Abstract
Human exposure to cold and heat stimulates cutaneous vasoconstriction and vasodilation via distinct sympathetic reflex and locally mediated pathways. The mechanisms mediating cutaneous vasoconstriction and vasodilation are impaired with primary aging, rendering the aged more vulnerable to hypothermia and cardiovascular complications from heat-related illness, respectively. This paper highlights recent findings discussing how age-related decrements in sympathetic neurotransmission contribute directly to thermoregulatory impairments, whereas changes in local intracellular signaling suggest a more generalized age-associated vascular dysfunction.
Keywords: Skin blood flow, aging, Temperature Regulation, Adrenergic, Rho Kinase, Cold, Heat, Nitric Oxide Prostaglandins, Arginase, Oxidant Stress, Cardiac Output, Regional Blood Flow, Review
2. CUTANEOUS VASOCONSTRICTION
2.1. Introduction
Cutaneous vasoconstriction is the initial thermoregulatory response to cold exposure, minimizing convective heat loss to the environment through distinct reflex and local pathways that work both independently and cooperatively to maximize vasoconstriction. Whole-body cooling evokes sympathetic reflex constriction, which is dependent on the release of norepinephrine (NE) and co-transmitters from perivascular sympathetic adrenergic nerve terminals (1, 2). In contrast, localized cooling of the cutaneous blood vessels and surrounding tissue engages local (i.e., non-reflex) cold-induced vasoconstriction that is mediated primarily by NE at α2-adrenoceptors (3–6) and by Rho kinase (7), along with a passive increase in constrictor tone via nitric oxide withdrawal (8).
Cutaneous vasoconstrictor responses to cold exposure are impaired in aged skin, leading to higher skin blood flows during cold exposure and rendering older humans more susceptible to excessive heat loss and, ultimately, hypothermia (9–12). Indeed, recent statistics indicate that people over the age of 65 account for fully half of all cold-exposure deaths each year (13–15). Over the past several years, researchers have systematically explored the mechanisms through which this heat-conserving response becomes impaired with aging, culminating in a working model that suggests that decrements in sympathetic neurotransmission contribute directly to thermoregulatory impairment, while changes in intracellular signaling indicate a more generalized age-associated vascular dysfunction (16).
2.2. Reflex Vasoconstriction
2.2.1. Young
Human cutaneous blood vessels are innervated by sympathetic adrenergic vasoconstrictor nerves that participate in the reflex vasoconstrictor response. The 19th-century French physiologist Claude Bernard, well-known for his study of vasomotor nerves, originally pioneered the notion of sympathetic innervation of the thermoregulatory vasculature (17). His conclusions laid the groundwork for later in vivo human research, which confirmed that reflex sympathetic outflow is responsible for not only resting vessel tone but also the pronounced cutaneous vasoconstriction observed during cooling of the skin (18, 19).
Cutaneous reflex vasoconstriction in humans occurs most often when mean skin temperature decreases below a thermoneutral point (~ 34ºC) due to either convective (cold air) or conductive (cold wet clothes, cold surfaces) heat transfer to the environment. A decrease in core temperature can also bring about reflex vasoconstriction, although core cooling in the absence of skin cooling only occurs under special medical circumstances, such as surgical anesthesia and cardiac surgery (20–22). Reflex constriction is a graded response, where the intensity of the response mirrors the intensity of the whole-body cold stimulus (quantified by whole-body mean skin temperature) until blood flow reaches a basement plateau, after which further cooling will not induce further constriction. In controlled laboratory experiments, the effects of whole-body cooling (reflex vasoconstriction) are isolated from any confounding effects of localized changes in skin temperature (locally mediated vasconstriction) at the site of skin blood flow measurement. Skin blood flow is recorded at a site where local skin temperature has been artificially maintained at 34ºC; thus, any constriction that occurs at those warmed sites could only be attributed to sympathetic reflex pathways, because local mechanisms would not be engaged.
Sympathetic reflex vasoconstriction is mediated by autonomic sympathetic efferent nerve signals traveling to cutaneous sympathetic axon terminals, stimulating the release of NE (the primary neurotransmitter in sympathetic adrenergic nerves) and co-transmitters from perivascular nerve endings. NE is synthesized in the cell body of noradrenergic neurons from the amino acid precursor L-tyrosine, with the help of the rate-limiting enzyme tyrosine hydroxylase and its essential cofactor, tetrahydrobiopterin (BH4) (23, 24). Although BH4 is particularly prone to deactivation by oxidation (via reactive oxygen species) (25), NE synthesis is rarely disrupted in healthy, young individuals due to a healthy balance of oxidant production vs. antioxidant activity.
Localized intradermal applications of bretylium tosylate, yohimbine, and propranolol (antagonists of pre-synaptic neurotransmitter release, α-adrenoceptors, and β-adrenoceptors, respectively) have revealed that while the vasoconstrictor response to whole-body cooling is entirely dependent on the release of sympathetic transmitters, only 60% of constriction is mediated by NE (1, 2, 11, 26). These findings provide in vivo evidence supporting the participation of sympathetic co-transmitter(s) in cutaneous reflex vasoconstriction to cold. Both neuropeptide Y (NPY) and adenosine triphosphate (ATP) act as sympathetic co-transmitters with NE in perivascular nerve endings in several vascular beds, acting post-junctionally through Y1 and P2X receptors, respectively (27–32). In skin-specific work, Stephens and colleagues (2004) confirmed that NPY operates as a sympathetic co-transmitter in the cutaneous circulation in response to whole-body cooling; further work is required to determine whether ATP also participates in this response in the cutaneous vasculature.
The effect of female reproductive hormones on the mechanisms driving reflex cutaneous vasoconstriction to cold remains unclear. There is evidence that women in the high-hormone phase of oral contraceptives exhibit the same ratio of NE-to-co-transmitter-mediated constriction that men do, while women in the low-hormone placebo phase of the pill tend to rely almost entirely on NE to effect the same magnitude of constriction (2). However, when normally menstruating women were tested under a similar protocol during the early follicular phase (low hormone state), there was no difference in either the magnitude of vasoconstriction or the individual contributions of sympathetic transmitters (11). This disparity might be attributed to the presence/absence of exogenous reproductive hormones, since oral contraceptive use can alter thermoregulatory function (33). Further work is warranted to fully clarify the effects of exogenous vs. endogenous reproductive hormones on reflex vasoconstriction.
2.2.2. Aging
In 1977, thermoregulatory researcher K.J. Collins and colleagues noted that hypothermia was an increasingly common medical concern among the elderly: “[A]ccidental hypothermia is now recognised as one of the natural hazards of old age. The problem is not simply one of unintentional accidental hypothermia resulting from a fall or accident at home and subsequent immobilisation and exposure, nor one entirely associated with concurrent illness; spontaneous hypothermia also occurs among apparently fit elderly people” (9). Their longitudinal study investigating the aging process as it specifically pertained to thermoregulation concluded that cutaneous vasoconstriction in response to cooling is impaired in older humans, predisposing them to hypothermia (9). Subsequent thermoregulatory studies not only confirmed these findings, but also characterized and documented this decrement in vasoconstrictor function in greater detail (10–12, 34–36). The cumulative finding of these studies and many others—that reflex cutaneous vasoconstriction is markedly impaired in aged skin, regardless of how the cold stress is induced or how skin blood flow is measured—suggests that pronounced cutaneous vasomotor dysfunction is wide-spread in older populations.
Building on this evidence of compromised thermoregulatory vasoconstriction in aged populations, researchers have identified elements of both neural and vascular dysfunction that likely mediate age-associated thermoregulatory failure. Cutaneous sympathetic constriction is compromised in aged skin due to impaired function at multiple points along the efferent arm of the reflex (see Figure 1): 1) the sympathetic autonomic nerve signal preceding constriction, 2) transmitter synthesis and release from the axon, and 3) post-junctional end-organ responsiveness (see Figure 1).
Figure 1.
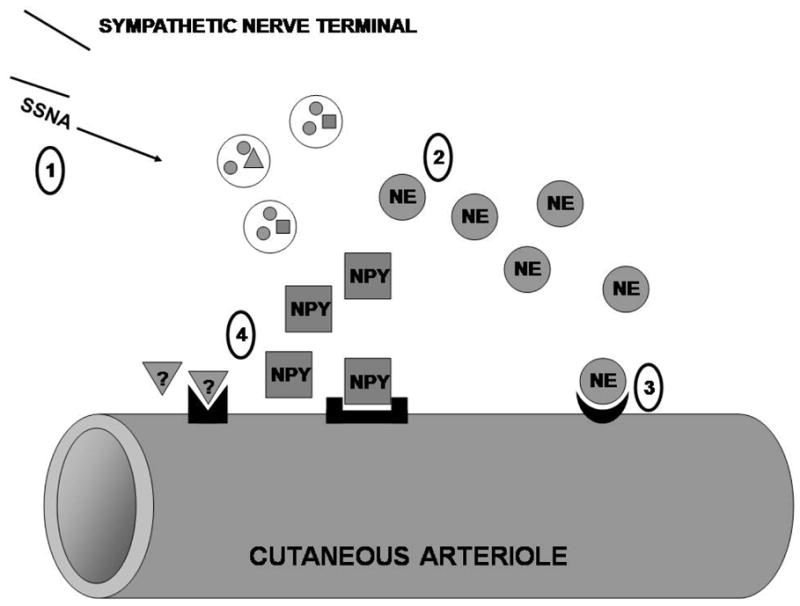
Schematic representation of sites of age-associated impairment in the sympathetic reflex thermoregulatory vasoconstriction response to cold. (A) Efferent skin sympathetic nerve activity (SSNA) is reduced, resulting in decreased perivascular nerve stimulation. (B) A decrease in axonal tetrahydrobiopterin (BH4) bioavailability may contribute to the documented decrease in neuronal synthesis and release of norepinephrine (NE). (C) Reduced synthesis and/or release of neuropeptide Y (NPY) and potentially adenosine triphosphate (ATP) may contribute to the documented complete loss of sympathetic co-transmitter function. (D) The end-organ vasoconstrictor response mediated by post-junctional α-adrenergic, Y1, and P2X receptors (for NE, NPY, and ATP, respectively) is reduced, although it is unclear whether this loss of sensitivity is due to changes in receptor population, intracellular second-messenger signaling pathways, or a combination of the two. Copyright permission obtained from Wolters Kluwer Health.
2.2.2.1. Sympathetic autonomic dysfunction
Sympathetic efferent outflow in response to a cold stimulus is decreased with aging, resulting in a dampened signal triggering the onset of cutaneous vasoconstriction. When skin sympathetic nerve activity was recorded in young (18–29 yrs), middle-aged (38–51 yrs), and elderly subjects (65–81 yrs), the cold-induced increase in vasomotor nerve activity (bursts/min) was depressed by approximately 60% in the elderly group compared to the other two groups (37). Thus, the sympathetic efferent signal for cold-induced cutaneous vasoconstriction is significantly weaker in aged skin.
2.2.2.2. Impaired transmitter synthesis/release
In addition to the sympathetic stimulation of perivascular nerves and subsequent release of sympathetic transmitters, the vasoconstrictor contributions of these transmitters is also impaired with aging. Reflex constriction in young skin is mediated by both NE (60%) and co-transmitters (40%). However, the co-transmitter portion of constriction is abolished in aged skin, indicating that older subjects rely entirely on NE to effect the reflex vascular response to cold; furthermore, this lone NE component of constriction is also blunted in aged skin (11). The decrements in NE- and co-transmitter-mediated constriction are due, in part, to age-related decreases in transmitter synthesis and/or release (36, 38, 39).
Although there are scant data available explaining the mechanisms driving the decrease in co-transmitter synthesis/release, recent research focusing on the vascular role of BH4 in the context of human aging has forwarded a plausible mechanism to explain the decrease in NE synthesis/release. Human aging is associated with a systemic net increase in reactive oxygen species (ROS) due to both increased production and decreased clearance (40), which leads to oxidant damage and various attendant pathologies. Because both de novo synthesis and recycling pathways for BH4 are particularly vulnerable to oxidation, BH4 bioavailability can be altered depending on the current redox state of the cell; thus, the pro-oxidant cellular environment associated with human aging likely reduces BH4 bioavailability (41, 42). This decrease in BH4 bioavailability may, in turn, decrease the tyrosine hydroxylase activity driving catecholamine synthesis, resulting in depressed NE synthesis in aged skin. In aging models, BH4 supplementation has markedly improved age-associated cutaneous vasodilatory dysfunction (where BH4 serves as an essential co-factor in nitric oxide (NO) synthesis) by increasing BH4 bioavaibility in the vascular endothelium (42–44). It is equally likely that BH4 supplementation may also augment NE synthesis and cutaneous vasoconstriction in aged populations by increasing BH4 bioavailability in sympathetic neurons.
