Abstract
Objective
To examine whether fatty liver, and abdominal visceral adipose tissue (VAT) are jointly associated with cardiometabolic abnormalities.
Methods and Results
African American participants were from the Jackson Heart Study (n=2882, 65% women) who underwent computed tomography. Fatty liver was measured by liver attenuation in Hounsfield Units (LA) and VAT was quantified volumetrically. Cross-sectional associations between LA, VAT, and cardiometabolic risk factors were assessed using linear and logistic regression, and their joint associations were further examined in 4 subgroups - (High-LA/Low-VAT [n=1704], Low-LA/Low-VAT [n=422], High-LA/High-VAT [n=436] and Low-LA/High-VAT [n=320]). Both LA and VAT were associated with most cardiometabolic traits (all p<0.0001), which persisted after additional adjustment for each other (LA, p < 0.01-0.0001 and VAT, p<0.0001). In bootstrap analyses, the regression coefficient of VAT was significantly greater than LA for triglycerides, HDL-C, impaired glucose and metabolic syndrome (MetS) (p range 0.009-0.0001). The interaction between LA and VAT was significant for HDL-C (p=0.002), impaired glucose (p=0.003) and MetS (p=0.04). Among 4 subgroups, participants with higher VAT and lower LA had higher prevalence of cardiometabolic traits than those with each condition alone.
Conclusion
Both fatty liver and VAT are independent correlates of cardiometabolic risk, but the associations are stronger for VAT than for fatty liver.
Keywords: Jackson Heart Study, Intrahepatic fat, abdominal adiposity and cardiometabolic risk factors
Both fatty liver and abdominal visceral adipose tissue (VAT) are important risk factors for the development of cardiometabolic complications due to obesity.1-4 Epidemiologic studies indicate that higher levels of VAT or fatty liver are associated with insulin resistance, metabolic syndrome, dyslipidemia, hypertension and diabetes.1, 2, 5 Moreover, because of the anatomic blood circulation between VAT and the liver, free fatty acids and inflammatory adipokines that are produced by VAT 6, 7 can be released into the portal vein and directly transported to the liver, causing fatty liver disease.3, 8, 9 These observations have led to a hypothesis that fatty liver may be another important characteristic of fat distribution that is associated with different metabolic risk profiles.3, 8
Although VAT and the liver are metabolically connected and both are associated with cardiometabolic risk factors,4, 6, 9 their joint associations with these risk factors remains unclear.10 In a cross-sectional study, cardiometabolic abnormalities were associated with increased intrahepatic triglyceride content, but not with high VAT volume, pointing to the possibility that fatty liver, not VAT, is linked to metabolic complications of obesity.10, 11 Other studies, however, have demonstrated that VAT and fatty liver are jointly associated with cardiometabolic abnormalities, suggesting that these two fat depots are both important with respect to cardiometabolic abnormalities.3, 4
African Americans are disproportionately affected by obesity, but the concomitant role of fatty liver and VAT remains unclear 12-14. Studies have consistently shown that African Americans have a lower quantity of VAT and fatty liver,12, 14 despite higher rates of insulin resistance, metabolic syndrome, dyslipidemia, hypertension and diabetes.15 This paradox suggests that either the associations of fatty liver or VAT with cardiometabolic risk factors vary across different ethnic groups or higher rates of cardiometabolic disorders in African Americans are due to factors above and beyond fatty liver and VAT. Therefore, it remains unclear whether fatty liver is an important correlate of cardiometabolic risk after accounting for VAT or whether fatty liver and VAT are jointly associated with cardiometabolic risk in African-American populations.
Thus, the purpose of the present study is to examine the associations among fatty liver, abdominal VAT and cardiometabolic abnormalities, and in particular to assess the association of fatty liver with cardiometabolic abnormalities above and beyond abdominal VAT in African Americans – the Jackson Heart Study (JHS).
Methods
Study Sample
The original JHS cohort enrolled participants from September 2000 to March 2004 and comprises 5301 participants between the ages of 21-94 years.16, 17 The present study includes a sub-set of participants (n=2884) who underwent multi-detector CT scanning from 2007 to 2009 as a part of the second JHS Examination (JHS Exam 2).
