Abstract
The present study has been undertaken to establish the therapeutic benefit of co-targeting epidermal growth factor receptor (EGFR) and sonic hedgehog pathways by using gefitinib and cyclopamine, respectively, for improving the efficacy of the current chemotherapeutic drug, docetaxel, to counteract the prostate cancer (PC) progression from locally invasive to metastatic and recurrent disease stages. The data from immuofluorescence analyses revealed that EGFR/Tyr1173-pEGFR, sonic hedgehog ligand (SHH), smoothened co-receptor (SMO) and GLI-1 were co-localized with the CD133+ stem cell-like marker in a small subpopulation of PC cells. These signaling molecules were also present in the bulk tumor mass of CD133− PC cells with a luminal phenotype detected in patient’s adenocarcinoma tissues. Importantly, the results revealed that the CD133+/CD44high/AR−/low side population (SP) cell fraction endowed with a high self-renewal potential isolated from tumorigenic and invasive WPE1-NB26 cells by Hoechst dye technique was insensitive to current chemotherapeutic drug, docetaxel. In contrast, the docetaxel treatment induced significant anti-proliferative and apoptotic effects on the CD133−/CD44low/AR+ non-SP cell fraction isolated from WPE1-NB26 cell line. Of therapeutic interest, the results have also indicated that combined docetaxel, gefitinib and cyclopamine induced greater anti-proliferative and apoptotic effects on SP and non-SP cell fractions isolated from WPE1-NB26 cells than individual drugs or two-drug combinations. Altogether, these observations suggest that EGFR and sonic hedgehog cascades may represent the potential therapeutic targets of great clinical interest to eradicate the total PC cell mass and improve the current docetaxel-based therapies against locally advanced and invasive PCs, and thereby prevent metastases and disease relapse.
Keywords: Prostate cancer, Prostate cancer stem/progenitor cells, EGF-EGFR system, Sonic hedgehog, Docetaxel, Treatment resistance, Molecular targeting, Combination cancer therapy
Introduction
Major progress in PC research has led to an earlier diagnosis and effective treatment of patients diagnosed with low-grade and organ-confined PCs by surgical tumor resection, anti-hormonal therapies and/or radiation therapy (1–3). Unfortunately, the disease progression to highly invasive and metastatic and hormone-refractory PCs (HRPCs), which are generally refractory to current conventional androgen ablation and chemotherapy therapies, generally culminate in the death of patients after about 12–19 months (1, 4–7). This inefficacy of current therapies against locally invasive and metastatic HRPCs underlines the urgent need to develop novel therapeutic strategies for counteracting disease progression and overcoming treatment resistance and disease relapse.
Recent lines of experimental evidence indicated that the accumulation of genetic and/or epigenetic alterations in multipotent CD133+/CD44+/high/androgen receptor (AR−low) adult prostatic stem/progenitor cells resident in the basal cell compartment and/or their early progenies, transit-amplifying (TA)/intermediate cells may culminate in their malignant transformation into highly tumorigenic PC stem/progenitor cells and tumor formation (1, 8–17). In support with the critical functions of PC stem/progenitor cells in PC initiation and progression, the small subpopulations of immature PC cells have been identified by immunohistochemical analyses as well as isolated from primary prostatic adenocarcinoma and metastatic tissue specimens of patients and well-established human PC cell lines (8–23). These PC stem/progenitor cells typically expressed different stem cell-like markers such as telomerase, CD133, CD44+/high, α2β1-integrinhigh, cytokeratin (CK5/14), CK18, CXC chemokine receptor 4 (CXCR4) and/or ATP-binding cassette (ABC) multidrug transporters. The highly tumorigenic PC stem/progenitor cells isolated from patient’s malignant tissues and PC cell lines were able to give rise to the total PC cell mass, including differentiated PC cells with a secretory luminal phenotype in vitro and in animal models in vivo that recapitulated the architectural phenotype of patient’s original tumors (8, 9, 12–14, 19–21, 23).
In addition, the PC progression to invasive and metastatic stages is typically characterized by a down-regulation of diverse tumor suppressor gene products combined with an up-regulation of the expression and/or activity of numerous oncogenic signaling elements in PC stem/progenitor cells and their progenies (1, 10, 11, 15, 24). In general, the interplay of a complex network of distinct oncogenic pathways initiated by hormones, growth factors, cytokines and chemokines through their cognate receptors is involved in sustained growth, survival, invasion and metastasis of PC cells as well as the development of an androgen-independent (AI) phenotype by tumor cells and treatment resistance (1, 10, 15).
Importantly, it has been reported that the persistent activation of EGFR and sonic hedgehog cascades frequently occurs in PC cells, including PC stem/progenitor cells, during PC initiation and progression to AI and metastatic stages (1, 10, 24–34). These tumorigenic cascades may cooperate in the acquisition of a more malignant behavior and resistance of PC cells to current clinical therapies, metastases at distant tissues and disease relapse (1, 10, 24–35). Consequently, the combination therapies targeting different oncogenic products, including EGFR and hedgehog pathways, in highly tumorigenic PC stem/progenitor cells and their differentiated progenies with a luminal phenotype, may represent more promising approaches than monotherapy to counteract disease progression and relapse (1, 10, 15, 16, 24, 32). In this regard, our recent works combined with several prior studies revealed that the blockade of the EGFR and hedgehog tumorigenic cascades resulted in a growth arrest and a massive rate of apoptotic death of metastatic PC cell lines (31–34). Importantly, we have shown that the co-targeting of EGFR and sonic hedgehog pathways by using gefitinib and cyclopamine with the chemotherapeutic drugs, docetaxel or mitoxantrone resulted in supra-additive anti-proliferative, anti-invasive and apoptotic effects on diverse metastatic parental PC cell lines as compared to individual agents and two-drug combinations (32, 33). Additional studies are required to ascertain the efficacy of these cytotoxic drugs to eradicate the PC-initiating cells and their differentiated progenies at earlier stages of prostate carcinogenesis, and thereby prevent the transition from localized PCs to invasive and metastatic HRPCs, disease recurrence and the death of patients.
The present investigation was undertaken to establish the therapeutic benefit of co-targeting EGFR and hedgehog cascades by using gefitinib and cyclopamine for eradicating the total PC cell mass, including PC-initiating cells and their progenies, and improving the current docetaxel-based chemotherapeutic treatments against locally advanced and invasive PCs. Therefore, the anti-proliferative and apoptotic effects of docetaxel, gefitinib and cyclopamine, alone or in combination, were estimated on SP cells and the non-SP cell fraction isolated from parental highly tumorigenic and invasive WPE1-NB26 cell line by the Hoechst dye exclusion method.
Materials and Methods
Materials
Human non-malignant immortalized RWPE-1 prostatic epithelial cell line and its PC cell line derivatives comprised of RWPE-2, WPE1-NA22, WPE1-NB14 and WPE1-NB26 as well as the metastatic and AI PC3 cell lines were originally purchased from American Type Culture Collection (Manassas, VA). All PC cells were maintained routinely in keratinocyte serum-free medium (SFM) supplemented with 1% L-glutamine, antibiotics (100 UI/ml penicillin-100 µg/ml streptomycin), bovine pituitary extract and EGF according to the instructions of the American Type Culture Collection in a 37°C incubator supplied with 5% CO2. Keratinocyte-SFM and all other culture materials were from Life Technologies (Carlsbad, CA). Cyclopamine was obtained from Toronto Research Chemicals, Inc. (Brisbane, Canada). Docetaxel, 3’,3’-dihexyloxacarbocyanine iodide (DiOC6(3)), (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and EGF were purchased from Sigma-Aldrich (St. Louis, MO), and broad caspase inhibitor, N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (Z-VAD-FMK) from Calbiochem Corp (San Diego, CA). The gefitinib was synthesized according to a modification of a described procedure (36). The mouse monoclonal anti-CK5 antibody (RCK103), rabbit polyclonal anti-CK18 antibody (H-80), rabbit polyclonal anti-EGFR antibody (1005), goat polyclonal anti-Tyr1173-phospho-EGFR antibody (1173) recognizing EGFR form phosphorylated at tyrosine 1173, rabbit polyclonal anti-SHH antibody (H-160) and goat polyclonal anti-GLI-1 antibody (H300) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The rabbit polyclonal anti-SMO antibody was provided by Abcam Inc. The phycoerythrin-conjugated monoclonal anti-CD133/2 antibody (293C3) was purchased from Miltenyi Biotec. Inc. and employed according to the manufacturer’s instructions. The Vectastain avidin-biotin complex method peroxidase kit and 3,3'-diaminobenzidine substrate kit were purchased from Vector Laboratories (Burlingame, CA). The amounts of proteins were estimated by using a detergent-compatible protein assay kit from Bio-Rad Laboratories, Inc. (Hercules, CA).
Immunohistochemical and Double-Immunohistofluorescence Analyses
Immunohistochemical studies on the localization of activated Tyr1173-pEGFR phosphorylated form and hedgehog signaling effector, GLI-1 transcription factor in non-malignant and malignant patient’s prostatic tissues were done as described previously using Vector avidin-biotin complex method kit as indicated in the manufacturer's instructions (33, 34). Briefly, the immunostaining was carried out on 32 pairs of AccuMax array tissue sections (Petagen, Inc., Shinchon-dong, Seoul, Korea) from patients with primary prostatic adenocarcinoma (Gleason scores 4–10) with their corresponding normal adjacent tissues from the same patients. A reddish brown color precipitate observed on tissue sections indicates a positive immunoreactivity with the tested primary antibody. For each tissue section, the intensity of immunoreactivity for each tested signaling element was semi quantitatively graded by a urological pathologist (S.L.J.) on a 0 to +3 scale (0 = no staining, 1+ = week staining, 2+ = moderately strong, and 3+ = strong staining). The staining intensity of Tyr1173-pEGFR or GLI-1 in prostatic adenocarcinoma samples was scored and compared with the corresponding non-neoplastic prostatic tissues, and the value was considered enhanced if the staining intensity was higher by one or more points.
