Abstract
Glucagon levels are elevated in diabetes and some liver diseases. Increased glucagon secretion leads to abnormal stimulation of glucagon receptors (GRs) and consequent elevated glucose production in the liver. Blocking glucagon receptor signaling has been proposed as a potential treatment option for diabetes and other conditions associated with hyperglycemia. Elucidating mechanisms of GR desensitization and downregulation may help identify new drug targets besides GR itself. The present study explores the mechanisms of GR internalization and the role of PKCα, GPCR kinases (GRKs) and β-arrestins therein. We have reported previously that PKCα mediates GR phosphorylation and desensitization. While the PKC agonist, PMA, did not affect GR internalization when tested alone, it increased glucagon-mediated GR internalization by 25–40% in GR-expressing HEK-293 cells (HEK-GR cells). In both primary hepatocytes and HEK-GR cells, glucagon treatment recruited PKCα to the plasma membrane where it colocalized with GR. We also observed that overexpression of GRK2, GRK3, or GRK5 enhanced GR internalization. In addition, we found that GR utilizes both clathrin- and caveolin-mediated endocytosis in HEK-GR cells. Glucagon triggered translocation of both β-arrestin1 and β-arrestin2 from the cytosol to the perimembrane region, and overexpression of β-arrestin1 and β-arrestin2 increased GR internalization. Furthermore, both β-arrestin1 and β-arrestin2 colocalized with GR and with Cav-1, suggesting the possible involvement of these arrestins in GR internalization.
Keywords: glucagon receptor, PKCα, GRK, β-arrestin
INTRODUCTION
Glucagon plays an important role in the regulation of blood glucose homeostasis. Dysregulation of glucagon metabolism is associated with several diseases, most importantly with diabetes, where the basal level of glucagon is elevated and the bihormonal insulin-to-glucagon relationship is altered [1–3]. Chronic hyperglucagonemia leads to increased hepatic production of glucose, aggravating hyperglycemia in diabetic patients. Suppression of glucagon secretion by somatostatin improves the condition of diabetic subjects. In recent years there has been an increased interest in the glucagon receptor (GR) as a therapeutic target in diabetes management [4]. However, application of GR antagonists in the chronic treatment of diabetes has had limited success thus far [5]. Therefore, gaining a better understanding of the mechanisms of GR desensitization is critical for evaluation of drug efficacy and identification of novel drug targets for diabetes.
The GR is a prototypical, Family B heptahelical G protein-coupled receptor (GPCR). Prolonged agonist stimulation attenuates signaling through GPCRs. This phenomenon, termed homologous desensitization, ensures precise spatiotemporal regulation of signal transduction by filtering input from multiple receptors and protecting against acute and chronic over-stimulation [6,7]. The first phase of desensitization involves phosphorylation of receptors by GPCR kinases (GRKs). GRK-mediated phosphorylation promotes binding of β-arrestins, which results in uncoupling of receptors from G proteins. In addition, it has been suggested that GRK2 and GRK3 can mediate GPCR desensitization through interaction with receptors in a phosphorylation-independent manner [8,9]. Other kinases, such as PKC and PKA, mediate desensitization and downregulation of some GPCRs, even in the absence of respective ligands (heterologous desensitization).
Previous studies from other laboratories and ours have shown that glucagon-stimulated cAMP production is diminished upon treatment of cells with glucagon and other hormones, such as angiotensin and vasopressin, as well as with PKC activating agents, suggesting that GRs undergo both homologous and heterologous internalization [10,11].
Desensitized receptors are sequestered in the cytoplasm within minutes of agonist stimulation. Internalization of receptors is the primary mechanism of signal transduction attenuation and is also crucial for receptor resensitization. Whereas most GPCRs utilize clathrin-mediated endocytosis, utilization of clathrin-independent pathways, including caveolar endocytosis, has been reported [12].
We and others have previously shown that, upon 30 minutes of glucagon stimulation, GRs are internalized in vitro [13,14] and in vivo [15]. The receptor’s carboxyl terminus is required for internalization of both the human [13]and rat GR [14]. Phosphorylation of certain Ser residues has been shown to be necessary for GR internalization [15]. However, the precise mechanisms of GR internalization are largely unknown. Therefore, the aim of the present study was to identify the key pathways of GR internalization. In this study, we have investigated (i) the role of PKC and GRK in GR internalization, (ii) utilization of different internalization pathways by GR, and (iii) ability of β-arrestin1 and β-arrestin2 to promote GR internalization.
MATERIALS AND METHODS
Materials
Cell culture media, Penicillin/Streptomycin, L-glutamine and amino acids were from Cellgro (Kansas City, MO). Nystatin, Filipin III, DABCO, Poly-L-lysine, phenylarsine oxide, Geneticin/G418, Tetracycline, Sodium-butyrate, bovine serum albumin (BSA), 1,4-diazobicyclo[2,2,2]octane (DABCO), paraformaldehyde, phenylmethylsulphonyl fluoride (PMSF), phosphate-buffered saline (PBS), Tris-buffered saline, and Di(N-succinimidyl) 3,3′-dithiodipropionate were from Sigma (St Louis, MO). Glucagon was purchased from Bachem (King of Prussia, PA). 125I-glucagon was purchased from Linco (St. Charles, MO). Phorbol 12-myristate 13-acetate (PMA), Forskolin, Phorbol 12,13-Dibutyrate (PDBu), 4α-phorbol ester, and Mowiol were purchased from Calbiochem (San Diego, CA). The transfection reagents Lipofectamine 2000 and OptiMEM were from Invitrogen (Rockville, MD). Lysotracker and Texas Red – Phalloidin and Hoescht 33258, as well as the fluorescently labeled secondary antibodies, goat anti-rabbit Alexa fluor 568, goat anti-mouse Alexa fluor 350, goat anti-mouse Alexa fluor 568 and goat anti-mouse Alexa fluor 488 were from Molecular Probes (Carlsbad, CA). The mouse anti-clathrin heavy chain antibody and the rabbit anti-EEA1 antibody were from Affinity Bioreagents (Golden, CO). The GR antibody (ST-18) was generated in rabbit for an epitope in the last 18 amino acids as previously reported [16]. The rabbit anti-caveolin (Cav)-1 antibody and the mouse anti-FLAG antibody were from Sigma (St. Louis, MO). The rabbit anti-PKCα antibody used for Western blots was from Research Diagnostics (Concord, MA). The rabbit anti-PKCα antibody used for immunoprecipitation experiments was from Cell Signaling Technology (Danvers, MA). The rabbit anti-PKCδ antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). The rabbit anti-GAPDH antibody was from Trevigen (Gaithersburg, MD).
Plasmids
β-arrestin1-GFP and β-arrestin2-GFP plasmids were a generous gift of Dr. Cornelius Krasel (University of Würzburg, Würzburg, Germany). Dominant negative (DN) β-arrestin1 (V53D), β-arrestin2 DN (arr2 319–418), GRK2, GRK3 and GRK5 were a generous gift from Dr. Jeffrey L. Benovic (Thomas Jefferson University, Philadelphia, PA). PKCα and PKCα DN were kindly provided by Dr. Jae-Won Soh (Inha University, Incheon, South Korea). PKCα-YFP was kindly provided by Dr. R. Kubitz (Heinrich-Heine University, Dusseldorf, Germany). Plasmid pcDNA 3.1, used as an empty vector control, was purchased from Invitrogen (Rockville, MD).
Cell culture and transfection
Hepatocytes were isolated by the collagenase perfusion technique as previously described [17] from male Golden Syrian hamsters (Harlan Sprague Dawley, Indianapolis, IN, 100–130g body wt) fed a rodent chow diet ad libitum. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, L-glutamine (200 μM), MEM non-essential and MEM essential amino acids, Penicillin (5000 I.U./mL) and Streptomycin (5000 μg/mL). HEK-293 cells stably expressing the rat glucagon receptor (HEK-GR, [18] were maintained under the culture conditions described above. HEK-293 cells stably expressing a tetracycline-inducible FLAG-tagged GR (HEK-FLAG-GR) [16]were maintained in DMEM/F-12 (50:50) supplemented with 200 μg/mL Geneticin/G418, 10% fetal calf serum and Penicillin/Streptomycin. The GR expression was induced by incubation of cells in an induction medium, DMEM/F-12 supplemented with sodium butyrate, tetracycline, 10% serum and Penicillin (5000 I.U./mL) and Streptomycin (5000 μg/mL) for 24–48 hours. The cells were transfected with 4–8 μg plasmid DNA using Lipofectamine 2000, following the manufacturer’s instructions. Control cells were transfected with the equivalent amount of vector plasmid (empty vector). Twenty-four hours after transfection, the cells were seeded onto poly-L-lysine coated coverslips and glass-bottom dishes (for microscopy) or onto 24-well plates (for binding). On average, the transfection efficiency was between 60–80%.
GR-GFP C-Terminus Fusion Plasmid Construction
The consecutive stop codons of the GR cDNA inserted in pGEM-2 plasmid were abolished by site-directed mutagenesis (QuikChange XL, Stratagene Corp., La Jolla, CA) using PAGE-purified primers (sense: 5′-GACAGCCCCACCTGTAAGCGGCCGCAGCTTCC-3′/antisense 5′-GGAAGCTGCGGCCGCTTACAGGTGGGGCTGTC-3′) (Sigma Genosys, The Woodlands, TX). The modified GR cDNA was amplified by PCR using primers (forward: 5′-CATCATCCATCTGACATTGGGACGCGTCG-3′, reverse: 5′-GAATACACGGAATTCCACCATGCTCCTCACC-3′) (Sigma Genosys, The Woodlands, TX). PCR products were resolved on a 1% TBE agarose gel and the mutated GR cDNA insert band was excised, purified and ligated into the pcDNA3.1/CT-GFP-TOPO expression vector (Invitrogen/Life Technologies, Carlsbad, CA) following the manufacturer’s protocol to create TOPO/GR. Ligation reactions were verified by EcoRI/NotI digestion as well as by sequencing.