2.2.2.3. Blunted end-organ responsiveness
In addition to a muted sympathetic signal and reduced NE/co-transmitter synthesis and release, the end-organ vasoconstrictor response is also attenuated in aged skin (11, 36, 45–48). By using direct administration of NE, NPY, and ATP (often in graded doses) under thermoneutral conditions, the upstream changes in sympathetic nerve activity and transmitter synthesis are bypassed, permitting direct evaluation of vasoconstriction to a given pharmacological stimulus. NE-mediated constriction is significantly impaired in aged skin, with elderly subjects exhibiting a blunted constrictor response to both physiologic and maximal doses of NE (11, 48). NPY-mediated vasoconstriction undergoes a similar reduction in older subjects (46), and ATP-mediated constriction in isolated rat arterioles is also profoundly depressed in vessels from aged rats (45).
Cumulatively, these findings suggest that the age-associated decrement in thermoregulatory reflex vasoconstriction is attributable to several factors: 1) reduced efferent sympathetic signal, 2) reduced sympathetic synthesis/release of NE (through a putative ROS-mediated decrease in BH4 bioavailability), 3) a complete loss of sympathetic co-transmitters function, and 4) a significant loss of both end-organ sensitivity and maximal response to NE and putative sympathetic co-transmitters (see Figure 1). It is unclear how second-messenger signaling pathways may be affected by aging, warranting further research to determine the intracellular mechanism(s) of blunted end-organ responsiveness in the cutaneous circulation.
2.2.3. Clinical Populations
Although the risk of hypothermia is increased in the very young and very old, there are certainly clinical conditions which also can also predispose individuals to hypothermia by exacerbating heat loss or inhibiting heat production on the cold. The ability to minimize skin blood flow through reflex sympathetic vasoconstriction is essential for thermoregulation in the cold, and clinical populations characterized by peripheral neuropathies (spinal cord injury, diabetes) are at greater risk for excessive heat loss due to inadequate vasoconstriction (49–51). Additionally, motion sickness can also pre-dispose individuals to excessive heat loss by altering patterns of neurally mediated cutaneous vasoconstriction and shivering in the cold (52), while alcohol consumption may particularly predispose the elderly to hypothermia by reducing oxygen consumption and metabolic rate (53).
2.3. Local vasoconstriction
2.3.1. Young
In contrast to reflex vasoconstriction that is elicited by whole-body cooling, localized cooling of the cutaneous blood vessels and surrounding tissue engages local constrictor mechanisms, independent of efferent sympathetic reflex activity (3, 4). During the early phase of localized skin cooling (0–10 min), vasoconstriction is dependent on NE (possibly from basal sympathetic activity) acting at α2-adrenoceptors (3). Early-phase constriction also appears to be dependent on intact sensory nerves (54), although the signaling pathway for this effect is still unclear. If localized cooling persists (> 15 min), maintenance constriction is primarily mediated by non-adrenergic, non-neuronal mechanisms, suggesting that cooling may alter signaling pathways within the vascular smooth muscle, including a downregulation of the endothelial nitric oxide synthase (eNOS) pathway (4, 6, 8).
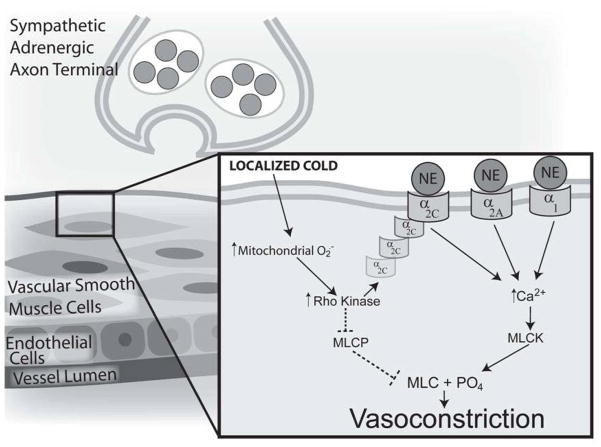
In vitro models of direct cutaneous vessel cooling have implicated Rho kinase (ROCK) as a key intracellular mediator of cold-induced vasoconstriction. Localized cutaneous vessel cooling stimulates the production of ROS (particularly mitochondrial superoxide), which upregulates the RhoA/ROCK pathway (55). ROS-activated ROCK, in turn, can augment constriction through two distinct mechanisms: 1) inhibition of myosin light chain phosphatase (MLCP), passively permitting phosphorylation of myosin light chain in the absence of a Ca2+ influx (also referred to as “Ca2+ sensitivity”); and 2) cyclic AMP- mediated translocation of α2C-adrenoceptors from the Golgi to the surface of the cell, leading to 5-fold increase in the adrenoceptor population available to bind with NE during cutaneous tissue cooling (55–59).
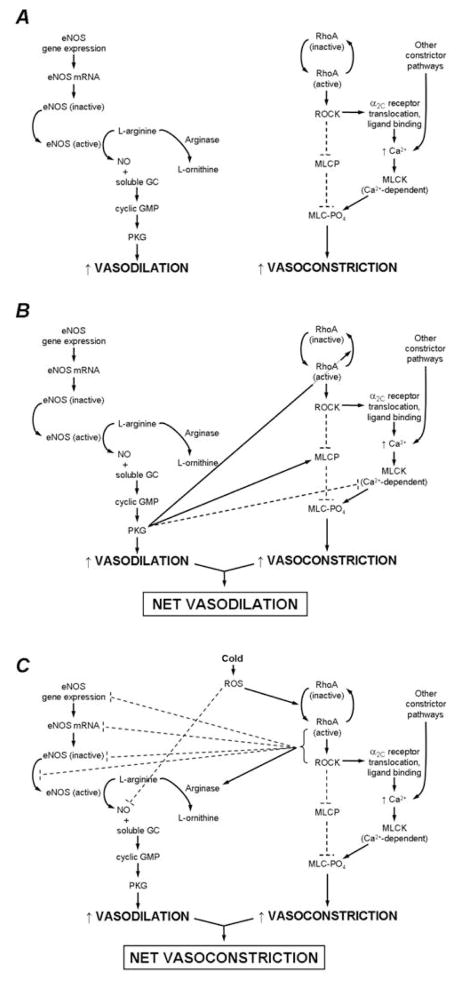
Recent in vivo work based on these in vitro findings has confirmed that ROCK participates in both adrenergic and non-adrenergic phases of locally mediated cutaneous vasoconstriction, likely through α2C translocation and Ca2+ sensitivity, respectively (7, 60). This finding also provides additional support for the putative cold-mediated downregulation of the eNOS pathway (8). The Rho/ROCK and eNOS pathways are mutually inhibitory; cGMP-dependent protein kinase (PKG, a downstream product of NO metabolism) inhibits Rho activation and ROCK phosphorylation of MLCP, while Rho and ROCK downregulate eNOS expression and activity (61, 62), maintaining a necessary balance between dilator and constrictor influences in the vasculature (see Figure 3). A cold-mediated increase in ROS production and ROCK activity would likely decrease NO production, further upregulating ROCK, thereby strengthening the effectiveness of cold-mediated constriction. However, the intricate interplay between these two signaling pathways in the cutaneous vascular response to cold requires further investigation.
Figure 3.
Pathway schematic of endothelial nitric oxide synthase (eNOS)-mediated vasodilation and RhoA/Rho kinase (ROCK)-mediated vasoconstriction in cutaneous vessels and the putative interactions between the two pathways. (A) eNOS-mediated vasodilation and ROCK-mediated vasoconstriction outlined as independent pathways. (B) The pro-dilator/anti-constrictor interactions of the two pathways that predominate in young, healthy skin under thermoneutral conditions. (C) The pro-constrictor/anti-dilator interactions of the two pathways that predominate during localized skin cooling and that are augmented in aged skin. --- = inhibitory effects; — = stimulatory effects. Copyright permission obtained from Wolters Kluwer Health.
Interestingly, localized skin cooling can also elicit a paradoxical transient vasodilation if sensory or constrictor adrenergic pathways are blocked, thus unmasking underlying dilatory mechanisms that may have been activated by cold (6–8, 54). Although it is unclear what mechanisms may be driving this response, recent in vitro evidence suggests that cold-induced vasodilation may be unmasked when constrictor pathways are blocked and attributed to the cold-mediated production of mitochondrial ROS—a mechanism common to cold-induced ROCK activation and subsequent vasoconstriction (55). ROS stimulate the localized and transient release of Ca2+ sparks from the sarcoplasmic reticulum in vascular smooth muscle cells, which in turn can activate Ca2+-activated K+ channels, leading to vascular smooth muscle relaxation (63). Although this elegant signaling cascade would confirm a vascular thermosensor (mitochondrial ROS) that contributes to cold-induced vasodilation and vasoconstriction, it requires further testing to confirm whether the transient dilation observed during localized skin cooling is, in fact, mediated by these mechanisms (64).
2.3.2. Aging
In contrast to the marked decrement in reflex cutaneous vasoconstriction that accompanies aging, the magnitude of local cold-induced constriction remains unaffected by age (47, 65). However, the balance of the underlying mechanisms that drive this response shifts with age, becoming less adrenergic and more dependent on ROCK signaling. The depressed cutaneous adrenergic response to a local cold stimulus in aged skin parallels similar findings in reflex vasoconstriction (whole-body cold stimulus) and exogenously administered NE (11, 48), while the overall local constrictor response to cold remains functionally unchanged due an apparent compensatory increase in ROCK-mediated constriction (47, 65).
Although the magnitude of local thermoregulatory responses to cold does not undergo significant change with aging, this increased dependence on Rho/ROCK signaling in a healthy aged population provides insight into the signaling changes that arise in conjunction with the development of cardiovascular disease in older humans. Greater dependence on the Rho/ROCK pathway with aging parallels the upregulation of the Rho/ROCK pathway that is seen in several age-associated vascular pathologies, including atherosclerosis, systemic hypertension, pulmonary hypertension, vascular remodeling, coronary and cerebral vasospasm, erectile dysfunction, and diabetes (61). These similar findings in healthy aged and clinical populations suggest that augmented Rho kinase-mediated vasoconstriction may be, at least in part, a function of aging per se rather than the diseases associated with aging. Thus, while advancing age does not affect the local cooling response from a thermoregulatory standpoint (i.e., magnitude of cutaneous constrictor activity is maintained in the face of cold stress), it is associated with pre-clinical pro-constrictor changes in signaling, suggesting that aging may serve as a prelude to more serious clinical vascular pathologies.
In addition to using local skin cooling as a thermoregulatory stimulus, it is likely that cutaneous vascular responses to local cooling may also be useful as an indirect measure of vascular (particularly endothelial) health. Because the Rho/ROCK and eNOS pathways are mutually inhibitory, a disruption of the healthy balance between the two systems can result in disproportionate dilation or constriction. In Figure 3A, the independent pathways for NO-mediated vasodilation and ROCK-mediated vasoconstriction are illustrated. Dilation occurs through eNOS activation, which catalyzes the conversion of L-arginine into NO. NO then binds to soluble guanylyl cyclase (GC) and increases cyclic GMP and cyclic GMP-dependent protein kinase (PKG) activity, ultimately resulting in cutaneous smooth muscle relaxation. Although arginase could potentially reduce nitric oxide production because it competes with eNOS for its substrate, arginase activity is negligible in young healthy vessels. Constriction through the RhoA/ROCK pathway occurs through RhoA/ROCK activation, which leads to 1) a decrease in myosin light chain (MLC) phosphatase (MLCP) activity, and 2) an increase in Ca2+-mediated MLC kinase (MLCK) activity via α2C-adrenoceptor translocation and norepinephrine binding (this pathway appears to be only active under cold conditions). Either the decrease in MLCP activity or the increase in MLCK activity leads to a net increase in MLC phosphorylation, ultimately resulting in smooth muscle contraction.
