Abstract
Glycoproteins are involved in many important molecular recognition processes including invasion, adhesion, differentiation, and development. To identify the glycoproteins of Toxoplasma gondii, a proteomic analysis was undertaken. T. gondii proteins were prepared and fractioned using lectin affinity chromatography. The proteins in each fraction were then separated using SDS-PAGE and identified by tryptic in gel digestion followed by tandem mass spectrometry. Utilizing these methods 132 proteins were identified. Among the identified proteins were 17 surface proteins, 9 microneme proteins, 15 rhoptry proteins, 11 heat shock proteins (HSP), and 32 hypothetical proteins. Several proteins had 1 to 5 transmembrane domains (TMD) with some being as large as 608.3 kDa. Both lectin-fluorescence labeling and lectin blotting were employed to confirm the presence of carbohydrates on the surface or cytoplasm of T. gondii parasites. PCR demonstrated that selected hypothetical proteins were expressed in T. gondii tachyzoites. This is data provides a large scale analysis of the T. gondii glycoproteome. Studies of the function of glycosylation of these proteins may help elucidate mechanism(s) involved in invasion improving drug therapy as well as identify glycoproteins that may prove to be useful as vaccine candidates.
Keywords: glycosylation, Toxoplasma, glycoproteome, membrane proteins, lectin chromatography
1. Introduction
Toxoplasma gondii is an obligate intracellular parasite of both humans and domestic animals. Infection in humans is common occurring via food-borne or waterborne transmission and via maternofetal transmission resulting in congenial infection [1, 2]. There are three major life stages of this Apicomplexan: the tachyzoite which is involved in acute infection and dissemination of the parasite in its host, the bradyzoite which is found in tissue cysts and latent infection, and the oocysts which is the sexual stage that develops in the feline gastrointestinal system[3]. Usually people become infected with T. gondii after ingestion of uncooked or undercooked meat products containing bradyzoites, or oocyst/sporozotes from contaminated water or soil[4]. This causes an acute infection, due to tachyzoites, which is occasionally symptomatic, but in the majority of cases resolves resulting in latent infection. Latent infection persists due to the formation of cysts, containing bradyzoites, in muscle, neurons and glia. It is capable of causing severe congenital neurological impairment in acquired in utero [1, 3]. Infection can reactivate from latent tissue cysts in patients with immune suppression, such as HIV infection, resulting in acute infections most often manifesting as encephalitis [4]. Reported T. gondii infection rates can be as high as 70%, depending on the population or geographic area studied[1]. T. gondii can infect all warm-blooded mammals although the definitive hosts are members of the cat family.
Glycosylation, both N-linked and O linked oligosaccharides, is one of the most common and important post-translation modifications seen in eukaryotic proteins [5–7]. This post translational modification can have significant effects on protein structure and function and is often a developmentally regulated process. Glycosylation pathways occur in the cytosol, endoplasmic reticulum, and the Golgi complex and involve transport steps, processing glycosidases, and glycosyltransferases[8, 9]. Glycosylation is often found on membrane and secreted proteins, being added onto these proteins during transport and synthesis in the ER (N-linked glycosylation) or during passage through the Golgi (O-linked glycosylation) Oligossacchrides greatly affect the physical properties and biological functions of many proteins playing critical roles in correct protein folding as well as cell-cell interactions.
Several studies had suggested that glycosylation was rarely seen in T. gondii proteins and this issue was considered controversial, but recent papers have clearly demonstrated glycoproteins in T. gondii RH strain tachyzoites [10–12]. Glycosylation has been demonstrated on proteins in the inner membrane complex associated with motility[10, 12]. Consistent with this observation, tunicamycin treated parasites have been demonstrated to have defects in invasion and motility [10, 12]. The tissue cyst wall (formed by modification of the parasitophorous vacuole by bradyzoites) of T. gondii has been demonstrated to react with the lectins Dolichos biflorus (DBA) and succinyl Wheat Germ Agglutinin (SWGA)[13]. Examination of the T. gondii genome (www.toxodB.org) demonstrates the presence of enzymes for the synthesis of both N-linked (dolichol-linked precursor oligosaccharides) and O-linked (UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferases) modifications[14, 15]. Cell free extracts of T. gondii have been demonstrated to have both N-glycosylation and O-glycosylation activity using synthetic peptide substrates [15–17]. In addition, studies using mass spectrometry have defined the presence of Man6(GlcNAc)2, Man7(GlcNAc)2, and Man8(GlcNAc)2 N-glycans in T. gondii [12]. Interestingly, there was an absence of glycans containing sialic acid, galactose and fucose residues in this analysis [12].
The development of new preventative and therapeutic strategies for pathogens should relay on an improved understanding of the interactions between pathogens and their hosts. Surface proteins are a potential target of many compounds aimed at preventing microbial infections and many of these proteins are glycosylated. Moreover, because surface proteins are likely to interact with the host immune system, they often become components of effective vaccines, many of which are based on glycoproteins. T. gondii, like other Apicomplexa, is surrounded by a triple membrane system, termed the pellicle, consisting of the plasmalemma and inner membrane complex. There are several approaches currently in practice to identify surface proteins. The first approach is based on surface protein prediction by genome analysis using algorithms such as PSORT [18]. The method is rapid but is not fully reliable nor is it quantitative. The second approach employs separation of membrane and cell wall fractions from the cytoplasmic fraction followed by the identification of proteins by 2D-gel electrophoresis or 2D-chromatography coupled to mass spectrometry [5]. This approach is reasonably quantitative. Subcellular extraction can simplify this type of analysis and enhance protein identification.
In this report we demonstrate a simple and efficient approach for determining the glycoproteome of T. gondii. A buffer containing detergent and a high salt concentration was utilized to extract surface and organelle glycoproteins using serial lectin affinity chromatography (SLAC). From this fraction, 132 glycoproteins were identified. Several methods were used to confirm the identification and the presence of carbohydrates on the surface or in cytoplasm of T. gondii.
2. Materials and methods
2.1 Host cells and parasites
Toxoplasma gondii RH strain was maintained by serial passage in confluent monolayers of human foreskin fibroblasts (HFF) incubated at 37°C under a 5% CO2 atmosphere in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS, Invitrogen, CA), 2mM glutamine and 20mM penicillin streptomycin. Cells were maintained as a contact-inhibited monolayer until required. Infection was maintained by inoculating flasks (T25 or 75) with freshly harvested tachyzoites. Parasites were purified by passing infected cells through a 25 gauge needle and then separated from host cell debris by passage through a 3μm Nucleopore membrane. Parasites were pelleted and then used for either protein or RNA purification. Total RNA was extracted using TRIzol (Invitrogen CA) as per the manufacturer’s recommendations.
2.2 Extraction of T. gondii proteins
T. gondii (2 × 1010) was suspended in 1ml PBS containing 0.5% Nonidt P40 and 500 mM NaCl and protease inhibitor cocktail (Complete Protease Cocktail, Roche Applied Science, Indianapolis, IN) and incubated for 2 hours at 4°C on ice. The tube was then centrifuged at 13600 × g in an Eppendorf microcentrifuge for 16 min and the supernatant collected for lectin affinity chromatography.
2.3. Serial lectin affinity chromatography (SLAC)
Spin columns of Agarose bound to Con A, WGA, or Jaclin (Vector Laboratories, Burlingame, CA) were equilibrated with buffer A (10 mM Tric Cl, pH 7.5, 150 mM NaCl, 1mM CaCl2, 1mM MnCl2). One ml of the T. gondii membrane extraction was first applied to the Con A column, this column was allowed to rotate at 4°C for 2 h and then the ConA column was then spun down (1000 × g for 1 min) and the pass through loaded into WGA column. The WGA column was then handled as noted for the Con A column with the pass-through being loaded onto the Jaclin column. Once the glycoproteins were bound to each column (i.e. ConA, WGA and Jaclin) the columns were washed extensively with buffer A until no proteins could be detected in washes. Elution was then carried out by incubation for 15 min with 200 mM α-methyl mannoside (α-MM) for the Con A column, 500 mM N-acetyglucosamine (NAGlc) for the WGA column, and 800 mM galactose for the Jaclin column, respectively followed by centrifugation (100 × g for 10 min). The eluates were then used for SDS-PAGE and subsequent mass spectrometry. This workflow is outlined in Figure 1. Changing the order of the columns might change where in the purification a protein appeared, as a protein could bind to multiple lectins, however, one would expect a similar overall list of proteins if the columns were used in a different order.