Confocal and fluorescence microscopy
The cells were seeded onto poly-L-lysine coated multi-chambered coverglass, coverslips or glass-bottom 35-mm dishes (for live cell microscopy). The cells were washed twice and serum-starved for one hour prior to treatment with drugs. After treatment, the cells were washed twice with PBS, fixed in 3.7% paraformaldehyde for 10 min, permeabilized with 1% Triton X-100 (Sigma, St Louis, MO), according to previously reported methods [19]. The cells were incubated with blocking buffer (8% BSA/PBS) for 1 hour. Primary and secondary antibodies were diluted in 2% BSA/PBS buffer and incubated with the cells for 1 hour, respectively. Between primary and secondary antibody incubations, the coverslips were washed 3 times for 5 min. The coverslips were mounted onto glass slides using Mowiol with DABCO. Images were taken either on a confocal laser scanning microscope (Olympus IX-70 microscope and Bio-Rad MRC 1024 confocal laser scanning system) or on a fluorescence microscope (Olympus IX-81 fluorescence microscope with a Cool Snap HG Photometrics CCD camera). For each frame, four to eight planes were acquired and the projection images were deconvolved using Slidebook 4.1 software (Intelligent Imaging Innovations, Denver, CO). In live cell time lapse experiments, the cells were maintained at 37°C using a heated microscope stage, and the images were acquired at 0.5–2 minute intervals.
Cell fractionation
Crude membrane and cytoplasmic fractions were purified from freshly isolated Golden Syrian hamster hepatocytes. Briefly, cells were washed twice on ice in cold PBS, centrifuged and resuspended in membrane isolation buffer (50 mM Tris HCl, pH 7.4, 2.5 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol (DTT), 5 mM NaF, 1 mM Na3VO4), supplemented with protease inhibitors (Roche Bioscience, Palo Alto, CA), and passed through a 26-gauge needle at least 10 times. Samples were cleared of unbroken cells and nuclei by centrifugation at 1000 × g for 5 minute and at 4°C. Crude membrane fractions were separated from cytoplasmic fractions by ultra-centrifugation at 100,000 × g for 50 min and at 4°C. Crude membrane fractions were resuspended in membrane isolation buffer and all samples were stored at −80°C.
Radioligand binding
In HEK-GR cells, two methods (Method A and B) were used to measure GR internalization by radioligand binding, as described previously [20]; [21]. These methods were used as complementary approaches. A third method (Method C), which is similar to Method A and to those described previously [20,21], was used with minor modifications in hepatocytes since these cells were in suspension rather than attached (as were HEK-GR cells) and thus, required a modified protocol. Method A: HEK-GR cells were washed twice and serum-starved in binding buffer for 1 hour and treated with 100 nM glucagon for the indicated time at 37°C. The cells were washed and binding was performed as previously described [14]. Each set of glucagon-treated cells was matched with a set of non-treated cells and the percentage GR internalization was calculated as the ratio of specific surface binding of 125I-glucagon in glucagon-treated and non-treated cells under the same conditions. Method B: HEK-GR cells seeded onto 24-well plates were washed twice and serum-starved in binding buffer (DMEM containing 0.1% BSA and 20 mM HEPES, pH 7.4) for 1 hour at 37 °C. 125I-glucagon was added (30,000 cpm per well) and incubated with the cells for 2 hours at 4°C to establish equilibrium. The labeled cells were then placed at 37°C for 30 min to allow for GR internalization. The unbound ligand was removed by washing with binding buffer on ice and surface-bound 125I-glucagon was stripped with a 5-minute incubation in cold glycine buffer (pH 3) and collected. The cells were then lysed in 0.8 M NaOH to collect the intracellular 125I-glucagon fraction. Nonspecific binding was determined as described above. The percentage of internalized receptors was calculated as a ratio of intracellular 125I-glucagon and the sum of intracellular and surface 125I-glucagon. Method C: Hepatocytes in suspension were serum starved for 1 hour in binding buffer and then treated with glucagon, PMA, or forskolin for the indicated time at 37°C. The cells were washed twice on ice in binding buffer, and then labeled with 125I-glucagon and binding was performed as previously described[14]. GR internalization was calculated as in Method A. Measurements for the three Methods were performed in triplicate or quadruplicate. All values were corrected for nonspecific binding, which accounted for 10–30% of total binding.
Immunoprecipitation and Western blotting
Immunoprecipitation and Western blotting were performed as previously described [14]. Briefly, after drug treatment, the cells were washed twice with cold PBS and then incubated with the cross-linker 1.25 mM di(N-succinimidyl) 3,3′-dithiodipropionate in serum-free culture medium for 30 min at room temperature. The cells were then washed twice, scraped and centrifuged. Cell pellets were resuspended in lysis buffer. The lysates containing 400 μg of protein were precleared with 50 μL of anti-mouse agarose beads (eBiosciences, San Diego, CA) for 30 min. Supernatants were incubated with the rabbit anti-GR (ST-18), mouse anti-FLAG or anti-PKCα antibodies and immunoprecipitation was performed. Immunoprecipitated proteins were separated on an 8–16% Tris-Glycine gradient gel (Invitrogen, Rockville, MD), and transferred to nitrocellulose Hybond membranes (Amersham Biosciences, Piscataway, NJ). Membranes were blocked in 5% milk dissolved in wash buffer containing 25 mM Tris, 150 mM NaCl and 0.1% Tween-20 for 1 h. Alkaline phosphatase activity was visualized with Western Lightning ECL detection reagent (Perkin Elmer, Boston, MA). The images were scanned and analyzed using ImageQuant software (GE Healthcare, Piscataway, NJ).
Statistical Analysis
The statistical significance was determined by one-way analysis of variance (ANOVA) followed by either Tukey post-hoc test or Bonferroni’s multiple comparison post-test. All calculations were performed using GraphPad Prism 4 (GraphPad Software, San Diego, CA). A p value of <0.05 was considered to be statistically significant.
RESULTS
Glucagon receptor internalization involves GRKs and PKCα
PKA and PKC mediate desensitization of many GPCRs through direct phosphorylation of receptors or indirectly through activation of GRKs [22,23]. GR’s C-terminus contains putative consensus sites for PKA and PKC. We have previously shown that PKCα promotes GR phosphorylation and desensitization [10]. To examine the respective roles of PKC and PKA in glucagon-stimulated GR internalization, we incubated HEK-293 cells stably expressing rat GR (HEK-GR cells) [18] in the presence or absence of phorbol 12-myristate 13-acetate (PMA), a PKC agonist that activates classical (α, β and γ) and novel (δ, ε, η and θ) PKC isoforms, or forskolin (FK), an adenylyl cyclase activator and indirect activator of PKA.
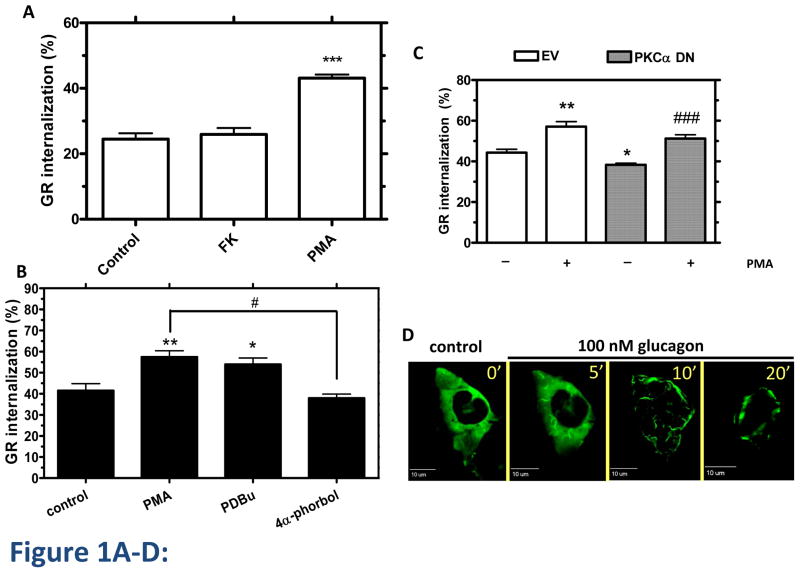
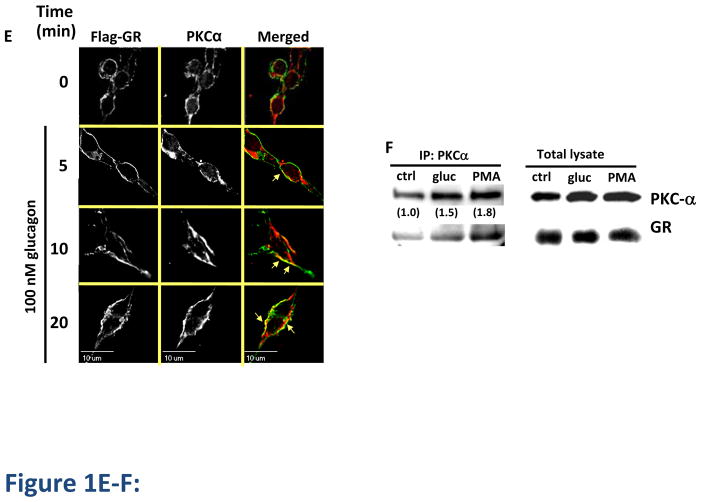
GR internalization after 30 min of glucagon treatment was measured by 125I-glucagon radioligand binding (Method A) as described in Materials in Methods. Treatment of HEK-GR cells with either PMA (200 nM) or forskolin (1 μM) in the absence of glucagon did not trigger GR internalization (data not shown). While activation of PKC with PMA increased glucagon-mediated GR internalization by approximately 20%, indirect activation of PKA with forskolin had no effect on glucagon-mediated GR internalization (Figure 1A). The data suggest that PKA does not play a role in GR internalization. To further explore the role of PKC, we treated HEK-GR cells with the PMA analog phorbol 12,13-dibutyrate (PDBu). The effect of PDBu was similar to that of PMA. In contrast, the inactive PMA analog, 4α-phorbol, had no effect (Figure 1B). As we have previously shown specific involvement PKCα in GR desensitization, we suspected that it may also play a role in internalization. Expression of a dominant negative (DN) mutant construct, PKCα DN, attenuated glucagon-induced GR internalization in HEK-GR cells, indicating that PKCα contributes to glucagon-stimulated GR internalization. In contrast, overexpression of PKCα DN did not affect the ability of PMA to enhance GR internalization induced by glucagon, suggesting that additional PKC isoforms that are activated by PMA may be involved in enhancing glucagon-stimulated GR internalization (Figure 1C). To further explore the involvement of PKCα in GR internalization, HEK-GR cells were transfected with PKCα-YFP and visualized by time-lapse fluorescence microscopy in live cells. At 10 and 20 min of glucagon treatment we observed translocation of PKCα-YFP from the cytoplasm to the plasma membrane, suggesting that glucagon triggers recruitment of PKC to the vicinity of GRs (Figure 1D). In HEK-293 cells stably expressing FLAG-GR (HEK-FLAG-GR), we observed colocalization of endogenous PKCα with GR in the plasma membrane at 5 and 10 min of glucagon treatment and in the perimembrane region at 10 and 20 min of treatment (Figure 1E, arrows). We confirmed that PKCα interacts with GR by co-immunoprecipitation of PKCα and GR from HEK-FLAG-GR cells. Relative to control conditions (untreated cells), association of PKCα with GR increased by 50% and 80% upon 30 min treatment with 100 nM glucagon and 200 nM PMA, respectively (Figure 1F). Taken together, these data support the hypothesis that PKCα interacts with GR after glucagon stimulation and after PMA stimulation in the absence of glucagon. It is worth mentioning that under basal conditions (control), there is already a certain level of interaction between PKCα and GR in HEK-GR cells, consistent with previous studies reporting the presence of PKCα in both the plasma membrane and cytoplasmic fractions of these cells [10].