The interaction between dilation and vasoconstriction in young, healthy vasculature results in a balance that is slightly skewed in favor of NO-mediated dilation, indicating the presence of a healthy functional endothelium (see Figure 3B). When eNOS activity predominates in these cutaneous vessels, NO drives dilation via its conventional pathway as well as through the anti-constrictor effects of PKG: directly deactivating RhoA and acting on both MLCP and MLCK to decrease the phosphorylation state of MLC.
The interaction between dilation and constriction in the context of human aging is biased towards constriction, indicating a loss of endothelial dilation and an increase in ROCK-mediated vasoconstriction (see Figure 3C). Age- and cold-induced increases in ROS likely both contribute to the activatation of RhoA/ROCK and lead to prolonged cutaneous constriction, which is mediated through ROCK’s established effects on MLCP and α2C translocation and possibly through its anti-dilator effects on the eNOS pathway. RhoA/ROCK can decrease NO bioavailability by decreasing eNOS transcription, translation, and activity and by activating arginase. It is likely that increased ROS production may also quench NO before it can bind with soluble GC.
Thus, age- and cold-induced increases in ROS production and RhoA/ROCK activity and decreases in NO production may perpetuate a cycle of pro-constrictor activity by stimulating RhoA/ROCK and removing NO-mediated inhibition, respectively. In particular, age-associated increases in ROS (which can both directly activate Rho and quench NO) and arginase activity (which limits NO production by preferentially metabolizing L-arginine, the substrate for eNOS) cumulatively result in reduced NO bioavailability (66); see Figure 3). The consequent reduction in NO metabolism and PKG activity may sufficiently disinhibit Rho and ROCK so as to create an ideal signaling environment for unchecked vasoconstriction. Although these interactions between eNOS and Rho/ROCK pathways require further testing in vivo, it is likely that age-related decrements in endothelial function may be reflected in Rho/ROCK contributions to local cold-mediated vasoconstriction.
2.3.3. Clinical populations
Raynaud’s phenomenon is a vascular disorder in which cutaneous arterioles, particularly those in the fingers and toes (and less commonly in the nose, lips, and ears), undergo painful vasospasm in response to acute cold exposure—an abnormally intense version of a normal physiological response. Raynaud’s phenomenon can be of idiopathic origin (primary Raynaud’s) or can occur in response to mechanical, immunologic, or chemical stress in cutaneous blood vessels (secondary Raynaud’s) (61).
The putative mechanisms that mediate Raynaud’s phenomenon have been investigated for decades, but the underlying pathophysiology is still incompletely understood; however, researchers postulate that the mechanisms involved in the normal vascular response to local cooling may also participate in Raynaud’s phenomenon, only with higher sensitivity and/or greater responsiveness (61). Because women of childbearing age and postmenopausal women taking estrogen replacement therapy (but not combined estrogen and progesterone) exhibit the highest incidence of Raynaud’s (58, 59), it is likely that female sex hormones may also participate in the etiology of the disease. Studies investigating sex differences in the normal response to localized cooling indicate that women rely more heavily on α2-adrenoceptors to achieve local cold-induced vasoconstriction compared to men (5). A recent study by Eid et al. (59) ties this observation into the sex difference observed with Raynaud’s incidence: estrogen, in the absence of cold, is a potent activator of α2C-adrenoceptor expression in vascular smooth muscle cells, and estrogen exposure during cutaneous vessel cooling further augments cold-induced vasoconstriction. Thus, estrogen may play a significant role in the etiology of Raynaud’s phenomenon in at-risk female populations. Certainly the notion of an estrogen-dependent augmentation of cold-induced α2C-adrenoceptor-mediated constriction complements current medical epidemiology and provides insight into potential therapeutic strategies, including the use of selective Rho kinase inhibitors or statins (which also inhibit the Rho kinase pathway) (61).
Contrary to the pronounced sex differences in Raynaud’s prevalence (arguably the most important factor in defining at-risk populations), the association of advancing age with incidence rates is less clearly defined, when it is studied at all. In the limited data available, aging does not appear to increase the prevalence or severity of Raynaud’s among women; once estrogen levels are accounted for (menarche, child-bearing years, menopause), age is no longer a significant predictor. In contrast, in men who suffer from Raynaud’s (incidence is lower but not altogether absent in men), older age is considered to be a risk factor for the onset of the disease (59).
2.4. Conclusions
Healthy human aging leads to compromised vascular thermoregulatory vasoconstriction to cold, predisposing older humans to hypothermia. This impaired response is predominately due to changes in sympathetic reflex vasoconstriction, where the efferent sympathetic signal, neural synthesis/release of NE, synthesis/contribution of co-transmitters, and vascular smooth muscle sensitivity are all significantly attenuated with aging. Additionally, it is possible that adrenoceptor-mediated effects are blunted due to changes in intracellular signaling pathways, although this hypothesis requires further investigation both in vitro and in vivo. The effects of aging on locally driven vasoconstriction responses to skin cooling are more subtle; the absolute magnitude of the response remains unchanged in healthy aged populations, while the mechanisms driving the response become increasingly dependent on intracellular pathways that are associated with vascular disease. In this context, aging itself is associated with pre-clinical pro-constrictor changes in signaling, suggesting that aging may serve as a prelude to more serious clinical vascular pathologies. Further research is warranted to explore the interaction between decreased NO bioavailability and increased ROCK-mediated vasoconstriction to more fully characterize the development of age-associated microvascular dysfunction.
3. REFLEX VASODILATION
3.1. Introduction
The human cardiovascular system has a tremendous capacity to increase skin blood flow (SkBF) in response to core and skin heating through an increase in cardiac output and a redistribution of blood flow from renal and splanchnic vascular beds (67). The significant increase in skin blood flow and sweating with increased body core temperature provide effective avenues of heat loss through convective and evaporative mechanisms, respectively. Reflex cutaneous vasodilation is neurogenically mediated by sympathetic cholinergic nerves that co-release acetylcholine and an unidentified cotransmitter(s) (68). Putative cholinergic neurotransmitters include vasoactive intestinal peptide (VIP) (69–71), substance P (72), calcitonin gene related peptide (CGRP) (71), and histamine and neurokinin receptor activation (70, 73, 74), which in-part mediate vasodilation through the activation of downstream second messenger pathway including nitric oxide (NO)- and cyclooxygenase (COX)-dependent mechanisms (70, 72, 73).
Human aging in the absence of overt pathology is associated with attenuated cutaneous vasodilation during thermal stress (66, 75–78) rendering aged humans more susceptible to heat-related cardiovascular complications with hyperthermia (79). This impaired vasodilatory response is apparent even when subjects are matched for fitness level (78) (VO2max), acclimation (80), and hydration status (81), suggesting that this is a primary aging phenomenon that is peripheral in origin (82). On average, healthy aged humans display 25–50% attenuation in SkBF during whole body (core) heating. Over the past several years, researchers have systematically explored the mechanisms underlying age-related impairments in reflex cutaneous vasodilation. Mechanisms mediating reduced NO bioavailability in the aged vasculature have been central to these investigations including 1) decreased NO synthesis through age-related decreases in functional L-arginine and BH4 availability and 2) increased NO degradation through oxidant stress.
3.1.1. Aging and the integrated cardiovascular response to heat stress
Age-related alterations in the control so skin blood flow occur presynaptically, postsynaptically, and in intracellular signaling pathways. However, it is important to recall that an integrated response of the entire cardiovascular system determines the available blood flow supply characteristics of the system, especially during passive hyperthermia, when SkBF can approach 8 L/min. While reflex and/or local skin vasodilation in response to whole-body or local heating is determined by those mechanisms detailed later in this chapter, the blood supply originates from (1) an increased cardiac output coupled with (2) redistribution of flow from other vascular beds. Perhaps not surprisingly, the supply side of this response, like the neurovascular mechanisms, is altered with aging.
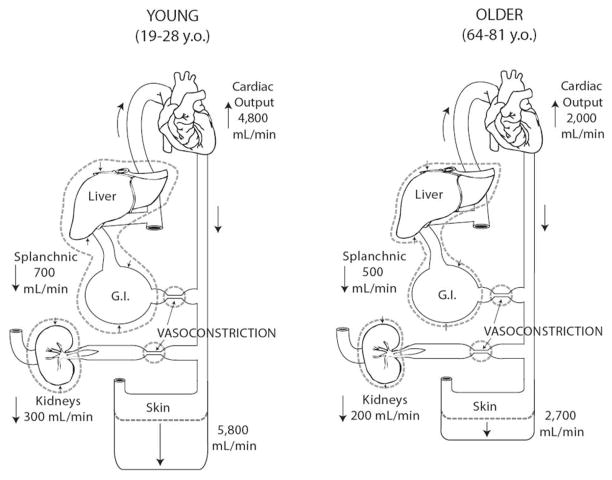
Minson et al. (1997) were the first to quantify the difference in SkBF between older (64–81 yrs) and young (19–28 yr) men by measuring cardiac output as well as its distribution to the major vascular beds (Figure 7) during prolonged passive heating. During supine rest, subjects were heated to the limit of their individual thermal tolerance using hot water perfused suits. Figure 4 displays the separate contributions of increased cardiac output and decreased splanchnic and renal blood flows to the total increase in SkBF from a thermoneutral baseline to the tolerated limit of passive heating. The young men increased SkBF by an average of 5.8 L/min, compared to an average increase of only 2.7 L/min by the older men. (Although the calculated increase in SkBF in these young subjects is lower than the mean increase reported by Rowell and colleagues, all subjects were well within their range of 3–11 L/min.)
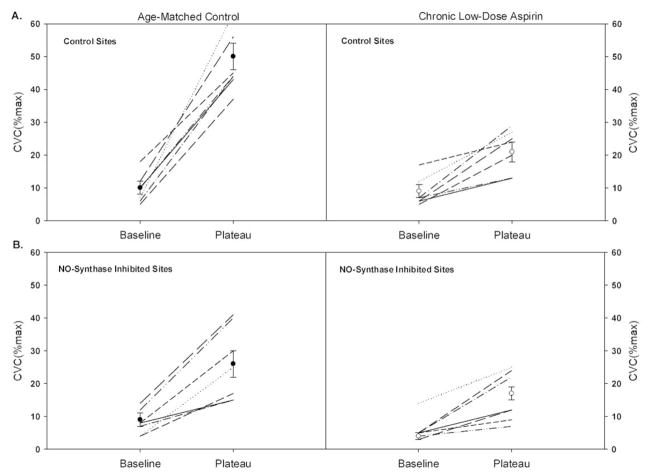
Figure 7.
Individual and mean subject responses at baseline and at the plateau in cutaneous vascular conductance (%CVCmax) as a percentage of maximum. Data from the age matched control subjects are displayed in the left hand panels and the chronic low-dose aspirin subjects are displayed in the right hand panels. Subjects taking chronic low-dose aspirin therapy displayed a significantly attenuated increase in skin blood flow during hyperthermia in both A. the control sites and B. the NO-synthase inhibited (L-NAME treated) sites. Copyright permission obtained from The American Physiological Society.
Figure 4.
During whole-body heating, cardiac output increases and blood flow to splanchnic and renal vascular beds decreases, redirecting flow to the cutaneous circulation. When healthy young men (left panel) were heated to the limit of their individual thermal tolerance using water-perfused suits, cardiac output essentially doubled and an additional liter of flow was redirected from visceral circulations, allowing skin blood flow to increase by 5.8 L/min. On average, healthy older men (right panel) responded with a much smaller increase in cardiac output coupled with a smaller decrease in flow from the combined visceral beds, providing only a 2.7 L/min increase in skin blood flow. These age differences in the integrated cardiovascular response to passive heating occurred despite similar increases in core temperature and decreases in central venous pressure.
However, a major finding of this investigation was that the orchestrated cardiovascular response to direct passive heating was altered as a function of age of the subject. Specifically, the increase in cardiac output was significantly smaller in the older men despite similar increases in skin and core temperatures. A lower stroke volume, most likely due to an attenuated β-adrenergic responsiveness, was the primary factor for the lower cardiac output observed in the older men. This age-difference in the stroke volume response occurred despite a similar fall in central venous pressure in all subjects. While the young men were able to maintain stroke volume fairly well despite the falling central venous pressure, stroke volume fell progressively in the older men. Both age groups had similar increases in HR during the heating protocol. However, when expressed as a percent of maximal HR, the older men responded with a lower %HRmax response, i.e., they relied on a greater proportion of their chronotropic reserve.
