Abstract
Given the important role of the dopamine transporter (DAT) in the addictive properties of cocaine, the development and use of compounds that target the DAT represents a reasonable approach for the pharmacological treatment of cocaine abuse. The present report describes a series of studies conducted in nonhuman primates that evaluated the effectiveness of DAT inhibitors in reducing cocaine self-administration. In addition, drug substitution studies evaluated the abuse liability of the DAT inhibitors. PET neuroimaging studies quantified DAT occupancy at behaviorally relevant doses, characterized the time-course of drug uptake in brain, and documented drug-induced changes in cerebral blood flow as a model of brain activation. Selective DAT inhibitors were effective in reducing cocaine use but high (>70%) levels of DAT occupancy were associated with significant reductions in cocaine self-administration. The selective DAT inhibitors were reliably self-administered but rates of responding were lower than those maintained by cocaine even at higher levels of DAT occupancy. A profile of slow rate of drug uptake in brain accompanied by a gradual increase in extracellular dopamine may account for the more limited reinforcing effectiveness of the DAT inhibitors. Selective serotonin transporter (SERT) inhibitors were also effective in reducing cocaine use and blocked cocaine-induced brain activation and increases in extracellular dopamine. Co-administration of SERT inhibitors with a selective DAT inhibitor was more effective than the DAT inhibitor administered alone, even at comparable levels of DAT occupancy. The results indicate that combined inhibition of DAT and SERT may be a viable approach to treat cocaine addiction.
Keywords: cocaine, dopamine transporter, serotonin transporter, PET imaging, nonhuman primates
Despite extensive efforts directed toward the development of medications to treat cocaine abuse, no effective pharmacotherapy is currently in clinical use. Given the obvious importance of dopaminergic mechanisms in the addictive properties of cocaine, the development and use of compounds that target dopaminergic systems represents a reasonable approach for the pharmacological treatment of cocaine abuse. The therapeutic approach of substitute agonist or replacement medication has been successful in the context of methadone maintenance for heroin dependence and nicotine replacement for tobacco use. These positive outcomes, combined with recent advances in the understanding of the neuropharmacology of cocaine, support efforts to develop a similar type of medication for cocaine abuse. Of the various types of medications being pursued, dopamine transporter (DAT) inhibitors represent a promising approach in drug development (Mello & Negus, 1996; Carroll, Howell, & Kuhar, 1999; Howell & Wilcox, 2001; Lindsey, et al., 2004; Howell, Carroll, Votaw, Goodman, & Kimmel, 2007). The DAT is an important recognition site for cocaine and likely mediates its reinforcing effects that contribute to significant abuse liability (Ritz, Lamb, Golderg, & Kuhar, 1987; Kuhar, Ritz, & Boja, 1991; Woolverton & Johnson, 1992). The affinities of several cocaine-like drugs for the DAT correlate well with their potencies for supporting self-administration behavior (Ritz et al., 1987; Bergman, Madras, Johnson, & Spealman, 1989; Wilcox, Paul, & Woolverton, 1999). Cocaine and selective DAT inhibitors exert similar effects on schedule-controlled behavior and are reliably self-administered in squirrel monkeys (Bergman et al., 1989; Howell & Byrd, 1991; Howell, Czoty, Kuhar, & Carroll, 2000; Kimmel, O’Connor, Carroll, & Howell, 2007) and rhesus monkeys (Nader, Grant, Davies, Mach, & Childers 1997; Lile et al., 2003; Lindsey et al., 2004; Wilcox et al., 2005, Howell et al., 2007; Kimmel et al., 2008). Importantly, a variety of preclinical studies in nonhuman primates provide evidence that DAT inhibitors can effectively attenuate cocaine self-administration (Glowa et al., 1995; Nader et al., 1997; Howell et al., 2000; Wilcox et al., 2002; Lindsey et al., 2004; Howell et al., 2007).
The relevance of the DAT in the reinforcing effects of cocaine is supported further by human and nonhuman primate neuroimaging studies. In human cocaine users, a significant correlation was observed between the level of DAT occupancy and the magnitude of the subjective high reported following administration of cocaine (Volkow et al., 1997) or the behavioral stimulant methylphenidate (Volkow et al., 1999). In rhesus monkeys, doses of cocaine that maintained peak responding in drug self-administration studies resulted in DAT occupancy greater than 65% (Wilcox et al., 2002; Lindsey et al., 2004). Doses of GBR12909 that decreased cocaine self-administration in rhesus monkeys resulted in DAT occupancy greater than 50% in baboons (Villemagne et al., 1999) and rhesus monkeys (Lindsey et al., 2004). Likewise, doses of cocaine analogs with selectivity for DAT decreased cocaine self-administration in rhesus monkeys at DAT occupancies greater than 70% (Wilcox et al., 2002; Lindsey et al., 2004; Howell et al., 2007). Collectively, these results indicate that DAT occupancy is closely associated with the reinforcing effects of cocaine and with the effectiveness of DAT inhibitors to reduce cocaine self-administration.
Preclinical studies have indicated that serotonin may also play an important role in the behavioral effects of psychomotor stimulants. For example, a negative relationship was observed between the potencies of several cocaine- and amphetamine-like drugs in self-administration studies and their binding potencies to serotonin uptake sites (Ritz et al., 1987; Ritz & Kuhar, 1989). Monoamine-releasing agents had decreased reinforcing effectiveness in rhesus monkeys when serotonin-releasing potency was increased relative to dopamine (Wee, et al., 2005). Moreover, a potent releaser of dopamine and serotonin lacked reinforcing effectiveness, yet it produced dose-dependent reductions in cocaine self-administration in rhesus monkeys (Rothman et al., 2005). The behavioral and neurochemical profile of DAT inhibitors also is influenced by actions at multiple monoamine transporters in squirrel monkeys (Ginsburg, Kimmel, Carroll, Goodman, & Howell, 2005). Consistent with these results, administration of the serotonin transporter (SERT) inhibitor fluoxetine decreased self-administration of cocaine in rodents (Carroll, Lac, Asencio, & Kragh, 1990) and rhesus monkeys (Kleven & Woolverton, 1993). In squirrel monkeys, the SERT inhibitors citalopram, fluoxetine, and alaproclate attenuated the behavioral-stimulant effects of cocaine (Spealman, 1993; Howell & Byrd, 1995). Alaproclate also attenuated cocaine self-administration and cocaine-induced increases in extracellular dopamine in squirrel monkeys (Czoty, Ginsburg, & Howell, 2002) and cocaine-induced activation of prefrontal activity in rhesus monkeys (Howell et al., 2002). Collectively, there is strong evidence to suggest that SERT inhibition can attenuate the neurochemical, behavioral-stimulant and reinforcing effects of psychomotor stimulants.