Figure 1.
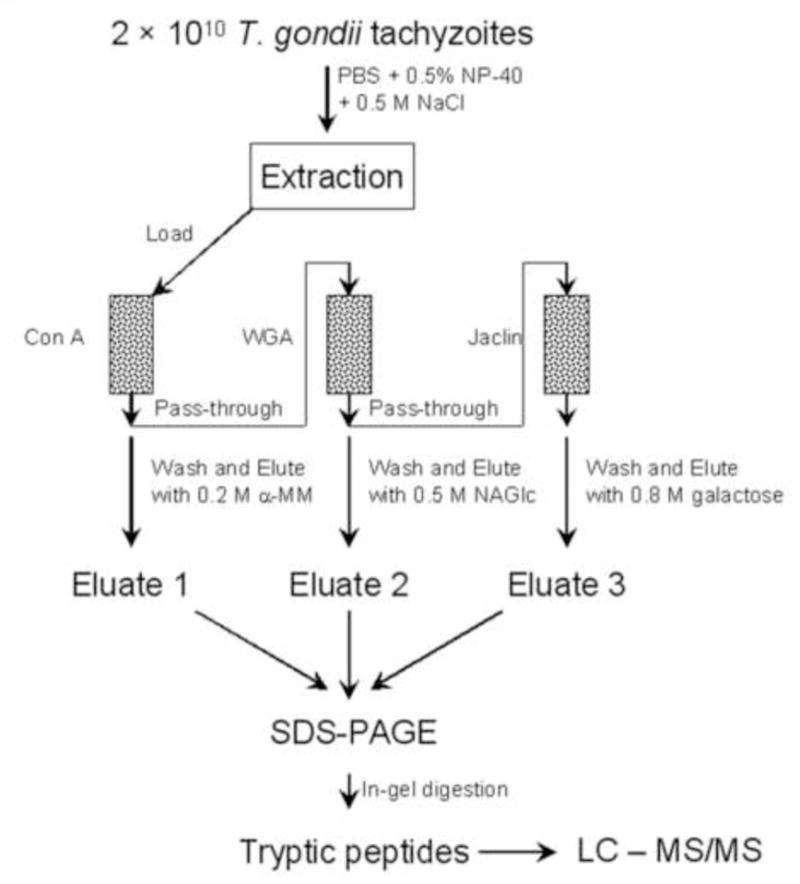
Schema for glycoprotein enrichment for proteomic analysis of T. gondii by LC-MS/MS.
2.4. SDS-PAGE and in-gel tryptic digestion
Protein eluates (30 μl per lane) were analyzed by electrophoreses in a 7.5 to 17.0 % gradient, 1 mm thick gel using a constant current of 400 mA for 1 h. The resolved proteins were visualized by GelCode Blue (Pierce, IL) and the corresponding bands were excised. The gel bands were destained with 60% acetonitrile solution and then the proteins in the gels were digested with trypsin[19]. Briefly, gel pieces were completely dried down in a vacuum centrifuge, rehydrated with a trypsin solution and allowed to incubate on ice for 45 min. After 45 min, the trypsin supernatant was removed and replaced with approximately 20 μl of digestion buffer without trypsin so that the gel pieces were covered. The gel pieces were kept wet at 37 °C overnight for digestion with mixing.
2.5. LC-MS Analysis
The labeled tryptic peptides were subjected to LC-MS/MS analysis on an LTQ mass spectrometer (Thermo Scientific, San Jose, CA). Chromatographic separation of peptides was performed on a nano HPLC System (LC Packings, San Francisco, CA, USA). The LC eluate from a 75 μm i.d. ×15 cm, PepMap C18 column (Dionex, Marlton, NJ) was directed to a micro-ionspray source. Throughout the LC gradient, MS and MS/MS data were recorded continuously using a 6-sec cycle time. With each cycle, MS data were accumulated for 1 s, followed by three CID acquisitions of 2.5 s each on ions selected by preset selection parameters of the data-dependant acquisition method. In general, the ions selected for CID were the 2 most abundant obtained from the survey MS spectrum, except that singly charged ions were excluded and dynamic exclusion was employed to prevent repetitive selection of the same ions within a preset time. Rolling collision energies were used to adjust automatically for the charge state and the mass/charge value of the precursor ion. Searches were performed using Mascot and a T. gondii protein database (http://toro.aecom.yu.edu/biodefense/) [19–21]. In all searches, oxidation of methionine, deamidation of asparagines and glutamine and conversion of glutamine to pyroglutamic acid (N-terminus) were selected as variable modifications and carboxyamidomethylation of cysteine residues was selected as a fixed modification, and a maximum of one missed cleavage. The peptide precursor mass tolerance was +/− 2 Da and the MS/MS product ions, +/− 0.8 Da. The data were also searched against a scrambled decoy database with false discovery rate of 1%.
2.6. Isolation of glycopeptides from intact T. gondii
In other experiments, trypsin was utilized to cut the surface membrane proteins free from T. gondii, (2 hr at 37°C), the T. gondii were then removed by centrifugation (10,000 × g for 3 min). The supernatant containing the liberated glycoproteins was then removed and was allowed to complete its proteolysis until tryptic peptides were obtained (12 hr at 37°C). The supernatant was diluted in buffer A and Con A conjugated beads (lectin affinity chromatography) were used to capture these glycopeptides. The column was washed three times with buffer A, twice with buffer B (25 mM Tris, 10 mM NaCl, pH7.36) and then the bound glycopeptides were released from the beads by incubation for 2 hr at 37 °C in buffer B containing peptide N-glycosidase F (PNGase F) and the column washed with 1 volume of buffer B. The last wash and PNGase F fractions were pooled and then strong cation exchange chromatography (SCX beads) was used to fractionate the deglycosylated peptide mixture into eight fractions by step-gradient elution using standard protocols. Nano-LC-MS/MS was then performed to analyze these eight fractions, and obtain amino acid sequences of the released glycopeptides.
2.7. Lectin blotting
For detection of lectin-binding sites on glycoproteins following SDS-PAGE and transfer to PFA membranes, the following HRP conjugated lectins (EY Laboratories) were used: Con A, high affinity for alpha-D-mannosyl residues; wheat germ agglutinin (WGA), affinity for D-N-acetylglucosamine; Jacalin, high affinity for N-acetylgalactosamine-beta-1,3-galactose. After blocking PFA membranes in 1% BSA/PBS containing 0.2 % Tween 20 for 4 h, they were incubated for 1 h at room temperature with lectins conjugated with HRP in PBS/0.2%BSA/0.2 Tween 20 at the following concentrations: 1 μg/mL Con A, 2 μg/mL WGA, 2μg/mL Jacalin, respectively. All incubations were accompanied by control incubations in the presence of corresponding inhibiting sugars: 0.4 M methyl-mannopyranose for Con A, 0.5 M N-acetylglucosamine for WGA, 0.8 M galactose for Jaclin. After rinsing using TBS/0.2% Tween 20, wash three times for 5 min in the same buffer, and reactive bands were detected according to the manufacturer’s instructions (Amersham Biosciences, Part of GE Healthcare).
2.8. Lectin-fluorescence labeling
For surfacing labeling of parasites, freshly purified T. gondii tachyzoites were suspended in fixation buffer (PBS/3% paraformaldehyde/0.05% glutaraldehyde, pH 7.2) at 105 parasites/ml and were then applied to poly-lysine coated (100 μg/ml) glass coverslips for 30 min. After adherence onto coverslips specimens were rinsed in PBS and placed in blocking solution (PBS/1%BSA/50 mM glycine) for 4h. Lectins (ConA, WGA, Jacalin) conjugated with FITC were diluted 1:200 in PBS and incubated with the coverslips for 1 h at 37°C. Specimens were rinsed five times in PBS for 5 min each time and then briefly rinsed in distilled water. For a negative control slides were incubated with anti mouse IgG -FITC at 1:500 for 1 hour and then rinsed five times in PBS. For a positive control slides were incubated with a mAb to SAG1 (p30 a major T. gondii surface antigen) at 1:500 for 1 hour followed by washing with PBS, then incubation with secondary anti-mouse IgG-FITC at 1:500 for 1 hour and then washed 5 times in PBS. The preparations were then examined on a Leitz Laborlux S fluorescence microscope.