Overall, 4203 participants attended JHS Exam 2 (from 2005 to 2008). Participants were excluded from the CT scan Exam if: 1) body weight was greater than 350 lbs (~160 kg) (n=41); 2) pregnant or unknown pregnancy status (n=13); 3) female participant < 40 years of age (n=128); 4) Male participant < 35 years of age (n=48). Of these, 2884 (65% women) underwent multi-detector CT assessment for fatty liver. Individuals imaged were further excluded if CT measurements were missing for total abdominal adipose tissue (n=1) or for VAT (n=1), resulting in a final sample size of 2882. The study protocol was approved by the institutional review board of the participating institutions: the University of Mississippi Medical Center, Jackson State University and Tugaloo College. All of the participants provided informed consent.
Multi-Detector CT Scan Protocol for Measuring Adiposity
The research CT protocol included the heart and lower abdomen using a 16 channel multi-detector computed tomography system equipped with cardiac gating (GE Healthcare Lightspeed 16 Pro, Milwaukee, Wisconsin). Quality control and image analysis was performed at a core reading center (Wake Forest University School of Medicine, Winston-Salem, NC). The protocol included scout images, one ECG gated series of the entire heart, and a series through the lower abdomen.
The acquired abdominal imaging slices covering the lower abdomen from L3 to S1 were used to quantify VAT. Briefly, 24 contiguous 2-mm thick slices centered on the lumbar disk space at L4-5 were used for this analysis; 12 images before the center of the L4 - L5 disk space and 12 images after the disk space were used for quantification of VAT. The abdominal muscular wall was first manually traced and the fat volumes in different compartments were measured by semiautomatic segmentation technique. Volume Analysis software (Advantage Windows, GE Healthcare, Waukesha, WI) was used to segment and characterize each individual voxel as a tissue attenuation of fat using a threshold range -190 to -30 Hounsfield units. The VAT volumes were the sum of VAT voxels over 24 slices. In this study, the interclass correlation coefficient for inter-reader comparisons was 0.95 for VAT in a random selected sample of 60 participants.
The CT diagnosis of fatty liver can be made by measuring CT attenuation in Hounsfield Units (HU) or the difference between the liver and spleen, which have been shown to be inversely correlated with the amount of fat in the liver seen on liver biopsy.18, 19 A more recent study demonstrates that a simple measurement of liver attenuation on unenhanced CT scans is the best method of predicting pathologic fat content in the liver.20 Thus, measurement of liver attenuation in HU (LA) was performed on multi-detector CT scans of the abdomen at the level of the T12 – L1 intervertebral space and was used to estimate fatty liver (low LA = high fatty liver). The LA was determined by calculating the mean HU of three regions of interest (ROI) in the parenchyma of the right lobe of the liver.19 One ROI randomly drawn, avoiding the large vessels and any focal lesions was considered and each ROI measured 100±13.31 mm2. The correlation coefficient between 2 different readers on a random selected sample of 60 participants was 0.98 for LA indicating reliable reproducibility of CT imaging measurements.
Risk Factors and Covariate Assessment
Risk factors and covariates were measured at Exam 2 (2005 - 2008). BMI was defined as weight (in kilograms) divided by the square of height (in meters). Two measures of the waist (at the level of the umbilicus, in the upright position) were averaged to determine waist circumference (WC) for each participant. Fasting blood samples were collected according to standardized procedures and the assessment of plasma glucose and lipids were processed at the Central Laboratory (University of Minnesota) as previously described.16,17 Sitting blood pressure was measured twice at 5-minutes intervals and the average of two measurements was used for analysis.
Participants were considered to have hypertension if they were taking antihypertensive medications, self-reported a diagnosis of hypertension, and/or if their systolic pressure was ≥ 140 mm Hg or diastolic pressure ≥ 90 mm Hg. Impaired fasting glucose was defined as fasting plasma glucose of 100-125 mg/dl among those not treated for diabetes. Diabetes was defined as a fasting plasma glucose level ≥ 126 mg/dl or treatment with insulin or hypoglycemic agents. High triglycerides level were defined as fasting plasma triglyceride level ≥ 150 mg/dl and low HDL-C levels were defined as fasting plasma HDL-C level < 40 mg/dl in men and < 50 in women. Participants were considered current smokers if they had smoked, used chewing tobacco or nicotine gum, or were wearing a nicotine patch at the time of interview. Daily alcohol consumption were assessed by the validated food frequency questionnaires21 and the alcohol drinkers were defined if they drank more than 14 per week (men) or more than 7 drinks per week in women. Obesity was defined by BMI of at least 30 kg/m2 and modified National cholesterol Education Program Adult Treatment Panel III criteria were used to define the metabolic syndrome.22
In order to define the individual with hepatic steatosis, a healthy referent sample was created by hierarchical exclusion of the presence of hypertension, triglycerides≥150 mg/dl or taking lipid medications, HDL-C< 40 mg/dl in men or < 50 in women; fasting glucose ≥126 mg/dl or diabetes (n=2551); prevalent cardiovascular disease (n=9); BMI < 18.5 kg/m2 (n=115) and alcohol drinkers (n=31), resulting in final healthy referent sample size of 178. The lowest 10th percentile was chosen as a cutoff point from this healthy referent sample to define the prevalence of hepatic steatosis23.