Secondly, the older subjects redistributed less blood flow from the combined splanchnic and renal circulations to the skin. While the young men decreased flow to these two circulations by 1 L/min, only 700 ml was redistributed in the older subjects. As inactive muscle flow does not change under these circumstances (supine passive heating), all of this additional flow was shunted to the skin.
When exercise is performed in hot environments, the need to deliver oxygenated blood to working muscle is added to the need to increase SkBF for heat dissipation, and these two circulations compete for a limited cardiac output. Similar to passive heating, during exercise in hot environments the smaller rise in SkBF in the aged is accompanied by a lower cardiac output (83). The combined decrease in splanchnic and renal flows – all of which is redirected to the skin – was some 35–40% less at age 65 than at age 25 (83) during work at 60% VO2max in the heat.
3.2. Reflex vasodilation
3.2.1. Young
Cutaneous active vasodilation is purportedly mediated by the co-transmission of acetycholine and an unknown neurotransmitter(s) from sympathetic cholinergic active vasodilator nerves, where acetylcholine primarily mediates the sweating response and the unknown neurotransmitter(s) co-released with acetylcholine mediates cutaneous vasodilation (Figure 5(79)). In support of this hypothesis, muscarinic receptor antagonism abolishes sweating and attenuates the initial rise in SkBF with rising Tc, while pre-synaptic blockade of cholinergic nerves with botulinum toxin abolishes both sweating and active vasodilation (68). However, the specific identity of the unknown neurotransmitter(s) mediating active vasodilation remains elusive with putative neurotransmitter candidates including the vasoactive neuropeptides: vasoactive intestinal peptide (VIP), substance P, and calcitonin gene-related peptide (CGRP). Immunoreactivity to these peptides has been demonstrated in human cutaneous nerves (85), and VIP (69), histamine receptor 1 (H1) (73), and neurokinin 1 (NK1) (72) receptor activation have all been shown to contribute to active vasodilation.
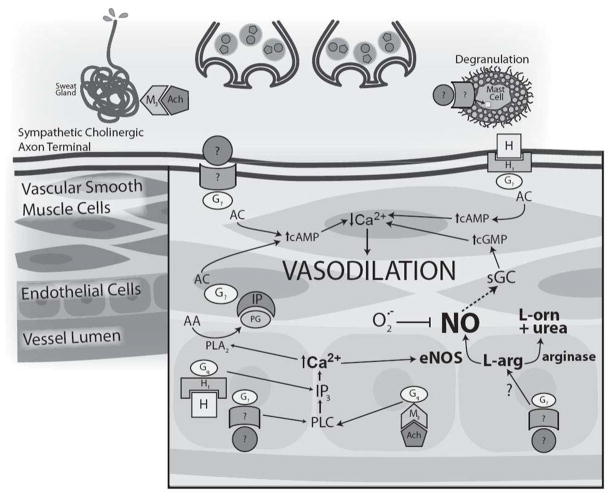
Figure 5.
Schematic representation of the putative mechanism of active vasodilation with relevant age-related alterations. The co-transmitter hypothesis, in which acetylcholine is co-released with an unknown neurotransmitter from sympathetic cholinergic nerves, is illustrated. Acetylcholine mediates the sweating response and may modulate the initial rise in active vasodilation through NO and COX-dependent prostanoid synthesis. In this schematic the unknown neurotransmitter and receptor (?) mediates vasodilation through adenylate cyclase mechanisms and may also increase NO synthesis through inositol triphosphate (IP3)-mediated increases in intracellular calcium. Histamine also contributes to active vasodilation through NO-dependent and NO-independent mechanisms. Putative neurotransmitters involved in active vasodilation include vasoactive intestinal peptide (VIP), substance P, and calcitonin gene related peptide (CGRP) which may induce histamine release through the degranulation of cutaneous mast cells. With aging there is a reduction in both the functional neurotransmitter and NO contributions. NO-dependent vasodilation is decreased by an age-related upregulation of arginase activity and increased oxidant stress. Copyright permission obtained from Wolters Kluwer Health.
Functional nitric oxide (NO) is required for full expression of active vasodilation (86) through activation of soluble guanalate cyclase-dependent mechanisms. Inhibition of NO-synthase (NOS) reduces active vasodilation by approximately 30–40% in young healthy skin. NO synthesis during active vasodilation may be stimulated from a variety of sources including direct stimulation from the putative neurotransmitters involved in active vasodilation. Acetylcholine (87), VIP (70), and substance P (72) partially mediate cutaneous vasodilation through NO-dependent mechanisms. Additionally, the contribution of each of these putative neurotransmitters to the different phases of the SkBF response during rising Tc creates to the potential for a great deal of redundancy in active vasodilation, especially with respect to NO mechanisms. For example, prolonged active vasodilation is insensitive to muscarinic receptor antagonism (88), but muscarinic receptor antagonisms inhibits ~30% of the initial rise in SkBF during hyperthermia. In addition acetylcholinesterase inhibition augments the initial rise in SkBF through NO-dependent mechanisms. Collectively these data suggest that acetylcholine is capable of modulating the initial rise in active vasodilation (Tc <0.5°C) through NO-dependent mechanisms(87) but not in the established plateau phase (Tc >0.6°C). Additionally, activation of histamine 1 (H1) receptors also contributes to the rise in SkBF through NO pathways, which has been linked to VIP signaling (73), but VIP also has an NO-dependent component which is independent of H1 receptor activation (70). One putative source of VIP-induced histamine and NO is the degranulation of cutaneous mast cells (Figure 4). However, the precise source and potential interplay among VIP, histamine and NO signaling and their contribution to the initial vs. late phases of active vasodilation remain unclear.
In addition to NO, cyclooxygenase (COX)-dependent second messenger systems also contribute to active vasodilation (89). Whether COX-derived vasodilators are independent of or interact with the NO pathway remains unclear, but combined inhibition of COX and NOS, which attenuates active vasodilation in an additive fashion, suggesting independent mechanisms. One potential stimulus for COX-derived vasodilator synthesis is through acetylcholine mechanisms. Acetylcholine-mediated vasodilation has significant prostanoid and NO components. In the context of active vasodilation, prostanoid production through acetylcholine may contribute to the early phase of AVD (90) and further prostanoid production in the later phases of AVD may be the result of shear stress mechanisms (89). An alternative stimulus for COX-derived vasodilators is through NK1 receptor activation increasing endothelial intracellular calcium and activating both NOS and COX pathways (72). Collectively, the putative neurotransmitters(s) mediate the synthesis of COX-derived vasodilators and NO which both contribute to active vasodilation.
3.2.2. Aging
Human aging in the absence of overt pathology is associated with attenuated cutaneous vasodilation during thermal stress (78). The origins of the age-related reduction in SkBF during hyperthermia appear to be of peripheral origin due to decreased sensitivity of the active vasodilator system and not age-related alteration in noradrenergic mechanisms (82). Although, noradrenergic mechanisms are clearly altered with primary aging (11), augmented vasoconstriction with increasing body core temperature is not responsible for attenuated SkBF with body core heating (82). Similar to VC mechanisms (11), the direct contribution of cholinergic active VD co-transmitters to reflex VD is functionally absent in aged skin (75) and instead the aged rely primarily on impaired NO-dependent mechanisms to increase SkBF during hyperthermia. Because of these alterations in co-transmitter and second messenger mechanisms, aged humans require a much greater increase in Tc (Δ Tc ≥0.9°C) to stimulate significant functional contributions from non-NO-dependent pathways compared to young subjects (Δ Tc ≥0.6°C). Although, NO-dependent vasodilation in aged cutaneous vessels was reduced overall (91) and the NO contribution to reflex vasodilation is reduced with mild increases in Tc (Δ Tc 0.3–0.6°C) compared to young subjects, NO plays a significant role in reflex vasodilation in aged skin especially with moderate increase in Tc (Δ Tc 0.6°C-0.9°C). Because (1) many of the putative cholinergic neurotransmitter(s) induce VD in part through NO- and COX-dependent mechanisms, and (2) the documented system wide impairments in NO bioavailability with primary human aging (92), the both of these pathways has been important molecular targets for investigating the mechanisms underlying the age-related attenuation in SkBF.
3.2.2.1. Acetycholine
Because acetylcholine is capable of modulating the initial rise in SkBF in the early phase of active vasodilation, and aged subjects display reduced SkBF in this Tc range, one potential mechanism to explain the impaired SkBF is decreased sensitivity of aged cutaneous vessels to acetylcholine or alterations in acetylcholine-mediated second messenger signaling. Acute exogenous infusion of acetylcholine at dosages inducing an increase in SkBF similar in magnitude to that observed during the initial phase of reflex vasodilation have been used to examine potential downstream impairments in NO and COX signaling with aging. Contrary to what was hypothesized, exogenous acetylcholine infusion in aged skin caused similar absolute increases in SkBF compared to young skin, however the contributions of the downstream second messenger systems were altered. Specifically, COX-dependent vasodilation was attenuated (90) and endothelium-derived hyperpolarization factor- (EDHF) dependent vasodilation was augmented. Interestingly, NO pathways did not directly contribute to the total vasodilation produced by this exogenous dose of acetylcholine in either age group. Collectively, these data suggest that altered acetylcholine-mediated prostanoid-dependent vasodilation may contribute to reduced reflex vasodilation in aged cutaneous vasculature, but the precise contributions of COX-dependent and EDHF pathways during reflex vasodilation in aged skin are still unclear.
3.2.2.2. NO-dependent mechanisms
NO production depends on adequate substrate (L-arginine) availability and the intracellular localization of NOS in relation to the L-arginine subdomains(93). One potential mechanism limiting L-arginine for NO synthesis and full expression of reflex cutaneous vasodilation in the aged is through upregulation of vascular arginase. Arginase is the last enzyme of the urea cycle catalyzing the conversion of L-arginine to L-ornithine and urea, which are important precursors for polyamine and collagen synthesis. In the vasculature arginase preferentially utilizes the common NOS substrate, L-arginine, limiting substrate availability for NO synthesis through NOS. Arginase is upregulated in several animal models of endothelial dysfunction including aging, atherosclerosis, and hypertension however the mechanisms underlying the arginase upregulation are pathology-specific. In line with these animal models, acute arginase inhibition or direct L-arginine supplementation to the cutaneous vasculature significantly augments reflex vasodilation in aged human subjects (66). Interestingly, arginase inhibition, L-arginine supplementation or both increased SkBF similarly (~45 %SkBFmax), suggesting that the intracellular concentration of L-arginine allowed the L-arg-NOS pathway to operate near Vmax, and/or the cutaneous vessels were maximally vasodilated for the given degree of hyperthermic stress, approaching a so called “ceiling effect”. Collectively, these results suggest that L-arginine deficiency through upregulated arginase limits NO production, and increasing L-arginine availability for NO synthesis can augment reflex vasodilation in aged skin.
Another potential mechanism contributing to reduced NO bioavailability with human aging is an increase in oxidant stress (see Figure 5) contributing to an increase in NO breakdown. In aged human skin, maximal cutaneous vasodilation to an exogenous NO donor is attenuated in aged skin, suggesting either an impaired ability of vascular smooth muscle to respond to NO through cyclic GMP mechanisms, or that NO may be rapidly degraded by reactive oxygen species possibly forming pro-constrictor oxidants (Figure 6). Endogenous reactive oxygen species increase with aging and are capable of readily reacting with NO to form peroxynitrite (ONOO• −), which limit NO-dependent vasodilation and may potentially serve as a vasoconstrictor stimulus (94).
Figure 6.
