the subset of 22 heavily pretreated patients with CLL, four achieved a PR (18%; 95% confidence interval [CI], 5%–40%). The median overall survival (OS) time was 10.5 months (95% CI, 4.9–20.7 months) and the median progression-free survival (PFS) interval was 5.1 months (95% CI, 2.3–8.3 months) [39]. The median decrease in clinically measurable lymph node size from baseline was 76% (range, 38%–93%) [39]. In addition, the absolute lymphocyte count increased in association with a decrease in lymphadenopathy in eight patients, suggesting mobilization of CLL cells from tissue into the circulation. The investigators suggested that mobilization of CLL cells by everolimus may have implications for combination therapy, potentially enhancing the cytotoxicity of agents such as alemtuzumab [39]. A phase II study of ridaforolimus monotherapy in 52 evaluable patients with relapsed or refractory hematologic malignancies demonstrated PRs in five patients (10%) and hematologic improvement or stable disease (SD) in 21 patients (40%) [40].
The PI3K/Akt/mTOR Pathway in Lymphoma
Persistent activation of mTOR signaling has been demonstrated in cell lines and primary tissues in diffuse large B-cell lymphoma (DLBCL) [19], mantle cell lymphoma (MCL) [41, 42], and Hodgkin's lymphoma (HL) [43]. High levels of active Rheb are expressed in some lymphomas, and Rheb expression is associated with greater mTOR activation [44]. In a syngeneic mouse model, introduction of Rheb via adoptive transfer of retrovirally modified hematopoietic progenitor cells induced rapid development of aggressive lymphomas [44]. The genetic alteration underlying MCL, a t(11;14)(q13;q32) chromosomal translocation, results in overexpression of cyclin D1, a cell cycle mediator regulated by the PI3K/Akt/mTOR pathway [45]. Thus, mTOR targeting is one of many therapeutic approaches being evaluated in lymphoma.
In Vitro Data with mTOR Inhibitors in Lymphoma
Results of in vitro and preclinical studies have demonstrated the potential value of mTOR inhibitors as single agents, in combination with other targeted agents, and in combination with conventional chemotherapy for the treatment of lymphoma. Administration of rapamycin in a mouse model that has an elevated incidence of MPD, AML, and T-cell lymphoma resulted in a longer time to disease development, lower incidence of T-cell lymphoma, and longer survival duration [22].
Treatment of DLBCL cell lines with the combination of rapamycin and the HDAC inhibitor panobinostat (LBH589), which also inhibits mTORC2, synergistically inhibited phosphorylation of p70S6K and 4E-BP1, as well as cell proliferation and survival [19, 46]. In other DLBCL cell lines, treatment with everolimus inhibited cell cycle progression and enhanced the toxicity of rituximab [47]. Everolimus also inhibited the proliferation of MCL cell lines, and synergistic cytotoxic effects were obtained with pairings of everolimus with rituximab, doxorubicin, vincristine, paclitaxel, vorinostat, and bortezomib [48]. The activity of everolimus in HL was suggested by induction of cell cycle arrest in HL cell lines and by results obtained in a xenotransplant model, in which treatment of mice with everolimus delayed tumor growth, decreased tumor size, and inhibited proliferation and metastasis [49].
Clinical Studies with mTOR Inhibitors in Lymphoma
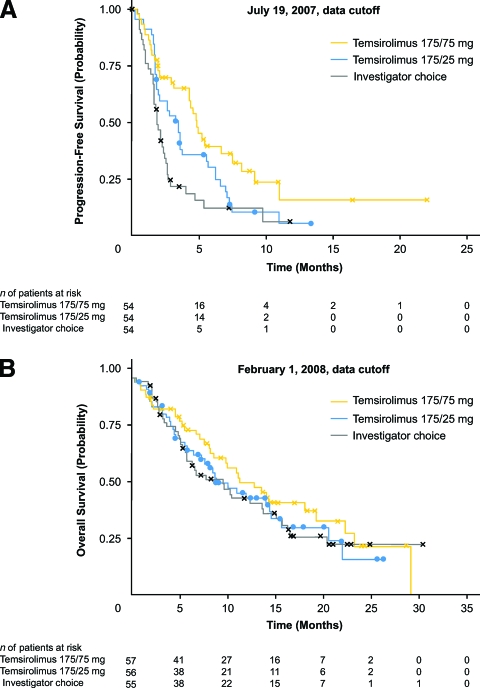
mTOR inhibitor therapy has achieved clinical responses in patients with relapsed or refractory lymphoma. In the first clinical evaluation of an mTOR inhibitor in MCL patients, temsirolimus was given as a single agent infused weekly at a dose of 250 mg to patients with relapsed or refractory disease in a phase II trial [50]. Patients were treated for up to six 4-week cycles; those who progressed or had SD at 6 months went off study whereas those who achieved a CR or PR at 6 months continued treatment (2 months for CRs and to a total of 12 months for PRs) [50]. The majority of patients (91%) had stage IV disease at baseline [50]. In the 34 patients assessed, the objective response rate was 38% (one CR, 12 PRs; 90% CI, 24%–54%). The median time to progression was 6.5 months (95% CI, 