In addition, the double-immunohistofluorescence analyses of the co-localization of stem cell-like marker, CD133 antigen (prominin-1) with unphosphorylated EGFR or its activated Tyr1173-pEGFR phosphorylated form and hedgehog signaling elements (SHH ligand, SMO co-receptor or GLI-1 transcription factor) were carried out on deparaffinized and rehydrated non-malignant and malignant human prostatic tissue specimens from the patients obtained from UNMC’s tissue bank. The tissue slides were blocked in the presence of 10% goat serum for 30 min followed by incubation with the phycoerythrin-conjugated anti-CD133 antibody plus anti-EGFR, anti-Tyr1173-pEGFR, anti-SHH, anti-SMO or anti-GLI-1 antibody for 2 h. The slides were washed twice with phosphate buffered saline (PBS) and processed for immunofluorescent detection as described below for the confocal microscopic analyses of fixed cells.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analyses
All PC cells were maintained in serum-free keratinocyte medium for 48 h. The expression levels of stem cell-like (CD133 and CD44), ABCG2 multidrug transporter, basal (CK5) and luminal (CK18 and AR) markers as well as EGF, EGFR, SHH, patched receptor 1 (PTCH-1), SMO and GLI-1 were estimated in total PC cell samples by RT-PCR. After incubation, the cells were collected by centrifugation, and the total cellular mRNA was extracted from cultured cell pellets using the RNeasy kit (Qiagen), according to the manufacturer's instructions. RT-PCR was performed with the Superscript™ II reverse transcriptase and Taq DNA Polymerase (Invitrogen) on 2 µg of total RNA. The reaction medium contained 2 µl of each primer. Equivalent amounts of primers (5 nmol/µl) were added to a 40.5 µl master mix of PCR reagents. After the denaturation of the aliquots at 95°C for 10 min, RT-PCR was performed as previously described (31,34). The samples were analyzed by electrophoresis on a 1.5% agarose gel staining with ethidium bromide. The primer sequences used to estimate the mRNA expression levels of human signaling products are presented in Table 1 (19, 29, 31, 32, 37–40).
TABLE 1.
Sequences of primers used for reverse transcriptase-polymerase chain reaction
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) | Ref. |
|---|---|---|---|
| CD133 | 5’-CACTTACGGCACTCTTCACCT-3’ | 5’-TGCACGATGCCACTTTCTCAC-3’ | (32) |
| CD44 | 5’-TCCATCAAAGGCATTGGGCAG-3’ | 5’-AACCTGCCGCTTTGCAGGTGT-3’ | (19) |
| ABCG2 | 5’-TGGCTGTCATGGCTTCAGTA- 3’ | 5’-GCCACGTGATTCTTCCACAA-3’ | (37) |
| CK5 | 5’-AGTCAACATCTCCGTCGTCAC-3’ | 5’-GGGACTGCCTAAAAGAAGCAG-3’ | (32) |
| CK18 | 5’-CAGCGCAGCCAGCGTCTATGC-3’ | 5’-CTTGCGGAGTCCATGGATGTCGC-3’ | (32) |
| AR | 5’-CTCTCTCAAGAGTTTGGATGGCT-3’ | 5’-CACTTGCACAGAGATGATCTCTGC -3’ | (38) |
| EGF | 5’-ACAGCCCTGAAGTGGATAGAG | 5’-GGGCTTCAGCATGCTGCCTTG-3’ | (39) |
| EGFR | 5’-ATGTCCGGGAACACAAAGAC-3’ | 5’-TTCCGTCATATGGCTTGGAT-3’ | (31) |
| SHH | 5’-GATGGCCACCACTCAGAGGAG-3’ | 5’-CGTCTCGATCACAGTAGAAGAC-3’ | (31) |
| PTCH-1 | 5’-TTCTCACAACCCTCGGAACCCA-3’ | 5’-CTGCAGCTCAATGACTTCCACCTTC-3’ | (40) |
| SMO | 5’-ATCTCCACAGGAGAGACTGGTTCGG-3’ | 5’-AAAGTGGGGCCTTGGGAACATG-3’ | (19) |
| GLI-1 | 5’-TACTCACGCCTCGAAACCT-3’ | 5’-GTCTGCTTTCCTCCCTGATG-3’ | (29) |
| β-actin | 5’-GTTGCTATCCAGGCTGTGC-3’ | 5’-GCATCCTGTCGGCAATGC-3’ | (31) |
Confocal Microscopy Analyses
All the cells were grown at a low density on sterilized cover slips for 24 h, washed with PBS, and fixed in ice-cold methanol at −20°C for 2 min (33, 34). The cells were blocked in 10% goat serum for 30 min and incubated with rabbit polyclonal anti-EGFR antibody (1105), goat polyclonal anti-Tyr1173-pEGFR antibody (1173), rabbit polyclonal anti-SHH antibody (H160), goat polyclonal anti-SMO antibody or goat polyclonal anti-GLI-1 antibody (N-16) diluted in PBS for 1 h at room temperature. After three washes with PBS, the cells were then incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse, FITC-conjugated donkey anti-goat and/or Texas red-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 1 h. Cells were washed again with PBS, nuclei counterstained with DAPI (diamidino-2-phenylindole) and mounted on glass slides in anti-fade Vestashield mounting medium (Vector Laboratories, Burlingame, CA). Immunofluorescence staining was observed under a confocal laser scanning microscope (LSM 410, Zeiss, Gottingen, Germany).
Isolation of the SP and non-SP Cell Fractions from the Human Tumorigenic and Invasive WPE1-NB26 Cell Line by Flow Cytometry and Colony-Forming Assays
The parental WPE1-NB26 cells (1 × 106 cells/ml) were stained with Hoechst buffer containing a final concentration of 2 µg/ml fluorescent Hoechst dye at 37°C for two hours in the absence or presence of a broad ABC transporter inhibitor, 50 µM verapamil and the small subpopulations of SP and non-SP cells were isolated by fluorescence-activated cell sorting (FACS) as previously described (22). The analyses and sorting of the viable SP and non-SP cell fractions were done using a FACS Aria flow cytometer with a DIVA software (Becton Dickinson Biosciences, San Jose, CA). The SP and non-SP cell fractions were collected after FAC sorting and the expression level of the CD133 marker without apparent further phenotypic and differentiation changes in these two cultured cell subpopulations was obtained by maintaining the cells in serum-free keratinocyte culture medium containing exogenous EGF (10 ng/ml) plus fibroblast growth factor (FGF) at 8 ng/ml before their use.
The monolayer clonogenic assays were then performed to estimate the self-renewal capacity of SP versus non-SP cell fractions isolated from the tumorigenic and invasive WPE1-NB26 cell line by FACS. For each assay, 500 viable SP or non-SP cells obtained after cell sorting were suspended in serum free-keratinocyte medium onto a 120 mm dish. All samples were plated in triplicate. After 14 days, the cultures were fixed and directly stained with a crystal violet solution and colonies were counted.
Cell Culture and Growth Assays
The SP and non-SP cell fractions isolated from the total WPE1-NB26 cell mass were maintained in serum-free keratinocyte culture medium. For growth assays, the cells were seeded on 96-well plates at a density of 3 × 104 cells/well in a total volume of 200 µl culture medium as previously mentioned (31, 33, 34, 41). After three days, the cell growth assays were performed in the serum-free medium. Different concentrations of 2 nM docetaxel, 0.5 µM gefitinib and 1 µM cyclopamine, alone or in combination, were also added to the culture medium. After incubation for 48 h, the rate of cell growth was estimated by a MTT colorimetric test (42).
Flow Cytofluorometric Analyses
The SP and non-SP cells were grown at a density of 5 × 105 cells on 25 cm2 dishes as described previously (31, 33, 34, 41). The cells were treated with different concentrations of docetaxel, gefitinib and cyclopamine, alone or in combination, in the absence or presence of broad caspase inhibitor, Z-VAD-FMK. In all experiments, the cells were kept at a sub-confluent level to avoid contact inhibition. More specifically, in order to determine the influence of drugs on the cellular cycle progression of SP and non-SP cell fractions, the cytometric analyses by FACS were performed 48 h after the addition of different concentrations of tested drugs, alone or in combination. Moreover, the apoptotic effect induced by the tested drugs, alone or in combination, on the SP and non-SP cell fractions were estimated by FACS analyses after four days of drug treatment initiation. The DNA content estimation of each sample was performed after staining with the propidium iodide by FACS™ analyses essentially as previously described (31, 33, 34, 41).