The ability to conduct neuroimaging studies in nonhuman primates provides a powerful translational approach to link findings from laboratory animal and human research. In nonhuman primates, experimental drugs under investigation can be evaluated in subjects with well-documented drug histories. As with all animal models, enhanced experimental control is a noted advantage over the necessary restrictions imposed in human clinical research. Nonhuman primates also offer several distinct advantages over other laboratory animal species. Their longevity is an important consideration. Nonhuman primates also provide unique relevance to understanding the neurochemical basis of substance abuse in humans. Species differences in the complex topographical organization of the ventral striatum and its connections with surrounding areas, for example, complicate extrapolations from rodents to primates (Haber, Kunishio, Mizobuchi, & Lynd-Balta, 1995; Lynd-Balta & Haber, 1994a,b). Neuroimaging studies have documented that the nonhuman primate brain differs markedly from the rodent brain in the cerebral metabolic response to cocaine (Lyons, Friedman, Nader & Porrino, 1996; Porrino et al., 2002). Compared to rodents, nonhuman primates are more similar to humans in the pharmacokinetics and metabolism of several drug classes including stimulants (Banks et al., 2007; see Weerts, Fantegrossi & Goodwin, 2007, for review). Collectively, these important species differences illustrate the importance of nonhuman primate models in neuroimaging and substance abuse research
The present series of studies characterized the effectiveness of several DAT inhibitors (Table 1) to suppress cocaine self-administration in rhesus monkeys. These effects were related to DAT occupancy determined with PET neuroimaging. A possible limitation to the use of selective DAT inhibitors as medications for treatment of cocaine addiction is their potential for abuse. Accordingly, the DAT inhibitors were substituted for cocaine in order to characterize their reinforcing effects and abuse liability. The influence of pharmacokinetics on reinforcing effectiveness was evaluated with PET neuroimaging and measures of rate of drug uptake and clearance from brain. The effectiveness of selective SERT inhibitors in suppressing cocaine self-administration was also evaluated and related to their effectiveness in blocking cocaine-induced elevations in extracellular dopamine determined with in vivo microdialysis, and cocaine-induce brain activation determined with PET neuroimaging of cerebral blood flow. Finally, studies were conducted to characterize the combined effects of selective DAT and selective SERT inhibitors on cocaine self-administration. The results indicate that combined inhibition of DAT and SERT is effective in reducing cocaine use and may limit the reinforcing effectiveness and abuse liability of DAT inhibitors.
Table 1. Monoamine Uptake Inhibition (IC50 nM).
| Compound | [3H]DA | [3H]5HT | Ratio 5HT/DA |
|---|---|---|---|
| Cocainea | 310 | 260 | 0.8 |
| RTI-112a | 1.1 | 1.4 | 1.3 |
| RTI-113a | 3.0 | 229 | 76 |
| RTI-177a | 1.9 | 235 | 124 |
| RTI-336b | 12 | 522 | 44 |
| GBR12909c | 1.0 | 170 | 170 |
Data from Kuhar et al. (1999). Uptake values were determined using rat tissue.
Values supplied by NIDA under the Cocaine Treatment Discovery Program (CTDP) . Uptake values were determined using cloned hDAT and hSERT.
Data from Anderson (1989). Uptake values were determined using rat tissue.
Method
Subjects
All studies were conducted at the Yerkes National Primate Research Center, Emory University, in adult male and female rhesus monkeys (Macaca mulatta) and adult male squirrel monkeys (Saimiri sciureus). Each subject was housed individually and fed Purina monkey chow (Ralston Purina, St. Louis, MO), fruits, and vegetables. Water was continuously available. Rhesus monkeys were prepared surgically with a chronic indwelling venous catheter under sterile conditions using a technique described previously (Howell & Wilcox, 2002; Lindsey et al., 2004). Catheters were flushed daily with 1.0 ml heparinized (100 units/ml) saline to maintain patency. A stereotaxic apparatus was used to implant CMA/11 guide cannulae (CMA/Microdialysis, Acton, MA) bilaterally to target both caudate nuclei in squirrel monkeys as described previously (Czoty, Justice, & Howell, 2000). Animal care procedures strictly followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Emory University.
Drug Self-administration
During behavioral testing, each rhesus monkey was seated in a commercially available primate chair (Primate Products, Redwood City, CA), and a response panel with one lever was mounted on the front of the chair. Located above the lever in the center of the response panel were red and white stimulus lights. Once the monkey was seated in the chair, a Huber needle (Access Technologies, Skokie, IL) was inserted into the venous access port. The polyvinyl-chloride tubing attached to the Huber needle was connected to a motor-driven syringe (Coulbourn Instruments, Allentown, PA) located outside of the chamber containing the drug solution. A volume of 2.0 ml/infusion was delivered over 7 sec. Testing during daily 1-hr sessions occurred in a ventilated, sound-attenuating chamber. IBM compatible computers controlled experimental events and recorded data.
Subjects responded for i.v. infusions of cocaine under a second-order schedule of reinforcement, as described previously (Lindsey et al., 2004; Howell et al., 2007). The training dose of cocaine was 0.1 mg/kg/infusion. When the daily session began, the red light on the response panel was illuminated and responding resulted in the delivery of a drug infusion and brief 2-sec illumination of the white light. Initially, the fixed ratio (FR) was one (FR 1) and gradually increased to FR 20. Ultimately, a second-order schedule of reinforcement was in effect, with the first FR 20 completed after 10 min (fixed-interval, FI 10-min) resulting in a drug infusion. FR 20 components completed within the 10-min FI resulted in illumination of the white light for 2 sec. There was a 30-sec limited hold for completion of the first FR 20 after the FI 10-min had elapsed, and a drug infusion was not delivered if the limited hold expired. Drug infusions were signaled by a change in the lights from red to white for 15 sec. Following each drug infusion there was a 1-min timeout during which responding on the lever had no programmed consequences. A total of 5 infusions could be delivered during a daily session comprising five FI 10-min (FR 20:S) components.
The training sequence remained in effect until responding for cocaine was stable (<20% variance in daily response rate over five consecutive days), after which saline was substituted for cocaine until responding decreased to below 30% of responding for the training dose of cocaine. After saline extinction, the maintenance dose (0.1 mg/kg/infusion) of cocaine was reinstated and responding was allowed to stabilize. For pretreatment studies, a given dose of drug was administered i.v. 15 min pre-session on five consecutive days. Vehicle was administered on all days that subjects did not receive a drug pretreatment, and these data contributed to ongoing calculations of baseline stability. Pretreatment doses were administered on two separate occasions in an ascending order. All doses of a particular drug were studied in combination with 0.1 mg/kg/infusion cocaine first. Subsequently, the maintenance dose of cocaine was changed to 0.3 mg/kg/infusion, and drug pretreatments were repeated as described above. For drug-substitution studies, each subject was allowed to self-administer several doses of a drug in a randomized order. Substitution for each drug dose continued for at least 5 consecutive sessions, or until responding stabilized (<20% variance in daily response rate).
PET Neuroimaging
All brain imaging was performed at the Emory University PET Center on a Siemens 951 scanner. A set of Ge-68 ring sources was used for attenuation correction before injection of radiolabeled compounds. All images were reconstructed with measured attenuation correction, zoom factor 8, and Shepp-Logan reconstruction filter cut off at 1 cycle/cm. This produced images with an inplane pixel size of 1.17- and 8-mm resolution. The axial slice thickness was 3.375 mm. All images were decay-corrected to the time of injection. Regions of interest were manually drawn on the late images over the caudate, putamen and cerebellum. The regions of interest were then overlaid on all images to obtain time-activity curves.