Figure 1. Involvement of PKC-α in GR internalization.
A, HEK-GR cells were serum-starved and incubated without (control) and with 200 nM PMA or 1 μM Forskolin (FK) plus 25 μM IBMX for 30 min, followed by 30 min incubation with 100 nM glucagon. GR internalization was determined by binding (Method A). Data represent the mean ± s.e.m. of 6 experiments. ***, p < 0.001, glucagon vs. glucagon+PMA. B, HEK-GR cells were serum-starved for 1 h and incubated without (control) and with 200 nM PMA, 1 μM phorbol-12-13-dibutyrate (PDBu), or 4α-phorbol for 15 min, followed by 30 min incubation with 100 nM glucagon. The internalization was determined by binding (Method B). Data represent the mean ± s.e.m. of 3 experiments. *, p < 0.05 vs. control, **; p < 0.01 vs. control; #, p < 0.05, 4α-phorbol vs. PMA. C, HEK-GR cells were transfected with dominant negative (DN) PKCα (checkered bars) or empty vector (EV; white bars). The cells were serum-starved for 1 h, incubated in the absence (−) or presence (+) of 200 nM PMA, and treated with 100 nM glucagon for 30 min. The GR internalization was determined by binding (Method A). Data represent the mean ± s.e.m. of 4–6 experiments. *, p < 0.05 vs. EV (−); **, p < 0.01 vs. EV (−); ###, p < 0.001 vs. PKCα DN (−). D, HEK-GR cells were transfected with PKCα-YFP and seeded onto glass-bottom 35-mm dishes. The cells were serum-starved for 1h and then treated with 100 nM glucagon. Translocation of PKCα-YFP was observed by time-lapse fluorescence microscopy. The cells were maintained on a heated stage during the time-lapse experiment. Images were taken at 0.5–2 minute intervals. Each fluorescence image is a deconvolved projection of 5–6 planes acquired using a 60 × objective. Data shown are representative of 3 independent experiments. E, HEK-FLAG-GR cells grown on coated glass coverslips were treated with 100 nM glucagon for the time indicated. The cells were then fixed, permeabilized and immunostained with anti-FLAG (green) and anti-PKCα (red) antibodies. Each fluorescence image is a deconvolved projection of 5–6 planes acquired using a 60 × objective. Representative images from 3 independent experiments are shown. Arrows indicate colocalization. F, HEK-FLAG-GR cells were incubated without (ctrl) or with either 100 nM glucagon (gluc) or 200 nM PMA for 30 min. Immunoprecipitation of PKCα and immunoblotting were performed as described in Materials and Methods. The mean values within parentheses represent the fold increase above control determined in the absence of either glucagon or PMA. The value for control was arbitrarily set as 1. The image shown is representative of 3 independent experiments.
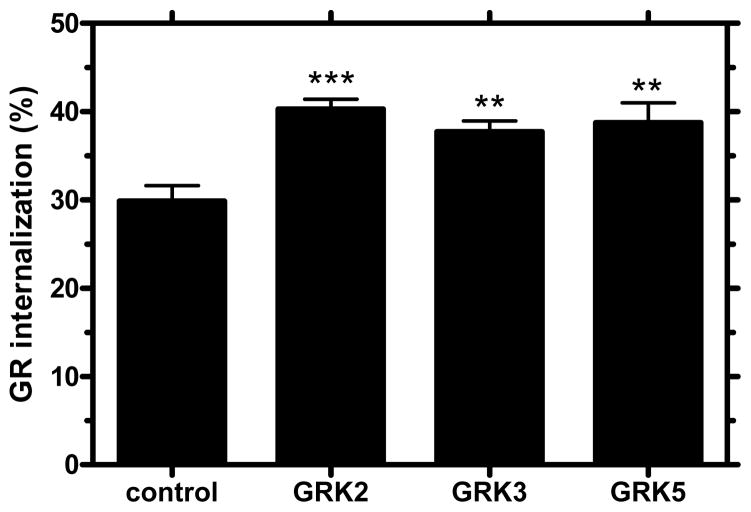
GRK-mediated phosphorylation is a critical step in GPCR desensitization. GRKs are involved in homologous desensitization, as well as internalization of many GPCRs [24]. Recent reports have shown that GRKs regulate receptors that are structurally similar to GR. For example, GRK isoforms 2, 3 and 5 desensitize the vasoactive intestinal polypeptide type-1 receptor [25]. GRKs represent a novel target for treatment of diabetes. Synthetic peptides have been used to block GRK2 and GRK3 and restore glucose homeostasis in mouse models of diabetes [26]. In order to investigate their possible role in GR internalization, we transfected GRK2, GRK3, and GRK5 into HEK-GR cells. Radioligand binding assays with 125I-glucagon demonstrated a significant increase (~10%) in glucagon-stimulated GR internalization in cells transfected with GRK2, GRK3, or GRK5 compared with glucagon-stimulated cells transfected with empty vector (control) (Figure 2). These findings suggest that GRKs are involved in internalization in HEK-GR cells.
Figure 2. Involvement of GRKs in GR internalization.
HEK-GR cells transiently transfected with GRK2, GRK3, GRK5 or empty vector plasmids (control) were serum-starved for 1 h and treated with 100 nM glucagon for 30 min. Receptor internalization was assessed by binding (Method B). Data represent the mean ± s.e.m. of 4 experiments. **; p < 0.01 vs. control; ***, p<0.001 vs. control.
Glucagon receptor utilizes both clathrin- and caveolin-mediated endocytosis
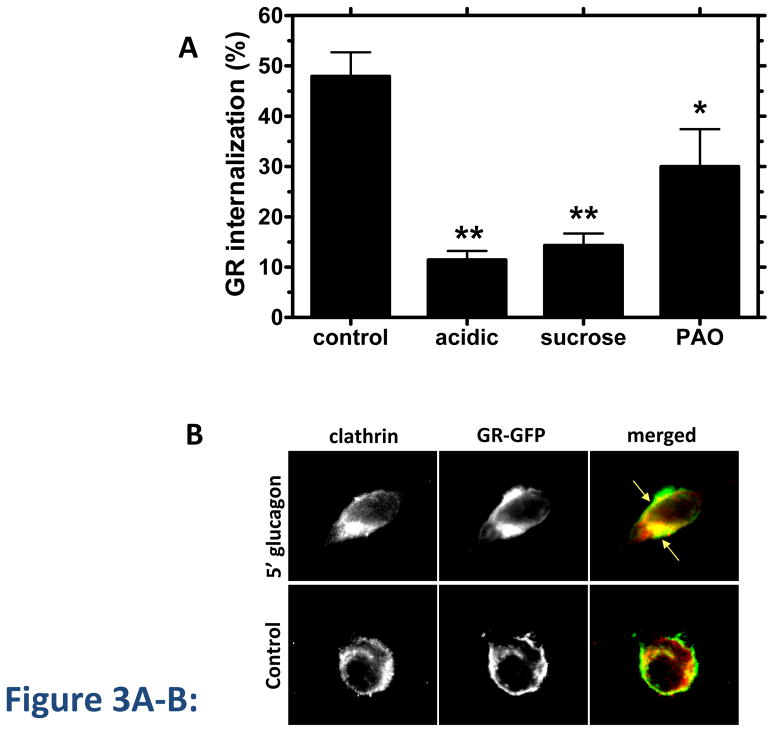
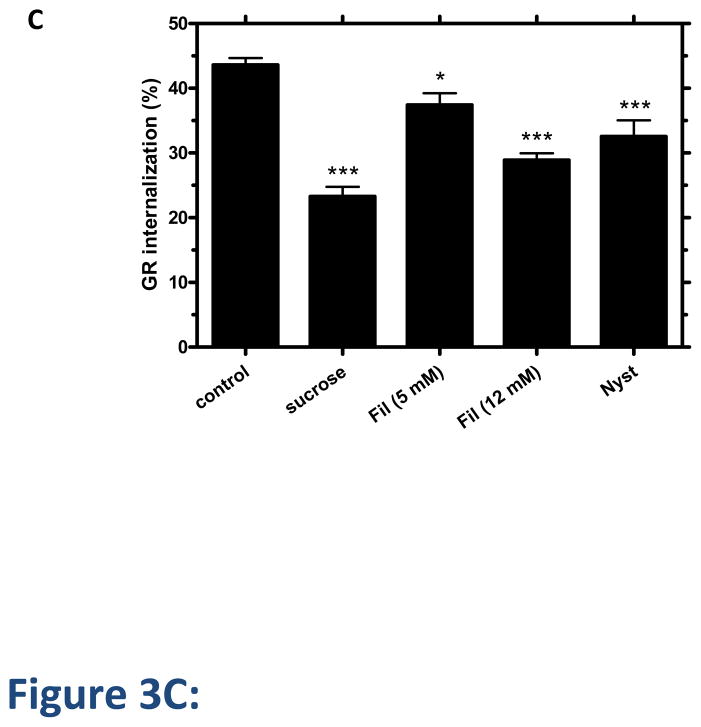
Disruption of clathrin-mediated endocytosis by well-established methods, including cytosolic acidification, treatment with 0.45 M sucrose and treatment with phenylarsine oxide (PAO), decreased GR internalization at 30 min by up to 70% in 125I-glucagon binding experiments (Figure 3A). Trypan blue staining was used to verify that the cell viability was not affected under these conditions. Furthermore, GR colocalized with clathrin as early as 5 min after glucagon treatment (Figure 3B). Given that the clathrin-disrupting agents failed to completely abolish GR internalization, we suspected that GR might utilize additional internalization pathway(s). To explore the involvement of clathrin-independent endocytosis, we used the cholesterol depleting agents, Filipin III and Nystatin, which are known to disrupt caveolae [27]. In our experiments, each reagent decreased GR internalization by 10–25% (Figure 3C).