Estimation of Mitochondrial Membrane Potential (MMP) and Cytosolic Cytochrome c Release
To determine whether the apoptotic effect induced by docetaxel, gefitinib and/or cyclopamine in the SP and non-SP fractions is mediated via a mitochondrial pathway, the MMP and the amount of cytosolic cytochrome c were estimated as previously described (31, 33, 34, 41). Briefly, the SP and non-SP cells were untreated (control) or treated with 2 nM docetaxel, 1 µM gefitinib, and 2 µM cyclopamine, either alone or in combination, for four days. The adherent and floating cells were collected and washed in PBS. The pellets corresponding to approximately 1 × 106 PC cells were resuspended in 1 ml PBS containing the cationic, lipophilic and fluorescent dye, 40 nM DiOC6(3), which specifically accumulates within the mitochondrial compartment in a MMP-dependent manner (43). After incubation at 37°C for 20 min, the accumulation of DiOC6(3) within the mitochondria of SP and non-SP cell fractions was measured by FACS analyses. Moreover, after 4 days of cell growth on 25 cm2 dishes in the absence or presence of different tested agents, which was performed under the same conditions as described above, the floating and adherent cells were collected by centrifugation, rinsed twice with PBS and centrifuged. Then, the amounts of cytochrome c present in the cytosolic extracts of each sample were estimated following the method described in the ELISA kit from Zymed Laboratories with a human anti-cytochrome c antibody.
Statistical Analyses
Statistical analyses were performed using the Student's t-test to compare the results with P values < 0.05 indicating statistically significant differences.
Results
Immunohistochemical Analyses of Tyr1173-pEGFR and GLI-1 Expression Levels in Non-malignant and Malignant Prostatic Tissues
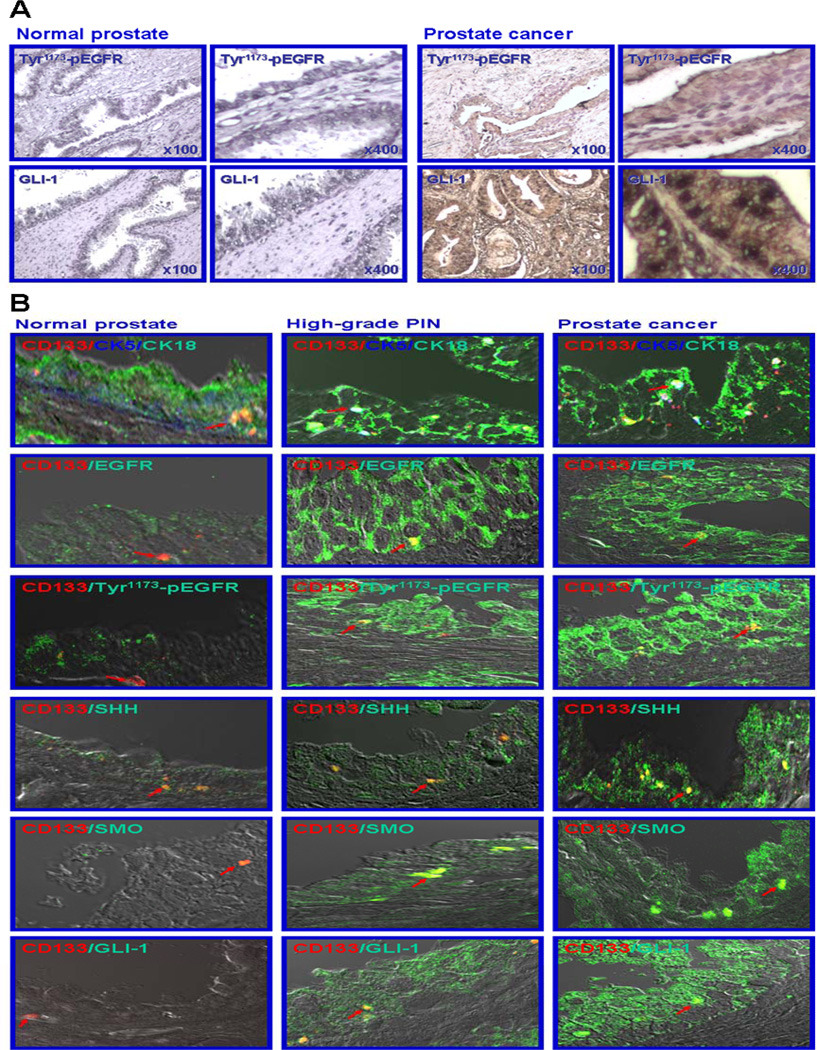
To establish the potential implication of the activation of EGFR and hedgehog cascades in PC cells during prostate carcinogenesis, the expression levels of the activated Tyr1173-pEGFR phosphorylated form of EGFR and hedgehog signaling effector, GLI-1 transcription factor were examined by immunohistochemical stains on non-malignant and malignant human prostatic tissue sections (Fig. 1A). The results from immunohistochemical analyses indicated a very weak cytoplasmic and membrane immunostaining for activated Tyr1173-pEGFR phosphorylated form of EGFR in certain prostatic epithelial cells in non-malignant prostatic tissues (Fig. 1A). In contrast, Tyr1173-pEGFR expression levels varied from weak to strong within the cytoplasm and at the membrane respectively, in the malignant epithelial cells localized in the intermediate and luminal compartments in a subset of primary prostatic adenocarcinomas as shown in Fig. 1A. The staining intensity associated with the Tyr1173-pEGFR protein expression was enhanced in 34% of tested primary prostatic adenocarcinomas (32 samples, 11 positive and 21 negative cases, Gleason scores= 4–10), as compared with the corresponding non-neoplastic tissues. In addition, a positive immunoreactivity for hedgehog signaling effector, GLI-1 transcription factor was also observed in the cytoplasm and nuclei of PC cells detected in primary prostatic adenocarcinoma tissues, while this protein was not expressed at significant levels in the cytoplasm and nuclei of epithelial cells detected in non-malignant prostatic tissues (Fig. 1A). The data from immunohistochemical analyses revealed that the expression of the hedgehog effector, GLI-1 transcription factor was enhanced in 38% of the primary prostatic adenocarcinomas analyzed (32 samples, 12 positive and 20 negative cases, Gleason scores= 4–10), relative to the corresponding non-malignant prostatic tissues from the same patients.
Figure 1.
Immunohistochemical and immunofluorescence analyses of expression levels of EGFR and hedgehog signaling elements in non-malignant and malignant prostatic tissues. (A) Immunohistochemical analyses of expression levels of activated Tyr1173-pEGFR phosphorylated form and sonic hedgehog effector, GLI-1 transcription factor in non-malignant and malignant prostatic tissues. AccuMax array sections of non-malignant prostatic tissues and adenocarcinomas from the same PC patients were probed with antibody directed against the activated Tyr1173-pEGFR phosphorylated form or GLI-1 transcription factor after blocking with serum as described in Materials and Methods. All tissue sections were examined under a microscope, and the Tyr1173-pEGFR or GLI-1 immunoreactivity was judged by dark brown staining. Representative pictures of stained tissue samples of normal prostate and adenocarcinoma are shown at original magnifications of ×100 and ×400. (B) Immunofluorescence analyses of the co-localization of the expression of the CD133 stem cell-like marker with CK5/18, EGFR and its activated Tyr1173-pEGFR phosphorylated form, and SHH hedgehog ligand, SMO co-receptor or GLI-1 transcriptional effector in non-malignant and malignant prostatic tissue specimens from patients. Double-immunofluorescence staining was simultaneously performed with the phycoerythrin-labeled anti-CD133 antibody (red color) plus Alexa 340-labeled anti-CK5 antibody (blue color) or fluorescein-labeled anti-CK18, EGFR, Tyr1173-pEGFR, -SHH, -SMO or -GLI-1 antibody (green color) after blocking with goat serum as described in Materials and Methods. Double staining (yellow/purple color) detected by confocal analyses, which is indicative of the co-localization of these markers, are shown with an arrow. Representative pictures are shown at original magnification ×630.
Immunohistofluorescence Confocal Microscopy Analyses of the Expression Level of CD133 Stem Cell-Like Marker and its Co-localization with EGFR and Hedgehog Signaling Elements in Non-malignant and Malignant Prostatic Tissues
To obtain further experimental evidence of the implication of EGFR and sonic hedgehog cascades in the malignant transformation of CD133+ adult prostatic stem/progenitor cells into CD133+ PC stem/progenitor cells, we have characterized the co-localization of the CD133 stem cell-like marker with the signaling effectors of these tumorigenic cascades in non-malignant and malignant prostatic tissues. The immunoconfocal co-analyses of the expression of the CD133 cell surface antigen with the basal CK5 and luminal CK18 markers, revealed that this stem cell-like marker is detectable only in a very rare subpopulation of CK5/18-expressing prostatic epithelial cells in the basal compartment in non-malignant prostatic tissue specimens (Fig. 1B). In contrast, the CD133 protein was principally detected in a small subset of intermediate PC cells (CK5/18) dispersed through the intermediate compartment in high-grade prostatic intraepithelial neoplasias (PINs) and prostatic adenocarcinoma tissues from patients (Fig. 1B).
As shown in Fig. 1B, the results from immunohistofluorescence analyses have also revealed that the expression levels of EGFR and hedgehog signaling elements were significantly enhanced in a small subset of CD133+ PC cells and the bulk tumor mass of CD133− PC cells in high-grade PINs and PCs relative to non-malignant prostatic tissue specimens from patients. Particularly, a positive immunoreactivity was observed for EGFR and its Tyr1173-pEGFR activated form as well as the SHH ligand and the SMO co-receptor in the cytoplasm and at the cell surface in intermediate and luminal CD133− tumor cells detected in high-grade PINs and prostatic adenocarcinoma specimens (Fig. 1B). Furthermore, a positive cytoplasmic and nuclear staining was detected for GLI-1, which acts as a transcriptional signaling effector of the activated hedgehog pathway, in intermediate and luminal CD133− tumor cells (Fig. 1B). Importantly, a double-positive immunostaining was also seen for the CD133 stem cell-like marker with EGFR, Tyr1173-pEGFR, SHH, SMO or GLI-1 signaling element in a similar small subset of PC cells dispersed through the intermediate compartment in malignant prostatic tissues (Fig. 1B).