DAT Occupancy
For DAT occupancy determinations, monkeys were anesthetized in the home cage with Telazol (4.0 mg/kg) and transported to the Emory University PET Center as described previously (Votaw et al., 2000; Lindsey et al., 2004; Howell et al., 2007). Subsequently, animals were intubated and anesthesia was maintained by isoflurane. Animals were positioned in the tomograph and a 15-minute transmission scan was obtained for attenuation correction. Thirty minutes after isoflurane anesthesia began, a slow bolus of approximately 5.0 mCi [18F]FECNT (specific activity = 1.5 Ci/μmole) was injected over five to six minutes at a rate of 1.0 ml/min. Within the first 90 min, [18F]FECNT was at or near quasi-equilibrium, after which a single dose of drug was injected. DAT occupancy was determined by displacement of [18F]FECNT binding during the 30-min period post-drug injection.
Time Course
The time course of uptake of the [11C]-labeled compounds was characterized in awake subjects as described previously (Kimmel et al., 2008). Subjects were positioned in the PET scanner and a 15-min transmission scan was obtained for attenuation correction. Subsequently, a tracer dose (5 mCi) of a [11C] labeled drug was administered as a rapid i.v. bolus in approximately 2.0 ml. Image acquisition began coincident with the start of the injection and continued for 90 min.
Cerebral Blood Flow
Functional changes in cerebral blood flow were determined in awake subjects with [15O] water following acute i.v. administration of cocaine as described previously (Howell et al., 2000; Howell, Hoffman, Votaw, Landrum, & Jordan, 2001). Experimental sessions comprised 8 consecutive i.v. injections of [15O] water at 10-min intervals. PET scans of 90 sec duration occurred 10 sec after each [15O] water injection.
In Vivo Microdialysis
At the time of testing, each squirrel monkey was seated in a commercially-available primate chair (MED Associates, Georgia, VT) as described previously (Czoty et al., 2002; Kimmel et al., 2007). Daily sessions lasted for approximately 4 hours and were conducted within a ventilated, sound-attenuating chamber. The chair limited movement of the animals and facilitated connections between the implanted probes and appropriate perfusion equipment. A Lexan plate positioned perpendicular to the medial plane of the body, just above shoulder height, ensured that animals could not contact the probe area. A microinjection pump (CMA/102) located outside the chamber continuously delivered artificial cerebrospinal fluid (Na2HPO4, 1.0 mM; NaCl, 150 mM; KCl, 3 mM; CaCl, 1.3 mM; MgCl, 1.0 mM; and ascorbic acid, 0.15 mM) via FEP Teflon tubing to the probe for perfusion at a flow rate of 2.0 μl/min.
During a 2-hour equilibrium period, animals sat in the chamber, and repeated 10-min samples were obtained. Subsequently, a single dose of drug was administered i.m. to determine drug-induced increases in extracellular dopamine. The order of drug testing was randomized and counterbalanced across treatment conditions, and at least one week separated repeated determinations. Samples were collected outside the test chamber at 10-min intervals to ensure that the monkeys were not disturbed during the experiments. Probes were tested in vitro to determine the suitability of probe efficiency and performance before and after in vivo experiments. Microbore HPLC and electrochemical detection quantitated extracellular levels of dopamine according to well-established analytical procedures (Church, Justice, & Byrd, 1987; Skirboll, Wang, Mefford, Hsiao, & Bankiewiicz, 1990; Parsons & Justice, 1993).
Results
DAT Inhibitor Pretreatments
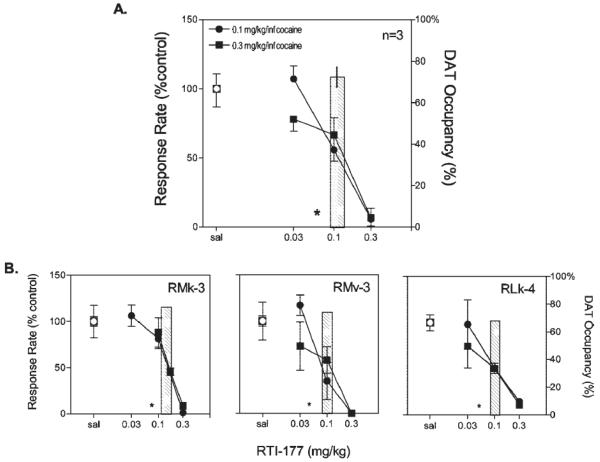
A variety of selective DAT inhibitors from distinct chemical classes have been reported to suppress i.v. cocaine self-administration in nonhuman primates. For example, the effects of the phenyltropane, RTI-177, on cocaine self-administration maintained under a second-order schedule in a group of three rhesus monkeys are shown in Figure 1. A range of doses of RTI-177 was administered in combination with two different unit doses of cocaine (0.1 and 0.3 mg/kg).These unit doses of cocaine were selected because they were positioned on the peak and descending limb of the cocaine dose-effect curve, respectively. Pretreatment with RTI-177 caused a dose-dependent reduction in cocaine-maintained responding in all subjects tested, and varying the maintenance dose of cocaine had no influence on the effectiveness of RTI-177 pretreatments.
Figure 1.
Effects of RTI-177 pretreatments on cocaine self-administration maintained by a second-order schedule in a group of three rhesus monkeys. The top panel shows data averaged for the group and the bottom panels show data for individual subjects. Abscissae: drug dose, log scale. Ordinates: response rate expressed as a percentage of control rate obtained following saline administration. The bar graphs (right ordinates) depict percentage of DAT occupancy by the doses of RTI-177 indicated on the abscissae. The asterisks represent occupancy values that were below the limit of detection. Adapted from Lindsey et al. (2004).
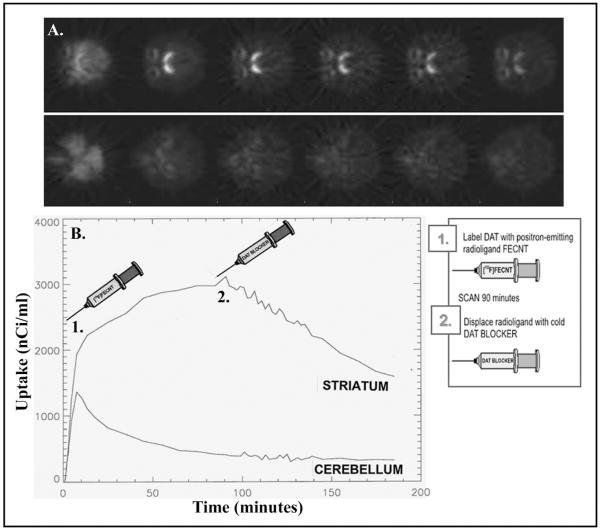
In subsequent studies, [18F]FECNT was used as a PET ligand to quantify levels of DAT occupancy at behaviorally relevant doses of selective DAT inhibitors. Note that [18F]FECNT has been validated for use as a PET ligand to label the DAT (Goodman et al., 2000; Votaw et al., 2002), and that it binds to a cocaine-sensitive binding site in a dose-dependent manner (Table 2). A typical [18F]FECNT time-activity curve and displacement of binding by cocaine is shown in an individual rhesus monkey in Figure 2. This approach was used to quantify the level of DAT occupancy associated with the ED50 doses of several DAT inhibitors (Table 3). DAT occupancy for RTI-177 and GBR 12909 was very similar and approximately 70%. The DAT inhibitor with the lowest potency to inhibit serotonin uptake, RTI-336, resulted in DAT occupancy of 90%. Interestingly, the ED50 dose of the DAT inhibitor with the highest potency to inhibit serotonin uptake, RTI-112, resulted in DAT occupancy below the limit of detection. However, the same dose resulted in SERT occupancy of 84% in the same group of subjects (Lindsey et al., 2004). Hence, it appears that drugs with greater selectivity for DAT require higher levels of DAT occupancy to suppress cocaine self-administration.