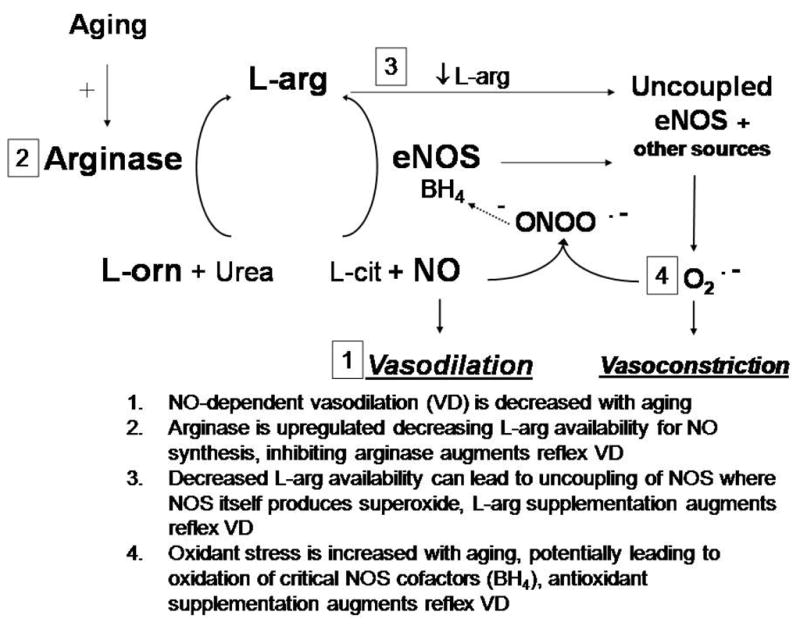
A schematic of the putative mechanisms affecting nitric oxide (NO) bioavailability in aged cutaneous vasculature. Primary human aging is associated with upregulated arginase activity which decreases L-arginine (L-arg) availability for NO synthesis through endothelial NO-synthase (eNOS). An age-associated increase in superoxide reacts with newly synthesized NO forming peroxynitrite (ONOO•−), which oxidizes the critical eNOS cofactor tetrahydrobiopterin (BH4). Decreased substrate (L-arg) and/or cofactor (BH4) availability leads to eNOS uncoupling where eNOS produces superoxide (O•−) instead of NO. Copyright permission obtained from Wolters Kluwer Health.
In humans, high dose non-specific antioxidant supplementation (ascorbate) is commonly used to examine effects of oxidant stress on NO-dependent vasodilation without affecting NOS activity. In aged human subjects localized high-dose ascorbate supplementation through intradermal microdialysis modestly increases SkBF during the secondary plateau phase of heat stress (ΔTc>0.7°C), however when ascorbate supplementation is combined with acute arginase inhibition there is a further increase in SkBF above what is observed with ascorbate alone(95). Localized ascorbate and/or arginase inhibition do not alter reflex vasodilation in young subjects. Collectively, these results suggest that 1) increased oxidant stress and upregulated arginase activity both limit NO bioavailability in aged skin and 2) reflex vasodilation can be augmented with ascorbate supplementation and acute arginase inhibition. Furthermore, these mechanisms may be functionally linked through the uncoupling of NO-synthase (NOS). Under circumstances where L-arginine and/or essential cofactors (tetrahydrobiopterin (BH4)) are limited, NOS can uncouple to produce superoxide instead of NO(94). In this deleterious cycle, NO reacts with superoxide forming peroxynitrite which then quickly oxidizes the critical NOS cofactor BH4 potentiating superoxide production through uncoupled NOS. Ascorbate is capable of increasing NO bioavailability by acting directly as an antioxidant and scavenging superoxide, and by stabilizing BH4. Because of the non-specific mechanism of action of ascorbate it is impossible to delineate whether the increase in NO-dependent vasodilation was through an antioxidant effect or through the corrections BH4 NOS uncoupling mechanisms. Future studies examining the relations between arginase, L-arginine and BH4 availability are necessary to more fully understand how NOS uncoupling may affect age-related reductions in cutaneous NO-dependent vasodilation.
3.2.2.3. Vessel structural alterations
Maximal cutaneous blood flow decreases linearly with age (96). This age-associated attenuation is observed in both absolute forearm maximal cutaneous vascular conductance (CVC) assessed with venous occlusion plethysmography and relative laser-Doppler flowmetry measures of SkBF. The reduction in maximal CVC with age presumably reflects structural changes to the vessel properties including 1) vascular smooth muscle hypertrophy and 2) a reduction in capillary density associated with flattening of the underside of the epidermis and reduction cutaneous microcirculatory capillary loops (97). While there are likely many signaling factors stimulating cutaneous microvascular smooth muscle hypertrophy and inward vessel remodeling, the candidate pathways include arginase (98) through the increase in polyamine and proline synthesis, Rho-kinase, and via constrictor factors produced through COX (99). Chronic activation of these pathways induces functional alterations in microvascular anatomy, which likely contributes to age-related cutaneous vessel structure alterations.
3.2.3. Clinical Populations
Healthy aged humans rely predominantly on compromised NO function to increase SkBF during hyperthermia, due to a reduced functional cotransmitter contribution (66, 75). Increasing NO bioavailability through a variety of mechanisms functionally augments reflex vasodilation in this healthy population. However, with endothelial dysfunction, vessel wall abnormalities, autonomic neuropathy and/or combinations of these neurovascular impairments that are manifested in a variety of clinically significant diseases which increase in frequency and severity concomitantly with aging.
3.2.3.1. Essential Hypertension
Essential hypertensive human subject exhibit an attenuated rise in skin blood flow during hyperthermia resulting in a functional reduction in heat transfer during exercise in the heat (100). While essential hypertension is associated with a significant increase in sympathetic activity the reduction in skin blood flow during hyperthermia is not due to an increase in sympathetic adrenergic vasoconstriction (101). Essential hypertensive humans exhibit a significant reductions in cutaneous NO-dependent vasodilation (102) and maximal cutaneous vasodilation reflecting endothelial dysfunction and inward vessel remodeling, respectively (102–104). Similar to primary aging, increased arginase activity and oxidant stress mechanisms contribute to reduced NO bioavailability in essential hypertensive cutaneous vasculature. In contrast the stimulus activating arginase and the intracellular regulation of NO substrate pools including impairments in cationic amino acid transporters (105) are different between essential hypertensive and primary aged humans (66, 76, 102, 104).
3.2.3.2. Type 2 Diabetes Mellitus
Type 2 diabetics also exhibit deficits in the reflex control of skin blood flow during whole body heat stress. Type 2 diabetics have an attenuated absolute reflex cutaneous vasodilator response reflecting a decreased sympathetic cholinergic neural stimulus (106). Additionally, the relative contribution of NO to the total lower skin blood flow response in type 2 diabetics is not different compared to age-matched control subjects (106). Together these findings indicate that in reflex cholinergic neural control, endothelial dysfunction, and inward vessel remodeling limit cutaneous vasodilator capacity during hyperthermia.
3.2.3.3. Congestive Heart Failure
The integrated cardiovascular response to hyperthermia includes an increase in skin blood flow that is facilitated by an increase in cardiac output coupled with a redistribution of blood flow from renal and splanchnic vascular beds. Congestive heart failure (CHF) patients have significant thermoregulatory challenges in the heat because of 1) central limitations to increase cardiac output and 2) impairments in microvascular function including substantial endothelial dysfunction. Patients with CHF have an attenuated cutaneous vasodilator response to both whole-body (107) and local heating (108). The later reflects both a reduction in NO-dependent vasodilation along with a reduction in maximal vasodilator capacity indicative of hypertrophic inward vessel remodeling. Interestingly, sweating responses are not different between CHF patients and age-matched controls, suggesting normal cholinergic skin sympathetic activity but a reduction in vascular end-organ responsiveness. The reduction in skin blood flow may serve as a counter-regulatory response to defend blood pressure regulation during heat exposure in subjects with cardiac insufficiency (107). Aspirin: In addition to microvascular pathology, commonly used pharmacological agents also affect reflex cutaneous vasodilator mechanisms. For example, chronic low-dose aspirin therapy (81mg daily) consistently and significantly attenuates reflex cutaneous vasodilation in middle-aged (58±3 years) human skin (109) which is unexpected considering the half-life and mechanisms of action on low-dose aspirin (Figure 7). While aspirin is an irreversible inhibitor of platelet and vascular COX I and II, at low doses aspirin acetylates platelet COX-1 in the presystemic (portal) circulation (110) inhibiting platelet production thromboxane A2 (TXA2) while sparing vascular endothelial production of COX-derived vasodilators. While precise mechanisms underlying attenuated reflex vasodilation in humans engaging in aspirin therapy are unclear several possibilities exist including: 1) platelet activation during hyperthermia may release substances that directly stimulate cutaneous vasodilator pathways, and/or 2) aspirin-induced differences in whole blood viscoelasticity may reduce shear-mediated cutaneous vasodilation. Considering the widespread use of low-dose aspirin and the unexpected potential for this therapy to impair thermoregulatory cutaneous vasodilation, further research examining the underlying neurovascular signaling mechanisms and potential functional consequences is necessary.
3.3. Local vasodilation
3.3.1. Young
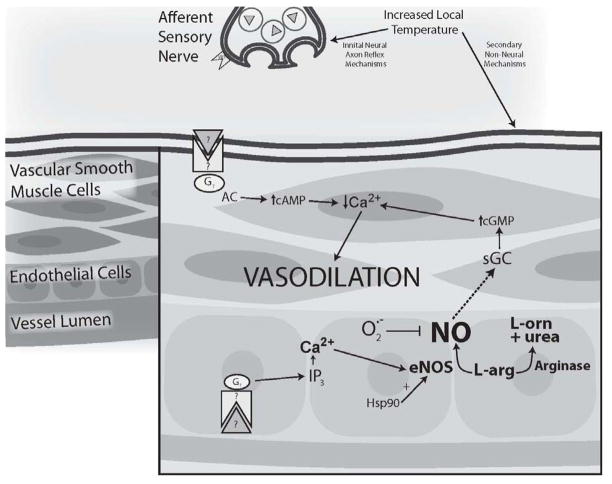
Local heating the skin to temperatures below the pain threshold (~43°C) produces a temperature-dependent (up to 42°C) increase in SkBF that is mechanistically distinct from the reflex mechanisms that increase SkBF in response to increased Tc (68). Skin heating induces a biphasic increase in SkBF that is mediated by two independent mechanisms (111). The initial rapid increase in SkBF is caused by a sensory axon reflex. The initial increase in SkBF is followed by a brief nadir, and then the secondary phase consists of a more slowly developing rise to a stable plateau in SkBF that is predominantly dependent on NO mechanisms (111) (Figure 8).
Figure 8.
Schematic representation of the putative mechanism of local heating-induced vasodilation with relevant age-related alterations. The initial vasodilation in response to local heating is mediated by a sensory axon reflex. Activation of valliniod type 1 receptors (VR1) has been implicated in the sensory nerve stimulation of the axon reflex. Putative neurtotransmitters (?) mediating the axon reflex include substance P and calcitonin gene related peptide (CGRP). The secondary (neurally independent) phase of local heating is predominantly (~70%) dependent on NO. Temperature-induced endothelial NOS (eNOS) activation and increased heat shock protein 90 have been implicated in the plateau phase. Aging-related alterations in the mechanism include a decreased axon reflex with a reduction in the capsaicin-sensitive primary afferent contribution. The NO-dependent plateau is also significantly reduced in aged skin. Copyright permission obtained from Wolters Kluwer Health.
The initial axon reflex phase of the local heating response is thought to be mediated by temperature-induced activation of C-fiber afferent neurons that release substance P and calcitonin gene related peptide (GCRP). Both of these vasoactive peptides are localized in sensory nerve terminals in human skin and are hypothesized to interact to modulate the amplitude and duration of cutaneous vasodilation; however, their precise role in the local heating response has not been established. NO also contributes modestly to the initial rise in SkBF with local heating. Heat sensitive vanilliod type 1 receptor (VR1) activation on sensory afferent nerves is also implicated in the initial axon reflex response to local heating. Sensitization of these receptors with capsaicin causes a dose-dependent leftward shift (toward lower temperatures) in the temperature at which the axon reflex is initiated (112).
NO mediates approximately 70% of the secondary prolonged plateau phase of the local heating response. Elevated temperature augments endothelial NOS (eNOS) mRNA expression and activity and increases synthesis of the eNOS molecular chaperone heat shock protein 90 (HSP90) which potentiates eNOS activity. Inhibition of HSP90 with geldanamyacin (specific to eNOS) decreased the plateau phase of the local heating response by ~20% (113), indicating that 1) eNOS appears to be the specific NOS isoform activated during local heating and 2) there is potential for other second messenger pathways to be involved in the response. Studies examining COX-dependent pathways suggest that COX products are not involved (89). The precise mechanisms mediating NO production and the other potential vasodilators contributing to the local heating response remain unclear.
