The sensory hair cells of amniote hearing organs are usually distributed in tonotopic array from low to high frequencies and are very sensitively and sharply tuned to acoustic stimulation. Frequency tuning and tonotopicity of non-mammalian auditory hair cells is due largely to intrinsic properties of the hair cells [1], but frequency tuning and tonotopic organisation of the mammalian cochlea has an extrinsic basis in the basilar membrane (BM); a spiralling ribbon of collagen-rich extracellular matrix which decreases in stiffness from the high frequency base of the cochlea to the low frequency apex [2, 3].
Sensitive frequency tuning is due to amplification, which specifically boosts low-level input to the mechanosensitive hair cells at their tonotopic location to overcome viscous damping [1–3]. In non-mammalian hearing organs, at least, amplification is attributed to calcium-mediated hair bundle motion [1]. In the mammalian cochlea, amplification is the remit of the sensory-motor outer hair cells (OHCs). These are located within the organ of Corti to exercise maximum mechanical effect on the motion of the BM and to transmit cochlear responses to the adjacent sensory inner hair cells (IHCs) and, consequently, to the auditory nerve [1–3] (Fig. 1A). OHCs behave like piezoelectric actuators, developing forces along their long axis in response to changes in membrane potential [2]. These forces are due to voltage-dependent conformational changes in the motor molecule prestin, which is densely distributed in the OHC lateral membranes [2].
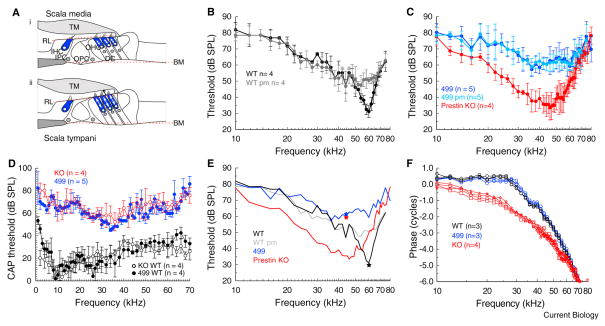
Figure 1. Basilar membrane and neural measurements from the mouse cochlea.
(A) Schematic cross-section of the organ of Corti of the cochlea illustrating hypothesised mechanical distortion of the structural supporting cells (inner pillar cells (IPC), outer pillar cells (OPC) and Deiters’ cells (DC) and major non-cellular elements (basilar membrane [BM], tectorial membrane [TM] and reticular laminar [RL]) when the outer hair cells (OHC) shorten (i) during maximum BM velocity towards scala media and lengthen (ii) during maximum velocity towards scala tympani. Inner hair cells (IHC).
(B) Means ± standard deviation of iso-displacement (0.2 nm) frequency tuning curves measured alive and post mortem (pm) from wild type (WT) mice. (C) Means ± standard deviation of iso-displacement (0.2 nm) frequency tuning curves measured alive and post mortem (pm) from prestin 499 mice (499) and alive from prestin KO mice. (D) CAP threshold from WT, prestin KO, and prestin 499 littermates as a function of stimulus frequency for the N1 peak of the auditory nerve. (E) Means of iso-displacement (0.2 nm) frequency tuning curves measured from WT, WT pm, homozygous prestin 499, and prestin KO mice taken from figures 1b, c. Stars: Neural thresholds at the characteristic frequency. (F) Phase of BM motion relative to the malleus as a function of stimulus frequency measured from WT, prestin 499, and prestin KO mice at 80 dB SPL. All measurements were made at similar locations on the BM (equivalent to the 60 kHz place in the WT mouse).