2.9–8.3 months), and the median OS time was 12 months (95% CI, 6.7 months to not reached). In the 13 responders, the median duration of response was 6.9 months (95% CI, 5.2–12.4 months) [50]. A second study of the 25-mg i.v. weekly dose found similar results with less myelosuppression [51]. Subsequently, a phase III open-label trial in patients with relapsed or refractory MCL was carried out to compare two regimens of temsirolimus (175 mg/week for 3 weeks followed by 25 mg/week and 175 mg/week for 3 weeks followed by 75 mg/week) with the investigator's choice of therapy, consisting of established treatment options (single-agent therapy with a cytotoxic agent or alemtuzumab) [52]. Temsirolimus was continued until disease progression or unacceptable toxicity [50]. Almost all (97%) patients had stage III or stage IV disease at baseline [50]. As shown in Figure 3A, the dosage of temsirolimus of 175 mg/week for 3 weeks followed by 75 mg/week achieved a significantly longer median PFS interval than with the investigator's choice of therapy (4.8 months versus 1.9 months, respectively; p = .0009; hazard ratio for progression, 0.44; 97.5% CI, 0.25–0.78). The lower dose of temsirolimus showed a trend for a longer PFS duration than with the investigator's choice of therapy, but the difference was not significant. The objective response rate also was significantly higher with temsirolimus (175 mg/75 mg) than with the investigator's choice of therapy (22% versus 2%, respectively; p = .0019). The median OS time did not differ significantly among groups (Fig. 3B) [52].
Figure 3.
Progression-free survival (A) and overall survival (B) with temsirolimus in patients with relapsed or refractory mantle cell lymphoma.
Reprinted from Hess G, Herbrecht R, Romaguera J et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol 2009;27:3822–3829. Reprinted with permission. © 2009 American Society of Clinical Oncology. All rights reserved.
A recent phase I and pharmacokinetic study of everolimus in patients with relapsed or refractory non-Hodgkin's lymphoma (NHL) determined that daily oral doses of everolimus up to 10 mg were well tolerated, with no dose-limiting toxicities [53]. Everolimus was investigated as single-agent therapy in an open-label phase II trial of patients with relapsed NHL and HL after a median of four prior therapies. Patients received everolimus (10 mg/day) until disease progression or unacceptable toxicity. The overall response rate (ORR) in 145 evaluable patients was 33% (five CRs, 43 PRs; 95% CI, 26%–41%). In patients with MCL, the ORR was 32% (95% CI, 13%–57%); in patients with DLBCL, the ORR was 30% (95% CI, 17%–45%); and in patients with T-cell lymphoma, the ORR was 63% (95% CI, 24%–91%). In the 48 responders, the median duration of response was 6.8 months (95% CI, 5.4–11.0 months) [54]. Patients with HL in that trial also were analyzed as a separate group. In all 19 HL patients, the ORR with everolimus was 47% (one CR, eight PRs; 95% CI, 24%–71%). The median PFS interval was 6.2 months (95% CI, 5.9–9.5 months), median OS time was 25.2 months (95% CI, 13.0 months to not reached), and median duration of response in nine responders was 7.1 months (95% CI, 3.9–14.8 months) [55]. Ongoing registered clinical trials of mTOR-targeted agents in lymphoma are summarized in Table 3.
Table 3.
Ongoing clinical trials of mTOR inhibitors in lymphoma, MM, WM
aAlso includes patients with relapsed or refractory chronic lymphocytic leukemia.
Abbreviations: AEs, adverse events; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CT, chemotherapy; DLBCL, diffuse large B-cell lymphoma; HL, Hodgkin's lymphoma; IGF-1R, insulin-like growth factor receptor 1; MCL, mantle cell lymphoma; MM, multiple myeloma; MTD, maximum tolerated dose; NHL, non-Hodgkin's lymphoma; PET, positron emission tomography; PFS, progression-free survival; WM, Waldenström's macroglobulinemia.
The PI3K/Akt/mTOR Pathway in MM
MM is a malignancy of terminal B cells and represents nearly 2% of all cancers [56]. The PI3K/Akt/mTOR pathway is constitutively activated in human myeloma cell lines and in freshly isolated plasmocytes from patients with MM [56].