For the detection of intracellular lectin binding sites, parasites were fixed in PBS/3% paraformaldehyde, and after adherence onto coverslips rinsed in PBS and subsequently permeabilized using 0.1% Triton X100 for 30 min. After fixation the coverslips were rinsed three times in PBS and were placed into blocking solution (PBS/1%BSA/50 mM glycine), washed with PBS and incubated with 1:200 lectin conjugated with FITC in PBS for 1 h at 37°C. Specimens were then rinsed in five times in PBS for 5 min each time and then briefly rinsed in distilled water. The preparations were then examined on a Leitz Laborlux S fluorescence microscope.
2.9. Hydropathy calculations and transmembrane mapping
All identified proteins were analyzed using the ProtParam program (available at http://www.expasy.ch/sprot/sprot-top.html), which allows the calculation of the grand average of hydrophobicity (GRAVY) value for a given protein. The proteins exhibiting positive GRAVY values were recognized as a hydrophobic. All identified proteins were examined by the TMHMM Server (Prediction of transmembrane helices in proteins; http://www.cbs.dtu.dk/services/TMHMM). The analysis was performed to map TMDs [22].
2.10. Polymerase Chain Reaction (PCR)
A total RNA of 5ug was used for the synthesis of cDNA using AMV reverse transcriptase (TAKARA USA BIO) following the protocol supplied with the reagent. One ul of the resultant cDNA was used as a template in a 50ul PCR reaction using the fast start high fidelity PCR system (Roche Applied Science, IN). Hot start amplification was initiated with 2 min 95°C denaturation, followed by 30 cycles (95°C for 15sec, 53°C for 15 sec, and 72°C for 3 min) of amplification which was terminated by a 72°C extension for 10 min. Gene products were cloned into the PCR8GWTOPO vector (Invitrogen, CA) by TOPO cloning and sequenced at the Einstein Sequencing Facility.
The following oligonucleotide primer pairs were used for proteins identified by SLAC (the ID number of the corresponding gene from ToxodB (www.toxodb.org) is listed followed by the primers used (5′-3′) for RT-PCR for each gene): 55.m00103 (TCATTCACCCCGTTTTT TGTGACTCTTGG::CTGACGTGACGCTGCGTACTGACTG); 41.m01274 (TCGGA CTCGGTCGGTCGAAATGTGC::CTCGGACTCACAAGCCAGTGAATACGTCG), 76.m01589 (GTGTGCCTCAGGCACTGGTGGCTC::GCCTCGCTGCATCGTCTCTCGA), 67.m00007 (TGGCGCATCTGGAGATGCCGGCTG::TCGCCTTGCGGAAACGTGTACGTCC), 49.m03169 (CAGGCGATAGCGCGGGGGGACCGC:: TTCGCTTGGTCTCTGGTAGCCCAGCC), 80.m02161(AGGGCGATCGTGGCATCGACGCAGCAG::GTTGTGTTTGCTGCCTGCAGAGCC GCGCA), 46.m01601 (AAGCCACAAGTTTTGTTCGGTCTTC::AACGTATTTCTTC AAAAGGTTGTCAAGGGTGG), 42.m03584 (TCGTCATCCAGATTGGTACTCGTTTCC::CCCC GTGACGGGGAAGTACGCAGTCAGTTGA), 31.m00928 (AGGACGTCACGTCTCTTGTG TGCATTTGG::CAGCACTTGTTGCATTG CGATTCCAGAAGC), 55.m08219 (AAAGTGACCA CGAAAGGGCTTGCTTT TGC:: CATCCGATGTGAAGAAAGTTCGGTAGTTGG), 50.m00023 (GCTGAAACTGCTCTGTACTAC CAGG::GTATTTGAAGTTCGGAGGCAACACAAACGC), 76.m01543 (TGTGTGTGGTACGGACAGGCACG::TATATTGTCATCTTGCTCGCCAGGA CCTGAAGCG), 59.m03403 (ATCCACACGGGCCTGGATCTGC::GTGAGCAGGAGCACCG GCCGGT)
3. Results
3.1 Glycoprotein identification
T. gondii membrane proteins were separated by SLAC followed by SDS-PAGE (Figs.1 and 2). Among the proteins identified from T. gondii by SLAC as having glycoepitopes were 9 microneme proteins, 7 dense granule proteins, 15 rhoptry proteins, 17 surface proteins, 19 enzymes, 11 heat shock proteins, 20 other proteins, and 32 hypothetical proteins (Table 1 and Supplemental Table 1). This data has been deposited at EPICdB (http://toro.aecom.yu.edu/cgi-bin/biodefense/main.cgi) and ToxodB. (http://toxodb.org/toxo/) [20]. Of these 132 glycoproteins some bound only to con A; some to both WGA and con A, or con A and Jacalin, or WGA and Jacalin; and some to all three lectins (see Table 1 final column). The majority of these proteins were purified using the Con A column. Of interest, is that one of the 32 novel hypothetical proteins, protein (80.m02347), with a molecular weight of 608.3 kDa was predicted by TMHHD to have a single TM helix domain with the majority of its structure being outside the membrane on the surface of the parasite. Table 1 presents all of the SLAC identified proteins organized according to presumed function. The majority of these proteins have predicted N-glycosylation sites (http://www.cbs.dtu.dk/services/NetNGlyc/). It was previously believed that glycosylation was rare in T. gondii proteins; however, our data provide evidence for a large number of previously unrecognized glycoproteins in this organism. Overall, this work demonstrates that SLAC combined with tandem MS is a powerful approach for glycoproteomic analysis in T. gondii and that glycosylation occurs in a significant number of the proteins.
Figure 2. SDS-PAGE of T. gondii glycoproteins isolated by lectin affinity chromatography.
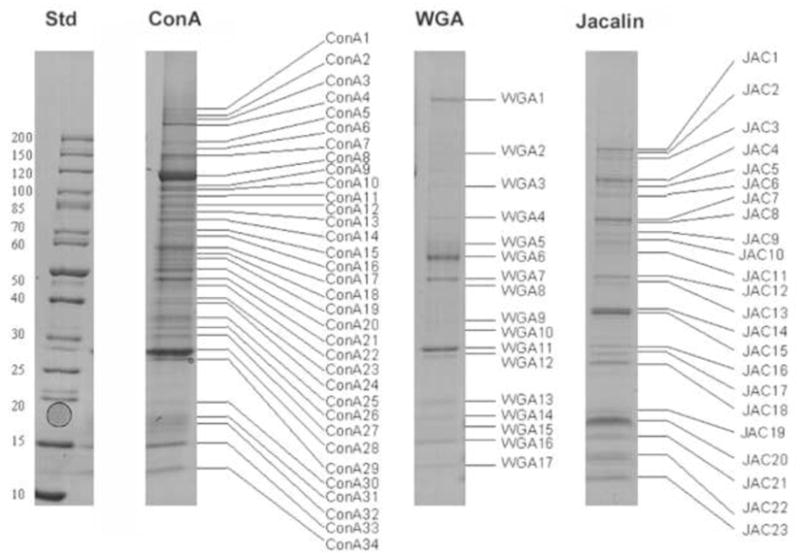
The protein molecular weight standards are shown on the left. The gel band numbers correspond to proteins identified in Table S1 and Table 2. Std: molecular weight standard.
Table 1.
The glycoproteins identified from T. gondii using lectin affinity chromatography.