3.3.2. Aging
When standardized local heating is performed in aged humans, they display attenuated vasodilation due to a decreased axon reflex contribution to the initial peak (91). The reduction in the axon reflex mediating the initial peak in aged skin is not due to a decrease in the modest NO-contribution to the initial rise in SkBF(91). These findings suggests that there is either 1) a decreased sensory component to the change in temperature, 2) a reduction in the release of neurotransmitter(s) from the sensory nerves mediating the axon reflex, and/or 3) a reduced vascular responsiveness to the neurotransmitter(s). Topical capsaicin pretreatment of the skin desensitizes the heat sensitive nociceptors (VR1) in cutaneous primary afferents and has been utilized to elucidate a possible sensory component contributing to the reduced axon reflex response in aged subjects. In aged skin capsaicin pretreatment does not alter the response to rapid non-painful local heating; however in young skin capsaicin pretreatment decreased the initial rise in SkBF during heating compared to control (non-capsaicin) conditions. These data suggesting that there is an age-related decrease in the capsaicin-sensitive primary afferents contribution to the initial axon with rapid local heating (114). Because the neurotransmitter(s) that mediate the axon reflex remains elusive, there is currently no evidence for reduced neurotransmitter(s) release or vascular responsiveness from sensory afferents.
In addition to an attenuated axon reflex, there is also a reduction in the NO-dependent plateau phase of the local heating response. Although the precise mechanisms mediating this reduction during local heating have not been systematically explored, upregulated arginase activity and an increase in oxidant stress may reduce the NO-contribution to the local heating response in aged skin. These mechanisms contribute to attenuated reflex cutaneous vasodilation with whole body heating and may also contribute to the age-related attenuation in SkBF during local heating. Furthermore, the NO-dependent plateau phase of the local heating response in aged skin can be augmented with exercise intervention strategies (115).
3.3.3. Clinical Populations
In recent years the cutaneous circulation has emerged as an accessible and potentially representative vascular bed to examine the mechanisms of microcirculatory function and dysfunction (116–119). Pathology-induced vascular dysfunction (including impaired endothelium-dependent vasodilation) is evident in the cutaneous circulation (102, 104, 120, 121), and may mirror generalized systemic vascular dysfunction in magnitude and underlying mechanisms (116, 117, 119, 122). Further, minimally-invasive skin-specific methodologies including local heating makes the cutaneous circulation a useful translational model for investigating mechanisms of vascular disease and providing preclinical data about the state of microcirculatory function in high risk populations. To date, the skin has been utilized as a model circulation to investigate vascular mechanisms in a variety of disease states, including: hypercholesterolemia (123), hypertension (121, 124), hyperhomocysteinemia (125), renal disease (119), Type II diabetes (126), peripheral vascular disease (127), atherosclerotic coronary artery disease (128), heart failure (107, 108), and systemic sclerosis (129).
3.4. Conclusions
In summary, the age-related attenuation in SkBF during whole body heating is due to impairments in the active vasodilator system. Aged humans have a reduced non-NO-dependent contribution to active vasodilation and rely primarily on impaired NO-dependent mechanisms to induce cutaneous vasodilation. Alterations in downstream acetylcholine-mediated vasodilation through COX may contribute to the reduced initial rise in SkBF in aged skin. NO-dependent vasodilation is limited in aged cutaneous vessels through upregulated arginase activity and an increase in oxidant stress limiting NO synthesis and increasing NO degradation, respectively. Cutaneous microvessel structural alterations including inward vessel remodeling also play a role in attenuated reflex vasodilation. Several clinical populations including essential hypertensives type 2 diabetics, and CHF patients are further at risk from suffering the consequences of attenuated reflex vasodilation due to pathology-, pharmacologic- and primary aged-related impairments in reflex vasodilator mechanisms.
In terms of the local heating response, both the axon reflex and the NO-dependent plateau phase of the local heating response are attenuated in aged skin. Because the SkBF response to local heating is predominately NO-dependent, local heating may be of value to non-invasively assess cutaneous vascular function and endothelium-dependent vasodilation in preclinical populations.
Figure 2.
Schematic model of the role of Rho kinase in local cold-induced vasoconstriction. Localized cooling of cutaneous vessels and surrounding tissue stimulates the production of mitochondrial superoxide. Superoxide (O2−) activates RhoA and Rho kinase, which can stimulate vasoconstriction through 2 distinct pathways: 1) Translocation of α2C-adrenoceptors from intracellular storage to the cell membrane, joining α2A- and α1-adrenoceptors to bind norepinephrine (NE), which leads to increased intracellular [Ca2+] and Ca2+-dependent vasoconstriction through phosphorylation of myosin light chain (MLC) by myosin light chain kinase (MLCK); and 2) Inhibition of myosin light chain phosphatase (MLCP) permits extant MLC phosphorylation to remain, thereby stimulating constriction in the absence of an increase in intracellular [Ca2+]. --- = inhibitory effects; — = stimulatory/activation effects. Copyright permission obtained from Wolters Kluwer Health.
References
- 1.Stephens DP, Aoki K, Kosiba WA, Johnson JM. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am J Physiol Heart Circ Physiol. 2001;280:H1496–504. doi: 10.1152/ajpheart.2001.280.4.H1496. [DOI] [PubMed] [Google Scholar]
- 2.Stephens DP, Bennett LA, Aoki K, Kosiba WA, Charkoudian N, Johnson JM. Sympathetic nonnoradrenergic cutaneous vasoconstriction in women is associated with reproductive hormone status. Am J Physiol Heart Circ Physiol. 2002;282:H264–72. doi: 10.1152/ajpheart.2002.282.1.H264. [DOI] [PubMed] [Google Scholar]
- 3.Ekenvall L, Lindblad LE, Norbeck O, Etzell BM. alpha-Adrenoceptors and cold-induced vasoconstriction in human finger skin. Am J Physiol. 1988;255:H1000–3. doi: 10.1152/ajpheart.1988.255.5.H1000. [DOI] [PubMed] [Google Scholar]
- 4.Pergola PE, Kellogg DL, Jr, Johnson JM, Kosiba WA, Solomon DE. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol. 1993;265:H785–92. doi: 10.1152/ajpheart.1993.265.3.H785. [DOI] [PubMed] [Google Scholar]
- 5.Cankar K, Finderle Z, Strucl M. The role of alpha1- and alpha2-adrenoceptors in gender differences in cutaneous LD flux response to local cooling. Microvasc Res. 2004;68:126–31. doi: 10.1016/j.mvr.2001.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Johnson JM, Yen TC, Zhao K, Kosiba WA. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am J Physiol Heart Circ Physiol. 2005;288:H1573–9. doi: 10.1152/ajpheart.00849.2004. [DOI] [PubMed] [Google Scholar]
- 7.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Cold-induced cutaneous vasoconstriction is mediated by Rho kinase in vivo in human skin. Am J Physiol Heart Circ Physiol. 2007;292:H1700–5. doi: 10.1152/ajpheart.01078.2006. [DOI] [PubMed] [Google Scholar]
- 8.Hodges GJ, Zhao K, Kosiba WA, Johnson JM. The involvement of nitric oxide in the cutaneous vasoconstrictor response to local cooling in humans. J Physiol. 2006;574:849–57. doi: 10.1113/jphysiol.2006.109884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins KJ, Dore C, Exton-Smith AN, Fox RH, MacDonald IC, Woodward PM. Accidental hypothermia and impaired temperature homoeostasis in the elderly. Br Med J. 1977;1:353–6. doi: 10.1136/bmj.1.6057.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenney WL, Armstrong CG. Reflex peripheral vasoconstriction is diminished in older men. J Appl Physiol. 1996;80:512–5. doi: 10.1152/jappl.1996.80.2.512. [DOI] [PubMed] [Google Scholar]
- 11.Thompson CS, Kenney WL. Altered neurotransmitter control of reflex vasoconstriction in aged human skin. J Physiol. 2004;558:697–704. doi: 10.1113/jphysiol.2004.065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degroot DW, Kenney WL. Impaired defense of core temperature in aged humans during mild cold stress. Am J Physiol Regul Integr Comp Physiol. 2007;292:R103–8. doi: 10.1152/ajpregu.00074.2006. [DOI] [PubMed] [Google Scholar]
- 13.Curriero FC, Heiner KS, Samet JM, Zeger SL, Strug L, Patz JA. Temperature and mortality in 11 cities of the eastern United States. Am J Epidemiol. 2002;155:80–7. doi: 10.1093/aje/155.1.80. [DOI] [PubMed] [Google Scholar]
- 14.Statistics, NCfH. Health, United States, 2005 with Chartbook on Trends in the Health of Americans. National Center on Health Statistics, Dept of Health and Human Services, CDC, Dept. of Health and Human Services, Centers for Disease Control and Prevention; 2005. pp. 1–550. [Google Scholar]
- 15.Hajat S, Kovats RS, Lachowycz K. Heat-related and cold-related deaths in England and Wales: who is at risk? Occup Environ Med. 2007;64:93–100. doi: 10.1136/oem.2006.029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson-Torgerson CS, Holowatz LA, Kenney WL. Altered mechanisms of thermoregulatory vasoconstriction in aged human skin. Exerc Sport Sci Rev. 2008;36:122–7. doi: 10.1097/JES.0b013e31817bfd47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernard C. An Introduction tot he Study of Experimental Medicine. Macmillan & Co; New York: 1865. [Google Scholar]
- 18.Green HD, Kepchar JH. Control of peripheral resistance in major systemic vascular beds. Physiol Rev. 1959;39:617–86. doi: 10.1152/physrev.1959.39.3.617. [DOI] [PubMed] [Google Scholar]
- 19.Fox RH, Edholm OG. Nervous control of the cutaneous circulation. Br Med Bull. 1963;19:110–4. doi: 10.1093/oxfordjournals.bmb.a070027. [DOI] [PubMed] [Google Scholar]
- 20.Campos JM, Paniagua P. Hypothermia during cardiac surgery. Best Pract Res Clin Anaesthesiol. 2008;22:695–709. doi: 10.1016/j.bpa.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Torossian A. Survey on intraoperative temperature management in Europe. Eur J Anaesthesiol. 2007;24:668–75. doi: 10.1017/S0265021507000191. [DOI] [PubMed] [Google Scholar]
- 22.Kurz A. Physiology of thermoregulation. Best Pract Res Clin Anaesthesiol. 2008;22:627–44. doi: 10.1016/j.bpa.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Sumi-Ichinose C, Urano F, Kuroda R, Ohye T, Kojima M, Tazawa M, Shiraishi H, Hagino Y, Nagatsu T, Nomura T, Ichinose H. Catecholamines and serotonin are differently regulated by tetrahydrobiopterin. A study from 6-pyruvoyltetrahydropterin synthase knockout mice. J Biol Chem. 2001;276:41150–60. doi: 10.1074/jbc.M102237200. [DOI] [PubMed] [Google Scholar]
- 24.Habecker BA, Klein MG, Sundgren NC, Li W, Woodward WR. Developmental regulation of neurotransmitter phenotype through tetrahydrobiopterin. J Neurosci. 2002;22:9445–52. doi: 10.1523/JNEUROSCI.22-21-09445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2006;26:2439–44. doi: 10.1161/01.ATV.0000243924.00970.cb. [DOI] [PubMed] [Google Scholar]