Akt hyperactivity is a frequent occurrence in myeloma [57], and the sensitivity of MM cells to the cytostatic effects of mTOR inhibitors is related to cellular levels of Akt. This is associated with Akt-dependent differential effects on cyclin D expression and translational efficiency as well as internal ribosome entry site activity that is mediated in part by regulation of extracellular signal–related kinase activity, another kinase regulator of cell proliferation [57].
In Vitro Data with mTOR Inhibitors in MM
Rapamycin has demonstrated in vitro activity against MM cell lines as a single agent and in combination with the immunomodulatory drug CC-5013 [6]. Exposure to rapamycin or temsirolimus prevents the proliferation of PTEN- and RAS-mutated myeloma cell lines. Temsirolimus was shown to inhibit the growth of human myeloma cell lines by inducing G1 cell cycle arrest, apoptosis, and tumor angiogenesis [6]. In a murine xenograft model of MM, temsirolimus demonstrated a dose-dependent inhibition of proliferation and angiogenesis and induced tumor cell apoptosis [58]. In a NOD-SCID mouse model of diffuse MM, everolimus suppressed MM tumor burden and led to a longer survival time (p < .01) [59].
Clinical Studies with mTOR Inhibitors in MM
Several clinical studies of mTOR inhibitors are ongoing in MM patients. In a phase I/II open label trial of everolimus, 17 patients with relapsed or refractory MM were enrolled after at least two lines of previous treatment. Following a dose-escalation protocol (5 mg, 7.5 mg, and 10 mg), patients received a fixed dose of oral everolimus for 6 months. No dose-limiting toxicities were observed at any dose level. Two of seven serious adverse events (pulmonary embolism and atypical pneumonia) during treatment were assessed as being possibly related to the study drug. Adverse events of grade ≥3 included two severe thrombocytopenias. Of 15 evaluable patients, one PR was reported in a heavily pretreated patient and SD was observed in seven additional patients (one maintained for >9 months) [60]. Ongoing registered studies of mTOR inhibitors in MM are summarized in Table 3.
The PI3K/Akt/mTOR Pathway in WM
WM is a rare, low-grade lymphoplasmacytic B-cell lymphoma accompanied by serum IgM monoclonal gammopathy [61]. mTOR inhibitors have been investigated in WM based on results of in vitro studies demonstrating activation of PI3K/Akt/mTOR signaling in WM cells. Results of one trial indicated that Akt is constitutively activated in WM cell lines and in bone marrow cells derived from WM patients. Further, results of a recent study demonstrated lower PTEN expression and constitutive activation of Akt and mTOR in patient-derived WM cells from bone marrow, further supporting the conclusion that the PI3K/Akt pathway is constitutively activated in WM [20].
In Vitro Data with mTOR Inhibitors in WM
The addition of the specific Akt inhibitor perifosine to WM cell lines resulted in antiproliferative effects and cytotoxicity [62]. In addition, when a mouse xenograft model containing tumors that developed from injected human WM cells was treated with oral perifosine, WM tumor growth was inhibited and the 12-week survival rate was greater than in vehicle-injected controls [62]. Dual targeting of the PI3K/Akt/mTOR pathway with the investigational agent NVP-BEZ235 in WM cell lines dose-dependently inhibited phosphorylation of Akt, glycogen synthase kinase (GSK)-3α, GSK-3β, ribosomal protein S6, mTOR, p70S6K, and 4E-BP1 [20]. NVP-BEZ235 also inhibited proliferation of WM cells and decreased their survival, without affecting normal cells [20].