Here we consider roles for prestin in harnessing the BM as the source of cochlear frequency tuning. The OHCs might be considered as active struts in the complex cellular architecture of the organ of Corti. The tubulin-packed cytoskeletons of the supporting cells behave as passive structures that are distorted by changes in OHC forces to interactively transmit sound induced vibrations between the mechanical elements of the cochlear partition (Fig. 1A). For this to happen, the mechanical impedance of the OHCs has to be matched to that of the surrounding mechanical elements of the cochlear partition [2]. We report measurements from homozygous prestin knockout (KO) mice, with compliant OHCs devoid of prestin [4], homozygous prestin 499 knockin mice, with stiff OHCs populated with nonmotile prestin [5], and wild type (WT) mice with stiff, motile, OHCs. We have tested the hypothesis that prestin, acting as both a motile and structural element of OHCs, is essential for both, power amplification [6] and mechanical coupling of BM vibrations to the organ of Corti and ultimately for auditory sensation.
Both prestin KO, and prestin 499 mice suffer significant loss of OHCs, at least from 28 days post partum [4, 5], from the basal turn of the cochlea, which is the locus of our measurements. We therefore made electrical and mechanical measurements only from 17 – 21 day-old mice. In these mice OHCs in the basal turn of the cochlea are viable and perform mechano-electrical transduction as indicated by the recording of compound receptor potentials (cochlear microphonic, CM) at the round-window membrane (see Supplemental Experimental Procedures). Round-window CM is dominated by receptor currents from the basal turn OHCs [7]. CM as a function of stimulus level is not significantly different between wild type (WT) and prestin 499 littermates (Figure S1) and WT and prestin KO littermates [4] in 17–21 day-old mice in response to 10 kHz tones. 10 kHz is about two octaves below the frequency range of basal turn OHCs and, therefore, the OHC responses were not subject to significant amplification [8].
Indication of the roles of prestin in amplifying and relaying mechanical responses between the BM, OHCs, and inner hair cells were obtained from measurements of BM mechanical responses to acoustic stimulation and compound action potential (CAP) threshold audiograms measured from the round window (see Methods, Fig. S1). BM, iso-displacement, frequency-tuning curves were obtained from WT mice (Fig. 1B) based on the sound pressure level (SPL re 2 × 10−5 Pa) required to cause 0.2 nm displacements of the BM in the ~60 kHz region of the cochlea. The curves were typical with a minimum at the characteristic frequency (CF) of the measurement location. Post mortem (pm), the tuning curve tips become broadened, desensitized by 20–30 dB SPL and moved to lower frequencies.
Tone-evoked BM displacements recorded from the cochleae of prestin KO and 499 mice, at BM spatial locations similar to those made from WT mice, were very broad, with minima shifted by about a half octave to ~45 kHz, and did not change significantly pm (Fig. 1C). The sensitivity of BM tuning curves measured from prestin KO mice, which is similar to that of WT mice, is attributed [4] to a reduction in mechanical coupling of the BM to other elements of the cochlear partition as a consequence of the greatly reduced axial stiffness of the OHCs [5]. Axial stiffness of OHCs of the prestin 499 mice are indistinguishable from those of WT mice [5] and the sensitivity of the BM tuning curves of these mice is similar to that of pm tuning curves of WT mice, as might be expected from a critically damped cochlea without amplification [3]. The significance of OHC axial stiffness for mechanically coupling the BM to other elements of the cochlear partition can be deduced from the close correspondence between CAP threshold audiograms (Fig. 1D) and BM iso-displacement thresholds for frequencies at the tip of the tuning curve for WT and prestin 499 mice, but not for prestin KO mice, where coupling between BM vibration and inner hair cell excitation is weak [4] (see stars representing CAP threshold at BM characteristic frequencies, Fig. 1E). BM responses to frequencies > 1 octave below the CF of the tuning curve, where BM motion is passive [3], are up to 20 dB more sensitive in prestin KO mice than in WT and prestin 499 mice (Figs. 1C, E); strong indication that the cochlear partition of prestin KO mice is more compliant. This conclusion is further supported by measurements of the phase of BM motion relative to that of the middle ear in response to high-level (80 dB SPL) stimulation, when BM motion is governed by passive forces [3]. Travelling waves along the BM in response to tones with frequencies well below the CF are stiffness dominated [3]. Changes in the phase angle with increasing frequency are expected to be smaller in this frequency region for faster travelling waves propagating along a stiffer cochlear partition, as indeed is the case for both WT and prestin 499 mice for frequencies between 10 kHz – 30 kHz (Fig. 1F). Over the same frequency range, BM vibrations measured from prestin KO mice lag by ~2 cycles (Fig. 1F); further indication that the cochlear partition of prestin KO mice is more compliant than those of WT and prestin KO mice.