Table 2. Percent DAT Occupancy following Cocaine Injection.
| Cocaine Dose |
||
|---|---|---|
| Subject | 0.1 mg/kg | 1.0 mg/kg |
| RMk-3 | 53 | 92.1 |
| RLm-1 | 56 | 95.5 |
| RLl-4 | 44 | 84.0 |
| RLk-4 | 53 | 83.6 |
| RSu-3 | 59 | 88.0 |
| Ave ± S.E.M | 53 ± 5 | 87 ± 5 |
Derived from Votaw et al. (2000).
Figure 2.
Typical time-activity curves for [18F]FECNT and displacement of binding by cocaine in an individual rhesus monkey. The data have been decay corrected to the time of injection. The displacement of [18F]FECNT binding is used to determine DAT occupancy by behaviorally-relevant doses of several DAT inhibitors. Abscissa: time in minutes. Ordinate: standard uptake value.
Table 3. Percent DAT Occupancy Associated with the ED50 Dose for Reducing Cocaine Self-administration.
| Subject | DAT Occupancy |
|---|---|
| GBR 12909 | |
| ROu-4 | 62 |
| RLk-4 | 68 |
| RKs-4 | 71 |
| Ave ± S.E.M. | 67 ± 5 |
| RTI-177 | |
| RMk-3 | 77 |
| RMv-3 | 73 |
| RLk-4 | 68 |
| Ave ± S.E.M. | 73 ± 5 |
| RTI-336 | |
| RVc-5 | 93 |
| ROk-5 | 91 |
| REl-5 | 99 |
| RNa-4 | 77 |
| Ave ± S.E.M. | 90 ± 5 |
Derived from Lindsey et al. (2004); Howell et al. (2007).
DAT Inhibitor Substitutions
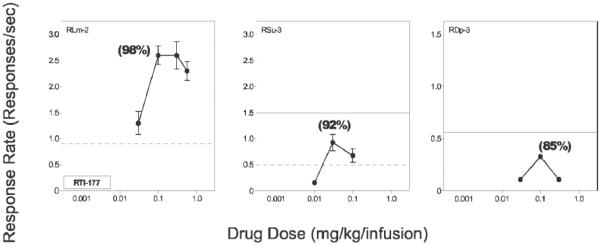
A variety of selective DAT inhibitors from distinct chemical classes have been reported to maintain i.v. drug self-administration in nonhuman primates. For example, the effects of substituting RTI-177 for cocaine under a second-order schedule of i.v. drug delivery in a group of three rhesus monkeys are shown in Figure 3. RTI-177 reliably maintained drug self-administration at levels greater than those maintained by saline in all subjects. Moreover, the shape of the dose effect curves resembled an inverted-U shape function typical of psychomotor stimulants. However, rates of responding were lower than those maintained by the training dose of cocaine (0.1 mg/kg/infusion). Interestingly, the least selective DAT inhibitor, RTI-112, with high affinity for the SERT, failed to maintain robust drug self-administration in any subject (Lindsey et al., 2004).
Figure 3.
Self-administration of RTI-177 maintained by a second-order schedule of i.v. drug delivery in individual rhesus monkeys. Abscissae: unit dose, log scale. Ordinates: response rate in responses/ sec. Solid lines indicate mean rates of responding maintained by the training dose of cocaine. Dashed lines indicate the upper limit of responding during saline extinction. Numbers in parentheses indicate the level of DAT occupancy associated with the unit doses that maintained peak rates of responding. Adapted from Lindsey et al. (2004).
In subsequent studies, [18F]FECNT was used as a PET ligand to quantify levels of DAT occupancy at doses of RTI-177 that maintained peak rates of responding. Unit doses were identified for individual subjects and then the average total dose the subject received during its self-administration sessions was determined and administered as a bolus injection in the PET experiments. DAT occupancy was 92% (range 85-98%) for the group (Figure 3). Note that DAT occupancy was reported to be between 65 and 76% for peak doses of cocaine and between 94 and 99% for peak doses of the selective DAT inhibitor RTI-113 based on total session intake as described above (Wilcox et al., 2002).
DAT Inhibitor Pharmacokinetics
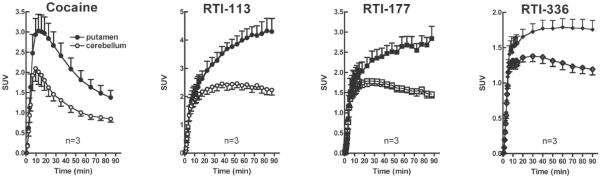
The time-course for drug uptake in putamen and cerebellum for each of the [11C]-labeled compounds is shown in Figure 4. Due to the short half-life of [11C], the PET scan was limited to 90 minutes post-injection of the labeled compounds. The average time to peak levels of cocaine in putamen was 9.5 minutes (Table 4), and cocaine levels dropped markedly after 40-50 minutes. In contrast, the time to peak levels of the selective DAT inhibitors was considerably greater, and drug levels were sustained for the duration of the 90-min session. The rank order of the time to peak uptake was cocaine < RTI-336 < RTI-113 < RTI-177. The time-course for drug uptake was very consistent across subjects for each of the compounds evaluated.
Figure 4.
Time course of uptake and clearance of [11C] labeled cocaine and selective DAT inhibitors. PET scans were acquired in awake subjects. Abscissa: time in minutes. Ordinate: standard uptake value (SUV). Each point shows the mean ± SEM in three monkeys. Adapted from Kimmel et al. (2008).
Table 4. Average Time to Peak Uptake of Drug in Putamen.
| Drug | Time of Peak Uptake (min) |
|---|---|
| Cocaine | 9.5 |
| RTI-336 | 40.0 |
| RTI-113 | 62.5 |
| RIT-177 | 87.5 |
Derived from Kimmel et al. (2008).