Figure 3. GR internalization involves clathrin and caveolae.
A, HEK-GR cells were serum-starved for 1 h and treated with inhibitors of clathrin-mediated endocytosis: acidic medium (pH 5.5), hypertonic medium (0.45 M sucrose), or 20 μM phenylarsine oxide (PAO) for 30 min, followed by 30 minute incubation with 100 nM glucagon. Control cells were incubated with glucagon alone. GR internalization was determined by binding (Method B). Data represent the mean ± s.e.m. of 3 independent experiments.*, p < 0.05 vs. control, **, p < 0.01 vs. control. B, HEK-293 transfected with GR-GFP were serum-starved for 1 h and incubated with or without (control) 100 nM glucagon for 5 min, fixed, permeabilized and immunostained for clathrin using an anti-clathrin heavy chain antibody. Each fluorescence image is a deconvolved projection of 5–6 planes acquired using a 60 × objective and is representative of 3 experiments. Arrows indicate colocalization of clathrin and GR-GFP. C, HEK-GR cells were pre-treated for 30 min with filipin III (Fil., 5 μM or 12 μM), 0.45 M sucrose or 5 μg/mL nystatin (Nyst). GR internalization after 100 nM glucagon incubation for 30 min was measured by binding (Method A). The data represent the mean ± s.e.m. of 4–5 independent experiments. *, p < 0.05, **, p < 0.01; ***, p < 0.001 vs. control.
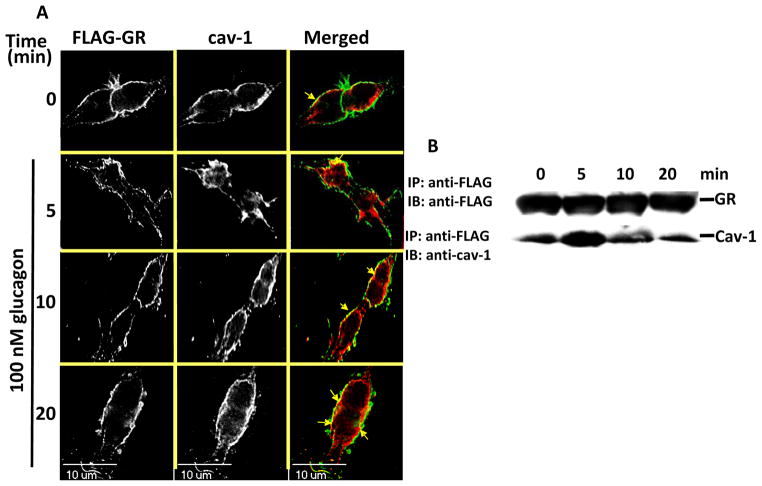
To confirm the involvement of caveolae in GR endocytosis, HEK-FLAG-GR cells were treated with glucagon and immunostained with the anti-caveolin (Cav)-1 antibody (Figure 4A and B). While in untreated cells Cav-1 colocalized with GR at the membrane, in glucagon-treated cells, it also colocalized with internalized GRs (Figure 4A). Furthermore, Cav-1 co-precipitated with GR when GR was immunoprecipitated using anti-FLAG, which we have previously shown to be specific for GR IP in HEK-FLAG-GR cells [14](Figure 4B). The association transiently increased, with a maximum observed at 5 min of glucagon stimulation (Figure 4B). This association of the GR and Cav-1 returned to the basal level by 20 min glucagon stimulation (Figure 4B).
Figure 4. GR associates with caveolin-1.
A, HEK-FLAG-GR cells were serum-starved for 1 h and treated with 100 nM glucagon for the time indicated, fixed, permeabilized and immunostained for GR using anti-FLAG antibody (FLAG-GR, green) and Cav-1 using the anti-Cav-1 antibody (cav-1, red). Each fluorescence image is a deconvolved projection of 5–6 planes acquired using a 60 × objective. Representative images of 3 independent experiments are shown. Arrows indicate colocalization of caveolin and FLAG-GR. B, HEK-FLAG-GR were treated with 100 nM glucagon for 0, 5, 10, or 20 min. FLAG-GR was immunoprecipitated using the anti-FLAG antibody. The immunoprecipitated proteins were analyzed by immunoblotting with the anti-GR antibody and the anti-Cav-1 antibody, respectively. The image shown is representative of 3 independent experiments.
Glucagon induces GR internalization and association of GR with PKCα and caveolin-1 in primary hepatocytes
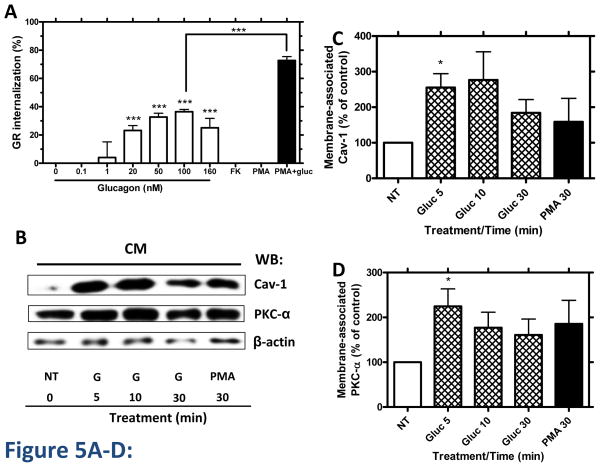
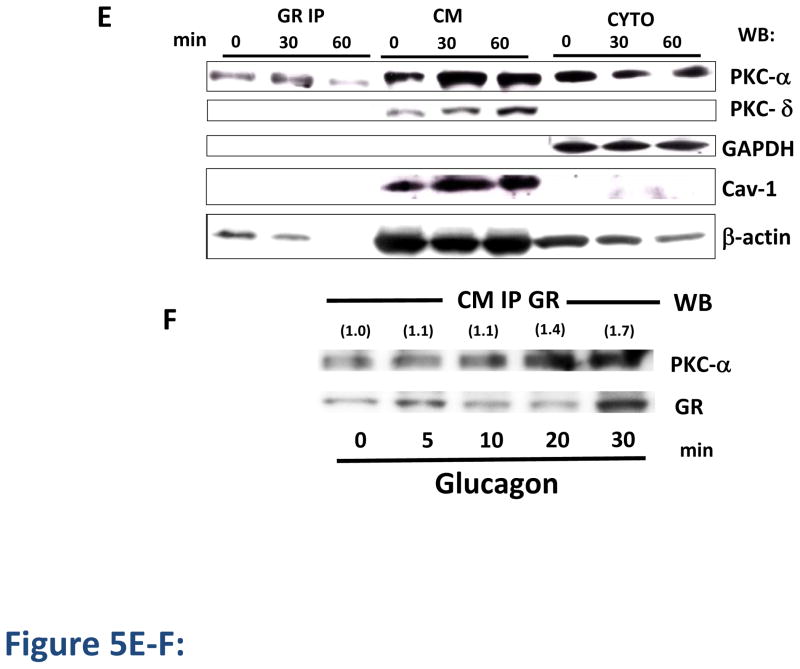
To investigate the physiological relevance of our findings, we performed radioligand binding assays with 125I-glucagon in primary hamster hepatocytes. In these studies, 30-minute incubation with glucagon (0.1–160 nM) resulted in a concentration-dependent decrease in cell surface GR (up to approximately 40%), indicating receptor internalization (Figure 5A). Hepatocytes were also incubated with 200 nM PMA in the presence or absence of glucagon (Figure 5A). PMA increased glucagon-stimulated GR internalization from 40% to 70% (Figure 5A), consistent with our results in HEK-GR cells (Figure 1A–C). As we found PKCα and Cav-1 translocation and association with the GR upon glucagon stimulation in HEK-GR cells (Figures 1E–F, 4A–B), we next examined whether Cav-1 and PKCα translocate to the membrane in hepatocytes upon glucagon stimulation. Upon incubation of the hepatocytes without and with either 100 nM glucagon or 200 nM PMA, the cytoplasmic and crude membrane fractions were separated and the membrane levels of Cav-1 and PKCα were determined by immunoblotting (Figure 5B). After a 5- to 10-min incubation of the cells with glucagon, the Cav-1 and PKCα membrane levels were increased by 2- to 3-fold and 1.5- to 2.5-fold, respectively (Figure 5B–D). Further, incubation of the hepatocytes with glucagon for 30 and 60 min led to increased membrane levels of PKCα, PKCδ and Cav-1, and decreased cytoplasmic levels of PKCα (Figure 5E, 6 right-hand lanes). Of note, Cav-1 was detectable in the cytoplasmic fraction only after an extended exposure time of the chemiluminescent signal. The blots were also probed with an antibody against the cytosolic marker glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to assess the purity of the cellular fractionation procedure, as has been reported by others [28,28]. In order to verify whether the accumulation of PKCα in the membrane leads to its interaction with the GR, the receptor was immunoprecipitated from the crude membrane (CM) fraction. PKCα and β-actin, but not PKCδ or Cav-1, co-immunoprecipitated with GR (Figure 5E, 3 left-hand lanes). In these experiments, PKCδ was examined to verify the specificity of involvement of PKCα in co-immunoprecipitation of the GR. The association of GR with PKCα peaked 30 min after the addition of glucagon, whereas the association with β-actin decreased over time and was undetectable after 60 min. The latter is possibly due to β-actin and F-actin distribution changes occurring with glucagon treatment, as we have previously reported [14]. As in HEK-GR cells (Figure 1F), a moderate level of interaction between PKCα and GR was observed under basal conditions in hepatocytes (Figure 5E), which is consistent with previous studies reporting the presence of PKCα in both the plasma membrane and cytoplasmic fractions of these cells [17]. To further examine the kinetics of GR association with PKCα in hepatocytes, we conducted an additional time-course experiment. After respective 20 and 30 min stimulation with 100 nM glucagon, the association of PKCα with GR reached a maximum of 40 and 70% above control (Figure 5F).