RT-PCR and Confocal Microscopy Analyses of the Expression Levels of Stem Cell-Like Markers and EGFR and Sonic Hedgehog Signaling Elements in Different Non-malignant and Malignant Prostatic Cell Lines and the SP and non-SP cell Fractions Isolated from the Parental WPE1-NB26 Cell Line
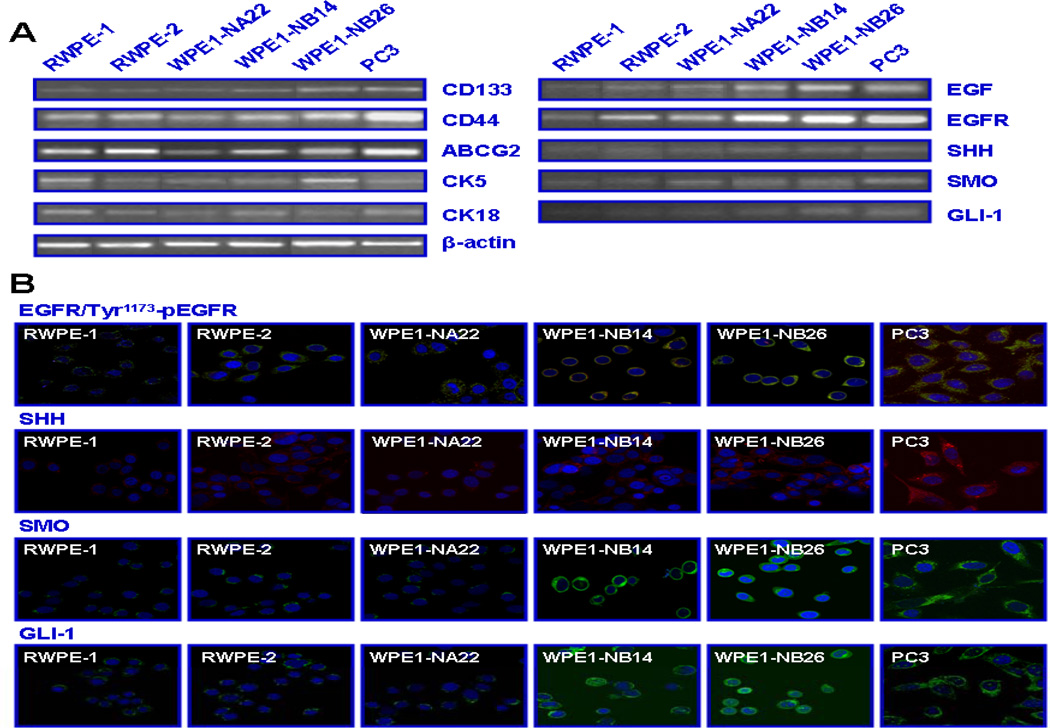
In order to further assess whether an enhanced expression and activation of EGFR and sonic hedgehog cascades occur in PC cells during disease progression, a characterization of the expression levels of distinct signaling components of these tumorigenic pathways was performed on well-established PC cell lines miming the multiple stages of prostate carcinogenesis and metastases at bone marrow. The data from RT-PCR and immunofluorescence analyses have indicated that the expression levels of EGF/EGFR and hedgehog signaling elements (SHH/SMO/GLI-1) were enhanced in tumorigenic and invasive WPE1-NB14 and WPE1-NB26 cell lines and metastatic PC3 cells as compared to weakly tumorigenic WPE1-NA22 and RWPE-2 and non-malignant RWPE-1 prostatic cell lines (Fig. 2). Moreover, all of the tested prostatic cell lines expressed significant levels of stem cell-like markers, including CD133, CD44 and ABCG2 transporter as well as CK5 (basal) and CK18 (luminal) markers, suggesting that these cell lines possess an intermediate phenotype (CK5/18) (Fig. 2).
Figure 2.
RT-PCR and immunoconfocal analyses of expression levels of stem cell-like markers, basal/luminal markers, EGFR and sonic hedgehog signaling elements in non-malignant and malignant prostatic cell lines. The cells were maintained for 48 hours in culture medium. Then, mRNA expression levels of stem cell-like (CD133, CD44 and ABCG2), basal (CK5) and luminal (CK18) markers as well as EGF/EGFR and sonic hedgehog signaling components were estimated by RT-PCR in human non-malignant RWPE-1, tumorigenic K-ras-transformed RWPE-2, MNU-transformed tumorigenic WPE1-NA22 and invasive WPE1-NB14 and WPE1-NB26, and metastatic PC3 prostatic cell lines. Immunofluorescence staining of methanol-fixed cells was done with anti-EGFR plus Tyr1173p-EGFR, -SHH, -SMO or -GLI-1 primary antibody plus fluorescein (green color) and/or Texas red secondary antibody and DAPI (nuclear blue color) after blocking with goat serum. Representative pictures obtained for the overlaps of EGFR/Tyr1173-pEGFR (hybrid yellow), SHH (red), SMO (green) and GLI-1 (green) are shown at original magnification ×630.
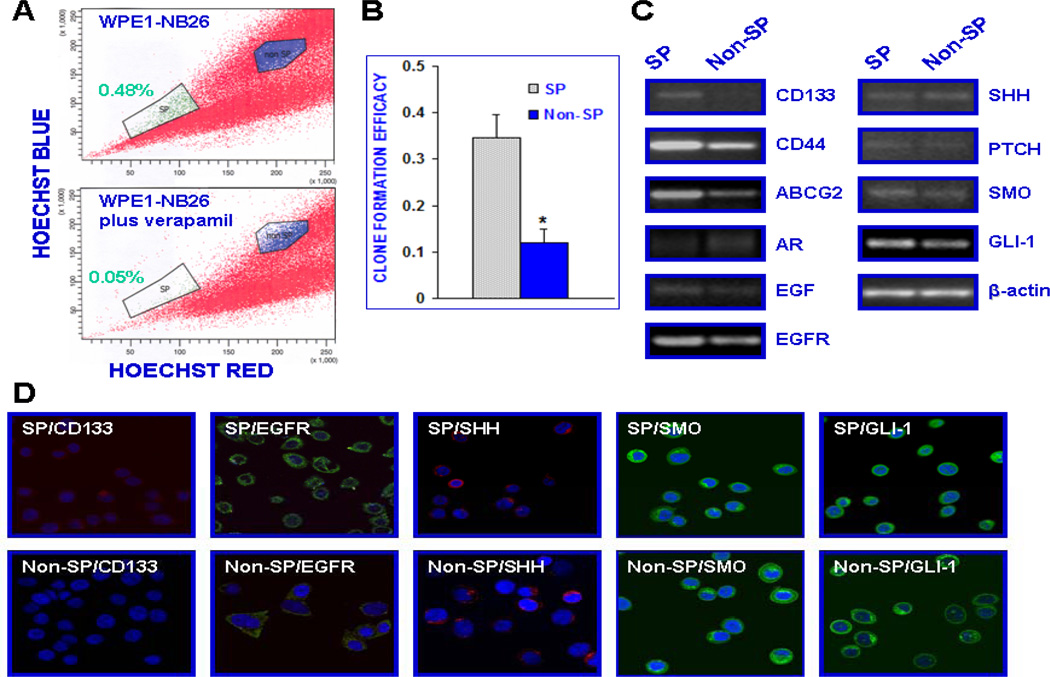
In addition, the results from RT-PCR and immunoconfocal microscopy have also revealed that the small SP cell fraction isolated from WPE1-NB26 cells by FACSorting was characterized by higher expression levels of different stem cell-like markers, including CD133, CD44 and ABCG2, but lower to undetectable level of AR relative to the non-SP cell subpopulation (Fig. 3C and D). Importantly, the SP and non-SP cell fractions isolated from the WPE1-NB26 cell line also expressed significant levels of EGF/EGFR and hedgehog signaling elements (Fig. 3C and D). Moreover, the results from the clonogenicity assays have also revealed that the SP cell subpopulation isolated from the WPE1-NB26 cell line displayed a higher self-renewal ability, which was retain upon serial passage, than the non-SP cell fraction in serum free-keratinocyte medium (Fig. 3B).
Figure 3.
Characterization of phenotypic features and the self-renewal ability of the SP and non-SP cell fractions isolated from parental tumorigenic and invasive WPE1-NB26 cells by Hoechst dye efflux technique and FAC sorting. (A) The WPE1-NB26 cells were stained with fluorescent Hoechst dye in the absence or presence of 50 µM verapamil and FACS analyses were performed. The Hoechst dye efflux profile shows the SP (green color), and non-SP fraction (blue color). The number of total PC cells localized in the SP fraction was significantly reduced in the presence of broad ABC transporter inhibitor, verapamil. (B) Clone formation efficacy of the SP and non-SP fractions from WPE1-NB26 corresponds to the ratio of the clone number to the plated cell numbers. (C and D) Comparative RT-PCR and immunoconfocal analyses of expression levels of prostatic stem cell-like markers (CD133, CD44 and ABCG2), AR, EGF/EGFR and sonic hedgehog signaling elements in the SP and non-SP cell fractions isolated from parental WPE1-NB26 cell line.