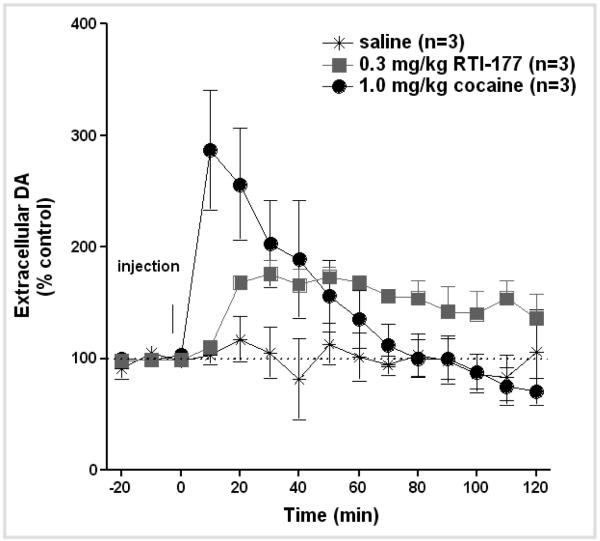
In vivo microdialysis experiments were conducted in a group of four awake squirrel monkeys in order to document drug-induced changes in extracellular dopamine in the caudate nucleus (Figure 5). As reported previously, cocaine had a rapid onset of action with peak effects observed during the first 10-min sample following drug administration. Dopamine levels returned to baseline within 60-min post-injection. In contrast, peak dopamine levels were not evident until 20-30 min following RTI-177 administration and they remained elevated for the duration of the 2-hr observation period. Hence, there was a close correspondence between the time course of drug uptake in brain and drug-induced increases in extracellular dopamine.
Figure 5.
Effects of cocaine and RTI-177 on extracellular dopamine in the caudate nucleus in a group of four awake squirrel monkeys. Drugs were administered i.m. at time point zero. Abscissa: time in minutes. Ordinate: extracellular dopamine expressed as a percentage of baseline values. Adapted from Kimmel et al. (2007).
SERT Inhibitor Pretreatments
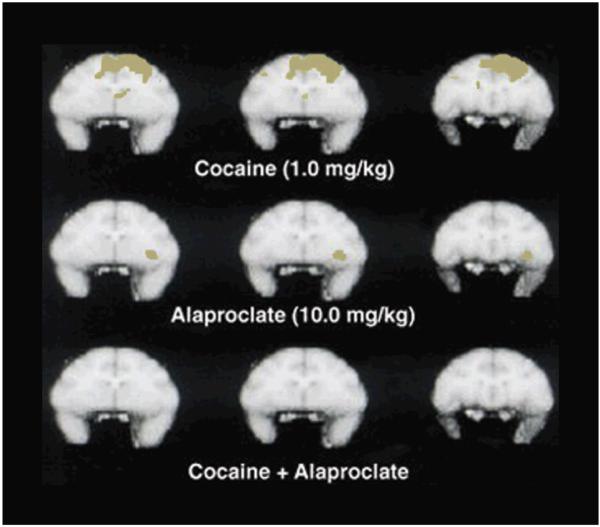
Previous reports have documented that selective SERT inhibitors can block the behavioral-stimulant effects of cocaine and attenuate i.v. cocaine self-administration in nonhuman primates. Related studies evaluated the effectiveness of a selective SERT inhibitor to attenuate cocaine-induced brain activation in a group of four awake rhesus monkeys. Cocaine had significant, dose-related effects on cerebral blood flow at 5 min post-injection that diminished relative to control (saline) conditions by 15 min post-injection. Brain activation maps normalized to global flow showed prominent cocaine-induced activation of prefrontal cortex localized primarily to dorsolateral regions (Figure 6). Importantly, cocaine-induced brain activation was blocked by pretreatment with the selective SERT inhibitor, alaproclate.
Figure 6.
Effects of cocaine and alaproclate administered alone or in combination on regional cerebral blood flow 5 min postinjection. Images for each condition represent sequential 1-mm sections obtained at the same time point. Although the effects of alaproclate alone were unremarkable, pretreatment with alaproclate blocked cocaine-induced brain activation. Data are derived from four monkeys. Adapted from Howell et al. (2002).
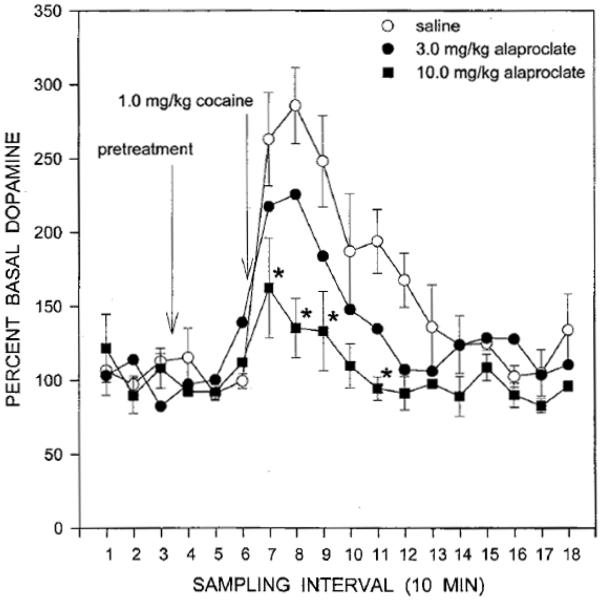
The neurochemical basis of the observed drug interactions on cerebral blood flow were investigated further with in vivo microdialysis in a group of three awake squirrel monkeys. As described above, cocaine increased extracellular dopamine in the caudate nucleus to approximately 300% of basal levels during the first 10-min sample following drug administration, and dopamine levels returned to baseline approximately 60 min post-injection (Figure 7). Importantly, when alaproclate was administered 30 min prior to cocaine, there was a dose-dependent attenuation of the effects of cocaine on extracellular dopamine. Collectively, the results suggest that increasing brain serotonin activity can attenuate the reinforcing and brain activating effects of cocaine, ostensibly by decreasing the ability of cocaine to elevate extracellular dopamine.
Figure 7.
Effects of cocaine on extracellular dopamine in the caudate nucleus in a group of three awake squirrel monkeys. Abscissa: 10-min sampling intervals beginning one hr after probe insertion. Ordinate: extracellular dopamine expressed as a percentage of baseline values. Adapted from Czoty et al. (2002).
Combined DAT and SERT Inhibitor Pretreatments
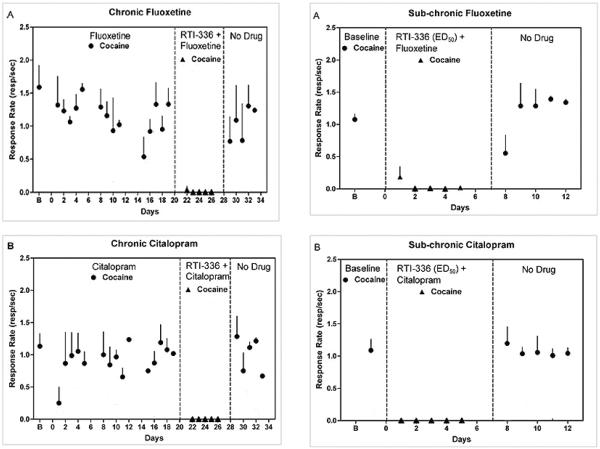
A final series of experiments co-administered the selective DAT inhibitor, RTI-336, with the selective SERT inhibitors, fluoxetine and citalopram, to evaluate their combined effects on cocaine self-administration maintained under a second-order schedule in a group of four rhesus monkeys. Pretreatments with RTI-336 produced dose-related reductions in cocaine self-administration, and the ED50 dose resulted in approximately 90% DAT occupancy. Interestingly, co-administration of the ED50 dose of RTI-336 in combination with either SERT inhibitor completely suppressed cocaine self-administration without affecting DAT occupancy (Figure 8). Hence, at comparable levels of DAT occupancy, co-administration of SERT inhibitors with RTI-336 produced more robust reductions in cocaine self-administration compared with RTI-336 administered alone.