| gene id | Release 6 Name | Protein Name | Percent Coverage | MOWSE | Mw (kDA) | Gel Band |
|---|---|---|---|---|---|---|
| MICRONEME PROTEINS | ||||||
| NR-2062142 | TGME49_091890 | microneme TgMIC1 | 46 | 4723 | 48.6 | ConA21 WGA7 JAC12 |
| 20.m00002 | TGME49_001780 | microneme protein, putative TgMIC2 | 11 | 49 | 124.8 | ConA12 |
| 641.m00002 | TGME49_119560 | MIC3 microneme protein TgMIC3 | 26 | 400 | 37.9 | ConA13 |
| 25.m00006 | TGME49_008030 | micronemal protein 4 TgMIC4 | 23 | 303 | 63 | ConA17 WGA5 JAC9 |
| NR-4704627 | TGME49_018520 | microneme protein, putative TgMIC6 | 19 | 50 | 36.6 | ConA29 WGA12 JAC17 |
| NR-118500931 | TGME49_004130 | perforin-like protein 1; PLP1 | 44 | 5922 | 124.6 | ConA8 |
| 20.m03849 | TGME49_004130 | membrane-attack complex/perforin domain-containing protein; PLP1 | 18 | 95 | 117.3 | ConA16 |
| 55.m00005 | TGME49_055260 | apical membrane antigen 1, putative AMA1 | 38 | 112 | 63 | ConA16 JAC9 |
| 20.m00387 | TGME49_004050 | subtilase family serine protease, putative TgSUB1 | 5 | 42 | 132.6 | JAC1 |
| DENSE GRANULE PROTEINS | ||||||
| 42.m00015 | TGME49_027620 | 28kd antigen GRA2 (or p28) | 30 | 313 | 19.8 | JAC18 |
| NR-161913 | TGME49_027620 | 28kd antigen; GRA2 (or p28) | 20 | 70 | 28 | JAC22 |
| NR-22652337 | TGME49_027280 | dense granule protein, putative GRA3 | 17 | 396 | 24.2 | ConA29 |
| 42.m00013 | TGME49_027280 | Dense granule protein 3 GRA3 | 16 | 61 | 22.2 | WGA12 |
| 63.m00002 | TGME49_075440 | dense granule antigen GRA6 | 27 | 49 | 24 | ConA27 |
| 20.m00005 | TGME49_003310 | granule antigen protein GRA7 | 86 | 701 | 25.8 | ConA28 |
| NR-2062409 | TGME49_003310 | 29kD excretory dense granule protein GRA7 | 34 | 88 | 25.8 | ConA29 JAC16 |
| RHOPTRY PROTEINS | ||||||
| NR-897823 | TGME49_109590 | rhoptry protein ROP1 | 18 | 1323 | 42.6 | ConA20 |
| NR-563627 | TGME49_015780 | rhoptry protein 2 ROP 2 (ROP2A) | 32 | 1585 | 64 | ConA19 |
| NR-52788873 | TGME49_095110 | rhoptry protein 4 ROP4 (ROP4/7 locus) | 28 | 289 | 64 | ConA17 JAC11 |
| NR-134035971 | TGME49_108080 | rhoptry protein 5 ROP5 | 13 | 80 | 60.8 | ConA22 JAC10 |
| 551.m00238 | TGME49_108080 | rhoptry protein 5 ROP5 | 28 | 2991 | 60.9 | ConA17 WGA5 |
| 83.m02145 | TGME49_095110 | rhoptry protein ROP7 (ROP4/7 locus) | 16 | 415 | 103.8 | ConA18 |
| 42.m03584 | TGME49_027810 | rhoptry protein 11 ROP11 | 7 | 48 | 58 | ConA21 |
| NR-71559154 | TGME49_112270 | rhoptry protein 13 ROP13 | 9 | 51 | 44.8 | ConA25 |
| 55.m08219 | TGME49_062730 | rhoptry protein 16 ROP16 | 17 | 1271 | 76.2 | JAC13 |
| NR-84618297 | TGME49_005250 | rhoptry protein 18 ROP18 | 21 | 276 | 62.3 | ConA19 |
| 583.m00597 | TGME49_110010 | rhoptry neck protein 1 TgRON1 | 8 | 43 | 118.8 | ConA13 |
| NR-71559160 | TGME49_100100 | rhoptry neck protein 2 TgRON2 | 31 | 389 | 155.5 | ConA7 |
| 583.m00636 | TGME49_111470 | rhoptry neck protein 5 TgRON5 | 27 | 190 | 56.2 | ConA22 JAC13 |
| 583.m00011 | TGME49_114500 | subtilisin-like protease TgSUB2 TgSUB2 | 9 | 57 | 141.5 | ConA8 JAC7 |
| 33.m02185 | TGME49_014080 | toxofilin | 21 | 83 | 27.1 | JAC17 |
| SURFACE PROTEINS | ||||||
| 76.m01626 | TGME49_085870 | SRS domain-containing surface antigen, putative; SRS20A | 68 | 459 | 34.9 | ConA27 |
| 44.m00008 | TGME49_033450 | GPI-anchored surface protein SRS29A (or SRS1) | 21 | 100 | 44.2 | ConA22 |
| NR-123186979 | TGME49_033460 | major surface antigen 1 SRS29B (SAG1 or p30) | 25 | 55 | 32.5 | ConA26 |
| NR-50082488 | TGME49_033460 | unnamed protein product SRS29B (SAG1 or p30) | 66 | 701 | 26.7 | ConA27 |
| NR-22219177 | TGME49_033460 | unnamed protein product; SRS29B (SAG1 or p30) | 86 | 29783 | 29.8 | ConA28 |
| NR-129348 | TGME49_033460 | major surface antigen P30 precursor; SRS29B (SAG1 or p30) | 55 | 2728 | 26.7 | WGA11 JAC16 |
| NR-10723 | TGME49_033460 | major surface antigen P30 precursor; SRS29B (SAG1 or p30) | 69 | 6669 | 34.8 | ConA29 |
| 44.m00010 | TGME49_033480 | SRS domain-containing protein SRS29C (or SRS2 or p35) | 16 | 105 | 39.1 | ConA25 |
| NR-2305260 | TGME49_033480 | SAG1-related sequence 2 SRS29C (or SRS2 or p35) | 20 | 367 | 39.3 | ConA26 |
| NR-5901701 | TGME49_033480 | P35 surface protein, putative; SRS29C (or SRS2 or p35) | 11 | 54 | 28.5 | ConA26 WGA9 |
| NR-161926 | TGME49_071050 | surface antigen P22; SRS34A (SAG2A or p22) | 48 | 3605 | 19 | ConA31 JAC19 |
| 57.m01840 | TGME49_067140 | SRS domain-containing protein; SRS38B | 7 | 68 | 40.9 | ConA26 |
| 583.m00001 | TGME49_108840 | conserved hypothetical protein; SRS51 (or SRS3) | 19 | 270 | 38 | ConA28 |
| 583.m05672 | TGME49_115320 | SRS domain-containing protein; SRS52A | 33 | 47 | 34.2 | ConA28 |
| NR-13447088 | TGME49_108010 | surface protein, putative | 40 | 844 | 41.7 | ConA25 |
| 641.m01520 | TGME49_119550 | transmembrane protein, putative | 14 | 58 | 76.1 | ConA14 |
| 129.m00256 | TGME49_099110 | cleft lip and palate transmembrane protein 1, putative | 9 | 65 | 68 | ConA15 |
| ENZYMES | ||||||
| 50.m03211 | TGME49_048160 | ATP-dependent DNA helicase II, 70 kDa subunit, putative; Ku70 | 1, 8 | 304 | 94.5 | ConA4 |
| 52.m01559 | TGME49_053030 | alpha-glucosidase II, putative | 18 | 140 | 183.2 | ConA4 |
| 42.m00006 | TGME49_028170 | serine/threonine protein phosphatase, putative | 25 | 218 | 170.7 | ConA5 JAC2 |
| 113.m00789 | TGME49_097650 | serine/threonine protein phosphatase, putative | 3 | 50 | 69 | ConA15 |
| NR-133990372 | TGME49_028170 | serine/threonine protein phosphatase, putative | 40 | 2048 | 168.2 | ConA7 |
| 44.m02735 | TGME49_032600 | patatin-like phospholipase domain-containing protein | 8 | 56 | 70.8 | ConA8 JAC5 |
| 583.m05329 | TGME49_110080 | long-chain-fatty-acid-CoA ligase, putative | 10 | 64 | 138.6 | ConA12 |
| 38.m01100 | TGME49_019130 | glutathione reductase, putative | 24 | 58 | 64 | ConA14 |
| 80.m02253 | TGME49_089940 | uroporphyrinogen decarboxylase, putative | 4 | 50 | 56.2 | ConA17 |
| 33.m00007 | TGME49_015260 | carbamoyl phosphate synthetase II | 4 | 44 | 186.9 | ConA17 |
| 83.m00004 | TGME49_094200 | putative glucose-6-phosphate-1-dehydrogenase | 23 | 80 | 62.7 | ConA18 WGA6 |
| 41.m01273 | TGME49_020940 | ribosomal RNA large subunit methyltransferase J, putative | 3 | 68 | 82.2 | ConA18 |
| 27.m00003 | TGME49_011680 | putative protein disulfide isomerase | 33 | 97 | 52.8 | ConA19 WGA6 |
| 55.m04808 | TGME49_059010 | vacuolar ATP synthase subunit D, putative | 33 | 106 | 44.9 | ConA22 |
| 20.m03680 | TGME49_001840 | eukaryotic aspartyl protease, putative; TgASP1 | 23 | 153 | 66.9 | ConA27 |
| 28.m00308 | TGME49_012310 | vacuolar ATP synthase 16 kDa proteolipid subunit, putative | 16 | 103 | 17.5 | ConA32 |
| 57.m01692 | TGME49_064650 | SRS domain-containing, N-acetylglucosamine-phosphate mutase, putative | 29 | 3655 | 324.2 | WGA1 |
| TgTigrScan_3843 | TGME49_078850 | glucose-6-phosphate dehydrogenase, putative | 12 | 196 | 197.8 | JAC5 |