Figure 5. Glucagon induces GR internalization, PKC-α and caveolin-1 translocation, and association of PKC with GR in cultured hepatocytes.
A, Golden Syrian hamster hepatocytes were incubated in the absence or presence of increasing concentrations (0.1–160 nM) of glucagon, 1 μM Forskolin (FK) + 50 μM IBMX, 200 nM PMA or PMA + 100 nM glucagon for 30 min at 37 °C. GR internalization was determined by binding (Method C). Data represent the mean ± s.e.m. of 3–5 experiments. ***, p < 0.001. B, Hepatocytes were incubated in the absence (NT, control) or presence of either 100 nM glucagon (G) or 200 nM PMA for up to 30 min and the crude membrane and cytoplasmic fractions were separated. The expression of caveolin (Cav)-1, PKCα and β-actin in the crude membrane (CM) fraction was determined by immunoblotting using specific antibodies. The image shown is representative of at least 3 independent experiments. C and D, Densitometric graphs for Cav-1 and PKC-α for combined results of at least 3 independent experiments for the representative Western blot shown in Figure 5B. Each bar represents the mean percent change for Cav-1 or PKC-α for each treatment relative to control (no treatment, NT), which was arbitrarily set as 100. The membrane-associated Cav-1 and PKCα values were calculated by dividing the Cav-1 or PKC-α band intensity obtained by densitometry by that of βactin. *, p < 0.05. E, Hepatocytes were incubated for 30 and 60 min with 100 nM glucagon and the crude membrane (CM) and cytoplasmic (CYTO) fractions were separated. The GR was immunoprecipitated (GR IP) from the CM fraction using the anti-GR antibody ST-18. The precipitated proteins as well as the proteins from the membrane and cytoplasmic fraction were analyzed by immunoblotting with anti-PKCα, anti-PKCδ, anti-GAPDH, anti-caveolin-1 and antiβ-actin antibodies. The blot shown is representative of 3 independent experiments. F, Hepatocytes were incubated for up to 30 min with 100 nM glucagon. GR from the crude membrane fraction was immunoprecipitated with the anti-GR antibody ST-18 and the precipitated proteins were analyzed by immunoblotting. The mean values within parentheses represent the fold increase above control (no glucagon stimulation), and were calculated as the ratio of PKCα to GR intensities determined by densitometry. The value for control was arbitrarily set as 1. The blot shown is representative of 3–4 independent experiments.
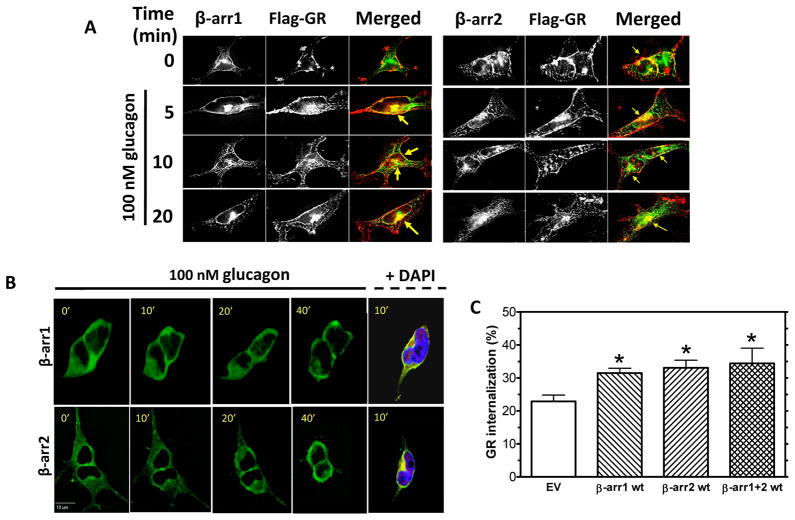
Figure 6. GR internalization involves β-arrestins.
A, HEK-FLAG-GR cells were transfected with either β-arrestin1-GFP (β-arr1, green) or β-arrestin2-GFP (β-arr2, green). The cells were serum-starved for 1 h prior to treatment with 100 nM glucagon for 0, 5, 10, or 20 min. At the indicated time, the cells were fixed, permeabilized and immunostained with anti-FLAG antibody (FLAG-GR, red). Representative images from 3 independent experiments are shown. Arrows indicate colocalization of GR and β-arrestin. Each fluorescence image is a deconvolved projection of 5–6 planes acquired using a 60× objective. B, HEK-GR cells were transfected with either β-arr1-GFP or β-arr2-GFP and seeded onto glass-bottom 35 mm dishes. The cells were serum-starved for 1 h and treated with 100 nM glucagon. β-arr1-GFP and β-arr2-GFP translocation were observed by time-lapse fluorescence microscopy. During the experiment, the cells were maintained on a heated stage. Images were taken at 0.5–2 minute intervals. The panel on the right shows β-arr1-GFP and β-arr2-GFP (green) colocalizing with FLAG-GR (immunostained with anti-FLAG antibody, red) near the nucleus labeled with DAPI (blue) in fixed and permeabilized HEK-FLAG-GR cells after a 10 min treatment with glucagon. Each fluorescence image is a deconvolved projection of 5–6 planes acquired using a 60× objective. Data shown are representative of 3 independent experiments. C, HEK-GR cells were transiently transfected with β-arr1 wild type (wt), β-arr2 wt, and β-arr1 wt + β-arr2 wt (β-arr1+2 wt) plasmids. Control cells were transfected with an empty vector (EV). After 1 h serum starvation, the cells were treated with 100 nM glucagon for 30 min. GR internalization was determined by radioligand binding (Method A). Data shown are the mean ± s.e.m. of quadruplicate determinations from 3–5 independent experiments. *, p < 0.05 vs. EV.
β-arrestins are involved in GR internalization
Clathrin-mediated internalization of most GPCRs requires β-arrestins. We have recently reported that, also referred to as arrestin-2 and -3, respectively [29], are required for recycling of GRs to the plasma membrane in HEK-GR cells [14]. Furthermore, co-transfection of dominant-negative constructs of β-arrestin1 and β-arrestin 2 decreased the amount of glucagon-stimulated GR internalization as compared to cells transfected with an empty vector. In the present study, to further examine their involvement in GR internalization. β-arrestin1-GFP and β-arrestin2-GFP were transfected into HEK-FLAG-GR cells. Upon 5–20 min of glucagon treatment, colocalization of FLAG-GR with both transfected β-arrestins was observed at the plasma membrane level and in the cytoplasm (Figure 6A, arrows), suggesting that the interaction with β-arrestins is maintained after receptor internalization. It appeared that, at 10 and 20 min of glucagon treatment, β-arrestins and GR colocalized predominantly in the perinuclear region (Figure 6A, arrows). This observation was supported by labeling the nuclei with DAPI (Figure 6B, right panel). Time-lapse fluorescence microscopy in live HEK-GR cells transfected with β-arrestins indicated that both β-arrestin1-GFP and β-arrestin2-GFP translocated from the cytoplasm to the perinuclear region after treatment with glucagon (Figure 6B, left panels). Binding experiments were conducted to further assess the involvement of β-arrestin isoforms in GR internalization (Figure 6C). GR internalization was significantly increased in cells transfected with either β-arrestin1 WT or β-arrestin2 WT, as compared to cells transfected with an empty vector (EV). However, co-transfection of β-arrestin1 WT and β-arrestin2 WT did not result in a further increase in GR internalization (Figure 6C).