Figure 8.
Effects of co-administration of fluoxetine or citalopram in combination with the ED50 dose of RTI-336 on cocaine self-administration maintained by a second-order schedule in a group of three rhesus monkeys. Fluoxetine or citalopram was administered i.m. either chronically for four consecutive weeks or subchronically for five consecutive days. Abscissae: consecutive days of treatment. Ordinates: response rate in responses/ sec. Adapted from Howell et al. (2007).
Discussion
The present series of studies compared the effects of several DAT inhibitors with varying selectivity for monoamine transporters in drug self-administration protocols. Direct, within-subject comparisons were made between drug effects on behavior and in vivo DAT occupancy measured with PET neuroimaging. Drugs with selectivity for DAT over SERT (RTI-177, RTI-336 and GBR 12909) exhibited high levels of DAT occupancy at doses that produced robust decreases in cocaine self-administration. RTI-336 has the greatest selectivity for DAT relative to SERT and it resulted in the highest level of DAT occupancy. In contrast, a mixed action inhibitor of DAT and SERT (RTI-112) did not exhibit levels of DAT occupancy above the threshold of detection at a dose that significantly reduced cocaine self-administration. Importantly, the same dose RTI-112 exhibited high levels of SERT occupancy, showing apparent in vivo selectivity for SERT over DAT at a behaviorally relevant dose. Hence, it appears that the greater the selectivity for DAT, the higher the level of DAT occupancy required to suppress cocaine self-administration. In drug substitution studies, the selective DAT inhibitors reliably maintained drug self-administration in all subjects, although rates of responding were lower than those maintained by the training dose of cocaine. In contrast, RTI-112 did not function as a robust reinforcer in any subject. Collectively, the results indicate that the behavioral profile of DAT inhibitors may be influenced by actions at other monoamine transporters.
A possible limitation to the use of selective DAT inhibitors as medications for treatment of cocaine addiction is their potential for abuse, given their documented reinforcing effects (Howell & Wilcox, 2001). In the present series of studies, the selective DAT inhibitors reliably maintained self-administration behavior in all subjects, consistent with results reported for phenyltropanes (Howell et al., 2000; Wilcox et al., 2002; Lindsey et al., 2004; Howell et al., 2007) and phenylpiperazines (Bergman et al., 1989; Howell and Byrd, 1991; Lindsey et al., 2004) in nonhuman primates. However, the selective DAT inhibitors maintained lower rates of responding compared with cocaine across a broad range of doses, even though DAT occupancy was equal to or greater than that observed for cocaine (Wilcox et al., 2002; Lindsey et al., 2004; Howell et al., 2007). In behavioral studies in rodents and nonhuman primates, these compounds had a slower onset and a longer duration of action compared with cocaine (Howell et al., 2000; Kimmel, Joyce, Carroll, & Kuhar, 2001). In PET neuroimaging studies which characterized the time-course of drug uptake in brain, there was a clear trend towards an inverse relationship between the time to peak uptake of [11C]-labeled drugs in putamen and the peak number of infusions received under a progressive ratio schedule of i.v. self-administration, such that the faster-onset drugs produced greater levels of responding relative to the slower-onset drugs (Kimmel et al., 2008). There also was a close correspondence between the time course of drug uptake in brain and drug-induced increases in extracellular dopamine in caudate (Ginsburg et al., 2005; Kimmel et al., 2007, 2008). Hence, the reinforcing effects and pattern of drug self-administration is likely influenced by pharmacokinetics in addition to steady-state levels of DAT occupancy.
The dopamine system is clearly an important site of action for cocaine, but preclinical studies have indicated that the serotonergic system can effectively modulate the behavioral effects of cocaine and related psychomotor stimulants. Although compounds that selectively increase serotonin neurotransmission lack behavioral-stimulant effects and do not reliably maintain self-administration behavior (Vanover, Nader, & Woolverton, 1992; Howell & Byrd, 1995), a negative relationship was observed between the potencies of several cocaine- and amphetamine-like drugs in self-administration studies and their binding affinities for serotonin uptake sites (Ritz et al., 1987; Ritz & Kuhar, 1989). Co-administration of agents that induce robust increases in both dopamine and serotonin produces minimal behavioral-stimulant effects (Baumann et al., 2000) and does not maintain self-administration behavior (Glatz et al., 2002) in rodents. Similarly, monoamine-releasing agents have decreased reinforcing efficacy in rhesus monkeys when serotonin-releasing potency is increased relative to dopamine (Wee et al., 2005). Consistent with these results, administration of the SERT inhibitor, fluoxetine, decreased self-administration of cocaine (Carroll et al., 1990) and amphetamine (Porrino et al., 1989) in rodents. In nonhuman primate studies, the SERT inhibitors citalopram, fluoxetine and alaproclate attenuated the behavioral-stimulant effects of cocaine on schedule-controlled behavior (Howell & Byrd, 1955; Spealman, 1993). Importantly, in the present series of studies, the serotonin uptake inhibitor alaproclate attenuated cocaine self-administration and cocaine-induced increases in extracellular dopamine in squirrel monkeys (Czoty et al., 2002) and cocaine-induced activation of prefrontal activity in rhesus monkeys (Howell et al., 2002). Hence, drugs that increase brain serotonin activity can effectively attenuate the behavioral and neurochemical effects of cocaine.
The present series of studies also evaluated the effects of combined DAT and SERT inhibitors on cocaine self-administration in rhesus monkeys (Howell et al., 2007). RTI-336 is a highly selective DAT inhibitor with greater than 1000- and 400-fold selectivity relative to SERT and norepinephrine transporter binding, respectively (Kuhar et al., 1999). Pretreatments with RTI-336 produced dose-dependent reductions in cocaine self-administration behavior, and RTI-336 maintained its effectiveness when the unit dose of cocaine was increased from 0.1 to 0.3 mg/kg/injection. Within subject comparisons were made between drug effects on behavior and in vivo DAT occupancy measured with PET neuroimaging. The ED50 dose of RTI-336 for reducing cocaine self-administration resulted in approximately 90% DAT occupancy for the group. Subsequently, the ED50 dose of RTI-336 was co-administered with the selective SERT inhibitors fluoxetine and citalopram. Administration of the SERT inhibitors alone at the doses selected had little effect on cocaine self-administration. However, co-administration of the ED50 dose of RTI-336 completely suppressed cocaine self-administration behavior in all subjects, and the drug interactions observed did not require chronic administration of the SERT inhibitors. Subchronic administration of either SERT inhibitor for five consecutive days during co-administration of the ED50 dose of RTI-336 also completely suppressed responding in all subjects. Likewise, co-administration of fluoxetine with the ED10 dose of RTI-336 had more pronounced effects compared with RTI-336 alone. When DAT occupancy for the ED50 dose of RTI-336 was redetermined during co-administration of fluoxetine, approximately 90% DAT occupancy was observed for the group. Hence, at comparable levels of DAT occupancy, co-administration of fluoxetine produced more robust reductions in cocaine self-administration compared with RTI-336 alone. Collectively, the results indicate that combined inhibition of DAT and SERT is effective in reducing cocaine self-administration and may limit the reinforcing effectiveness of DAT inhibitors.