| 44.m02735 | TGME49_032600 | patatin-like phospholipase domain-containing protein | 12 | 81 | 70.8 | JAC5 |
| HEAT SHOCK PROTEINS | ||||||
| 49.m00060 | TGME49_044560 | heat shock protein 90, putative; HSP90 | 23 | 70 | 99.1 | ConA1 WGA3 JAC5 |
| 80.m00001 | TGME49_088380 | heat shock protein 90; HSP90 | 9 | 43 | 89.5 | ConA3 |
| 583.m00009 | TGME49_111720 | heat shock protein 70, putative; HSP70 | 16 | 301 | 73.2 | ConA9 WGA4 JAC4 |
| 59.m00003 | TGME49_073760 | heat shock protein 70, putative; HSP70 | 22 | 508 | 72.8 | ConA14 WGA4 JAC8 |
| NR-3323502 | TGME49_073760 | heat shock protein, putative; HSP70 | 23 | 508 | 72.3 | ConA14 JAC7 |
| 50.m00085 | TGME49_051780 | heat shock protein 70kD, putative; HSP70 | 17 | 43 | 70.6 | ConA14 WGA4 JAC8 |
| NR-12248795 | TGME49_073760 | hsp70; HSP70 | 39 | 421 | 73.4 | ConA15 |
| 42.m03533 | TGME49_026830 | DnaK family protein; HSP70 | 33 | 617 | 101.6 | ConA7 |
| 49.m00030 | TGME49_040600 | TCP-1/cpn60 chaperonin family protein, putative | 30 | 84 | 72.2 | ConA15 |
| 50.m00006 | TGME49_047550 | heat shock protein, putative; HSP60 | 11 | 86 | 60.9 | ConA17 WGA5 JAC9 |
| 55.m00016 | TGME49_058390 | DnaJ protein, putative | 19 | 61 | 44.6 | ConA23 |
| OTHERS | ||||||
| NR-2209250 | TGME49_035470 | myosin A, putative; TgMyoA | 30 | 379 | 93.2 | ConA11 |
| 25.m00007 | TGME49_009030 | actin | 39 | 224 | 41.9 | JAC13 |
| 59.m00006 | TGME49_070240 | cyst matrix protein | 12 | 48 | 62.4 | ConA15 |
| 46.m01699 | TGME49_036540 | RRM domain-containing protein | 21 | 377 | 65.7 | ConA18 |
| 76.m00016 | TGME49_094800 | elongation factor 1-alpha, putative | 23 | 304 | 49 | ConA20 |
| 50.m05680 | TGME49_050770 | eukaryotic translation initiation factor 4A | 20 | 48 | 46.6 | ConA20 |
| 50.m00069 | TGME49_049270 | thioredoxin, putative | 18 | 148 | 46.9 | ConA22 |
| 42.m00047 | TGME49_025800 | ABC transporter, putative | 35 | 46 | 35.6 | ConA24 |
| 38.m00002 | TGME49_018410 | 60S acidic ribosomal protein P0 | 28 | 111 | 34.1 | ConA25 |
| 64.m00002 | TGME49_076140 | ADP-ribosylation factor, arf, putative | 37 | 147 | 21 | ConA30 |
| 49.m00025 | TGME49_039890 | SCP-like extracellular domain-containing protein | 15 | 178 | 26.6 | ConA31 |
| 55.m00221 | TGME49_063700 | 40s ribosomal protein S14, putative | 25 | 91 | 16.3 | ConA32, JAC20 |
| 541.m01147 | TGME49_105010 | RNA binding protein, putative | 24 | 84 | 24.4 | ConA32 |
| 583.m00614 | TGME49_109740 | small nuclear ribonucleoprotein, putative | 17 | 43 | 28.2 | ConA33 |
| 59.m03439 | TGME49_069180 | crooked neck-like protein 1, putative | 6 | 53 | 93.7 | WGA6 |
| 76.m01689 | TGME49_087170 | kinesin motor domain-containing protein, putative | 14 | 49 | 142.3 | WGA7 |
| 42.m00069 | TGME49_026410 | elongation factor 1-beta, putative | 28 | 64 | 36 | WGA10 |
| 641.m01588 | TGME49_120600 | glycine-rich protein 2, putative | 30 | 323 | 23 | WGA15 |
| 80.m02347 | TGME49_092020 | cysteine repeat modular protein, putative | 6, 7 | 593 | 608.3 | ConA1 |
| 641.m01513 | TGME49_119340 | kelch motif domain-containing protein | 18 | 77 | 44 | JAC15 |
| HYPOTHETICAL PROTEINS | ||||||
| 20.m03804 | TGME49_003520 | hypothetical protein | 6 | 43 | 256.2 | ConA4 |
| 42.m00026 | TGME49_023920 | hypothetical protein | 14 | 61 | 214.7 | ConA6 |
| 50.m07132 | TGME49_047210 | hypothetical protein, conserved | 7 | 57 | 50.7 | ConA6 |
| 55.m00103 | TGME49_058870 | hypothetical protein | 17 | 94 | 88.1 | ConA9 |
| 80.m02216 | TGME49_089500 | hypothetical protein | 34 | 2170 | 100.2 | ConA11 |
| 20.m00331 | TGME49_002200 | hypothetical protein | 22 | 84 | 80.1 | ConA13 |
| 583.m05446 | TGME49_111710 | hypothetical protein | 22 | 345 | 133 | ConA15 |
| 49.m00054 | TGME49_043930 | hypothetical protein | 12 | 1155 | 101.3 | ConA16 JAC8 |
| 41.m01274 | TGME49_020950 | hypothetical protein | 16 | 146 | 35.4 | ConA17 |
| 76.m01589 | TGME49_085180 | hypothetical protein | 22 | 158 | 66.1 | ConA18 |
| 41.m01337 | TGME49_022100 | hypothetical protein | 11 | 82 | 128.2 | ConA19 |
| 67.m00007 | TGME49_079100 | hypothetical protein | 38 | 214 | 47.7 | ConA20 |
| 49.m03169 | TGME49_039740 | hypothetical protein | 20 | 63 | 44.7 | ConA22 |
| 80.m02161 | TGME49_088650 | hypothetical protein | 43 | 1289 | 47.8 | ConA23 |
| 583.m05736 | TGME49_116250 | hypothetical protein | 32 | 308 | 44.7 | ConA24 |
| 20.m08222 | TGME49_003990 | hypothetical protein, conserved | 17 | 91 | 25.5 | ConA24 |
| 80.m02287 | TGME49_090730 | hypothetical protein, conserved | 48 | 1777 | 37.2 | ConA25 |
| 46.m01601 | TGME49_034380 | hypothetical protein | 17 | 144 | 39.4 | ConA26 |
| 31.m00928 | TGME49_013280 | hypothetical protein | 25 | 359 | 20.8 | ConA30 |
| 41.m00025 | TGME49_022880 | hypothetical protein | 3 | 47 | 27.5 | ConA31 |
| 583.m05298 | TGME49_109600 | hypothetical protein | 4 | 45 | 106.5 | WGA3 |
| NR-95007087 | TGME49_095100 | hypothetical protein | 5 | 43 | 152.2 | WGA10 |
| 583.m00652 | TGME49_112420 | hypothetical protein | 5 | 57 | 85.9 | WGA12 JAC13 |
| 69.m00143 | TGME49_079420 | hypothetical protein | 33 | 2572 | 144.6 | JAC1 |
| 42.m03456 | TGME49_025860 | hypothetical protein | 15 | 604 | 102.4 | JAC3 |
| 50.m00023 | TGME49_045610 | hypothetical protein, conserved | 20 | 378 | 79.1 | JAC13 |
| 76.m01543 | TGME49_083540 | hypothetical protein, conserved | 34 | 2959 | 47.1 | JAC15 |
| 59.m03403 | TGME49_068760 | hypothetical protein | 18 | 320 | 32 | JAC17 |
| 583.m05643 | TGME49_114840 | hypothetical protein, conserved | 9 | 131 | 228.8 | ConA3 |
| 50.m03374 | TGME49_050820 | hypothetical protein, conserved | 6 | 52 | 259.3 | ConA3 WGA4 |
| 31.m00856 | TGME49_012790 | hypothetical protein | 6 | 268 | 230.5 | JAC3 |
| NR-35187725 | TGME49_015980 | hypothetical protein, conserved | 10 | 339 | 20.2 | WGA14 |
3.2. Glycopeptide Isolation from Intact T. gondii
A total of 30 T. gondii glycoproteins were identified using an approach which employed trypsin to “shave” surface proteins off of T. gondii followed by completion of the proteolysis of the liberated proteins until tryptic peptides were obtained. The glycopeptides were then purified by Con A lectin affinity chromatography and the bound glycopeptides released from the affinity column by PNGase F and analyzed by mass spectrometry. As expected, most of the identified glyoproteins were putative membrane proteins based on BLAST similarities (Table 2), some, however, were enzymes and hypothetical proteins of unknown function and localization. All of the proteins had N-linked glycoprotein domains (indicated by bold type in Table 2) consistent with the presence of glycoepitopes in these proteins. This simplified approach of “protein shaving”, could prove to have significant utility for the identification of unknown or previously unsuspected T. gondii surface membrane proteins.