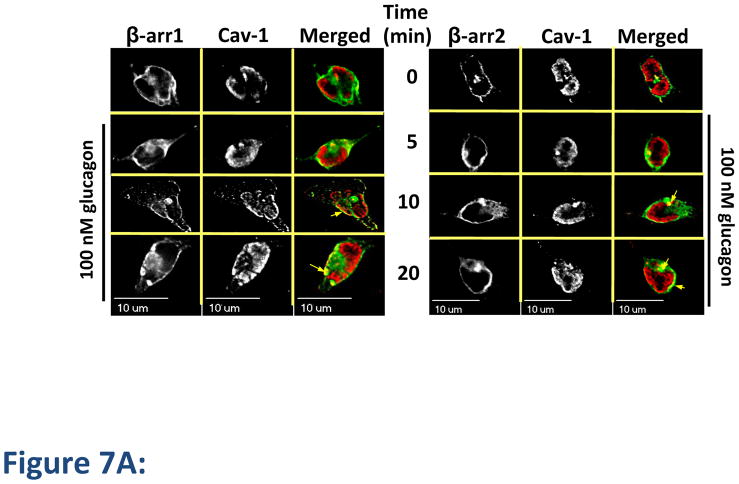
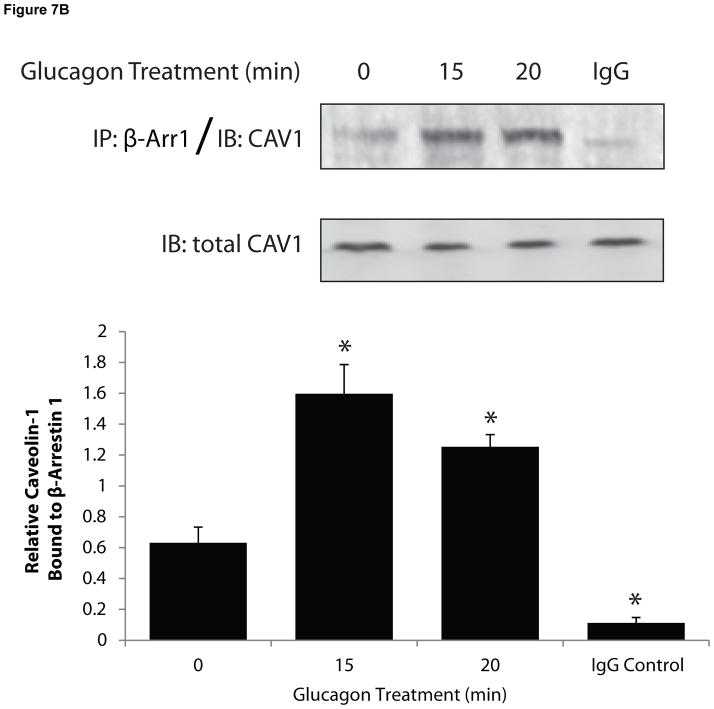
The role of β-arrestins in caveolae-mediated internalization is unknown. Given that our findings indicated that both clathrin- and caveolin-mediated pathways are utilized by GR, we considered the possibility that β-arrestins could be a common component of the two internalization routes. We observed colocalization of Cav-1 with both β-arrestin1 and β-arrestin2 by fluorescence microscopy at 10 and 20 min after treatment with glucagon for 10 and 20 min (Figure 7A, arrows). Co-immunoprecipitation (Co-IP) experiments in total cell lysates confirmed an interaction between β-arrestins and Cav-1. In these experiments, glucagon stimulation for 10 or 15 min induced a time-dependent 2- to 3-fold increase in Co-IP of Cav-1 with β-arrestin1 (Figure 7B). Similar results were observed in Co-IP experiments with Cav-1 with β-arrestin2 following glucagon stimulation (2-fold increase following 10 and 15 min stimulation; data not shown). As expected, Co-IP experiments with an IgG control did not result in appreciable detection of Cav-1 (Figure 7B).
Figure 7. Caveolin-1 colocalizes with β-arrestins.
HEK-FLAG-GR cells were transfected with either β-arrestin1-GFP (β-arr1, green) or β-arrestin2-GFP (β-arr2, green). The cells were starved for 1 h prior to treatment with glucagon. A, After treatment, the cells were fixed, permeabilized and immunostained with anti-Cav-1 antibody (cav-1, red). Each fluorescence image is a deconvolved projection of 5–6 planes acquired using a 60× objective. Representative images from 3 independent experiments are shown. Arrows indicate colocalization of caveolin-1 and β-arrestins. B, After treatment, cells were lysed and β-Arr1 was immunoprecipitated with an anti-GFP antibody. Immunoprecipitated lysates were immunoblotted for caveolin-1 (CAV1). In parallel experiments, an IgG control was used for immunoprecipitation followed by immunoblotting for CAV1. Top panel is a representative blot; bottom panel represents the mean + S.E.M. of 3 independent experiments. *Significantly different from the 0 time point (P < 0.05).
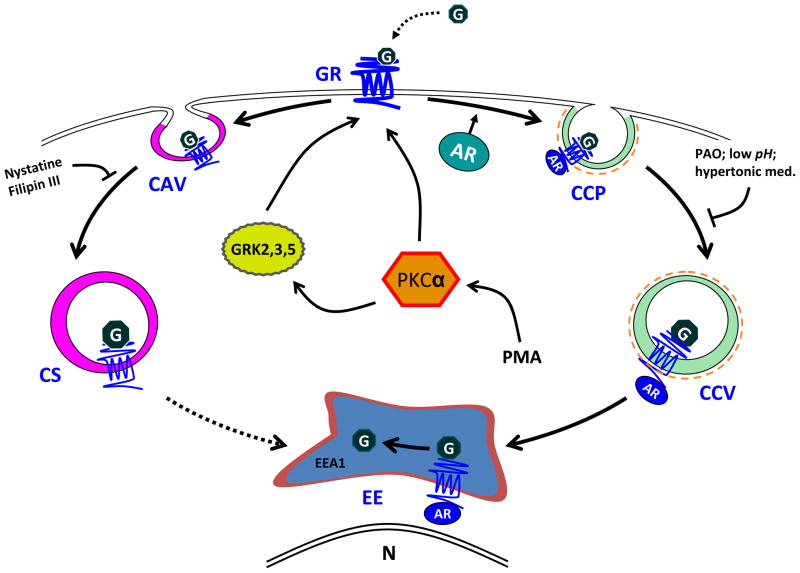
Based on the results of our study, we propose the following hypothetical model for GR internalization in GR-expressing HEK-293 cells (Figure 8). Upon treatment with glucagon, PKCα is recruited to the plasma membrane to facilitate GR desensitization and internalization. GRK2, GRK3, and GRK5 may all play a role in GR desensitization. PKCα possibly facilitates recruitment of GRK2 to the membrane, as has been suggested in the literature [24]. Desensitized GRs interact with β-arrestin1 and/or β-arrestin2, and the complexes are internalized via clathrin-coated pits and eventually targeted to early endosomes. Internalized receptors are recycled to the plasma membrane in a β-arrestin-dependent manner, as we have previously established [14]. Alternatively, GRs are internalized through caveolae. The two internalization pathways are either utilized in parallel or the receptors shuttle from one pathway to the other. The possible point of interaction between the two endocytic routes might be vesicles in which β-arrestins and Cav-1 colocalize.
Figure 8. Hypothetical model of GR internalization.
Upon ligand binding, GRs are desensitized and targeted for internalization through either a clathrin-mediated pathway or through caveolae. The internalization involves β-arrestins, which colocalize with the receptors at the membrane and intracellularly. The internalized receptors rapidly enter early endosomes. Second messengers GRK2, GRK3 and GRK5 as well as PKCα stimulate receptor internalization. Abbreviations: G, glucagon; GR, glucagon receptor; AR, β-arrestins; CCP, clathrin coated pit; CCV, clathrin-coated vesicle; EE, early endosome; CAV, caveolae; CS, caveosome; GRK, G protein-coupled receptor kinase; PAO, phenylarsine oxide; N, nucleus; DN, dominant negative.
DISCUSSION
In the present study, we confirmed that the GR is internalized upon stimulation with glucagon in GR-transfected HEK-293 cells and in cultured hepatocytes. We found that GR utilizes both clathrin- and caveolin-mediated endocytosis in HEK-GR cells. Our observation that both β-arrestin1 and β-arrestin2 colocalize with GR suggests that these β-arrestins are involved in GR internalization. The two arrestins also colocalized with caveolin-1 upon treatment of cells with glucagon. To our knowledge, this is the first reported putative interaction between the caveolin- and clathrin-mediated receptor internalization pathways. We have previously shown that PKC plays a role in GR phosphorylation and desensitization [19]. The results of the present study suggest that PKC also enhances glucagon-stimulated GR internalization. GRKs are known to promote internalization of GPCRs through enhancing β-arrestin recruitment and binding to clathrin, but their role in GR internalization has not been explored to date. We found that overexpression of either GRK2, GRK3, or GRK5 enhanced glucagon-stimulated GR internalization in HEK-GR cells, indicating that these three kinases may be important for GR internalization.
In both HEK-GR cells and hepatocytes, significant GR internalization was observed with 30 min of glucagon (100 nM) stimulation. We and others have reported that PMA stimulates GR desensitization in cultured hepatocytes as well as in HEK-293 cells [10], but the effect of PMA on GR internalization has not been addressed. In the present study we determined that PMA alone did not affect GR internalization, but it enhanced glucagon-stimulated GR internalization in cultured hepatocytes. This phenomenon can be interpreted as a positive feedback mechanism through PKC, which is activated through GR coupling to Gq. Another feedback pathway could take place through coupling of GR to Gs and activation of PKA. Previous studies suggested that PKA does not play a role in GR desensitization [30]. These earlier findings are supported by the present study, in which PKA stimulation, mediated by adenylyl cyclase activation by forskolin, also does not affect GR internalization.