Clinical Studies with mTOR Inhibitors in WM
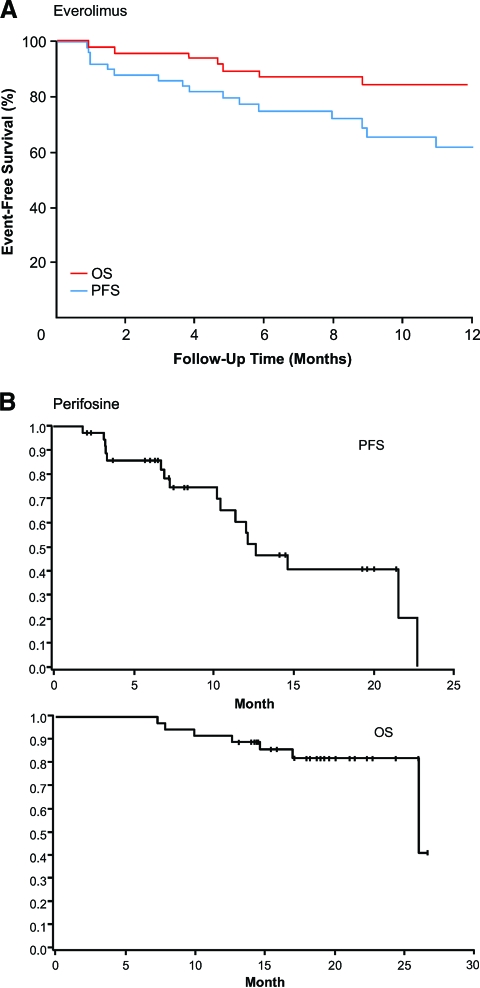
In the first clinical evaluation of an mTOR inhibitor in WM patients, single-agent everolimus was given to patients with symptomatic, relapsed or refractory WM in a phase II study [63]. Half of the patient population was classified as intermediate or high risk based on the international scoring system for WM, and the median number of prior therapies was three [63]. Patients were treated with oral everolimus (10 mg/day) in 4-week cycles. Those who progressed at any time or had SD at 6 months went off study, whereas those with SD or better after six cycles continued treatment based on the physician discretion and until disease progression or toxicity [63]. In the 50 evaluable patients, the ORR was 42% (all PRs; 95% CI, 26%–55%) and the CR plus PR plus minimal response (MR) rate was 70% (95% CI, 55%–82%) [63]. The median PFS and OS times had not been reached after 12 months of treatment (Fig. 4A) [64]. The estimated PFS rate was 75% (95% CI, 64%–89%) at 6 months and 62% (95% CI, 48%–80%) at 12 months [63]. Everolimus demonstrated acceptable tolerability, with manageable toxicities [63]. These favorable results prompted further study of everolimus in WM patients.
Figure 4.
Progression-free survival (PFS) and overall survival (OS) with everolimus (A) and perifosine (B) in patients with relapsed or refractory Waldenström's macroglobulinemia.
(A) Reprinted from Ghobrial IM, Gertz M, LaPlant B et al. Phase II trial of the oral mammalian target of rapamycin inhibitor everolimus in relapsed or refractory Waldenström macroglobulinemia. J Clin Oncol 2010;28:1408–1414. Reprinted with permission. © 2010 American Society of Clinical Oncology. All rights reserved.
(B) Adapted and reprinted by permission from the American Association for Cancer Research: Ghobrial IM, Roccaro A, Hong F et al. Clinical and translational studies of a phase II trial of the novel oral Akt inhibitor perifosine in relapsed or relapsed/refractory Waldenström's macroglobulinemia. Clin Cancer Res 2010;16:1033–1041.
Perifosine was evaluated in a phase II trial of 37 patients with symptomatic, relapsed or refractory WM (median of two prior therapy lines). Over half (51%) of the patient population was classified as intermediate or high risk based on the international scoring system for WM. Patients received oral perifosine at a dose of 150 mg daily in 28-day cycles. Those with disease progression after two cycles went off study, and those with SD or a response to treatment received six cycles of therapy and could continue therapy beyond six cycles until disease progression [64]. The ORR obtained was 35% (four PRs, 11%; nine MRs, 24%), with an SD rate of 54%. The median PFS interval was 12.6 months (90% CI, 10.2–22.7 months), and the median OS time was 26 months (90% CI, 26.0 to no estimate) (Fig. 4B) [64]. Ongoing trials of mTOR-targeted agents in patients with WM are summarized in Table 3.
Conclusions
Many hematologic malignancies involve tumor cells with hyperactive PI3K/Akt/mTOR pathways, making mTOR a rational target for therapy. Inhibition of mTOR achieves antiproliferative effects and cytotoxic effects in a variety of leukemias, lymphomas, MM, and WM, as demonstrated in in vitro and preclinical studies. Clinical activity with mTOR inhibitors has been achieved in patients with relapsed or refractory AML, CLL, MCL, DLBCL, T-cell lymphoma, HL, MM, and WM. Ongoing clinical trials are extensions of this work and are showing promise in patients with a variety of hematologic malignancies. Combinations of mTOR inhibitors with other targeted agents and with cytotoxic drugs may achieve additive or synergistic effects to maximize outcomes for patients with hematologic malignancies.
Acknowledgment
Funding for research support from Novartis Oncology.
Author Contributions
Conception/Design: Anas Younes, Nousheen Samad
Collection and/or assembly of data: Anas Younes, Nousheen Samad
Data analysis and interpretation: Anas Younes, Nousheen Samad
Manuscript writing: Anas Younes, Nousheen Samad
Final approval of manuscript: Anas Younes, Nousheen Samad
The authors take full responsibility for the content of the paper but thank Amy Zannikos, Scientific Connexions, Newtown, PA, supported by Novartis Pharmaceuticals, for editorial assistance in preparing this manuscript for submission.