Compelling data have emerged from clinical research supporting indirect agonist-like pharmacotherapy for stimulant abuse and dependence (Grabowski, Shearer, Merrill, & Negus, 2004). The concept of indirect agonist pharmacotherapy implies that the medication will exhibit some cocaine-like properties at a neurochemical and behavioral level. In this regard, selective SERT inhibitors do not exhibit cocaine-like behavioral effects and do not seem appropriate as indirect agonist pharmacotherapies (Grabowski et al., 1995). In contrast, selective DAT inhibitors clearly exhibit cocaine-like behavioral effects and seem promising as cocaine medications based on preclinical evaluations. However, their reinforcing effects in animal models still indicate that they may exhibit high abuse liability that could limit their clinical utility. In addition, the effects of high levels of DAT occupancy that are required to reduce cocaine use are unknown. RTI-336 alone may prove to be a suitable pharmacotherapy, but an even better approach may be to use RTI-336 in combination with a SERT inhibitor. Alternatively, development of medications involving dual actions at DAT and SERT could lead to compounds with cocaine-like properties appropriate in indirect agonist pharmacotherapy but with limited abuse liability.
Acknowledgements
The author gratefully acknowledges the technical assistance of Peggy M. Plant in the preparation of the manuscript. Research from the laboratory of the author and preparation of the manuscript were supported in part by U.S. Public Health Service Grants DA010344, DA012514, DA016589, DA000517, DA013326 and RR000165 (Division of Research Resources, National Institutes of Health).
References
- Anderson PH. The dopamine uptake inhibitor GBR 2909: selectivity and molecular mechanism of action. European Journal of Pharmacology. 1989;166:495–504. doi: 10.1016/0014-2999(89)90363-4. [DOI] [PubMed] [Google Scholar]
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metabolism and Disposition. 2007;35(10):1840–1845. doi: 10.1124/dmd.107.016261. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, Brockington A, Rice KC, Rothman RB. Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: Therapeutic implications. Synapse. 2000;36:102–113. doi: 10.1002/(SICI)1098-2396(200005)36:2<102::AID-SYN3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. Journal of Pharmacology and Experimental Therapeutics. 1989;251:150–155. [PubMed] [Google Scholar]
- Carroll FI, Howell LL, Kuhar MJ. Pharmacotherapies for treatment of cocaine abuse: Preclinical aspects. Journal of Medicinal Chemistry. 1999;42:2721–2736. doi: 10.1021/jm9706729. [DOI] [PubMed] [Google Scholar]
- Carrol ME, Lac ST, Asencio M, Kragh R. Fluoxetine reduces intravenous cocaine self-administration in rats. Pharmacology Biochemistry and Behavior. 1990;35:237–244. doi: 10.1016/0091-3057(90)90232-7. [DOI] [PubMed] [Google Scholar]
- Church WH, Justice JB, Jr., Byrd LD. Extracellular dopamine in rat striatum following uptake inhibition by cocaine, nomifensine and benzotropine. European Journal of Pharmacology. 1987;139:345–348. doi: 10.1016/0014-2999(87)90592-9. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. Journal of Pharmacology and Experimental Therapeutics. 2002;300:831–837. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Justice JB, Jr., Howell LL. Cocaine-induced changes in extracellular dopamine determined by microdialysis in awake squirrel monkeys. Psychopharmacology. 2000;148:299–306. doi: 10.1007/s002130050054. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Kimmel HL, Carroll FI, Goodman MM, Howell LL. Interaction of cocaine and dopamine transporter inhibitors on behavior and neurochemistry in monkeys. Pharmacology Biochemistry and Behavior. 2005;80:481–491. doi: 10.1016/j.pbb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Glatz AC, Ehrlich M, Bae RS, Clarke MJ, Quinlan PA, Brown EC, et al. Inhibition of cocaine self-administration by fluoxetine or p-fenfluramine combined with phentermine. Pharmacology Biochemistry and Behavior. 2002;71:197–204. doi: 10.1016/s0091-3057(01)00657-8. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Wojnicki FHE, Matecka D, Bacher JJ, Mansbach RS, Balster RL, et al. Effects of dopamine reuptake inhibitors on food- and cocaine-maintained responding. I. Dependence on unit dose of cocaine. Experimental and Clinical Psychopharmacology. 1995;3:219–231. [Google Scholar]
- Goodman MM, Kilts CD, Keil R, Shi B, Martarello L, Xing D, et al. 18F-Labeled FECNT: A selective radioligand for PET imaging of brain dopamine transporters. Nuclear Medicine and Biology. 2000;27:1–12. doi: 10.1016/s0969-8051(99)00080-3. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus S. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addictive Behaviors. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Elk R, Schmitz J, Davis J, Creson D, et al. Fluoxetine is ineffective for treatment of cocaine dependence or concurrent opiate and cocaine dependence: Two placebo-controlled double-blind trials. Journal of Clinical Psychopharmacology. 1995;15:163–174. doi: 10.1097/00004714-199506000-00004. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. Journal of Neuroscience. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Characterization of the effects of cocaine and GBR 12909, a dopamine uptake inhibitor, on behavior in the squirrel monkey. Journal of Pharmacology and Experimental Therapeutics. 1991;258:178–185. [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Serotonin modulation of the behavioral effects of cocaine in the squirrel monkey. Journal of Pharmacology and Experimental Therapeutics. 1995;275:1551–1559. [PubMed] [Google Scholar]
- Howell LL, Czoty PW, Kuhar MJ, Carroll FI. Comparative behavioral pharmacology of cocaine and the selective dopamine uptake inhibitor, RTI-113, in the squirrel monkey. Journal of Pharmacology and Experimental Therapeutics. 2000;292:521–529. [PubMed] [Google Scholar]
- Howell LL, Hoffman JM, Votaw JR, Landrum AM, Jordan JF. An apparatus and behavioral training protocol to conduct positron emission tomography (PET) neuroimaging in conscious rhesus monkeys. Journal of Neuroscience Methods. 2001;106:161–169. doi: 10.1016/s0165-0270(01)00345-4. [DOI] [PubMed] [Google Scholar]
- Howell LL, Hoffman JM, Votaw JR, Landrum AM, Wilcox KM, Lindsey KP. Cocaine-induced brain activation determined by positron emission tomography neuroimaging in conscious rhesus monkeys. Psychopharmacology (Berl.) 2002;159:154–160. doi: 10.1007/s002130100911. [DOI] [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 2007;320:757–765. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. The dopamine transporter and cocaine medication development: Drug self-administration in nonhuman primates. Journal of Pharmacology and Experimental Therapeutics. 2001;298:1–6. [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. Functional imaging and neurochemical correlates of stimulant self-administration in primates. Psychopharmacology. 2002;163:352–361. doi: 10.1007/s00213-002-1207-y. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Joyce AR, Carroll FI, Kuhar MJ. Dopamine D1 and D2 receptors influence dopamine transporter synthesis and degradation in the rat. Journal of Pharmacology and Experimental Therapeutics. 2001;298:129–140. [PubMed] [Google Scholar]