Our immunoblotting and time-lapse fluorescence microscopy studies demonstrated that glucagon triggered the accumulation of PKCα in the plasma membrane of HEK-GR cells and in the isolated crude membrane fraction of cultured hepatocytes. The significance of this translocation is twofold: first, it represents a step in the activation of PKCα mediated by the interaction with membrane-bound phosphatidylserine and diacylglycerol, and second, it brings PKCα into proximity of GR, allowing for interaction of the two proteins. Indeed, we verified that GR associated with PKCα under basal conditions in both HEK-GR cells and hepatocytes. The association transiently increased to a maximum observed 30 min after stimulation with glucagon. In HEK-GR cells PMA alone also triggered translocation of PKCα and its association with GR in the plasma membrane. Given that our binding assays show lack of effect of PMA on GR internalization, it appears that, in the absence of glucagon, PKCα does not facilitate GR internalization.
We observed no association of GR with PKCδ in hepatocytes, suggesting that PKCδ is not directly involved or associated with GR during glucagon stimulation. Furthermore, we also did not observe any increase in glucagon-stimulated GR internalization when PKCδ and PKCζ, representative of novel and classical PKCs, respectively, were overexpressed in HEK-GR cells (data not shown). At this time, we cannot rule out involvement of other classic and atypical PKC isoforms in GR internalization following glucagon treatment alone or in combination with PMA.
GRKs are indispensable for homologous desensitization of GPCRs. Recent studies have shown that GRKs not only phosphorylate receptors but also modulate signaling through interaction with numerous other proteins, including Gα, Gβγ, caveolin, clathrin, PI3K, MEK and Akt [24]. The effect of GRKs on GR desensitization has been addressed to a certain extent [31,32]. These reports examined the role of GRK2 in GR desensitization in the liver in vivo and the roles of GRK2 and GRK3 in PMA-mediated GR desensitization in vitro. To our knowledge, this is the first report implicating the GRK2, GRK3 and GRK5 in GR internalization.
Depending on the interplay between the receptor, cell type, receptor ligand, and the nature of desensitization, receptors may utilize multiple internalization pathways and even switch from one pathway to another during endocytosis. Most GPCRs utilize a clathrin-mediated internalization pathway, although usage of alternative pathways, such as internalization through caveloae/lipid rafts, has been described [6,33] and may be associated with different receptor functions, e.g., promotion of cell signaling versus receptor degradation, respectively, as reported for the EGF receptor [34]. In the present study, pharmacological disruption of clathrin-coated pits resulted in only partial inhibition of GR internalization in HEK-GR cells, suggesting that the receptor utilizes additional internalization routes. It should be noted that the use of pharmacological agents to characterize GR internalization should be interpreted with caution as they may lack selectivity and may affect membrane structure and function. Examined under a light microscope, the cell shape appeared normal after pharmacological disruption of clathrin-coated pits. While the pharmacological inhibition regimens chosen in our study are widely used in the literature, hypertonic sucrose and PAO are considered non-selective inhibitors of clathrin-mediated endocytosis [35]. The cholesterol-depleting agents Nystatin and Filipin III disrupt caveolae and have been utilized to demonstrate the role of caveolae in GPCR internalization. A significant reduction of GR endocytosis/degradation in the presence of these drugs was observed.
We found that in HEK-FLAG-GR cells, GR colocalizes with Cav-1, a major constituent of caveolae, at the plasma membrane and, after stimulation with glucagon, in the cytoplasm. A similar interaction pattern with Cav-1 was previously observed for the structurally related glucagon-like peptide 1 (GLP-1) receptor expressed in HEK-293 cells [36]. Glucagon triggered recruitment of Cav-1 to the membrane in both hepatocytes and HEK-GR cells, with the same peak time of Cav-1 plasma membrane accumulation (5 min). Additionally, in co-immunoprecipitation studies in HEK-FLAG-GR cells, we found that GR associates with Cav-1 in a time-dependent manner. However, under the conditions tested, co-immunoprecipitation of GR and Cav-1 was not found in hepatocytes. This discrepancy could be due to the use of different time points examined in HEK-GR cells (5, 10 and 20 min) versus hepatocytes (30 and 60 min) and different fractions analyzed (total lysate for HEK cells versus crude membrane for hepatocytes).
As a scaffolding protein, Cav-1 directly interacts with several signaling molecules, including PKA, PKC, and Gαs [12,37]. At the plasma membrane, receptors that interact with caveolae are often part of lipid rafts, which serve as distinct signaling platforms. Our results suggest, for the first time, that GR may be partitioned between lipid rafts and non-raft plasma membrane domains. Further studies are needed to assess the importance of putative lipid raft localization for downstream signaling, in particular ERK1/2 activation, as observed with the GLP-1R [36].
β-arrestins are required for clathrin-mediated internalization and many GPCRs are desensitized and internalized in a β-arrestin-dependent manner [38]. Our results suggest that GR interacts with both β-arrestin1 and β-arrestin2 in HEK-GR cells at the plasma membrane and in the cytoplasm. Of note, Merlen et al. [15] did not find evidence of β-arrestin1 or β-arrestin2 involvement in GR internalization in liver cells isolated from rats injected with glucagon. The authors suggested that clathrin-independent internalization pathways (such as lipid rafts) may be relevant in vivo. While there are earlier reports on the requirement for β-arrestins in clathrin-independent internalization [39,40], this is the first study to demonstrate colocalization of β-arrestin1 and β-arrestin2 with Cav-1. These results are intriguing, however, we recognize that β-arrestins cannot be definitively linked to caveolae-mediated internalization through colocalization and co-immunoprecipitation experiments alone. As an example, desensitization, but not internalization, of the M2 muscarinic acetylcholine receptor was found to require arrestins [41] Therefore, it is possible that the interaction of caveolin with β-arrestins occurs at the level of receptor desensitization. However, the fact that the proteins appear to colocalize in the cytosol, suggests that β-arrestins may indeed be involved in caveolae-mediated internalization, at least under conditions of β-arrestin overexpression. We do recognize that it is plausible that alternate internalization pathways predominate under β-arrestin1 and β-arrestin2 basal expression levels, as has been seen for the M2 muscarinic acetylcholine receptor [42].
In the present studies, β-arrestins rapidly accumulated in the perinuclear region in response to glucagon treatment, which presumably denotes a recycling compartment, as reported for the N-formyl peptide receptor [43]. Moreover, we recently reported that recycling of GR is abolished by downregulation of β-arrestin1 and β-arrestin2 [14]. Perinuclear targeting of β-arrestins may also be correlated with their ability to act as signaling scaffolds and facilitate activation of ERK1/2 by glucagon, which has been reported to require clathrin-dependent GR internalization [44]. We also speculate that β-arrestins could mediate GR signaling that is independent of G protein activation, as recently reported for the beta2-adrenergic receptor [38].
In conclusion, the present study demonstrates that GRK2, GRK3, GRK5, and PKCα stimulate agonist-induced GR internalization in HEK-GR cells. PKCα interacts with the GR in both hepatocytes and HEK-293 cells. Endocytosis of GRs proceeds through both clathrin- and caveolae- dependent pathways. However, further experiments are needed to clarify whether the receptor shuttles between the two pathways, as has been observed for the AT1 receptor [45,46] and the β2-adrenergic receptor [47]. GR internalization in HEK-GR cells involves both β-arrestin1 and β-arrestin2. The intracellular components of GR desensitization and internalization identified in this study represent potential drug targets for novel diabetes therapies focused on antagonizing GR signaling.
Acknowledgments
The authors wish to thank Drs. Marianne David and Jianping Ming for their technical advice and assistance. We are also grateful to Drs. Jeffrey L. Benovic, Marc Caron, Cornelius Krasel, Ralf Kubitz, and Jae-Won Soh, for providing us with the specific plasmids. We thank the Center for Microscopy and Image Analysis, The George Washington University, for the use of the confocal microscope.