Table 2.
The glycoproteins identified from T. gondii using “membrane shaving” PNGF technique
| EPICdB ID | ToxodB version 6.0 | Identified Peptide | Protein Name |
|---|---|---|---|
| TgGlmHMM_0006 | TGME49_037860 | QMNISLAAA SAFR | DNA polymerase I, putative |
| TgTigrScan_4836 | TGME49_118470 | AGNVGLNLTR | DNA repair protein, putative |
| TgTigrScan_1309 | TGME49_117700 | NQTTASSAQLR | enoyl-CoA hydratase, putative |
| TgGlmHMM_1305 | TGME49_017680 | GVNVTIDR | hypothetical protein, conserved |
| TgGlmHMM_0380 | TGME49_097110 | NLTNVYMNAFAGTQPSR | kinesin motor domain containing protein |
| 541.m01146 | TGME49_104990 | AHAQSVNATSTLPQ | HSEK guanylate binding protein, putative |
| 49.m00054 | TGME49_043930 | TMNSEGVISDGLQSQLPVNHTR | hypothetical protein TGME49_043930 |
| TgTigrScan_5612 | TGME49_014560 | AIGENGSCMHKSTSPAR | hypothetical protein TGME49_014560 |
| TgTigrScan_5939 | TGME49_019320 | NYTSEALR | GAP50 membrane anchor for myosin XIV |
| TgGlmHMM_4009 | TGME49_058770 | QRENGSGSSQLPGAANGR | UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase T2 |
| TgTigrScan_7263 | TGME49_005410 | MEALSNESRSSFHDVCDETAQK | RNA pseudouridylate synthase, putative |
| TgGlmHMM_2200 | TGME49_112480 | MEANSSSSSLPSVQK | uracil phosphoribosyltransferase |
| 129.m00256 | TGME49_099110 | NNTTLYVHVR | cleft lip and palate transmembrane protein 1, putative |
| TgTigrScan_4899 | TGME49_000250 | CWKTSKCVFMoHFNNDGCTLSGINATAQTDANSK | PAN domain-containing protein |
| TgGlmHMM_0029 | TGME49_087480 | NASPSATSGLLKQLK | hypothetical protein TGME49_087480 |
| TgTigrScan_5651 | TGME49_110750 | GNETALMPK | hypothetical protein, conserved |
| TgTigrScan_0391 | TGME49_042890 | ENQSANACIENNR | hypothetical protein TGME49_042890 |
| TgTigrScan_5004 | |TGME49_082030 | EQIVEKNDTLELHDR | hypothetical protein TGME49_082030 |
| 57.m03967 | TGME49_066660 | NLTARQEGLSK | hypothetical protein TGME49_066660 |
| TgTigrScan_1934 | TGME49_047260 | GGAQNASEAIRTESDK | retinoblastoma-binding protein, putative |
| TgTigrScan_7907 | TGME49_115760 | TNTEGNATPVDSQSSPPSK | hypothetical protein, conserved |
| TgGlmHMM_0680 | TGGT1_018880 | NLSTTPSAVQTEER | conserved hypothetical protein |
| TgTigrScan_4198 | TGME49_002980 | LATQQVTAQTSNVSQALGDRK | hypothetical protein TGME49_002980 |
| TgTwinScan_3338 | TGME49_080730 | NRSLCAGGAAAEAAVAQK | nucleotide-binding protein, putative |
| TgTigrScan_4829 | TGME49_118370 | AMNPSSPPHPNHPVDNVSQ | hypothetical protein TGME49_118370 |
| TgTigrScan_2810 | TGME49_066670 | LASQSMPTDAENTSFTLQGGSVGMGLGGRER | hypothetical protein TGME49_066670 |
| TgGlmHMM_0694 | TGME49_002540 | TEQGTALLTGAPPSANELEAASAMANPT‘“NSS”’R | 3′,5′-cyclic nucleotide phosphodiesterase, putative |
| TgTigrScan_5174 | TGME49_079340 | NQQETEMNGSPHNAAR | hypothetical protein TGME49_079340 |
| TgTigrScan_4790 | TGME49_120590 | EMENVSGDARAGGIGASDK | glycosyl hydrolases family 35 domain-containing protein |
| TgTigrScan_7969 | TGME49_095710 | SPTSQGNNASAVCRASRAPSGQEAEMR | ubiquitin-transferase domain-containing protein |
3.3. Detection of lectin binding in T. gondii using florescence microscopy
FITC-conjugated Con A, WGA, and Jacalin were used to examine the microscopic localization of these lectins in RH strain tachyzoites (i.e. a lectin IFA technique). Con A produced a bright and uniform staining on the entire surface of every tachyzoites (see Fig. 3A) as did WGA and Jacalin. When these organisms were permeablized with 0.1% Triton X-100 diffuse staining of the cytoplasm was seen. No specific structures (i.e. micronemes, rhoptries, dense granules, etc.) were uniquely stained by any of these lectins. This suggests that glycoepitopes binding these lectins are present in both membrane bound and cytoplasmic proteins (Fig. 3A).
Figure 3. Lectin binding to T. gondii.
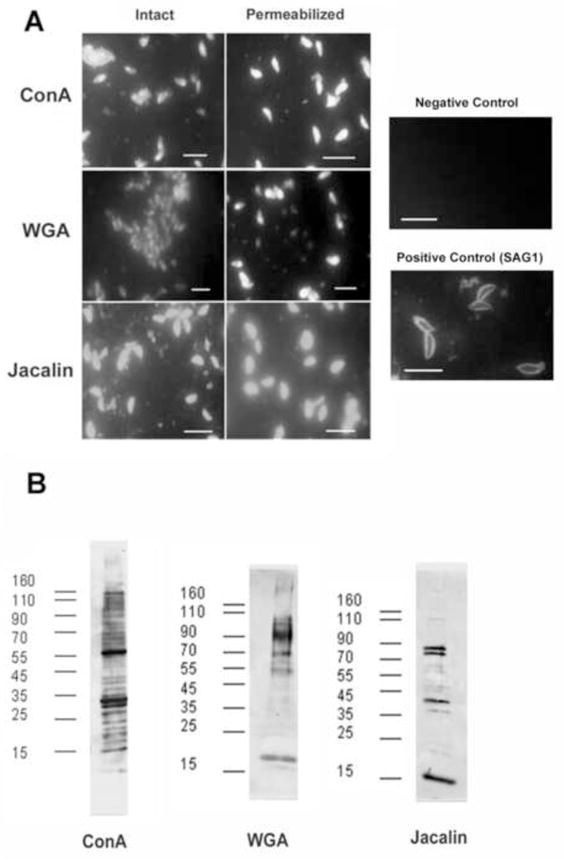
A. Fluorescence microscopy of lectin-stained T. gondii. Con A-FITC, WGA-FITC, and Jacalin-FITC was employed to examine both fixed and permeabized (0.2% Triton X100) T. gondii. There was no change in staining with permabilization. All three lectins bound to the parasite consistent with the presence of glycoproteins. Negative control: antimouse-FITC, Positive control: mAb to SAG1 (p30) with secondary anti-mouse IgG-FITC. Bar length in each panel is 5 μm.