References
- 1.Altman JK, Platanias LC. Exploiting the mammalian target of rapamycin pathway in hematologic malignancies. Curr Opin Hematol. 2008;15:88–94. doi: 10.1097/MOH.0b013e3282f3deaa. [DOI] [PubMed] [Google Scholar]
- 2.Teachey DT, Grupp SA, Brown VI. Mammalian target of rapamycin inhibitors and their potential role in therapy in leukaemia and other haematological malignancies. Br J Haematol. 2009;145:569–580. doi: 10.1111/j.1365-2141.2009.07657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopelovich L, Fay JR, Sigman CC, et al. The mammalian target of rapamycin pathway as a potential target for cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2007;16:1330–1340. doi: 10.1158/1055-9965.EPI-07-0045. [DOI] [PubMed] [Google Scholar]
- 4.Le Tourneau C, Faivre S, Serova M, et al. mTORC1 inhibitors: Is temsirolimus in renal cancer telling us how they really work? Br J Cancer. 2008;99:1197–1203. doi: 10.1038/sj.bjc.6604636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manning BD. Balancing Akt with S6K: Implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witzig TE, Kaufmann SH. Inhibition of the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway in hematologic malignancies. Curr Treat Options Oncol. 2006;7:285–294. doi: 10.1007/s11864-006-0038-1. [DOI] [PubMed] [Google Scholar]
- 7.Costa LJ. Aspects of mTOR biology and the use of mTOR inhibitors in non-Hodgkin's lymphoma. Cancer Treat Rev. 2007;33:78–84. doi: 10.1016/j.ctrv.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Gingras AC, Kennedy SG, O'Leary MA, et al. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyuhas O. Synthesis of the translational apparatus is regulated at the translational level. Eur J Biochem. 2000;267:6321–6330. doi: 10.1046/j.1432-1327.2000.01719.x. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt AP, Bhende PM, Sin SH, et al. Dual inhibition of PI3K and mTOR inhibits autocrine and paracrine proliferative loops in PI3K/Akt/mTOR-addicted lymphomas. Blood. 2010;115:4455–4463. doi: 10.1182/blood-2009-10-251082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 12.Engelman JA. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 13.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 14.Bhagwat SV, Crew AP. Novel inhibitors of mTORC1 and mTORC2. Curr Opin Investig Drugs. 2010;11:638–645. [PubMed] [Google Scholar]
- 15.Kojima K, Shimanuki M, Shikami M, et al. The dual PI3 kinase/mTOR inhibitor PI-103 prevents p53 induction by Mdm2 inhibition but enhances p53-mediated mitochondrial apoptosis in p53 wild-type AML. Leukemia. 2008;22:1728–1736. doi: 10.1038/leu.2008.158. [DOI] [PubMed] [Google Scholar]
- 16.Fan QW, Knight ZA, Goldenberg DD, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janes MR, Limon JJ, So L, et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010;16:205–213. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman ME, Apsel B, Uotila A, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta M, Ansell SM, Novak AJ, et al. Histone deacetylase inhibition with LBH589 inhibits the rapamycin insensitive rictor-mTOR (mTORC2) complex and translation initiation factor eIF4E activation in diffuse large B-cell lymphoma [abstract 603]. Presented at the Annual Meeting of the American Society of Hematology; December 6–9, 2008; San Francisco, CA. [Google Scholar]
- 20.Roccaro AM, Sacco A, Husu EN, et al. Dual targeting of the PI3K/Akt/mTOR pathway as an antitumor strategy in Waldenstrom macroglobulinemia. Blood. 2010;115:559–569. doi: 10.1182/blood-2009-07-235747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kornblau SM, Womble M, Qiu YH, et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108:2358–2365. doi: 10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kharas MG, Okabe R, Ganis JJ, et al. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood. 2010;115:1406–1415. doi: 10.1182/blood-2009-06-229443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva A, Yunes JA, Cardoso BA, et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118:3762–3774. doi: 10.1172/JCI34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Récher C, Dos Santos C, Demur C, et al. mTOR, a new therapeutic target in acute myeloid leukemia. Cell Cycle. 2005;4:1540–1549. doi: 10.4161/cc.4.11.2159. [DOI] [PubMed] [Google Scholar]
- 25.Zeng Z, Sarbassov dos D, Samudio IJ, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–3512. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiarini F, Falí F, Tazzari PL, et al. Dual inhibition of class IA phosphatidylinositol 3-kinase and mammalian target of rapamycin as new therapeutic option for T-cell acute lymphoblastic leukemia. Cancer Res. 2009;69:3520–3528. doi: 10.1158/0008-5472.CAN-08-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamburini J, Chapuis N, Bardet V, et al. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: Rationale for therapeutic inhibition of both pathways. Blood. 2008;111:379–382. doi: 10.1182/blood-2007-03-080796. [DOI] [PubMed] [Google Scholar]