This work was supported by the National Institutes of Health, grant DK56108 to BB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1987;64:106–110. doi: 10.1210/jcem-64-1-106. [DOI] [PubMed] [Google Scholar]
- 2.Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2000;85:4053–4059. doi: 10.1210/jcem.85.11.6993. [DOI] [PubMed] [Google Scholar]
- 3.Unger RH. Role of glucagon in the pathogenesis of diabetes: the status of the controversy. Metabolism: clinical and experimental. 1978;27:1691–1709. doi: 10.1016/0026-0495(78)90291-3. [DOI] [PubMed] [Google Scholar]
- 4.Petersen KF, Sullivan JT. Effects of a novel glucagon receptor antagonist (Bay 27-9955) on glucagon-stimulated glucose production in humans. Diabetologia. 2001;44:2018–2024. doi: 10.1007/s001250100006. [DOI] [PubMed] [Google Scholar]
- 5.McCormack JG, Westergaard N, Kristiansen M, Brand CL, Lau J. Pharmacological approaches to inhibit endogenous glucose production as a means of anti-diabetic therapy. Curr Pharm Des. 2001;7:1451–1474. doi: 10.2174/1381612013397393. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 7.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 8.Dhami GK, Anborgh PH, Dale LB, Sterne-Marr R, Ferguson SS. Phosphorylation-independent regulation of metabotropic glutamate receptor signaling by G protein-coupled receptor kinase 2. J Biol Chem. 2002;277:25266–25272. doi: 10.1074/jbc.M203593200. [DOI] [PubMed] [Google Scholar]
- 9.Freedman NJ, Ament AS, Oppermann M, Stoffel RH, Exum ST, Lefkowitz RJ. Phosphorylation and desensitization of human endothelin A and B receptors. Evidence for G protein-coupled receptor kinase specificity. J Biol Chem. 1997;272:17734–17743. doi: 10.1074/jbc.272.28.17734. [DOI] [PubMed] [Google Scholar]
- 10.Ikegami T, Krilov L, Meng J, Patel B, Chapin-Kennedy K, Bouscarel B. Decreased glucagon responsiveness by bile acids: a role for protein kinase Calpha and glucagon receptor phosphorylation. Endocrinology. 2006;147:5294–5302. doi: 10.1210/en.2006-0516. [DOI] [PubMed] [Google Scholar]
- 11.Murphy GJ, Hruby VJ, Trivedi D, Wakelam MJ, Houslay MD. The rapid desensitization of glucagon-stimulated adenylate cyclase is a cyclic AMP-independent process that can be mimicked by hormones which stimulate inositol phospholipid metabolism. Biochem J. 1987;243:39–46. doi: 10.1042/bj2430039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chini B, Parenti M. G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J Mol Endocrinol. 2004;32:325–338. doi: 10.1677/jme.0.0320325. [DOI] [PubMed] [Google Scholar]
- 13.Buggy JJ, Heurich RO, MacDougall M, Kelley KA, Livingston JN, Yoo-Warren H, Rossomando AJ. Role of the glucagon receptor COOH-terminal domain in glucagon-mediated signaling and receptor internalization. Diabetes. 1997;46:1400–1405. doi: 10.2337/diab.46.9.1400. [DOI] [PubMed] [Google Scholar]
- 14.Krilov L, Nguyen A, Miyazaki T, Unson CG, Bouscarel B. Glucagon receptor recycling: role of carboxyl terminus, beta-arrestins, and cytoskeleton. Am J Physiol Cell Physiol. 2008;295:C1230–C1237. doi: 10.1152/ajpcell.00240.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merlen C, Fabrega S, Desbuquois B, Unson CG, Authier F. Glucagon-mediated internalization of serine-phosphorylated glucagon receptor and Gsalpha in rat liver. FEBS Letters. 2006;580:5697–5704. doi: 10.1016/j.febslet.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Unson CG. Expression of glucagon receptors in tetracycline-inducible HEK293S GnT1- stable cell lines: an approach toward purification of receptor protein for structural studies. Biopolymers. 2008;90:287–296. doi: 10.1002/bip.20951. [DOI] [PubMed] [Google Scholar]
- 17.Bouscarel B, Gettys TW, Fromm H, Dubner H. Ursodeoxycholic acid inhibits glucagon-induced cAMP formation in hamster hepatocytes: a role for PKC. Am J Physiol. 1995;268:G300–G310. doi: 10.1152/ajpgi.1995.268.2.G300. [DOI] [PubMed] [Google Scholar]
- 18.Ikegami T, Cypess AM, Bouscarel B. Modulation of glucagon receptor expression and response in transfected human embryonic kidney cells. Am J Physiol Cell Physiol. 2001;281:C1396–C1402. doi: 10.1152/ajpcell.2001.281.4.C1396. [DOI] [PubMed] [Google Scholar]
- 19.Tao YX, Segaloff DL. Functional characterization of melanocortin-4 receptor mutations associated with childhood obesity. Endocrinology. 2003;144:4544–4551. doi: 10.1210/en.2003-0524. [DOI] [PubMed] [Google Scholar]
- 20.Hipkin RW, Wang Y, Schonbrunn A. Protein kinase C activation stimulates the phosphorylation and internalization of the sst2A somatostatin receptor. J Biol Chem. 2000;275:5591–5599. doi: 10.1074/jbc.275.8.5591. [DOI] [PubMed] [Google Scholar]
- 21.Innamorati G, Le Gouill C, Balamotis M, Birnbaumer M. The long and the short cycle. Alternative intracellular routes for trafficking of G-protein-coupled receptors. J Biol Chem. 2001;276:13096–13103. doi: 10.1074/jbc.M009780200. [DOI] [PubMed] [Google Scholar]
- 22.Winstel R, Freund S, Krasel C, Hoppe E, Lohse MJ. Protein kinase cross-talk: membrane targeting of the beta-adrenergic receptor kinase by protein kinase C. Proceedings of the National Academy of Sciences of the United States of America; 1996. pp. 2105–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cong M, Perry SJ, Lin FT, Fraser ID, Hu LA, Chen W, Pitcher JA, Scott JD, Lefkowitz RJ. Regulation of membrane targeting of the G protein-coupled receptor kinase 2 by protein kinase A and its anchoring protein AKAP79. J Biol Chem. 2001;276:15192–15199. doi: 10.1074/jbc.M009130200. [DOI] [PubMed] [Google Scholar]
- 24.Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F., Jr The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochimica et Biophysica Acta. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Shetzline MA, Walker JK, Valenzano KJ, Premont RT. Vasoactive intestinal polypeptide type-1 receptor regulation. Desensitization, phosphorylation, and sequestration. J Biol Chem. 2002;277:25519–25526. doi: 10.1074/jbc.M201815200. [DOI] [PubMed] [Google Scholar]
- 26.Anis Y, Leshem O, Reuveni H, Wexler I, Ben Sasson R, Yahalom B, Laster M, Raz I, Ben Sasson S, Shafrir E, Ziv E. Antidiabetic effect of novel modulating peptides of G-protein-coupled kinase in experimental models of diabetes. Diabetologia. 2004;47:1232–1244. doi: 10.1007/s00125-004-1444-1. [DOI] [PubMed] [Google Scholar]
- 27.Prevostel C, Alice V, Joubert D, Parker PJ. Protein kinase C(alpha) actively downregulates through caveolae-dependent traffic to an endosomal compartment. J Cell Sci. 2000;113 ( Pt 14):2575–2584. doi: 10.1242/jcs.113.14.2575. [DOI] [PubMed] [Google Scholar]
- 28.Ronnebaum SM, Ilkayeva O, Burgess SC, Joseph JW, Lu D, Stevens RD, Becker TC, Sherry AD, Newgard CB, Jensen MV. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem. 2006;281:30593–30602. doi: 10.1074/jbc.M511908200. [DOI] [PubMed] [Google Scholar]
- 29.Kim YM, Benovic JL. Differential roles of arrestin-2 interaction with clathrin and adaptor protein 2 in G protein-coupled receptor trafficking. J Biol Chem. 2002;277:30760–30768. doi: 10.1074/jbc.M204528200. [DOI] [PubMed] [Google Scholar]
- 30.Heurich RO, Buggy JJ, Vandenberg MT, Rossomando AJ. Glucagon induces a rapid and sustained phosphorylation of the human glucagon receptor in Chinese hamster ovary cells. Biochem Biophys Res Commun. 1996;220:905–910. doi: 10.1006/bbrc.1996.0504. [DOI] [PubMed] [Google Scholar]
- 31.Charbonneau A, Unson CG, Lavoie JM. High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: potential interaction with acute exercise. J Physiol. 2007;579:255–267. doi: 10.1113/jphysiol.2006.121954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobias ES, Rozengurt E, Connell JM, Houslay MD. Co-transfection with protein kinase D confers phorbol-ester-mediated inhibition on glucagon-stimulated cAMP accumulation in COS cells transfected to overexpress glucagon receptors. Biochem J. 1997;326 ( Pt 2):545–551. doi: 10.1042/bj3260545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 34.Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15:209–219. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Ivanov AI. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol Biol. 2008;440:15–33. doi: 10.1007/978-1-59745-178-9_2. [DOI] [PubMed] [Google Scholar]
- 36.Syme G, Rowe P, Martin D, Daly G. Disability in patients with chronic patellofemoral pain syndrome: a randomised controlled trial of VMO selective training versus general quadriceps strengthening. Man Ther. 2009;14:252–263. doi: 10.1016/j.math.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 38.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 39.Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Ferguson SS, Barak LS, Menard L, Caron MG. Dynamin and beta-arrestin reveal distinct mechanisms for G protein-coupled receptor internalization. J Biol Chem. 1996;271:18302–18305. doi: 10.1074/jbc.271.31.18302. [DOI] [PubMed] [Google Scholar]
- 41.Pals-Rylaarsdam R, Gurevich VV, Lee KB, Ptasienski JA, Benovic JL, Hosey MM. Internalization of the m2 muscarinic acetylcholine receptor. Arrestin-independent and -dependent pathways. J Biol Chem. 1997;272:23682–23689. doi: 10.1074/jbc.272.38.23682. [DOI] [PubMed] [Google Scholar]
- 42.Lee KB, Ptasienski JA, Pals-Rylaarsdam R, Gurevich VV, Hosey MM. Arrestin binding to the M(2) muscarinic acetylcholine receptor is precluded by an inhibitory element in the third intracellular loop of the receptor. J Biol Chem. 2000;275:9284–9289. doi: 10.1074/jbc.275.13.9284. [DOI] [PubMed] [Google Scholar]
- 43.Vines CM, Revankar CM, Maestas DC, LaRusch LL, Cimino DF, Kohout TA, Lefkowitz RJ, Prossnitz ER. N-formyl peptide receptors internalize but do not recycle in the absence of arrestins. J Biol Chem. 2003;278:41581–41584. doi: 10.1074/jbc.C300291200. [DOI] [PubMed] [Google Scholar]
- 44.Dalle S, Longuet C, Costes S, Broca C, Faruque O, Fontes G, Hani EH, Bataille D. Glucagon promotes cAMP-response element-binding protein phosphorylation via activation of ERK1/2 in MIN6 cell line and isolated islets of Langerhans. J Biol Chem. 2004;279:20345–20355. doi: 10.1074/jbc.M312483200. [DOI] [PubMed] [Google Scholar]
- 45.Ishizaka N, Griendling KK, Lassegue B, Alexander RW. Angiotensin II type 1 receptor: relationship with caveolae and caveolin after initial agonist stimulation. Hypertension. 1998;32:459–466. doi: 10.1161/01.hyp.32.3.459. [DOI] [PubMed] [Google Scholar]
- 46.Wyse BD, Prior IA, Qian H, Morrow IC, Nixon S, Muncke C, Kurzchalia TV, Thomas WG, Parton RG, Hancock JF. Caveolin interacts with the angiotensin II type 1 receptor during exocytic transport but not at the plasma membrane. J Biol Chem. 2003;278:23738–23746. doi: 10.1074/jbc.M212892200. [DOI] [PubMed] [Google Scholar]
- 47.Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of beta -adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]