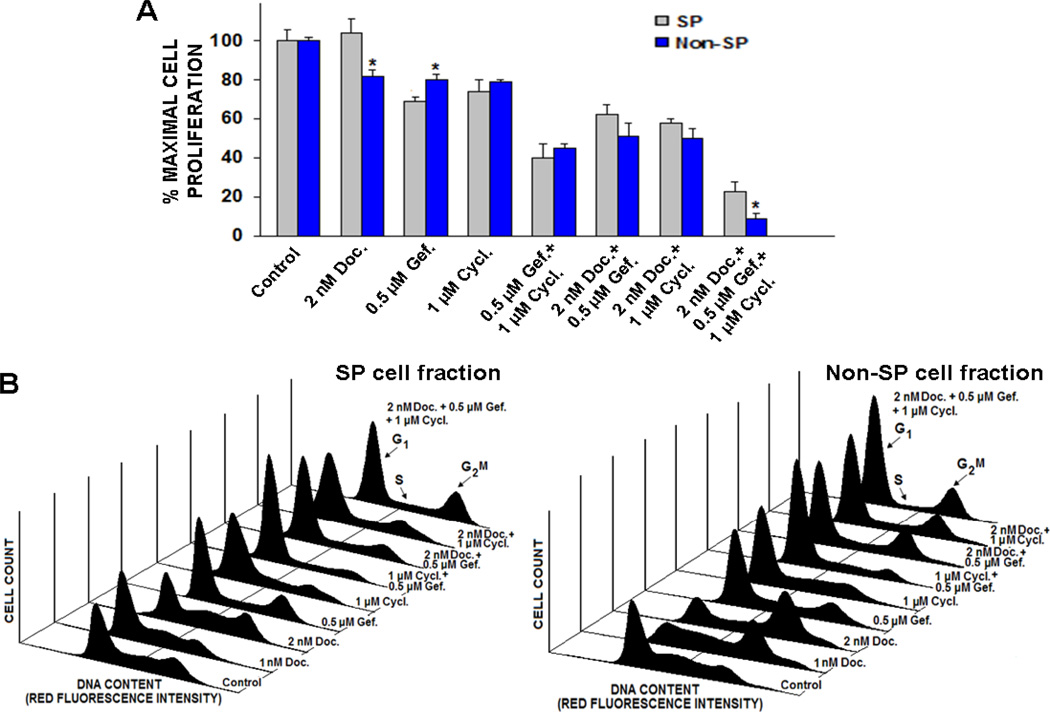
Anti-proliferative Effects Induced by Docetaxel, Gefitinib and Cyclopamine on the SP and Non-SP Cell Fractions Isolated from the Parental Tumorigenic and Invasive WPE1-NB26 Cell Line
To establish whether the anti-proliferative effect induced by docetaxel on the PC cell proliferation may be improved by combined use of gefitinib and cyclopamine, the growth-inhibitory effects either of a single agent, two-agent or triple drug combinations, were evaluated on SP and non-SP cell fractions from parental tumorigenic and invasive WPE1-NB26 cells (Fig. 4). The low concentrations of tested drugs, which induced about 15–25% inhibition, were used in experiments in the present combination study with other drugs. As shown in Fig. 4A, the results from MTT assays revealed that 2 nM docetaxel induced a significant anti-proliferative effect on non-SP cells while the SP cell fraction was insensitive to a treatment with 2 nM docetaxel. Interestingly, 1 µM gefitinib or 2 µM cyclopamine was however effective to induce the anti-proliferative effect on both SP and non-SP cell fractions from WPE1-NB26 cells (Fig. 4A). Moreover, a bi-combination of 2 nM docetaxel plus 0.5 µM gefitinib or 1 µM cyclopamine induced a greater growth inhibitory effect on SP and non-SP cell fractions relative to individual drugs. Of therapeutic interest, the SP and non-SP cell growth was more markedly inhibited by a triple drug combination of docetaxel, gefitinib and cyclopamine as compared to two-drug combinations (Fig. 4A). As shown in Fig. 4B, the results from FACS analyses have also indicated that the combined use of 2 nM docetaxel, 0.5 µM gefitinib plus 1 µM cyclopamine, caused a marked reduction of the growth of the SP and non-SP cell fractions isolated from the WPE1-NB26 cell line via a blockade in the G1 and G2M phases of the cellular cycle.
Figure 4.
Anti-proliferative effects induced by docetaxel, gefitinib, and cyclopamine on SP and non-SP cell fractions isolated from parental tumorigenic and invasive WPE1-NB26 cells. The SP and non-SP cells were treated with the indicated concentrations of docetaxel (Doc.), gefitinib (Gef.), and cyclopamine (Cycl.), alone or in combination, for two days. (A) The effects of tested agents are expressed as the percentage of inhibition of SP and non-SP cell proliferation compared with the growth of untreated cells (control). The data are the means of three to four different experiments done in triplicate. *P < 0.05, indicates a significant difference of the anti-proliferative effects induced by docetaxel, gefitinib, and cyclopamine, alone or in combination, on the SP and non-SP cell fractions. (B) FACS analyses of the growth inhibitory effect induced by mixed docetaxel, gefitinib, and cyclopamine on SP and non-SP cell fractions. The inhibitory effect induced by drugs on the progression of PC cells in the cellular cycle was investigated by flow cytometric analyses. The SP and non-SP cell cells were untreated or treated with the indicated concentrations of 2 nM docetaxel, 0.5 µM gefitinib, and 1 µM cyclopamine, alone or in combination, for two days. At the end of incubation time, the cells were prepared as described in Materials and Methods and the cell cycle distributions were assessed by FACS analyses. Representative results obtained from three separate experiments are shown.
Apoptotic Effect Induced by Docetaxel, Gefitinib and Cyclopamine on the SP and non-SP Cell Fractions Isolated from the Parental Tumorigenic and Invasive WPE1-NB26 Cell Line
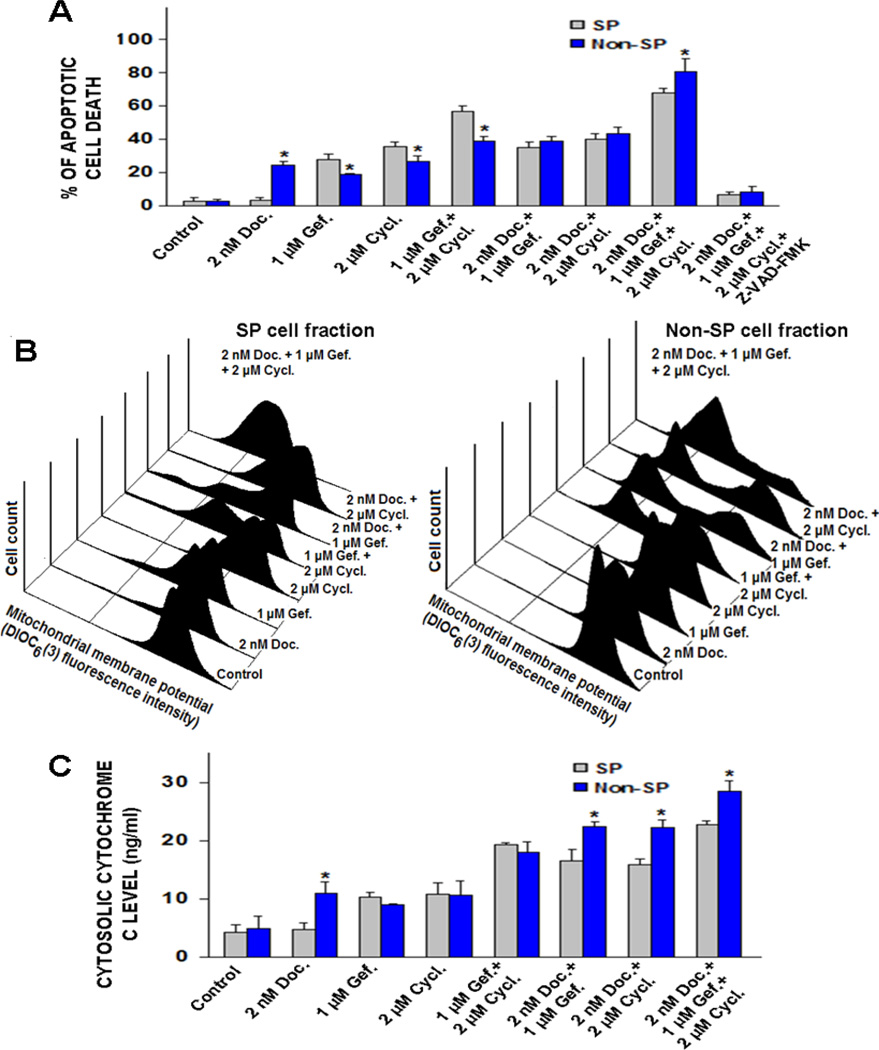
To determine the benefit of combining gefitinib and cyclopamine to improve the efficacy of current chemotherapeutic drug, docetaxel, the percentages of apoptotic cell death induced by docetaxel, either alone or in drug combinations, were estimated by the flow cytometric analyses and the apoptotic cell number in the sub-G1 phase was quantified. The lowest effective concentrations for each tested drug which can trigger apoptotic death in PC cells were used. As shown in Fig. 5A, the results of FACS analyses revealed that SP cell fraction was insensitive to a treatment with 2 nM docetaxel. In contrast, 2 nM docetaxel alone caused a significant increase in apoptotic population of non-SP cell fraction as compared to untreated non-SP cells (control) after four days of treatment. Moreover, the bi-combination of 2 nM docetaxel plus 1 µM gefitinib or 2 µM cyclopamine resulted in a higher rate of apoptotic death of SP and non-SP cells as compared to individual drugs (Fig. 5A). Importantly, the triple combination of docetaxel, gefitinib and cyclopamine was also more effective than two-drug combinations and caused the death of the majority of SP and non-SP cells (Fig. 5A).