- 26.Kellogg DL, Jr, Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol. 1989;257:H1599–606. doi: 10.1152/ajpheart.1989.257.5.H1599. [DOI] [PubMed] [Google Scholar]
- 27.Ekblad E, Edvinsson L, Wahlestedt C, Uddman R, Hakanson R, Sundler F. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul Pept. 1984;8:225–35. doi: 10.1016/0167-0115(84)90064-8. [DOI] [PubMed] [Google Scholar]
- 28.Burnstock G. Mechanisms of interaction of peptide and nonpeptide vascular neurotransmitter systems. J Cardiovasc Pharmacol. 1987;10(Suppl 12):S74–81. [PubMed] [Google Scholar]
- 29.Lundberg JM. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol Rev. 1996;48:113–78. [PubMed] [Google Scholar]
- 30.Han S, Yang CL, Chen X, Naes L, Cox BF, Westfall T. Direct evidence for the role of neuropeptide Y in sympathetic nerve stimulation-induced vasoconstriction. Am J Physiol. 1998;274:H290–4. doi: 10.1152/ajpheart.1998.274.1.H290. [DOI] [PubMed] [Google Scholar]
- 31.Burnstock G. Purinergic cotransmission. Brain Res Bull. 1999;50:355–7. doi: 10.1016/s0361-9230(99)00103-3. [DOI] [PubMed] [Google Scholar]
- 32.Bradley E, Law A, Bell D, Johnson CD. Effects of varying impulse number on cotransmitter contributions to sympathetic vasoconstriction in rat tail artery. Am J Physiol Heart Circ Physiol. 2003;284:H2007–14. doi: 10.1152/ajpheart.01061.2002. [DOI] [PubMed] [Google Scholar]
- 33.Stachenfeld NS, Silva C, Keefe DL. Estrogen modifies the temperature effects of progesterone. J Appl Physiol. 2000;88:1643–9. doi: 10.1152/jappl.2000.88.5.1643. [DOI] [PubMed] [Google Scholar]
- 34.Khan F, V, Spence A, Belch JJ. Cutaneous vascular responses and thermoregulation in relation to age. Clin Sci (Lond) 1992;82:521–8. doi: 10.1042/cs0820521. [DOI] [PubMed] [Google Scholar]
- 35.Richardson D, Tyra J, McCray A. Attenuation of the cutaneous vasoconstrictor response to cold in elderly men. J Gerontol. 1992;47:M211–4. doi: 10.1093/geronj/47.6.m211. [DOI] [PubMed] [Google Scholar]
- 36.Frank SM, Raja SN, Bulcao C, Goldstein DS. Age-related thermoregulatory differences during core cooling in humans. Am J Physiol Regul Integr Comp Physiol. 2000;279:R349–54. doi: 10.1152/ajpregu.2000.279.1.R349. [DOI] [PubMed] [Google Scholar]
- 37.Grassi G, Seravalle G, Turri C, Bertinieri G, Dell’Oro R, Mancia G. Impairment of thermoregulatory control of skin sympathetic nerve traffic in the elderly. Circulation. 2003;108:729–35. doi: 10.1161/01.CIR.0000081769.02847.A1. [DOI] [PubMed] [Google Scholar]
- 38.Connat JL, Busseuil D, Gambert S, Ody M, Tebaldini M, Gamboni S, Faivre B, Quiquerez AL, Millet M, Michaut P, Rochette L. Modification of the rat aortic wall during ageing; possible relation with decrease of peptidergic innervation. Anat Embryol (Berl) 2001;204:455–68. doi: 10.1007/s429-001-8002-0. [DOI] [PubMed] [Google Scholar]
- 39.Donoso V, Gomez CR, Orriantia MA, Perez V, Torres C, Coddou C, Nelson P, Maisey K, Morales B, Fernandez R, Imarai M, Huidobro-Toro JP, Sierra F, Acuna-Castillo C. The release of sympathetic neurotransmitters is impaired in aged rats after an inflammatory stimulus: a possible link between cytokine production and sympathetic transmission. Mech Ageing Dev. 2008;129:728–34. doi: 10.1016/j.mad.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Droge W. Oxidative stress and aging. Adv Exp Med Biol. 2003;543:191–200. doi: 10.1007/978-1-4419-8997-0_14. [DOI] [PubMed] [Google Scholar]
- 41.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–54. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 42.Delp MD, Behnke BJ, Spier S, Wu G, Muller-Delp J. Ageing diminishes endothelium-dependent vasodilation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol. 2007 doi: 10.1113/jphysiol.2007.147686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin Augments Endothelium-Dependent Dilation in Sedentary but Not Habitually Exercising Older Adults. J Physiol. 2005;568:1057–65. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, Jitsuiki D, Goto C, Oshima T, Chayama K, Yoshizumi M. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis. 2006;186:390–5. doi: 10.1016/j.atherosclerosis.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 45.Konishi C, Naito Y, Ohara N. Age-related changes in adenosine 5′-triphosphate-induced constriction of isolated, perfused mesenteric arteries of rats. Life Sci. 1999;64:1265–73. doi: 10.1016/s0024-3205(99)00061-2. [DOI] [PubMed] [Google Scholar]
- 46.Lambert ML, I, Callow D, Feng QP, Arnold JM. The effects of age on human venous responsiveness to neuropeptide Y. Br J Clin Pharmacol. 1999;47:83–9. doi: 10.1046/j.1365-2125.1999.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson CS, Holowatz LA, Kenney WL. Attenuated noradrenergic sensitivity during local cooling in aged human skin. J Physiol. 2005;564:313–9. doi: 10.1113/jphysiol.2004.080788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson CS, Holowatz LA, Kenney WL. Cutaneous vasoconstrictor responses to norepinephrine are attenuated in older humans. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1108–13. doi: 10.1152/ajpregu.00839.2004. [DOI] [PubMed] [Google Scholar]
- 49.Applebaum GD, Kim B. A case of recurrent and fatal hypothermia in a man with diabetic neuropathy. Diabetes Care. 2002;25:2108–9. doi: 10.2337/diacare.25.11.2108. [DOI] [PubMed] [Google Scholar]
- 50.Kitamura A, Hoshino T, Kon T, Ogawa R. Patients with diabetic neuropathy are at risk of a greater intraoperative reduction in core temperature. Anesthesiology. 2000;92:1311–8. doi: 10.1097/00000542-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 51.Kalincik T, Jozefcikova K, Waite PM, Carrive P. Local response to cold in rat tail after spinal cord transection. J Appl Physiol. 2009;106:1976–85. doi: 10.1152/japplphysiol.00095.2009. [DOI] [PubMed] [Google Scholar]
- 52.Nobel G, Eiken O, Tribukait A, Kolegard R, Mekjavic IB. Motion sickness increases the risk of accidental hypothermia. Eur J Appl Physiol. 2006;98:48–55. doi: 10.1007/s00421-006-0217-6. [DOI] [PubMed] [Google Scholar]
- 53.Murphy MT, Lipton JM. Effects of alcohol on thermoregulation in aged monkeys. Exp Gerontol. 1983;18:19–27. doi: 10.1016/0531-5565(83)90047-5. [DOI] [PubMed] [Google Scholar]
- 54.Hodges GJ, Traeger JA, 3rd, Tang T, Kosiba WA, Zhao K, Johnson JM. Role of sensory nerves in the cutaneous vasoconstrictor response to local cooling in humans. Am J Physiol Heart Circ Physiol. 2007;293:H784–9. doi: 10.1152/ajpheart.00323.2007. [DOI] [PubMed] [Google Scholar]
- 55.Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol. 2005;289:H243–50. doi: 10.1152/ajpheart.01305.2004. [DOI] [PubMed] [Google Scholar]
- 56.Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent alpha (2C)-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol. 2000;278:H1075–83. doi: 10.1152/ajpheart.2000.278.4.H1075. [DOI] [PubMed] [Google Scholar]
- 57.Jeyaraj SC, Chotani MA, Mitra S, Gregg HE, Flavahan NA, Morrison KJ. Cooling evokes redistribution of alpha2C-adrenoceptors from Golgi to plasma membrane in transfected human embryonic kidney 293 cells. Mol Pharmacol. 2001;60:1195–200. doi: 10.1124/mol.60.6.1195. [DOI] [PubMed] [Google Scholar]
- 58.Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of alpha2C-adrenoceptor translocation. Circ Res. 2004;94:1367–74. doi: 10.1161/01.RES.0000128407.45014.58. [DOI] [PubMed] [Google Scholar]
- 59.Eid AH, Maiti K, Mitra S, Chotani MA, Flavahan S, Bailey SR, Thompson-Torgerson CS, Flavahan NA. Estrogen increases smooth muscle expression of alpha2C-adrenoceptors and cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol. 2007;293:H1955–61. doi: 10.1152/ajpheart.00306.2007. [DOI] [PubMed] [Google Scholar]
- 60.Honda M, Suzuki M, Nakayama K, Ishikawa T. Role of alpha2C-adrenoceptors in the reduction of skin blood flow induced by local cooling in mice. Br J Pharmacol. 2007;152:91–100. doi: 10.1038/sj.bjp.0707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol. 2006;290:C661–8. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Somlyo AV. Cyclic GMP regulation of myosin phosphatase: a new piece for the puzzle? Circ Res. 2007;101:645–7. doi: 10.1161/CIRCRESAHA.107.161893. [DOI] [PubMed] [Google Scholar]
- 63.Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res. 2005;97:354–62. doi: 10.1161/01.RES.0000177669.29525.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flavahan NA. Regulation of vascular reactivity in scleroderma: new insights into Raynaud’s phenomenon. Rheum Dis Clin North Am. 2008;34:81–7. vii. doi: 10.1016/j.rdc.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Rho kinase-mediated local cold-induced cutaneous vasoconstriction is augmented in aged human skin. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.00152.2007. [DOI] [PubMed] [Google Scholar]
- 66.Holowatz LA, Thompson CS, Kenney WL. L-Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J Physiol. 2006;574:573–81. doi: 10.1113/jphysiol.2006.108993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- 68.Kellogg DL, Jr, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995;77:1222–8. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- 69.Bennett LA, Johnson JM, Stephens DP, Saad AR, Kellogg DL., Jr Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J Physiol. 2003;552:223–32. doi: 10.1113/jphysiol.2003.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilkins BW, Chung LH, Tublitz NJ, Wong BJ, Minson CT. Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. J Appl Physiol. 2004;97:1291–8. doi: 10.1152/japplphysiol.00366.2004. [DOI] [PubMed] [Google Scholar]
- 71.Savage MV, Brengelmann GL, Buchan AM, Freund PR. Cystic fibrosis, vasoactive intestinal polypeptide, and active cutaneous vasodilation. J Appl Physiol. 1990;69:2149–54. doi: 10.1152/jappl.1990.69.6.2149. [DOI] [PubMed] [Google Scholar]
- 72.Wong BJ, Minson CT. Neurokinin-1 Receptor Desensitisation Attenuates Cutaneous Active Vasodilatation in Humans. J Physiol. 2006 doi: 10.1113/jphysiol.2006.112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol. 2004;560:941–948. doi: 10.1113/jphysiol.2004.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilkins BW, Holowatz LA, Wong BJ, Minson CT. Nitric oxide is not permissive for cutaneous active vasodilatation in humans. J Physiol. 2003;548:963–9. doi: 10.1113/jphysiol.2002.035931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol. 2003;284:H1662–7. doi: 10.1152/ajpheart.00871.2002. [DOI] [PubMed] [Google Scholar]
- 76.Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am J Physiol Heart Circ Physiol. 2006 doi: 10.1152/ajpheart.00648.2006. [DOI] [PubMed] [Google Scholar]
- 77.Holowatz LA, Thompson-Torgerson CS, Kenney WL. Altered mechanisms of vasodilation in aged human skin. Exerc Sport Sci Rev. 2007;35:119–25. doi: 10.1097/jes.0b013e3180a02f85. [DOI] [PubMed] [Google Scholar]
- 78.Kenney WL. Control of heat-induced cutaneous vasodilatation in relation to age. Eur J Appl Physiol Occup Physiol. 1988;57:120–5. doi: 10.1007/BF00691250. [DOI] [PubMed] [Google Scholar]
- 79.McGeehin MA, Mirabelli M. The potential impacts of climate variability and change on temperature-related morbidity and mortality in the United States. Environ Health Perspect. 2001;109(Suppl 2):185–9. doi: 10.1289/ehp.109-1240665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Armstrong CG, Kenney WL. Effects of age and acclimation on responses to passive heat exposure. J Appl Physiol. 1993;75:2162–7. doi: 10.1152/jappl.1993.75.5.2162. [DOI] [PubMed] [Google Scholar]