- Kimmel HL, Negus SS, Wilcox KM, Ewing SB, Stehouwer J, Goodman MM, et al. Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys. Behavioral Pharmacology and Behavior. 2008;90:453–462. doi: 10.1016/j.pbb.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, O’Connor JA, Carroll FI, Howell LL. Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacology Biochemistry and Behavior. 2007;86:45–54. doi: 10.1016/j.pbb.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Woolverton WL. Effects of three monoamine uptake inhibitors on behavior maintained by cocaine or food presentation in rhesus monkeys. Drug and Alcohol Dependence. 1993;31:149–158. doi: 10.1016/0376-8716(93)90067-z. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends in Neurosciences. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, McGirr KM, Hunter RG, Lambert PD, Garrett BE, Carroll FI. Studies of selected phenyltropanes at monoamine transporters. Drug and Alcohol Dependence. 1999;56:9–15. doi: 10.1016/s0376-8716(99)00005-8. [DOI] [PubMed] [Google Scholar]
- Lile JA, Wang Z, Woolverton WL, France JE, Gregg TC, Davies HM, et al. The reinforcing efficacy of psychostimulants in rhesus monkeys: The role of pharmacokinetics and pharmacodynamics. Journal of Pharmacology and Experimental Therapeutics. 2003;307:356–366. doi: 10.1124/jpet.103.049825. [DOI] [PubMed] [Google Scholar]
- Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, et al. Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: Relationship to transporter occupancy determined by positron emission tomography neuroimaging. Journal of Pharmacology and Experimental Therapeutics. 2004;309:959–969. doi: 10.1124/jpet.103.060293. [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN. The organization of midbrain projections to the striatum in the primate: Sensorimotor-related striatum versus ventral striatum. Neuroscience. 1994a;59(3):625–640. doi: 10.1016/0306-4522(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN. The organization of midbrain projections to the ventral striatum in the primate. Neuroscience. 1994b;59(3):609–623. doi: 10.1016/0306-4522(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Lyons D, Friedman DP, Nader MA, Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. Journal of Neuroscience. 1996;6(3):1230–1238. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Nader MA, Grant KA, Davies HM, Mach RH, Childers SR. The reinforcing and discriminative stimulus effects of the novel cocaine analog 2beta-propanoyl-3beta-(4-tolyl)-tropane in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 1997;280:541–550. [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr. Serotonin and dopamine sensitization in the nucleus accumbens, ventral tegmental area, and dorsal raphe nucleus following repeated cocaine administration. Journal of Neurochemistry. 1993;61:1611–161. doi: 10.1111/j.1471-4159.1993.tb09794.x. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. Journal of Neuroscience. 2002;22(17):7687–7694. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Ritz MC, Goodman NL, Sharpe LG, Kuhar MJ, Goldberg SR. Differential effects of the pharmacological manipulation of serotonin systems on cocaine and amphetamine self-administration in rats. Life Sciences. 1989;45:1529–1535. doi: 10.1016/0024-3205(89)90418-9. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Kuhar MJ. Relationship between self-administration of amphetamine and monoamine receptors in brain: Comparison with cocaine. Journal of Pharmacology and Experimental Therapeutics. 1989;248:1010–1017. [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, et al. Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. Journal of Pharmacology and Experimental Therapeutics. 2005;313:1361–1369. doi: 10.1124/jpet.104.082503. [DOI] [PubMed] [Google Scholar]
- Skirboll S, Wang J, Mefford I, Hsiao J, Bankiewicz KS. In vivo changes of catecholamines in hemiparkinsonian monkeys measured by microdialysis. Experimental Neurology. 1990;110:187–193. doi: 10.1016/0014-4886(90)90029-r. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Modification of behavioral effects of cocaine by selective serotonin and dopamine uptake inhibitors in squirrel monkeys. Psychopharmacology (Berl.) 1993;112:93–99. doi: 10.1007/BF02247368. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Nader MA, Woolverton WL. Evaluation of the discriminative stimulus and reinforcing effects of sertraline in rhesus monkeys. Pharmacology Biochemistry and Behavior. 1992;41:789–793. doi: 10.1016/0091-3057(92)90228-8. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Rothman RB, Yokoi F, Rice KC, Matecka D, Dannals RF, et al. Doses of GBR12909 that suppress cocaine self-administration in non-human primates substantially occupy dopamine transporters as measured by [11C] WIN35,428 PET scans. Synapse. 1999;32:44–50. doi: 10.1002/(SICI)1098-2396(199904)32:1<44::AID-SYN6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, et al. Blockade of striatal dopamine transporters by intravenous methylphenidate is not sufficient to induce self-reports of “high”. Journal of Pharmacology and Experimental Therapeutics. 1999;288:14–20. [PubMed] [Google Scholar]
- Votaw JR, Howell LL, Martarello L, Hoffman JM, Kilts CD, Lindsey KP, et al. Measurement of dopamine transporter occupancy for multiple injections of cocaine using a single injection of [F-18]FECNT. Synapse. 2002;44:203–210. doi: 10.1002/syn.10068. [DOI] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WA. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. Journal of Pharmacology and Experimental Therapeutics. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. The value of nonhuman primates in drug abuse research. Experimental and Clinical Psychopharmacology. 2007;15(4):309–327. doi: 10.1037/1064-1297.15.4.309. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Kimmel HL, Lindsey KP, Votaw JR, Goodman MM, Howell LL. In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse. 2005;58:220–228. doi: 10.1002/syn.20199. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Lindsey KP, Votaw JR, Goodman MM, Martarello L, Carroll FI, et al. Self-administration of cocaine and the cocaine analog RTI-113: Relationship to dopamine transporter occupancy determined by PET neuroimaging in rhesus monkeys. Synapse. 2002;43:78–85. doi: 10.1002/syn.10018. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Paul IA, Woolverton WL. Comparison between dopamine transporter affinity and self-administration potency of local anesthetics in rhesus monkeys. European Journal of Pharmacology. 1999;367:175–181. doi: 10.1016/s0014-2999(98)00967-4. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Johnson KM. Neurobiology of cocaine abuse. Trends in Pharmacological Sciences. 1992;13:193–200. doi: 10.1016/0165-6147(92)90063-c. [DOI] [PubMed] [Google Scholar]