Figure 5.
Assessment of the stimulatory effect induced by docetaxel, gefitinib, and cyclopamine on the apoptotic death, mitochondrial membrane depolarizing and cytosolic cytochrome c releasing in the SP and non-SP cell fractions isolated from parental tumorigenic and invasive WPE1-NB26 cells. The PC cells were untreated (control) or treated with the indicated concentrations of docetaxel (Doc.), gefitinib (Gef.), and cyclopamine (Cycl.), alone or in combination, for four days. (A) The cell nuclei were stained with propidium iodide and the number of apoptotic PC cells detected in the sub-G1 phase was analyzed by flow cytometry. Plots showing the percentages of apoptotic PC cell death induced by tested drugs, alone or in combination, obtained from three separate experiments. *p < 0.05, indicates a significant difference between the apoptotic effects induced by docetaxel, gefitinib, and cyclopamine on the SP and non-SP cell fractions. After the treatments, the cells were prepared by staining with 40 nM DIOC6(3) for analyses of MMP by flow cytometry. Moreover, the amounts of cytochrome c released into cytosol were estimated by ELISA as described in Materials and Methods. (B) Representative profiles of effects induced by tested drugs, alone or in combination, on MMP in SP and non-SP cell fractions isolated from WPE1-NB26 cells are shown. (C) Plots showing the percentages of the stimulatory effects induced by tested drugs, alone or in combination on cytochrome c release in SP and non-SP cell fractions. *p < 0.05, indicates a significant difference between the stimulatory effect induced by docetaxel, gefitinib and cyclopamine, alone or in combination, on cytochrome c release in the SP and non-SP cell fractions.
Estimation of the Role of the Caspase Pathway in the Apoptotic Effect Induced by Docetaxel, Gefitinib and Cyclopamine on the SP and non-SP cell Fractions Isolated from the WPE1-NB26 Cell Line
To assess whether the cytotoxic effects induced by the tested drugs on the SP and non-SP cell fractions from the WPE1-NB26 cell line is mediated through a mitochondrial pathway-dependent caspase activation, an estimation of the effects of drug treatment on MMP and cytosolic cytochrome c was performed by FAC analyses and ELISA assays. As shown in Fig. 5B, the continuous treatment of the non-SP cells for four days with 2 nM docetaxel induced a small decrease of MMP while SP cell fraction was non responsive to docetaxel treatment. Furthermore, 1 µM gefitinib, or 2 µM cyclopamine alone caused only a slight decrease of MMP, as indicated by the weak shoulder of peak as compared to the stained cells that were untreated (control). The treatment of the SP and non-SP cell fractions with 2 nM docetaxel plus 1 µM gefitinib or 2 µM cyclopamine, however, was accompanied with a marked decrease of MMP. The triple drug combination of 2 nM docetaxel plus 1 µM gefitinib and 2 µM cyclopamine also induced a higher mitochondrial membrane depolarizing effect and cytochrome c amount released in the cytosol in the SP and non-SP cells as compared to the treatment of cells with the two-drug combinations (Fig. 5B and C). Consistent with the drug-induced caspase activation, we have also observed that the broad spectrum caspase inhibitor, Z-VAD-FMK at 50 µM abrogated the rate of apoptotic death induced by these cytotoxic agents on the SP and non-SP cell fractions (Fig. 5A).
Discussion
Recent accumulating lines of experimental evidence have revealed that the highly tumorigenic CD133+/CD44+/high/AR−/low PC stem/progenitor cells and/or their early progenies endowed with the stem cell-like properties can provide critical role for PC initiation and progression (1, 8–17, 21, 23, 44). Moreover, the PC transition to invasive and metastatic disease stages is typically associated with the deregulation of diverse signaling cascades, such as hedgehog and EGFR pathways, in PC stem/progenitor cells and their progenies that may confer them with more malignant phenotypes and survival advantages contributing to treatment resistance, metastases and disease relapse (1, 10, 11, 15, 44, 45). In this regard, the data from the present investigation indicated that EGFR and its activated Tyr1173-pEGFR phosphorylated form as well as hedgehog signaling transduction elements (SHH/SMO/GLI-1) were undetectable or expressed at low levels in CD133+ cells in basal compartment in non-malignant prostatic tissue specimens. In contrast, higher expression levels of these signaling components were detected in a small subset of CD133+ PC cells and the bulk tumor mass of CD133− PC cells with a luminal phenotype in certain cases of PC patients as compared to non-malignant prostatic tissues (Fig. 1). These data support numerous previous studies indicating a small subpopulation of prostatic stem/progenitor cells within basal cell compartment of prostate gland and expressing stem-cell like markers including CD133 may contribute to regenerate all of the bulk mass of differentiated CD133− epithelial cells (10, 17, 46–48). Moreover, although a typical prostate structural change associated with primary PC development is a complete loss of the basal epithelial layer, there is evidence for a maintenance of PC-initiating cells expressing the stem cell-like markers including CD133 protein and gene products normally restricted to basal cells found through the bulk tumor mass (8–10, 12, 14–17). More specifically, the results from previous immunohistochemical staining investigations indicated that the stem cell-like marker, CD133 protein was detected only in a very small subpopulation of basal epithelial cells in normal prostatic tissues (12, 48). In contrast, a significant expression level of CD133 stem cell-like marker was seen in a small subset of AR− PC cells dispersed through the total tumor cell mass in clinical PIN and PC tissue specimens (12). Similarly, the CD44 stem cell-like surface marker has also been detected in basal cells in normal prostatic tissues and only in a minority of PC cells localized through the bulk tumor mass in certain cases of low- and high-grade patient’s prostatic adenocarcinoma tissues (27). Moreover, a co-localization of the CD44 protein with hedgehog signaling element, PTCH-1 or GLI-1, has been observed in a very small subset of normal and hyperplastic basal cells in non-malignant prostatic tissues as well as in certain PC cells in tumor tissue specimens from patients (27).
In addition, a progressive increase of the expression levels of EGF/EGFR and hedgehog signaling components were also detected in WPE1-NA22, WPE1-NB14 and WPE1-NB26 established cell lines possessing enhanced tumorigenic and invasive properties, respectively, relative to the non-malignant RWPE-1 prostatic epithelial cell line (Fig. 2). Importantly, we have also observed that the CD133+/CD44high/AR−/low SP and CD133−/CD44low/AR+/high non-SP cell fractions isolated from the WPE1-NB26 cell line expressed significant levels of EGF/EGFR and hedgehog signaling elements and ABCG2 multidrug transporter (Fig. 3). These data are in agreement with numerous prior studies have revealed that higher expression levels of EGFR, SHH, PTCH-1, GLI-1 and/or GLI-2 signaling components were detected in high-grade PIN and localized advanced PC, AI and metastatic tumor specimens as well as PC cell lines relative to non-malignant prostatic tissues and prostatic epithelial cell lines (10, 24–26, 28, 29, 31–35, 49–56). Especially, an increase in the number of PC cells expressing EGFR and basal and luminal cell-specific markers CK5/18, respectively, has been observed in a subset of patients with HRPCs (26, 55). This observation suggests that the proportion of EGFR- and CK5/18 positive PC cells with an intermediate phenotype, may rise during androgen deprivation, supporting the benefit of targeting EGFR for eliminating this small neoplastic cell subpopulation. Moreover, higher expression levels of SMO hedgehog co-receptor and β-catenin were also detected in CD44+ DU145 progenitor cells displaying a higher tumorigenic potential as compared to the corresponding CD44−/low DU145 cell subpopulation (19). It is worth mentioning here that a small subpopulation of CD133+/CD44+/α2β1-integrinhigh DU145 stem/progenitor cells purified from the CD44+ DU145 total population has also been shown to possess a higher tumorigenic potential in vivo than the CD133−/CD44+/α2β1-integrinhigh cell fraction (21). On the other hand, it has also been reported that the putative prostatic stem/progenitor cells and their malignant counterpart, PC-initiating cells in non-malignant and malignant primary prostatic tissues from patients may be detected within the SP cells endowed with stem cell-like properties (13, 18, 57). Collectively, these observations suggest that an enhanced expression and sustained activation of EGFR and sonic hedgehog oncogenic signaling elements in CD133+/CD44+/high/AR−/low PC stem/progenitor cells and their differentiated CD133−/CD44−/low/AR+ PC progenies may contribute to PC initiation and disease progression to locally invasive, AI, metastatic and recurrent disease stages.