Statistical Analysis
LA and triglycerides were normalized by logarithmic transformation. Age-adjusted Pearson correlations of log LA was performed with each of the metabolic risk factors including VAT, BMI, systolic and diastolic blood pressure, fasting plasma glucose, triglycerides, and HDL-C. VAT and log LA were standardized to a mean of 0 and a standard deviation (SD) of 1. A multivariable regression model was constructed with VAT or log LA as the independent variable and each of metabolic risk factors as the dependent variable. Three models were generated in stages: (1) the multivariable-adjusted model, with covariates including age, gender, smoking and alcohol consumption, medications for hypertension, diabetes and dyslipidemia; (2) the second model in which the first model was additionally adjusted for BMI. (3) the third model in which the first model was additionally adjusted for VAT. Interactions between LA and VAT were examined for each of the outcomes after accounting for VAT in the model. In order to further assess whether VAT or LA was more strongly associated with risk factors, a bootstrap analysis were performed and the differences in standardized regression coefficients for VAT and LA were compared. Specifically, 5000 samples with replacement were randomly selected from original sample. An overall estimate of the VAT and LA regression coefficients, their variance and covariance were obtained from two multivariable linear regressions (one with VAT and another with log LA) on the 5000 samples. A Z statistics was used to test the absolute difference in regression coefficients between VAT and LA.24
In addition, secondary analyses were conducted to examine the joint associations of LA and VAT with metabolic parameters. Study participants were stratified into four groups based on the 75th percentile of VAT and the 25th percentile of LA, in which the highest risk categories had VAT in the 75th percentile and LA in the 25th percentile (low LA = high fatty liver).19 Therefore, four groups: High-LA/Low-VAT, Low-LA/Low-VAT, High-LA/High-VAT and Low-LA/High-VAT were derived. A multivariable logistic regression model was used to assess these phenotypes in association with cardiometabolic risk factors as compared to a reference group (High-LA/Low-VAT).
All computations were performed by SAS software version 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
Study Sample Characteristics by Tertiles of LA
Overall, 2882 participants (65% women) with an average age of 60 years were available for analysis. In the lowest LA tertile, cardiometabolic risk factor prevalence was generally higher, compared to the highest LA tertile (Table 1). Approximately, 55% were obese, 59% had metabolic syndrome and 27.8% had hepatic steatosis.
Table 1.
Clinical Characteristics (Mean ± SD) of Study Participants who underwent Computed Tomography Exam by Tertiles of Liver Attenuation (LA) (low liver attenuation=high liver fat)
| Tertile 1 (n=961) | Tertile 2 (n=960) | Tertile 3 (n=961) | p for diff | |
|---|---|---|---|---|
| Covariates | ||||
| Age (years) | 59±10 | 59±11 | 59±11 | 0.82 |
| Women % | 60 | 64 | 71 | 0.0001 |
| Smoking Status % | 7.4 | 6.8 | 6.0 | 0.49 |
| Alcohol Drinker | 40.9 | 28.8 | 30.3 | 0.06 |
|
| ||||
| Fat-related | ||||
| LA (HU) | 49.4±8.6 | 60.5±1.7 | 67.3±3.4 | 0.0001 |
| Abdominal VAT (cm3) | 971.1±404.0 | 790.6±363.7 | 730.1±338.8 | 0.0001 |