B. Lectin overlay with ConA,, WGA and Jacalin. Blots were probed with horseradish peroxidase conjugated Con A, WGA, and jacalin, respectively. Numerous bands were present demonstrating the presence of both N- and O-glycoroteins in T. gondii tachyzoites. No bands were seen when the sugars binding each lectin (e.g. α-methyl mannoside for ConA, N-acetylglucosamnine for WGA and galactose for jacalin) were added to the elution buffer.
3.4. Detection of glycoproteins using lectin-blotting
Further information on the presence of glycoproteins in T. gondii was obtained by employing HRP-conjugated lectins Con A, WGA, and Jacalin. Following SDS-PAGE and transfer to nitrocellulose, the same lectins used for fluorescence studies were used to probe the three different fractions obtained after NP-40 extraction of T. gondii tachyzoites (Fig. 3B). This lectin overlay procedure demonstrated multiple bands in the solubized membrane preparations. Binding of the lectins to these proteins could be blocked by the addition of the corresponding saccharide used for SLAC elution in the lectin-HRP binding solution. These results demonstrate that the membrane protein preparation contains glycoproteins with both mannose and N-acetylglucosamine modifications.
3.5. PCR validation
To test for the presence of expression of hypothetical proteins identified by mass spectrometric techniques, we chose 13 candidate proteins which were annotated as hypothetical proteins in the T. gondii genome database. Of these, 8 were eluted with α-methyl mannoside from the ConA column and 4 were identified in the galactose eluted fraction from the jacalin column. A forward and reverse primer was designed to the beginning and end of the target gene coding region as predicted in the T. gondii database. As shown in Fig. 4, out of 13 target genes there were specific PCR products of the predicted size for 12 of the candidate genes confirming their expression at the transcriptional level in T. gondii RH strain validating the presence and active expression of the majority of the genes corresponding to the hypothetical proteins identified by mass spectrometry.
Figure 4. RT-PCR of the genes corresponding to hypothetical glycoproteins identified by mass spectrometry.
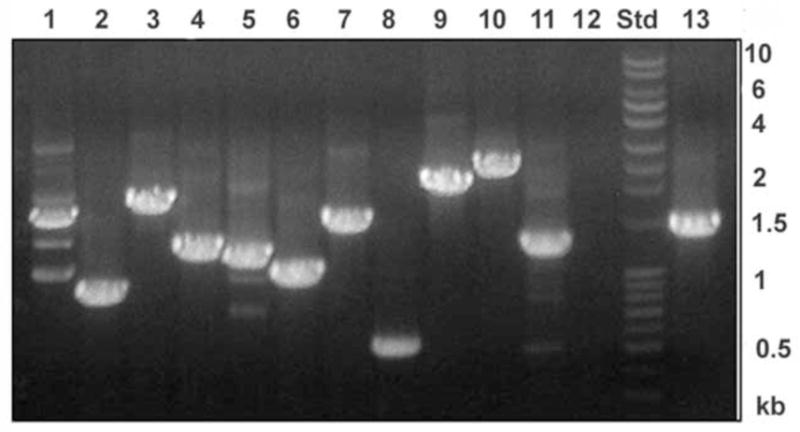
Lanes 1–8 and lane 13 are the gene products in which the corresponding protein was eluted from Con A column by α methyl mannoside and the lanes 9–12 belongs to gene products corresponds to proteins eluted from jacalin column by galactose. Lane 1: 55.m00103, Lane 2: 41.m01274, Lane 3: 76.m01589, Lane 4: 67.m00007, Lane 5: 49.m03169, Lane 6: 80.m02161, Lane 7: 46.m01601, Lane 8: 42.m03584, Lane 9: 31.m00928, Lane 10: 55.m08219, Lane 11: 50.m00023, lane 12:76.m01543, Lane 13: 59.m03403. Std: DNA ladder.
4. Discussion
The identification of an entire proteome, regardless of its origin, is a daunting task for several reasons. The dynamic range of current instrumentation is limited by fluctuating protein expression levels, which may span more than six orders of magnitude. In addition, the limited sensitivity and ability of contemporary proteomics to characterize proteins with high molecular masses, extreme isoelectric points, or extremes in hydrophobicity precludes complete coverage of a given proteome. One approach to circumvent these difficulties is to reduce sample complexity by fractionation. The enrichment of proteins from a selected part of proteome is expected to facilitate the identification of low abundance proteins and assist characterization of proteins related to specific organelles or structures. Fractionation of tachyzoites using the non-ionic detergent NP-40 and 500 mM NaCl demonstrated that the majority of glycoproteins were identified in the NP-40-soluble, hydrophobic, fraction, which is enriched for membrane proteins; however, it should be appreciated that this method may not detect all of the integral glycoproteins. In addition, while the method used should limit protein complex formation during SLAC it is still feasible that some of the identified proteins may have been purified due to their binding to glycoprotein(s) which bound to the columns.
In the current manuscript we present several lines of evidence that indicate the presence of a significant number of glycoproteins in T. gondii tachyzoites. This data confirmed that a significant number of proteins involved in invasion and motility are probably glycoproteins. This is consistent with observations from other investigators [10–13, 15, 16, 23] as well as the presence of the enzymatic machinery for both N-linked and O-linked glycosylation in the T. gondii genome (www.toxodB.org)[14]. Both lectin overlay (i.e. lectin blotting) and lectin IFA techniques confirmed the presence of glycoproteins and provided independent validation of the SLAC results. In addition, it has been previously reported that tunicamycin treatment of T. gondii decreased the number of proteins purified by ConA affinity chromatography [12]. Many of the glyoproteins that were identified were membrane-associated proteins. We identified several surface antigens (SAGs and SRS domain proteins) by SLAC as being probable glycoproteins. By microscopy, ConA, WGA and jacalin fluorescent conjugates labeled the surface of T. gondii.
T. gondii has several specialized organelles associated with invasion and its ability to establish a parasitophorous vacuole in which it replicates within its host cell [1, 3, 24]. The apical end of this parasite contains an elaborate cytoskeletal structure and regulated secretory organelles, the micronemes and rhoptries, which function in host cell invasion discharging their contents from the apical end of the parasite. Microneme proteins are released first, upon contact with the host cell and are thought to function in host cell and are thought to function in host cell recognition and attachment. The content of rhoptries are released next and may function in the formation of the parasitophorous vacule (PV). Another secretory vacuole, the dense granules, discharge from the apical, lateral, and posterior surfaces of the parasite. Dense granule proteins are released next during the formation of the parasitophorous vacuole. Several of the glycoproteins we identified were from these organelles, suggesting that this post translational modification may be important in the process of invasion and establishment of the parasitophorous vacuole. This is consistent with data on the ability of tunicamycin to inhibit invasion[10, 12]. In addition, many of the identified proteins in these structures had TMDs, suggesting that they were membrane associated. Microneme proteins (MIC) 1, 2, 3, 4, 6 were identified as glycoproteins and MIC 2 and MIC6 also had predicted TMDs. Fifteen rhoptry proteins (ROPs and RONs) were identified as glycosylated and four have at least one TMD. This is consistent with a previous publication that also found evidence for glycosylation in RON2 and AMA [12], which are key components in the moving junction formed during cell invasion. Seven dense granule proteins (GRA) proteins were identified as glycoproteins and four GRAs have two TMD. Consistent with these findings, it had been previously demonstrated that GRA2 is O-glycosylated[25], Several cytoskeletal components were also identified as glycoproteins consistent with data that demonstrated that membrane anchor myosin XIV (GAP50) and TgMyoA were glycoproteins and involved in the effect of tunicamycin on invasion [10, 12]. These data are suggestive that glycoproteins are components of the glideosome. Our data confirmed that myosin, despite lacking an obvious ER motif, is consistently purified by lectin affinity chromatography. In other eukaryotes there are examples of proteins being glycosylated without such ER motifs [26, 27]. Overall, our data suggest that glycoproteins are key constituents in host-parasite interactions during invasion and establishment of the parasitophorous vacuole.