Although a high curative rate is associated with the treatment of patients diagnosed with PCs confined to prostate gland by prostatectomy, anti-androgen deprivation therapy and/or radiation therapy, the locally advanced, invasive and metastatic PCs remain incurable with the current therapeutic regimens (1, 4, 6, 7). Moreover, the first-line systemic docetaxel-based chemotherapies used as care for patients with high-risk or metastatic HRPCs are only palliative and typically culminate in the development of diverse mechanisms of resistance by PC cells, disease relapse and the death of PC patients after a short time period of about 15–19 months after treatment initiation (1, 4, 5, 7). Of particular interest, the results from the present study have revealed that the co-targeting EGFR and hedgehog cascades could constitute a potential therapeutic strategy for reversing docetaxel resistance and improving its efficacy to eradicating the total tumor cell mass in organ-confined PCs, and thereby prevent PC progression to metastatic, recurrent and lethal disease stages. Indeed, despite the SP cells isolated from WPE1-NB26 cell line was insensitive to 2 nM docetaxel treatment as compared to the non-SP cell fraction, a combination of 2 nM docetaxel with selective inhibitor of EGFR or SMO hedgehog co-receptor, orally active gefitinib and cyclopamine, resulted in significant anti-proliferative and apoptotic effects on this immature PC cell subset. Moreover, the combined use of low concentrations of gefitinib and cyclopamine cooperatively improved the anti-proliferative and apoptotic effects induced by docetaxel on the SP and non-SP cell fractions as compared to two drug combinations (Figs. 4 and 5). More specifically, the anti-proliferative effect induced by the combined drugs, docetaxel plus gefitinib and cyclopamine was mediated through a blockade of SP and non-SP cells in the G1 and G2M phases of the cell cycle (Fig. 4). Moreover, the results have also revealed that this triple drug combination mediated a massive rate of apoptotic cell death, at least in part, though a depolarization of the mitochondrial membrane, cytochrome c release into cytosol and activation of caspase pathway (Fig. 5). Hence, together these data revealed a beneficial effect of combining EGFR and hedgehog signaling inhibitors, gefitinib and cyclopamine, with current clinical chemotherapeutic drug, docetaxel, to improve the anti-proliferative and apoptotic responses induced by low drug concentrations on the total PC cell mass.
These data support several studies conducted by us and other research groups that indicated that the treatment of metastatic PC cells with docetaxel and/or specific inhibitors of EGFR and SMO hedgehog signaling cascades was associated with an inhibition of growth, invasion and metastatic spread of PC cells in vitro and animal model in vivo (1, 10, 28, 29, 31, 33, 34, 49, 50, 58–62). More specifically, gefitinib has been observed to sensitize the PC3 cells to ionizing radiations and diverse chemotherapeutic agents such as docetaxel, paclitaxel and platinum compounds, cisplatin and carboplatinum (1, 33, 34, 63). The continuous cyclopamine treatment of PC3 cell-derived tumor xenografts in nude mice in vivo also led to the apoptotic death of PC3 cells and tumoral endothelial cells whose molecular events were accompanied by a tumor growth regression and without major toxicity on normal cells (29). Additionally, our prior studies have also revealed that the simultaneous blockade of EGFR and hedgehog cascades significantly improved the cytotoxic effects induced by docetaxel or mitoxantrone on metastatic PC cells including enriched CD44+/high PC3 and DU145 cell fractions (32;33).
Taken together, the results from the present investigation revealed that an enhanced expression and activation of EGFR and sonic hedgehog signaling elements may occur in CD133+ PC cells and their differentiated CD133− progenies during disease progression to locally invasive PCs. Moreover, the co-targeting of EGFR and hedgehog cascades was effective for improving the efficacy of current chemotherapeutic drug, docetaxel to eradicate the total PC cell mass, including SP cells with stem cell-like properties and the non-SP cell fraction from tumorigenic and invasive WPE1-NB26 cells. Further in vivo studies should allow us to corroborate these in vitro data supporting the benefit of co-targeting EGFR and hedgehog pathways for improving the efficacy of current clinical docetaxel-based chemotherapeutic regimens against locally invasive PCs and/or metastatic HRPCs and prevent disease recurrence.
Supplementary Material
Acknowledgments
We thank Ms. Kristi L. Berger for editing the manuscript. We thank the Molecular Biology Core Facility at the University of Nebraska Medical Center for FACS analyses.
Grant support: U.S. Department of Defense grants PC04502 and PC074289.
Abbreviations
- ABC
ATP-binding cassette
- AI
androgen-independent
- AR
androgen receptor
- CK
cytokeratin
- CXCR4
CXC chemokine receptor 4
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- FACS
fluorescence-activated cell sorting
- HRPCs
hormone-refractory prostate cancers
- MNU
N-methyl-N-nitrosourea
- PBS
phosphate buffered saline
- PC
prostate cancer
- PTCH
patched receptor
- RT-PCR
reverse transcriptase-polymerase chain reaction
- SHH
sonic hedgehog ligand
- SMO
smoothened hedgehog co-receptor and SP, side population
References
- 1.Mimeault M, Batra SK. Recent advances on multiple tumorigenic cascades involved in prostatic cancer progression and targeting therapies. Carcinogenesis. 2006;27:1–22. doi: 10.1093/carcin/bgi229. [DOI] [PubMed] [Google Scholar]
- 2.Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109:2239–2247. doi: 10.1002/cncr.22676. [DOI] [PubMed] [Google Scholar]
- 3.Gignac GA, Morris MJ, Hussain M. Castration resistant, taxane naive metastatic prostate cancer: Current clinical approaches and future directions. J Urol. 2007;178:S30–S35. doi: 10.1016/j.juro.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 7.Grubb RL, III, Kibel AS. Prostate cancer: screening, diagnosis and management in 2007. Mo Med. 2007;104:408–413. [PubMed] [Google Scholar]
- 8.Maitland NJ, Bryce SD, Stower MJ, Collins AT. Prostate cancer stem cells: a target for new therapies. Ernst Schering Found Symp Proc. 2006;5:155–179. doi: 10.1007/2789_2007_050. [DOI] [PubMed] [Google Scholar]
- 9.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 10.Mimeault M, Mehta PP, Hauke R, Batra SK. Functions of normal and malignant prostatic stem/progenitor cells in tissue regeneration and cancer progression and novel targeting therapies. Endocr Rev. 2008;29:234–252. doi: 10.1210/er.2007-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mimeault M, Hauke R, Mehta PP, Batra SK. Recent advances on cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. J Mol Cell Med. 2007;11:981–1011. doi: 10.1111/j.1582-4934.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miki J, Furusato B, Li H, et al. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res. 2007;67:3153–3161. doi: 10.1158/0008-5472.CAN-06-4429. [DOI] [PubMed] [Google Scholar]
- 13.Brown MD, Gilmore PE, Hart CA, et al. Characterization of benign and malignant prostate epithelial Hoechst 33342 side populations. Prostate. 2007;67:1384–1396. doi: 10.1002/pros.20620. [DOI] [PubMed] [Google Scholar]
- 14.Gu G, Yuan J, Wills M, Kasper S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 2007;67:4807–4815. doi: 10.1158/0008-5472.CAN-06-4608. [DOI] [PubMed] [Google Scholar]
- 15.Birnie R, Bryce SD, Roome C, et al. Gene expression profiling of human prostate cancer stem cells reveals a pro-inflammatory phenotype and the importance of extracellular matrix interactions. Genome Biol. 2008;9:R83. doi: 10.1186/gb-2008-9-5-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubrovska A, Kim S, Salamone RJ, et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci USA. 2009;106:268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shepherd CJ, Rizzo S, Ledaki I, et al. Expression profiling of CD133+ and C. Prostate. 2008;68:1007–1024. doi: 10.1002/pros.20765. [DOI] [PubMed] [Google Scholar]
- 18.Oates JE, Grey BR, Addla SK, et al. Hoechst 33342 side population identification is a conserved and unified mechanism in urological cancers. Stem Cells Dev. 2009;18:1515–1522. doi: 10.1089/scd.2008.0302. [DOI] [PubMed] [Google Scholar]
- 19.Patrawala L, Calhoun T, Schneider-Broussard R, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 20.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: The CD44+{alpha}2{beta}1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796–6805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 21.Wei C, Guomin W, Yujun L, Ruizhe Q. Cancer stem-like cells in human prostate carcinoma cells DU145: The seeds of the cell line? Cancer Biol Ther. 2007;6:763–768. doi: 10.4161/cbt.6.5.3996. [DOI] [PubMed] [Google Scholar]
- 22.Mimeault M, Batra SK. Characterization of non-malignant and malignant prostatic stem/progenitor cells by Hoecsht side population method. Methods Mol Biol. 2009;568:139–149. doi: 10.1007/978-1-59745-280-9_8. [DOI] [PubMed] [Google Scholar]
- 23.Rowehl RA, Crawford H, Dufour A, Ju J, Botchkina GI. Genomic analysis of prostate cancer stem cells isolated from a highly metastatic cell line. Cancer Genomics Proteomics. 2008;5:301–310. [PubMed] [Google Scholar]