| Abdominal SAT (cm3) | 2489.2±1051 | 2268.3±985 | 2232.7±987.8 | 0.0001 |
| BMI (kg/m2) | 33.2±6.7 | 31.2±6.1 | 30.5±6.2 | 0.0001 |
| WC (cm) | 105.9±15.2 | 100.6±14.4 | 100.3±80.0 | 0.014 |
| Obesity % | 65.1 | 53.8 | 46.0 | 0.0001 |
| Hepatic Steatosis% | 84.5 | 0 | 0 | 0.0001 |
|
| ||||
| BP-related | ||||
| Systolic BP (mm Hg) | 127±17 | 127±18 | 127±34 | 0.92 |
| Diastolic BP (mm Hg) | 77±10 | 77±10 | 77±10 | 0.30 |
| Hypertension % | 76.6 | 71.8 | 69.3 | 0.002 |
|
| ||||
| Glucose-related | ||||
| Fasting Glucose (mg/dl) | 111.7±39.6 | 101.7±31.4 | 103.3±30.1 | 0.0001 |
| HbA1C % | 6.3±1.2 | 6.0±1.0 | 5.9±0.9 | 0.0001 |
| Impaired Glucose % | 20.0 | 17.6 | 16.1 | 0.08 |
| Diabetes Mellitus % | 35.5 | 22.0 | 19.7 | 0.0001 |
|
| ||||
| Lipid-related | ||||
| TRG (mg/dl)* | 99(70, 146) | 84(61, 117) | 81(60, 117) | 0.0001 |
| High TRG % | 19.0 | 9.6 | 10.6 | 0.0001 |
| HDL-C (mg/dl) | 51.3±15.0 | 55.7±15.5 | 56.5±0.5 | 0.0001 |
| Low HDL % | 48.8 | 37.7 | 39.7 | 0.0001 |
|
| ||||
| Syndrome-related | ||||
| Metabolic Syndrome % | 69.1 | 55.5 | 51.8 | 0.0001 |
presented as median (25%, 75% percentile)
VAT: visceral adipose tissue; SAT: subcutaneous adipose tissue; BMI: body mass index; WC: waist circumference; BP: blood pressure; HbA1C: hemoglobin A1C; TRG: triglyceride; LA(HU): liver attenuation in Hounsfield Units.
Correlations with Liver Attenuation
Age-adjusted correlations of log LA with metabolic risk factors are displayed in Table 2. Log LA was inversely associated with all cardiometabolic risk factors tested including VAT, BMI, WC, triglycerides, fasting plasma glucose, hemoglobin A1C (HbA1C) and positively associated with HDL-C.
Table 2.
Age-Adjusted Pearson Correlation Coefficients between Log LA and Metabolic risk Factors
| Log LA | p | VAT | p | |
|---|---|---|---|---|
| Log LA | - | - | -0.30 | 0.0001 |
| VAT | -0.30 | 0.0001 | - | - |
| SAT | -0.09 | 0.0001 | 0.35 | 0.0001 |
| BMI | -0.19 | 0.0001 | 0.55 | 0.0001 |
| WC | -0.04 | 0.05 | 0.23 | 0.0001 |
| Systolic BP | -0.01 | 0.52 | 0.04 | 0.10 |
| Diastolic BP | -0.01 | 0.45 | 0.06 | 0.01 |
| Log TRG | -0.22 | 0.0001 | 0.32 | 0.0001 |
| HDL-C | 0.16 | 0.0001 | -0.34 | 0.0001 |
| Fasting | 0.23 | 0.0001 | ||
| Glucose | -0.16 | 0.0001 | ||
| HbA1C | -0.20 | 0.0001 | 0.27 | 0.0001 |
VAT: visceral adipose tissue; SAT: subcutaneous adipose tissue; LA: liver attenuation in Hounsfield Units; BMI: body mass index; WC: waist circumference; BP: blood pressure; HbA1C: hemoglobin A1C; TRG: triglyceride.
Multivariable-Adjusted Regression model with LA and Metabolic Risk Factors
The results of the multivariable-adjusted regressions for the association of log LA are summarized with continuous variables in Table 3 and dichotomous metabolic risk factors in Table 4. We observed strong and consistent associations between log LA and most cardiometabolic outcomes. For example, lower log LA (per 1-SD decrement) was associated with higher fasting plasma glucose levels (3.79±0.7 mg/dl, p < 0.0001) after multivariable adjustment. The association persisted after additional adjustment for BMI (p=0.0001) and VAT (p=0.004). When comparing the association with cardiometabolic outcomes between VAT and log LA using bootstrap with 5000 replications, the regression coefficients were stronger for VAT with triglycerides (VAT: 0.18±0.1 vs. log LA: -0.12±0.0; p<0.0008 for difference) and HDL-C(VAT: -4.74±0.3 vs. log LA: 2.00±0.3; p<0.0001) than for log LA (Table 3).
Table 3.