- 28.Xu Q, Simpson SE, Scialla TJ, et al. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–980. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 29.Janus A, Linke A, Cebula B, et al. Rapamycin, the mTOR kinase inhibitor, sensitizes acute myeloid leukemia cells, HL-60 cells, to the cytotoxic effect of arabinozide cytarabine. Anticancer Drugs. 2009;20:693–701. doi: 10.1097/CAD.0b013e32832e89b4. [DOI] [PubMed] [Google Scholar]
- 30.VanderWeele DJ, Zhou R, Rudin CM. Akt up-regulation increases resistance to microtubule-directed chemotherapeutic agents through mammalian target of rapamycin. Mol Cancer Ther. 2004;3:1605–1613. [PubMed] [Google Scholar]
- 31.Saunders PO, Bradstock KF, Bendall LJ. Combining RAD001 (everolimus) with proteasome inhibitors bortezomib (Velcade) or MG132 significantly enhances pre-B ALL cell death in vitro [abstract 2743]. Presented at the Annual Meeting of the American Society of Hematology; December 5–8, 2009; New Orleans, LA. [Google Scholar]
- 32.Mancini M, Petta S, Martinelli G, et al. RAD 001 (everolimus) prevents mTOR and Akt late re-activation in response to imatinib in chronic myeloid leukemia. J Cell Biochem. 2010;109:320–328. doi: 10.1002/jcb.22380. [DOI] [PubMed] [Google Scholar]
- 33.Mancini M, Corradi V, Petta S, et al. mTOR inhibitor RAD001 (everolimus) enhances the effects of imatinib in chronic myeloid leukemia by raising the nuclear expression of c-ABL protein. Leuk Res. 2010;34:641–648. doi: 10.1016/j.leukres.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Minami Y, Minami M, Kuwatsuka Y, et al. Treatment with mTOR inhibitor, everolimus (RAD001) overcomes resistance to imatinib in Ph-leukemia quiescent or T315I-mutated cells [abstract 3277]. Presented at the Annual Meeting of the American Society of Hematology; December 5–8, 2009; New Orleans, LA. [Google Scholar]
- 35.Nagai T, Ohmine K, Fujiwara S, et al. Combination of tipifarnib and rapamycin synergistically inhibits the growth of leukemia cells and overcomes resistance to tipifarnib via alteration of cellular signaling pathways. Leuk Res. 2010;34:1057–1063. doi: 10.1016/j.leukres.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Boehm A, Mayerhofer M, Herndlhofer S, et al. Evaluation of in vivo antineoplastic effects of rapamycin in patients with chemotherapy-refractory AML. Eur J Intern Med. 2009;20:775–778. doi: 10.1016/j.ejim.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Perl AE, Kasner MT, Tsai DE, et al. A phase I study of the mammalian target of rapamycin inhibitor sirolimus and MEC chemotherapy in relapsed and refractory acute myelogenous leukemia. Clin Cancer Res. 2009;15:6732–6739. doi: 10.1158/1078-0432.CCR-09-0842. [DOI] [PubMed] [Google Scholar]
- 38.Yee KW, Zeng Z, Konopleva M, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12:5165–5173. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- 39.Zent CS, LaPlant BR, Johnston PB, et al. The treatment of recurrent/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) with everolimus results in clinical responses and mobilization of CLL cells into the circulation. Cancer. 2010;116:2201–2207. doi: 10.1002/cncr.25005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizzieri DA, Feldman E, Dipersio JF, et al. A phase 2 clinical trial of deforolimus (AP23573, MK-8669), a novel mammalian target of rapamycin inhibitor, in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2008;14:2756–2762. doi: 10.1158/1078-0432.CCR-07-1372. [DOI] [PubMed] [Google Scholar]
- 41.Dal Col J, Zancai P, Terrin L, et al. Distinct functional significance of Akt and mTOR constitutive activation in mantle cell lymphoma. Blood. 2008;111:5142–5151. doi: 10.1182/blood-2007-07-103481. [DOI] [PubMed] [Google Scholar]
- 42.Peponi E, Drakos E, Reyes G, et al. Activation of mammalian target of rapamycin signaling promotes cell cycle progression and protects cells from apoptosis in mantle cell lymphoma. Am J Pathol. 2006;169:2171–2180. doi: 10.2353/ajpath.2006.051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dutton A, Reynolds GM, Dawson CW, et al. Constitutive activation of phosphatidyl-inositide 3 kinase contributes to the survival of Hodgkin's lymphoma cells through a mechanism involving Akt kinase and mTOR. J Pathol. 2005;205:498–506. doi: 10.1002/path.1725. [DOI] [PubMed] [Google Scholar]
- 44.Mavrakis KJ, Zhu H, Silva RLA, et al. Tumorigenic activity and therapeutic inhibition of Rheb GTPase. Genes Dev. 2008;22:2178–2188. doi: 10.1101/gad.1690808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hipp S, Ringshausen I, Oelsner M, et al. Inhibition of the mammalian target of rapamycin and the induction of cell cycle arrest in mantle cell lymphoma cells. Haematologica. 2005;90:1433–1434. [PubMed] [Google Scholar]