Heat shock- or stress induced activation of a set of heat shock protein genes is a characteristic of eukaryotic and prokaryotic cells. The heat shock proteins have been implicated as chaperons for protein folding and transport [28–30]. Heat shock proteins fall into several subfamilies, the low molecular weight (16–35 kDa) or small heat shock proteins (smHsps), the hsp60 family, the hsp70 family (68–78 kDa), and the high molecular weight (89–110 kDa) heat shock protein families (hsp90 and hsp100) [28, 29]. In recent years, it has become clear that heat shock proteins are not just restricted to stress responses, but are also regulated developmentally [28, 29]. In protozoan systems, a link between heat shock proteins and development and intracellular survival has been established [29]. We identified 11 heat shock proteins as possible glycoproteins. In examination of the identified protein gene predictions, there appear to be two different types of hsp90 one with TMD and one without TMD, five isotypes of hsp70 of which one has TMD and two hsp60s one with and one without a TMD. Consistent with our data Tomavo and his collaborators also have demonstrated that a number of heat shock proteins were present in their analysis of N-glycosylation in T. gondii [12].
Overall, this work clearly demonstrates that SLAC combined with tandem MS is a powerful approach for glycoproteomics in T. gondii and that glycosylation is not a rare modification but occurs in a significant number of the proteins of this important human and veterinary pathogen.
Supplementary Material
Supplemental Table 1. Details of the peptides identified from T. gondii using lectin affinity chromatography and mass spectrometry.
Acknowledgments
Supported by NIH/NIAID grant AI31744 (LMW), NIH/NIAID contract HHSN266200400054C, NIH/NIAID R01AI087625 (KK) and and RC4AI092801 (KK)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiss LM, Kim K. Toxoplasma gondi: The Model Apicomplexan: Perspectives and Methods. Elsevier (Academic Press); Amsterdam; Boston: 2007. [Google Scholar]
- 2.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubey JP. Advances in the life cycle of Toxoplasma gondii. Int J Parasitol. 1998;28:1019–1024. doi: 10.1016/s0020-7519(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 4.Wong SY, Remington JS. Biology of Toxoplasma gondii. AIDS. 1993;7:299–316. doi: 10.1097/00002030-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Marino K, Bones J, Kattla JJ, Rudd PM. A systematic approach to protein glycosylation analysis: a path through the maze. Nat Chem Biol. 2010;6:713–723. doi: 10.1038/nchembio.437. [DOI] [PubMed] [Google Scholar]
- 6.Vagin O, Kraut JA, Sachs G. Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am J Physiol Renal Physiol. 2009;296:F459–469. doi: 10.1152/ajprenal.90340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian E, Ten Hagen KG. Recent insights into the biological roles of mucin-type O-glycosylation. Glycoconj J. 2009;26:325–334. doi: 10.1007/s10719-008-9162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bill RM, Revers L, Wilson IBH, editors. Protein glycosylation. Kluwer Academic Publishers; Boston: 1998. [Google Scholar]
- 9.Taylor ME, Drickamer K, editors. Introduction to glycobiology. Oxford University Press; Oxford; New York: 2006. [Google Scholar]
- 10.Luk FC, Johnson TM, Beckers CJ. N-linked glycosylation of proteins in the protozoan parasite Toxoplasma gondii. Mol Biochem Parasitol. 2008;157:169–178. doi: 10.1016/j.molbiopara.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garenaux E, Shams-Eldin H, Chirat F, Bieker U, Schmidt J, Michalski JC, Cacan R, Guerardel Y, Schwarz RT. The dual origin of Toxoplasma gondii N-glycans. Biochemistry. 2008;47:12270–12276. doi: 10.1021/bi801090a. [DOI] [PubMed] [Google Scholar]
- 12.Fauquenoy S, Morelle W, Hovasse A, Bednarczyk A, Slomianny C, Schaeffer C, Van Dorsselaer A, Tomavo S. Proteomics and glycomics analyses of N-glycosylated structures involved in Toxoplasma gondii--host cell interactions. Mol Cell Proteomics. 2008;7:891–910. doi: 10.1074/mcp.M700391-MCP200. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YW, Halonen SK, Ma YF, Wittner M, Weiss LM. Initial characterization of CST1, a Toxoplasma gondii cyst wall glycoprotein. Infect Immun. 2001;69:501–507. doi: 10.1128/IAI.69.1.501-507.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuelson J, Banerjee S, Magnelli P, Cui J, Kelleher DJ, Gilmore R, Robbins PW. The diversity of dolichol-linked precursors to Asn-linked glycans likely results from secondary loss of sets of glycosyltransferases. Proc Natl Acad Sci U S A. 2005;102:1548–1553. doi: 10.1073/pnas.0409460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stwora-Wojczyk MM, Kissinger JC, Spitalnik SL, Wojczyk BS. O-glycosylation in Toxoplasma gondii: identification and analysis of a family of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Int J Parasitol. 2004;34:309–322. doi: 10.1016/j.ijpara.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Dieckmann-Schuppert A, Bause E, Schwarz RT. Glycosylation reactions in Plasmodium falciparum, Toxoplasma gondii, and Trypanosoma brucei brucei probed by the use of synthetic peptides. Biochim Biophys Acta. 1994;1199:37–44. doi: 10.1016/0304-4165(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 17.Stwora-Wojczyk MM, Dzierszinski F, Roos DS, Spitalnik SL, Wojczyk BS. Functional characterization of a novel Toxoplasma gondii glycosyltransferase: UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase-T3. Arch Biochem Biophys. 2004;426:231–240. doi: 10.1016/j.abb.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Nakai K, Horton P. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci. 1999;24:34–36. doi: 10.1016/s0968-0004(98)01336-x. [DOI] [PubMed] [Google Scholar]
- 19.Che FY, Madrid-Aliste C, Burd B, Zhang H, Nieves E, Kim K, Fiser A, Angeletti RH, Weiss LM. Comprehensive proteomic analysis of membrane proteins in Toxoplasma gondii. Mol Cell Proteomics. 2011;10:M110.00745. doi: 10.1074/mcp.M110.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madrid-Aliste CJ, Dybas JM, Angeletti RH, Weiss LM, Kim K, Simon I, Fiser A. EPIC-DB: a proteomics database for studying Apicomplexan organisms. BMC Genomics. 2009;10:38. doi: 10.1186/1471-2164-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dybas JM, Madrid-Aliste CJ, Che FY, Nieves E, Rykunov D, Angeletti RH, Weiss LM, Kim K, Fiser A. Computational analysis and experimental validation of gene predictions in Toxoplasma gondii. PLoS One. 2008;3:e3899. doi: 10.1371/journal.pone.0003899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Yu P, Luo J, Jiang Y. Secreted protein prediction system combining CJ-SPHMM, TMHMM, and PSORT. Mamm Genome. 2003;14:859–865. doi: 10.1007/s00335-003-2296-6. [DOI] [PubMed] [Google Scholar]
- 23.Odenthal-Schnittler M, Tomavo S, Becker D, Dubremetz JF, Schwarz RT. Evidence for N-linked glycosylation in Toxoplasma gondii, Biochem J. 1993;291:713–721. doi: 10.1042/bj2910713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11:267–299. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercier C, Lecordier L, Darcy F, Deslee D, Murray A, Tourvieille B, Maes P, Capron A, Cesbron-Delauw MF. Molecular characterization of a dense granule antigen (Gra 2) associated with the network of the parasitophorous vacuole in Toxoplasma gondii. Mol Biochem Parasitol. 1993;58:71–82. doi: 10.1016/0166-6851(93)90092-c. [DOI] [PubMed] [Google Scholar]
- 26.Blachly-Dyson E, Stevens TH. Yeast carboxypeptidase Y can be translocated and glycosylated without its amino-terminal signal sequence. J Cell Biol. 1987;104:1183–1191. doi: 10.1083/jcb.104.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silve S, Monod M, Hinnen A, Haguenauer-Tsapis R. The yeast acid phosphatase can enter the secretory pathway without its N-terminal signal sequence. Mol Cell Biol. 1987;7:3306–3314. doi: 10.1128/mcb.7.9.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tutar L, Tutar Y. Heat shock proteins; an overview. Curr Pharm Biotechnol. 2010;11:216–222. doi: 10.2174/138920110790909632. [DOI] [PubMed] [Google Scholar]
- 29.Shonhai A. Plasmodial heat shock proteins: targets for chemotherapy. FEMS Immunol Med Microbiol. 2010;58:61–74. doi: 10.1111/j.1574-695X.2009.00639.x. [DOI] [PubMed] [Google Scholar]
- 30.Csermely P, Korcsmáros T, Sulyok K, editors. Stress responses in biology and medicine: stress of life in molecules, cells, organisms, and psychosocial communities. Blackwell Pub. (New York Academy of Sciences); Boston, Mass: 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Details of the peptides identified from T. gondii using lectin affinity chromatography and mass spectrometry.