A Multivariable-Adjusted# Regression coefficient of Continuous Variables for VAT and Log LA (per 1 SD Increment) with Cardiometabolic Risk Factors
| MV# Regression coefficients | p for Significance | p for difference btw VAT & LA | MV# Regression Coefficients after BMI Adjustments | p for significance | MV# Regression Coefficients after VAT or LA Adjustments | p for significance | p for VAT & LA interaction | ||
|---|---|---|---|---|---|---|---|---|---|
| SBP | LogLA | -0.47±0.3 | 0.17 | 0.79 | -0.34±0.3 | 0.32 | -0.21 ±0.4 | 0.38 | 0.14 |
| VAT | 0.59±0.4 | 0.09 | - | 0.46 ±0.4 | 0.28 | 0.30 ±0.4 | 0.17 | - | |
| DBP | LogLA | -0.31±0.2 | 0.11 | 0.84 | -0.28±0.2 | 0.15 | -0.23 ±0.2 | 0.26 | 0.82 |
| VAT | 0.34±0.2 | 0.10 | - | 0.39 ±0.2 | 0.10 | 0.27 ±0.2 | 0.20 | - | |
| FPG | LogLA | -3.79±0.7 | 0.0001 | 0.37 | -3.21±0.8 | 0.0001 | -2. 70±0.8 | 0.0004 | 0.22 |
| VAT | 4.62±0.7 | 0.0001 | - | 4.86±0.9 | 0.0001 | 3.84 ±0.8 | 0.0001 | - | |
| HbA1C | LogLA | -0.15±0.0 | 0.0001 | 0.22 | -0.13±0.0 | 0.0001 | -0.11 ±0.0 | 0.0001 | 0.36 |
| VAT | 0.18±0.0 | 0.0001 | - | 0.16 ±0.0 | 0.0001 | 0.15 ±0.0 | 0.0001 | - | |
| LogTRG | LogLA | -0.12±0.0 | 0.0001 | 0.0008 | -0.11 ±0.0 | 0.0001 | -0. 07±0.0 | 0.0001 | 0.11 |
| VAT | 0.18±0.1 | 0.0001 | - | 0.20 ±0.1 | 0.0001 | 0.15±0.1 | 0.0001 | - | |
| HDL-C | LogLA | 2.00±0.3 | 0.0001 | 0.0001 | 1.39 ±0.3 | 0.0001 | 0.76 ±0.3 | 0.01 | 0.002 |
| VAT | -4.74±0.3 | 0.0001 | - | -3.65 ±0.4 | 0.0001 | -4.51 ±0.3 | 0.0001 | - |
MV indicates multivariable.
Adjusted for age, gender, smoking, alcohol and medications for HTN, DM or dyslipidemia.
VAT: visceral adipose tissue; LA: liver attenuation in Hounsfield Units; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FPG: Fasting plasma glucose; HbA1C: hemoglobin A1C; TRG: Triglyceride.
Table 4.
A Multivariable-Adjusted# Odds Ratio of Dichotomous Variables for VAT and Log LA (per 1 SD Increment) with Cardiometabolic Risk Factors
| MV# Regression coefficients | p for Significance | p for difference btw VAT & LA | MV# Regression Coefficients after BMI Adjustments | p for significance | MV# Regression Coefficients after VAT or LA Adjustments | p for significance | p for VAT & LA interaction | ||
|---|---|---|---|---|---|---|---|---|---|
| HTN | LogLA | 0.84(0.7-0.9) | 0.04 | 0.85 | 0.87(0.7-1.1) | 0.11 | 0.89(0.7-1.1) | 0.14 | 0.24 |
| VAT | 1.21(1.0-1.4) | 0.03 | - | 1.17(0.9-1.4) | 0.13 | 1.14(0.9-1.4) | 0.10 | - | |
| Impaired G | LogLA | 0.85(0.7-0.8) | 0.0009 | 0.009 | 0.88(0.7-0.9) | 0.01 | 0.94(0.8-1.0) | 0.21 | 0.003 |
| VAT | 1.46(1.3-1.6) | 0.0001 | - | 1.39(1.2-1.6) | 0.0001 | 1.36(1.2-1.6) | 0.0001 | - | |
| DM | LogLA | 0.70(0.6-0.8) | 0.0001 | 0.79 | 0.76(0.6-0.9) | 0.001 | 0.75(0.6-0.9) | 0.001 | 0.64 |
| VAT | 1.45(1.2-1.7) | 0.0001 | - | 1.18(0.9-1.5) | 0.17 | 1.10(0.8-1.4) | 0.48 | - | |
| High TRG | LogLA | 0.72(0.6-0.8) | 0.0001 | 0.0004 | 0.74(0.6-0.8) | 0.0001 | 0.81(0.7-0.9) | 0.0001 | 0.66 |
| VAT | 1.73(1.6-1.9) | 0.0001 | - | 1.84(1.6-1.9) | 0.0001 | 1.62(1.4-1.8) | 0.0001 | - | |
| Low HDL | LogLA | 0.81(0.7-0.9) | 0.0001 | 0.0003 | 0.84(0.7-0.9) | 0.0001 | 0.89(0.8-0.9) | 0.008 | 0.56 |
| VAT | 1.51(1.4-1.6) | 0.0001 | - | 1.43(1.3-1.6) | 0.0001 | 1.45(1.3-1.6) | 0.0001 | - | |
| MetS | LogLA | 0.64(0.6-0.7) | 0.0001 | 0.0001 | 0.74(0.6-0.8) | 0.0001 | 0.82(0.7-0.9) | 0.004 | 0.04 |
| VAT | 3.32(2.9-3.9) | 0.0001 | - | 2.54(2.1-3.0) | 0.0001 | 3.16(2.7-3.7) | 0.0001 | - |
MV indicates multivariable.