- 46.Gupta M, Ansell SM, Novak AJ, et al. Inhibition of histone deacetylase overcomes rapamycin-mediated resistance in diffuse large B-cell lymphoma by inhibiting Akt signaling through mTORC2. Blood. 2009;114:2926–2935. doi: 10.1182/blood-2009-05-220889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wanner K, Hipp S, Oelsner M, et al. Mammalian target of rapamycin inhibition induces cell cycle arrest in diffuse large B cell lymphoma (DLBCL) cells and sensitizes DLBCL cells to rituximab. Br J Haematol. 2006;134:475–484. doi: 10.1111/j.1365-2141.2006.06210.x. [DOI] [PubMed] [Google Scholar]
- 48.Haritunians T, Mori A, O'Kelly J, et al. Antiproliferative activity of RAD001 (everolimus) as a single agent and combined with other agents in mantle cell lymphoma. Leukemia. 2007;21:333–339. doi: 10.1038/sj.leu.2404471. [DOI] [PubMed] [Google Scholar]
- 49.Jundt F, Raetzel N, Ml̈ler C, et al. A rapamycin derivative (everolimus) controls proliferation through down-regulation of truncated CCAAT enhancer binding protein β and NF-kB activity in Hodgkin and anaplastic large cell lymphomas. Blood. 2005;106:1801–1807. doi: 10.1182/blood-2004-11-4513. [DOI] [PubMed] [Google Scholar]
- 50.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 51.Ansell SM, Inwards DJ, Rowland KM, Jr, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: A phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113:508–514. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 53.Ogura M, Uchida T, Maruyama D, et al. Phase I and pharmacokinetic (PK) study of everolimus (RAD001) in patients with relapsed or refractory non-Hodgkin's lymphoma (NHL) [abstract 1712]. Presented at the Annual Meeting of the American Society of Hematology; December 5–8, 2009; New Orleans, LA. [Google Scholar]
- 54.Witzig T, Habermann T, Reeder C, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed non-Hodgkin lymphoma (NHL) and Hodgkin disease (HD) [abstract 1081]. Presented at the Congress of the European Hematology Association; June 4–7, 2009; Berlin, Germany. [Google Scholar]
- 55.Johnston PB, Inwards DJ, Colgan JP, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol. 2010;85:320–324. doi: 10.1002/ajh.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pene F, Claessens YE, Muller O, et al. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene. 2002;21:6587–6597. doi: 10.1038/sj.onc.1205923. [DOI] [PubMed] [Google Scholar]
- 57.Frost P, Shi Y, Hoang B, et al. Regulation of D-cyclin translation inhibition in myeloma cells treated with mammalian target of rapamycin inhibitors: Rationale for combined treatment with extracellular signal-regulated kinase inhibitors and rapamycin. Mol Cancer Ther. 2009;8:83–93. doi: 10.1158/1535-7163.MCT-08-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frost P, Moatamed F, Hoang B, et al. In vivo antitumor effects of the mTOR inhibitor CCI-779 against human multiple myeloma cells in a xenograft model. Blood. 2004;104:4181–4187. doi: 10.1182/blood-2004-03-1153. [DOI] [PubMed] [Google Scholar]
- 59.Mitsiades N, McMullan C, Poulaki V, et al. The mTOR inhibitor RAD001 (everolimus) is active against multiple myeloma cells in vitro and in vivo [abstract 1496] Blood. 2004;104:418a. [Google Scholar]
- 60.Guenther A, Baumann P, Burger R, et al. Single-agent everolimus (RAD001) in patients with relapsed or refractory multiple myeloma: Final results of a phase I study [abstract 8137] J Clin Oncol. 2010;28:7s. [Google Scholar]
- 61.Leleu X, Gay J, Roccaro AM, et al. Update on therapeutic options in Waldenström macroglobulinemia. Eur J Haematol. 2009;82:1–12. doi: 10.1111/j.1600-0609.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leleu X, Jia X, Runnels J, et al. The Akt pathway regulates survival and homing in Waldenstrom macroglobulinemia. Blood. 2007;110:4417–4426. doi: 10.1182/blood-2007-05-092098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghobrial IM, Gertz M, LaPlant B, et al. Phase II trial of the oral mammalian target of rapamycin inhibitor everolimus in relapsed or refractory Waldenström macroglobulinemia. J Clin Oncol. 2010;28:1408–1414. doi: 10.1200/JCO.2009.24.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghobrial IM, Roccaro A, Hong F, et al. Clinical and translational studies of a phase II trial of the novel oral Akt inhibitor perifosine in relapsed or relapsed/refractory Waldenstrom's macroglobulinemia. Clin Cancer Res. 2010;16:1033–1041. doi: 10.1158/1078-0432.CCR-09-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ballou LM, Lin RZ. Rapamycin and mTOR kinase inhibitors. J Chem Biol. 2008;1:27–36. doi: 10.1007/s12154-008-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.ClinicalTrials.gov. Panobinostat and Everolimus in Treating Patients With Relapsed or Refractory Lymphoma or Multiple Myeloma. ClinicalTrials.gov Identifier, NCT00962507. [accessed February 23, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00962507?term=NCT00962507&rank=1.