- 24.Shah RB, Ghosh D, Elder JT. Epidermal growth factor receptor (ErbB1) expression in prostate cancer progression: correlation with androgen independence. Prostate. 2006;66:1437–1444. doi: 10.1002/pros.20460. [DOI] [PubMed] [Google Scholar]
- 25.Di Lorenzo G, Tortora G, D'Armiento FP, et al. Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin Cancer Res. 2002;8:3438–3444. [PubMed] [Google Scholar]
- 26.van Leenders GJ, Aalders TW, Hulsbergen-van de Kaa CA, Ruiter DJ, Schalken JA. Expression of basal cell keratins in human prostate cancer metastases and cell lines. J Pathol. 2001;195:563–570. doi: 10.1002/path.993. [DOI] [PubMed] [Google Scholar]
- 27.Chen BY, Liu JY, Chang HH, et al. Hedgehog is involved in prostate basal cell hyperplasia formation and its progressing towards tumorigenesis. Biochem Biophys Res Commun. 2007;357:1084–1089. doi: 10.1016/j.bbrc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 28.Sheng T, Li C, Zhang X, Chi S, et al. Activation of the hedgehog pathway in advanced prostate cancer. Mol Cancer. 2004;3:29. doi: 10.1186/1476-4598-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karhadkar SS, Bova GS, Abdallah N, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez P, Clement V, Altaba AR. Therapeutic targeting of the Hedgehog-GLI pathway in prostate cancer. Cancer Res. 2005;65:2990–2992. doi: 10.1158/0008-5472.CAN-05-0439. [DOI] [PubMed] [Google Scholar]
- 31.Mimeault M, Moore E, Moniaux N, et al. Cytotoxic effects induced by a combination of cyclopamine and gefitinib, the selective hedgehog and epidermal growth factor receptor signaling inhibitors, in prostate cancer cells. Int J Cancer. 2006;118:1022–1031. doi: 10.1002/ijc.21440. [DOI] [PubMed] [Google Scholar]
- 32.Mimeault M, Mehta PP, Hauke R, et al. Improvement of cytotoxic effects of mitoxantrone on hormone-refractory metastatic prostate cancer cells by co-targeting epidermal growth factor receptor and hedgehog signaling cascades. Growth Factors. 2007;25:400–416. doi: 10.1080/08977190801930935. [DOI] [PubMed] [Google Scholar]
- 33.Mimeault M, Johansson SL, Venkatraman G, et al. Combined targeting of epidermal growth factor receptor and hedgehog signaling by gefitinib and cyclopamine cooperatively improves the cytotoxic effects of docetaxel on metastatic prostate cancer cells. Mol Cancer Ther. 2007;6:967–978. doi: 10.1158/1535-7163.MCT-06-0648. [DOI] [PubMed] [Google Scholar]
- 34.Mimeault M, Venkatraman G, Johansson SL, et al. Novel combination therapy against metastatic and androgen-independent prostate cancer by using gefitinib, tamoxifen and etoposide. Int J Cancer. 2007;120:160–169. doi: 10.1002/ijc.22268. [DOI] [PubMed] [Google Scholar]
- 35.Datta S, Datta MW. Sonic Hedgehog signaling in advanced prostate cancer. Cell Mol Life Sci. 2006;63:435–448. doi: 10.1007/s00018-005-5389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barker AJ, Gibson KH, Grundy W, et al. Studies leading to the identification of ZD1839 (IRESSA): an orally active, selective epidermal growth factor receptor tyrosine kinase inhibitor targeted to the treatment of cancer. Bioorg Med Chem Lett. 2001;11:1911–1914. doi: 10.1016/s0960-894x(01)00344-4. [DOI] [PubMed] [Google Scholar]
- 37.Dai CL, Tiwari AK, Wu CP, et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsieh TC, Lu X, Guo J, et al. Effects of herbal preparation Equiguard on hormone-responsive and hormone-refractory prostate carcinoma cells: mechanistic studies. Int J Oncol. 2002;20:681–689. [PubMed] [Google Scholar]
- 39.Saruc M, Standop S, Standop J, et al. Pancreatic enzyme extract improves survival in murine pancreatic cancer. Pancreas. 2004;28:401–412. doi: 10.1097/00006676-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mimeault M, Jouy N, Depreux P, Henichart JP. Synergistic antiproliferative and apoptotic effects induced by mixed epidermal growth factor receptor inhibitor ZD1839 and nitric oxide donor in human prostatic cancer cell lines. Prostate. 2005;62:187–199. doi: 10.1002/pros.20138. [DOI] [PubMed] [Google Scholar]
- 42.Hirst SJ, Barnes PJ, Twort CH. Quantifying proliferation of cultured human and rabbit airway smooth muscle cells in response to serum and platelet-derived growth factor. Am J Respir Cell Mol Biol. 1992;7:574–581. doi: 10.1165/ajrcmb/7.6.574. [DOI] [PubMed] [Google Scholar]
- 43.Castedo M, Ferri K, Roumier T, Metivier D, Zamzami N, Kroemer G. Quantitation of mitochondrial alterations associated with apoptosis. J Immunol Methods. 2002;265:39–47. doi: 10.1016/s0022-1759(02)00069-8. [DOI] [PubMed] [Google Scholar]
- 44.Mimeault M, Batra SK. Recent insights into the molecular mechanisms involved in aging and the malignant transformation of adult stem/progenitor cells and their therapeutic implications. Ageing Rev Rev. 2009;8:94–112. doi: 10.1016/j.arr.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fioriti D, Mischitelli M, Di Monaco F, et al. Cancer stem cells in prostate adenocarcinoma: a target for new anticancer strategies. J Cell Physiol. 2008;216:571–575. doi: 10.1002/jcp.21493. [DOI] [PubMed] [Google Scholar]
- 46.Hudson DL, O'Hare M, Watt FM, Masters JR. Proliferative heterogeneity in the human prostate: evidence for epithelial stem cells. Lab Invest. 2000;80:1243–1250. doi: 10.1038/labinvest.3780132. [DOI] [PubMed] [Google Scholar]
- 47.Collins AT, Habib FK, Maitland NJ, Neal DE. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J Cell Sci. 2001;114:3865–3872. doi: 10.1242/jcs.114.21.3865. [DOI] [PubMed] [Google Scholar]
- 48.Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 49.Fan L, Pepicelli CV, Dibble CC, et al. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology. 2004;145:3961–3970. doi: 10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez P, Hernandez AM, Stecca B, et al. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci USA. 2004;101:12561–12566. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernes E, Fossa SD, Berner A, Otnes B, Nesland JM. Expression of the epidermal growth factor receptor family in prostate carcinoma before and during androgen-independence. Br J Cancer. 2004;90:449–454. doi: 10.1038/sj.bjc.6601536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schafer W, Funke PJ, Kunde D, Rausch U, Wennemuth G, Stutzer H. Intensity of androgen and epidermal growth factor receptor immunoreactivity in samples of radical prostatectomy as prognostic indicator: correlation with clinical data of long-term observations. J Urol. 2006;176:532–537. doi: 10.1016/j.juro.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 53.Zellweger T, Ninck C, Bloch M, et al. Expression patterns of potential therapeutic targets in prostate cancer. Int J Cancer. 2005 Nov 7;113:619–628. doi: 10.1002/ijc.20615. [DOI] [PubMed] [Google Scholar]
- 54.Bartlett JM, Brawley D, Grigor K, Munro AF, Dunne B, Edwards J. Type I receptor tyrosine kinases are associated with hormone escape in prostate cancer. J Pathol. 2005;205:522–529. doi: 10.1002/path.1735. [DOI] [PubMed] [Google Scholar]
- 55.Gil-Diez de Medina S, Salomon L, Colombel M, et al. Modulation of cytokeratin subtype, EGF receptor, and androgen receptor expression during progression of prostate cancer. Hum Pathol. 1998;29:1005–1012. doi: 10.1016/s0046-8177(98)90208-8. [DOI] [PubMed] [Google Scholar]
- 56.Klarmann GJ, Hurt EM, Mathews LA, et al. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin Exp Metastasis. 2009;26:433–446. doi: 10.1007/s10585-009-9242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhatt RI, Brown MD, Hart CA, et al. Novel method for the isolation and characterisation of the putative prostatic stem cell. Cytometry A. 2003;54:89–99. doi: 10.1002/cyto.a.10058. [DOI] [PubMed] [Google Scholar]
- 58.Levitt RJ, Zhao Y, Blouin MJ, Pollak M. The hedgehog pathway inhibitor cyclopamine increases levels of p27, and decreases both expression of IGF-II and activation of Akt in PC-3 prostate cancer cells. Cancer Lett. 2007 Jun 27;255:300–306. doi: 10.1016/j.canlet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Karashima T, Sweeney P, Slaton JW, et al. Inhibition of angiogenesis by the antiepidermal growth factor receptor antibody ImClone C225 in androgen-independent prostate cancer growing orthotopically in nude mice. Clin Cancer Res. 2002;8:1253–1264. [PubMed] [Google Scholar]
- 60.Sirotnak FM, She Y, Lee F, Chen J, Scher HI. Studies with CWR22 xenografts in nude mice suggest that ZD1839 may have a role in the treatment of both androgen-dependent and androgen-independent human prostate cancer. Clin Cancer Res. 2002;8:3870–3876. [PubMed] [Google Scholar]
- 61.Vicentini C, Festuccia C, Gravina GL, Angelucci A, Marronaro A, Bologna M. Prostate cancer cell proliferation is strongly reduced by the epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 in vitro on human cell lines and primary cultures. J Cancer Res Clin Oncol. 2003;129:165–174. doi: 10.1007/s00432-003-0420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Angelucci A, Gravina GL, Rucci N, et al. Suppression of EGF-R signaling reduces the incidence of prostate cancer metastasis in nude mice. Endocr Relat Cancer. 2006;13:197–210. doi: 10.1677/erc.1.01100. [DOI] [PubMed] [Google Scholar]
- 63.Sirotnak FM, Zakowski MF, Miller VA, Scher HI, Kris MG. Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res. 2000;6:4885–4892. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.