We conclude that prestin evolved in the mammalian cochlea to provide the basis for the amplified, impedance-matching, mechanical link that enabled the OHCs of the organ of Corti to devolve responsibility for frequency tuning to the potentially enormous frequency range of the graded mechanical properties of the BM. In this scenario, prestin provides the rapid, voltage-dependent, conformational changes that amplify and closely couple the movements of the BM to those of the OHCs, as part of a mechanosensory feedback loop, and the essential mechanical link between the movements of the BM and the excitatory shear of the inner hair cells [4,9]. Prestin is therefore the key molecular element that has enabled the organ of Corti of the mammalian cochlea to exploit a mechanically-tuned, extracellular-matrix to gain mammals the enormous apparent benefit of being able to listen to frequencies way beyond the auditory ranges of other amniotes [10].
Supplementary Material
Document S1. Experimental Procedures and One Figure
Acknowledgments
We thank Ben Warren for comments on the manuscript, James Hartley for designing and constructing electronic equipment, and Jennifer Dearman for genotyping. This work was supported by grants from the Medical Research Council and DC006471, ALSAC, CA21765, N000140911014. M.M-L. is a Sir Henry Wellcome Research Fellow.
Footnotes
Supplemental Information includes experimental procedures and one figure and can be found with this article online at *bxs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrei N. Lukashkin, Email: A.Lukashkin@sussex.ac.uk.
Jian Zuo, Email: Jian.Zuo@STJUDE.ORG.
Ian J. Russell, Email: I.J.Russell@sussex.ac.uk.
References
- 1.Fettiplace R, Hackney CM. The sensory and motor roles of auditory hair cells. Nat Rev Neurosci. 2006;7:19–29. doi: 10.1038/nrn1828. [DOI] [PubMed] [Google Scholar]
- 2.Ashmore J. Cochlear outer hair cell motility. J Physiol Rev. 2008;88:173–210. doi: 10.1152/physrev.00044.2006. [DOI] [PubMed] [Google Scholar]
- 3.Robles L, Ruggero MA. Mechanics of the mammalian cochlea. Physiol Rev. 2001;81:1305–1352. doi: 10.1152/physrev.2001.81.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellado Lagarde MM, Drexl M, Lukashkin AN, Zuo J, Russell IJ. A role for prestin in the frequency tuning of cochlear mechanical responses and their transmission to neural excitation. Curr Biol. 2008;18:200–202. doi: 10.1016/j.cub.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Dallos P, et al. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron. 2008;58:333–9. doi: 10.1016/j.neuron.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lukashkin AN, Walling MN, Russell IJ. Power amplification in the mammalian cochlea. Curr Biol. 2007;17:1340–1344. doi: 10.1016/j.cub.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 7.Patuzzi RB, Yates GK, Johnstone BM. The origin of the low-frequency microphonic in the first cochlear turn of guinea-pig. Hear Res. 1989;39:177–88. doi: 10.1016/0378-5955(89)90089-0. [DOI] [PubMed] [Google Scholar]
- 8.Patuzzi RB, Yates GK, Johnstone BM. Changes in cochlear microphonic and neural sensitivity produced by acoustic trauma. Hear Res. 1989a;39:189–202. doi: 10.1016/0378-5955(89)90090-7. [DOI] [PubMed] [Google Scholar]
- 9.Santos-Sacchi J. Cochlear mechanics: no shout but a twist in the absence of prestin. Curr Biol. 2008;18:R304–6. doi: 10.1016/j.cub.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Vater M, Kössl M. Comparative aspects of cochlear functional organization in mammals. Hear Res. 2011;273:89–99. doi: 10.1016/j.heares.2010.05.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Document S1. Experimental Procedures and One Figure