- 67.ClinicalTrials.gov. Panobinostat and Everolimus in Treating Patients With Recurrent Multiple Myeloma, Non-Hodgkin Lymphoma, or Hodgkin Lymphoma. ClinicalTrials.gov Identifier, NCT00918333. [accessed February 23, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00918333?term=NCT00918333&rank=1.
- 68.ClinicalTrials.gov. A Phase I/II Study of BEZ235 in Patients With Advanced Solid Malignancies Enriched by Patients With Advanced Breast Cancer. ClinicalTrials.gov Identifier, NCT00620594. [accessed September 8, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00620594?term=NCT00620594&rank=1.
- 69.Gharbi SI, Zvelebil MJ, Shuttleworth SJ, et al. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007;404:15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.ClinicalTrials.gov. RAD001 in Combination With PKC412 in Patients With Relapsed, Refractory or Poor Prognosis AML or MDS. ClinicalTrials.gov Identifier, NCT00819546. [accessed February 23, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00819546?term=NCT00819546&rank=1.
- 71.ClinicalTrials.gov. Sirolimus in Combination With MEC in High Risk Myeloid Leukemias (UPCC 02407) ClinicalTrials.gov Identifier, NCT00780104. [accessed February 23, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00780104?term=NCT00780104&rank=1.
- 72.ClinicalTrials.gov. Combination of Nilotinib (AMN107) and RAD001 in Patients With Acute Myeloid Leukemia. ClinicalTrials.gov Identifier, NCT00762632. [accessed February 23, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00762632?term=NCT00762632&rank=1.
- 73.ClinicalTrials.gov. CCI-779 in B-Cell Lymphoma and Chronic Lymphocytic Leukemia (CLL) ClinicalTrials.gov Identifier, NCT00290472. [accessed February 23, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00290472?term=NCT00290472&rank=1.
- 74.ClinicalTrials.gov. Safety and Efficacy of RAD001 in Patients With Mantle Cell Lymphoma Who Are Refractory or Intolerant to Velcade® Therapy (PILLAR-1) ClinicalTrials.gov Identifier, NCT00702052. [accessed February 23, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00702052?term=PILLAR-1&rank=1.
- 75.ClinicalTrials.gov. Phase III Study of RAD001 Adjuvant Therapy in Poor Risk Patients With Diffuse Large B-Cell Lymphoma (DLBCL) of RAD001 Versus Matching Placebo After Patients Have Achieved Complete Response With First-Line Rituximab-Chemotherapy (PILLAR-2) ClinicalTrials.gov Identifier, NCT00790036. [accessed February 23, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00790036?term=PILLAR&rank=5.
- 76.ClinicalTrials.gov. Everolimus Plus Rituximab for Relapsed/Refractory Diffuse Large B Cell Lymphoma. ClinicalTrials.gov Identifier, NCT00869999. [accessed February 23, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00869999?term=NCT00869999&rank=1.
- 77.ClinicalTrials.gov. Everolimus in Treating Older Patients With Mantle Cell Lymphoma Previously Treated With First-Line or Second-Line Chemotherapy. ClinicalTrials.gov Identifier, NCT00727207. [accessed February 23, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00727207?term=NCT00727207&rank=1.
- 78.ClinicalTrials.gov. IMC-A12 in Combination with Temsirolimus (CCI-779) in Patients with Advanced Cancers. ClinicalTrials.gov Identifier, NCT00678769. [accessed April 6, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00678769?term=NCT00678769&rank=1.
- 79.ClinicalTrials.gov. Sorafenib and Everolimus in Treating Patients With Relapsed or Refractory Lymphoma or Multiple Myeloma. ClinicalTrials.gov Identifier, NCT00474929. [accessed February 23, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00474929?term=NCT00474929&rank=1.
- 80.ClinicalTrials.gov. Everolimus in Treating Patients With Lymphoma That Has Relapsed or Not Responded to Previous Treatment. ClinicalTrials.gov Identifier, NCT00436618. [accessed February 23, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00436618?term=NCT00436618&rank=1.