- 81.Kenney WL, Tankersley CG, Newswanger DL, Hyde DE, Puhl SM, Turner NL. Age and hypohydration independently influence the peripheral vascular response to heat stress. J Appl Physiol. 1990;68:1902–8. doi: 10.1152/jappl.1990.68.5.1902. [DOI] [PubMed] [Google Scholar]
- 82.Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA. Decreased active vasodilator sensitivity in aged skin. Am J Physiol Heart Circ Physiol. 1997;272:H1609–14. doi: 10.1152/ajpheart.1997.272.4.H1609. [DOI] [PubMed] [Google Scholar]
- 83.Ho CW, Beard JL, Farrell PA, Minson CT, Kenney WL. Age, fitness, and regional blood flow during exercise in the heat. J Appl Physiol. 1997;82:1126–35. doi: 10.1152/jappl.1997.82.4.1126. [DOI] [PubMed] [Google Scholar]
- 84.Johnson JM, PDW . Cardiovascular Adjustments to Heat Stress. In: Fregly MJ, Blatteis CM, editors. Section 4: Environmental Physiology. Bethesda: 1996. [Google Scholar]
- 85.Schulze E, Witt M, Fink T, Hofer A, Funk RH. Immunohistochemical detection of human skin nerve fibers. Acta Histochem. 1997;99:301–9. doi: 10.1016/S0065-1281(97)80024-4. [DOI] [PubMed] [Google Scholar]
- 86.Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998;85:824–9. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- 87.Shibasaki M, Wilson TE, Cui J, Crandall CG. Acetylcholine released from cholinergic nerves contributes to cutaneous vasodilation during heat stress. J Appl Physiol. 2002;93:1947–51. doi: 10.1152/japplphysiol.00036.2002. [DOI] [PubMed] [Google Scholar]
- 88.Shastry S, Minson CT, Wilson SA, Dietz NM, Joyner MJ. Effects of atropine and L-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol. 2000;88:467–72. doi: 10.1152/jappl.2000.88.2.467. [DOI] [PubMed] [Google Scholar]
- 89.McCord GR, Cracowski JL, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol. 2006 doi: 10.1152/ajpregu.00710.2005. [DOI] [PubMed] [Google Scholar]
- 90.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol. 2005;563:965–73. doi: 10.1113/jphysiol.2004.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93:1644–9. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- 92.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–9. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 93.Topal JL, Brunet A, Walch L, Boucher JL, David-Dufilho M. Mitochondrial Arginase II Modulates Nitric-Oxide Synthesis through Nonfreely Exchangeable L-Arginine Pools in Human Endothelial Cells. J Pharmacol Exp Ther. 2006;318:1368–74. doi: 10.1124/jpet.106.103747. [DOI] [PubMed] [Google Scholar]
- 94.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–14. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 95.Holowatz LA, Thompson CS, Kenney WL. L-arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J Physiol. 2006 doi: 10.1113/jphysiol.2006.108993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin HL, Loomis JL, Kenney WL. Maximal skin vascular conductance in subjects aged 5–85 yr. J Appl Physiol. 1995;79:297–301. doi: 10.1152/jappl.1995.79.1.297. [DOI] [PubMed] [Google Scholar]
- 97.Li H, Witte K, August M, Brausch I, Godtel-Armbrust U, Habermeier A, Closs EI, Oelze M, Munzel T, Forstermann U. Reversal of endothelial nitric oxide synthase uncoupling and up-regulation of endothelial nitric oxide synthase expression lowers blood pressure in hypertensive rats. J Am Coll Cardiol. 2006;47:2536–44. doi: 10.1016/j.jacc.2006.01.071. [DOI] [PubMed] [Google Scholar]
- 98.Peyton KJ, Ensenat D, Azam MA, Keswani AN, Kannan S, Liu XM, Wang H, Tulis DA, Durante W. Arginase promotes neointima formation in rat injured carotid arteries. Arterioscler Thromb Vasc Biol. 2009;29:488–94. doi: 10.1161/ATVBAHA.108.183392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yi SL, Kantores C, Belcastro R, Cabacungan J, Tanswell AK, Jankov RP. 8-Isoprostane-induced endothelin-1 production by infant rat pulmonary artery smooth muscle cells is mediated by Rho-kinase. Free Radic Biol Med. 2006;41:942–9. doi: 10.1016/j.freeradbiomed.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 100.Kenney WL. Decreased core-to-skin heat transfer in mild essential hypertensives exercising in the heat. Clin Exp Hypertens A. 1985;7:1165–72. doi: 10.3109/10641968509073582. [DOI] [PubMed] [Google Scholar]
- 101.Kellogg DL, Jr, Morris SR, Rodriguez SB, Liu Y, Grossmann M, Stagni G, Shepherd AM. Thermoregulatory reflexes and cutaneous active vasodilation during heat stress in hypertensive humans. J Appl Physiol. 1998;85:175–80. doi: 10.1152/jappl.1998.85.1.175. [DOI] [PubMed] [Google Scholar]
- 102.Holowatz LA, Kenney WL. Up-regulation of arginase activity contributes to attenuated reflex cutaneous vasodilatation in hypertensive humans. J Physiol. 2007;581:863–72. doi: 10.1113/jphysiol.2007.128959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kenney WL, Kamon E, Buskirk ER. Effect of mild essential hypertension on control of forearm blood flow during exercise in the heat. J Appl Physiol. 1984;56:930–5. doi: 10.1152/jappl.1984.56.4.930. [DOI] [PubMed] [Google Scholar]
- 104.Holowatz LA, Kenney WL. Local ascorbate administration augments NO- and non-NO-dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.00295.2007. [DOI] [PubMed] [Google Scholar]
- 105.Schlaich MP, Parnell MM, Ahlers BA, Finch S, Marshall T, Zhang WZ, Kaye DM. Impaired L-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjects. Circulation. 2004;110:3680–6. doi: 10.1161/01.CIR.0000149748.79945.52. [DOI] [PubMed] [Google Scholar]
- 106.Sokolnicki LA, Strom NA, Roberts SK, Kingsley-Berg SA, Basu A, Charkoudian N. Skin blood flow and nitric oxide during body heating in type 2 diabetes mellitus. J Appl Physiol. 2009;106:566–70. doi: 10.1152/japplphysiol.91289.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Green DJ, Maiorana AJ, Siong JH, Burke V, Erickson M, Minson CT, Bilsborough W, O’Driscoll G. Impaired skin blood flow response to environmental heating in chronic heart failure. Eur Heart J. 2006;27:338–43. doi: 10.1093/eurheartj/ehi655. [DOI] [PubMed] [Google Scholar]
- 108.Cui J, Arbab-Zadeh A, Prasad A, Durand S, Levine BD, Crandall CG. Effects of heat stress on thermoregulatory responses in congestive heart failure patients. Circulation. 2005;112:2286–92. doi: 10.1161/CIRCULATIONAHA.105.540773. [DOI] [PubMed] [Google Scholar]
- 109.Holowatz LA, Kenney WL. Chronic low-dose aspirin therapy attenuates reflex cutaneous vasodilation in middle-aged humans. J Appl Physiol. 2008;106:500–5. doi: 10.1152/japplphysiol.91215.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pedersen AK, FitzGerald GA. Dose-related kinetics of aspirin. Presystemic acetylation of platelet cyclooxygenase. N Engl J Med. 1984;311:1206–11. doi: 10.1056/NEJM198411083111902. [DOI] [PubMed] [Google Scholar]
- 111.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–26. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 112.Stephens DP, Charkoudian N, Benevento JM, Johnson JM, Saumet JL. The influence of topical capsaicin on the local thermal control of skin blood flow in humans. Am J Physiol Regul Integr Comp Physiol. 2001;281:R894–901. doi: 10.1152/ajpregu.2001.281.3.R894. [DOI] [PubMed] [Google Scholar]
- 113.Shastry S, Joyner MJ. Geldanamycin attenuates NO-mediated dilation in human skin. Am J Physiol Heart Circ Physiol. 2002;282:H232–6. doi: 10.1152/ajpheart.2002.282.1.H232. [DOI] [PubMed] [Google Scholar]
- 114.Munce TA, Kenney WL. Age-specific modification of local cutaneous vasodilation by capsaicin-sensitive primary afferents. J Appl Physiol. 2003;95:1016–24. doi: 10.1152/japplphysiol.00934.2002. [DOI] [PubMed] [Google Scholar]
- 115.Black MA, Green DJ, Cable NT. Exercise prevents age-related decline in nitric-oxide-mediated vasodilator function in cutaneous microvessels. J Physiol. 2008 doi: 10.1113/jphysiol.2008.153742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abularrage CJ, Sidawy AN, Aidinian G, Singh N, Weiswasser JM, Arora S. Evaluation of the microcirculation in vascular disease. J Vasc Surg. 2005;42:574–81. doi: 10.1016/j.jvs.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 117.RG IJ, de Jongh RT, Beijk MA, van Weissenbruch MM, Delemarre-van de Waal HA, Serne EH, Stehouwer CD. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur J Clin Invest. 2003;33:536–42. doi: 10.1046/j.1365-2362.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 118.Sax FL, Cannon RO, 3rd, Hanson C, Epstein SE. Impaired forearm vasodilator reserve in patients with microvascular angina. Evidence of a generalized disorder of vascular function? N Engl J Med. 1987;317:1366–70. doi: 10.1056/NEJM198711263172202. [DOI] [PubMed] [Google Scholar]
- 119.Stewart J, Kohen A, Brouder D, Rahim F, Adler S, Garrick R, Goligorsky MS. Noninvasive interrogation of microvasculature for signs of endothelial dysfunction in patients with chronic renal failure. Am J Physiol Heart Circ Physiol. 2004;287:H2687–96. doi: 10.1152/ajpheart.00287.2004. [DOI] [PubMed] [Google Scholar]
- 120.Lindstedt IH, Edvinsson ML, Edvinsson L. Reduced responsiveness of cutaneous microcirculation in essential hypertension--a pilot study. Blood Press. 2006;15:275–80. doi: 10.1080/08037050600996586. [DOI] [PubMed] [Google Scholar]
- 121.Carberry PA, Shepherd AM, Johnson JM. Resting and maximal forearm skin blood flows are reduced in hypertension. Hypertension. 1992;20:349–55. doi: 10.1161/01.hyp.20.3.349. [DOI] [PubMed] [Google Scholar]
- 122.Rossi M, Carpi A, Galetta F, Franzoni F, Santoro G. The investigation of skin blood flowmotion: a new approach to study the microcirculatory impairment in vascular diseases? Biomed Pharmacother. 2006;60:437–42. doi: 10.1016/j.biopha.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 123.Khan F, Litchfield SJ, Stonebridge PA, Belch JJ. Lipid-lowering and skin vascular responses in patients with hypercholesterolaemia and peripheral arterial obstructive disease. Vasc Med. 1999;4:233–8. doi: 10.1177/1358836X9900400405. [DOI] [PubMed] [Google Scholar]
- 124.Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–5. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 125.Abahji TN, Nill L, Ide N, Keller C, Hoffmann U, Weiss N. Acute hyperhomocysteinemia induces microvascular and macrovascular endothelial dysfunction. Arch Med Res. 2007;38:411–6. doi: 10.1016/j.arcmed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 126.Sokolnicki LA, Roberts SK, Wilkins BW, Basu A, Charkoudian N. Contribution of nitric oxide to cutaneous microvascular dilation in individuals with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2006 doi: 10.1152/ajpendo.00365.2006. [DOI] [PubMed] [Google Scholar]
- 127.Rossi M, Carpi A. Skin microcirculation in peripheral arterial obliterative disease. Biomed Pharmacother. 2004;58:427–31. doi: 10.1016/j.biopha.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 128.Shamim-Uzzaman QA, Pfenninger D, Kehrer C, Chakrabarti A, Kacirotti N, Rubenfire M, Brook R, Rajagopalan S. Altered cutaneous microvascular responses to reactive hyperaemia in coronary artery disease: a comparative study with conduit vessel responses. Clin Sci (Lond) 2002;103:267–73. doi: 10.1042/cs1030267. [DOI] [PubMed] [Google Scholar]
- 129.Boignard A, Salvat-Melis M, Carpentier PH, Minson CT, Grange L, Duc C, Sarrot-Reynauld F, Cracowski JL. Local hyperemia to heating is impaired in secondary Raynaud’s phenomenon. Arthritis Res Ther. 2005;7:R1103–12. doi: 10.1186/ar1785. [DOI] [PMC free article] [PubMed] [Google Scholar]