Adjusted for age, gender, smoking, alcohol and medications for HTN, DM or dyslipidemia.
VAT: visceral adipose tissue; LA: liver attenuation in Hounsfield Units; HTN: Hypertension; DM: Diabetes mellitus; MetS: Metabolic syndrome; TRG: Triglyceride.
For dichotomous variables, significant associations with log LA were also observed for impaired glucose, high triglycerides, low HDL-C, hypertension, diabetes, and the metabolic syndrome (Table 4). These associations persisted after additional adjustment for BMI or for VAT, with the exception of hypertension and impaired glucose. For differences in the regression coefficients between log LA and VAT with all risk factors examined, the magnitude of the associations was consistently stronger for VAT than for log LA with impaired glucose, high triglyceride, low HDL-C and metabolic syndrome (p-value range 0.009-0.0001) (Table 3 and 4).
We also observed significant interactions between log LA and VAT for HDL-C (p = 0.009), impaired fasting glucose (p = 0.003) and metabolic syndrome (p = 0.04) (Table 3 and 4), suggesting that participants with higher VAT and lower log LA had lower HDL-C levels, more impaired fasting glucose and metabolic syndrome compared to those with each condition alone.
Multivariable-Adjusted Association of Four Stratified Log LA / VAT Patterns and Cardiometabolic Risk Factors
We assessed the joint association of log LA and VAT with cardiometabolic risk factors. When the study sample was derived into four groups based on 25th percentile of log LA and 75th percentile of VAT in secondary analyses, significant differences were observed for all risk factors examined among 4 groups except for blood pressure and hypertension (all p<0.0001; Table 5).
Table 5.
Multivariable-Adjusted# Means ± SD of Continuous Variables or Odds Ratio of Dichotomous Variables for Four VAT/LA Pattern
| High-LA/Low-VAT (n=1704) | Low-LA/Low-VAT (n=422) | High-LA/High-VAT (n=436) | Low-LA/High-VAT (n=320) | p for difference | |
|---|---|---|---|---|---|
| Continuous Variables | |||||
| Age | 59±11 | 57±10 | 63±10 | 61±9 | 0.0001 |
| VAT | 630.6±216 | 739.0±205 | 1307.0±281 | 1373.7±291 | 0.0001 |
| LA(HU) | 63.2± 4.8 | 48.6±7.8 | 62.4± 4.5 | 45.1±9.4 | 0.0001 |
| BMI | 29.9±5.6 | 31.9±6.1 | 35.2±6.5 | 36.2±6.8 | 0.0001 |
| SBP | 126±19 | 126±17 | 128±18 | 129±17 | 0.89 |
| DBP | 77±10 | 77±11 | 77±11 | 77±10 | 0.67 |
| FPG | 100.4±28 | 109.8±34 | 113.7±41 | 118.5±46 | 0.0001 |
| HbA1C | 5.86±1.0 | 6.25±1.1 | 6.31±1.1 | 6.61±1.4 | 0.0001 |
| LogTRG | 4.4±0.5 | 4.6±0.9 | 4.7±0.9 | 4.8±1.8 | 0.0001 |
| HDL | 57.2±15 | 51.9±15 | 50.5±13 | 48.1±13 | 0.0001 |
|
| |||||
| Dichotomous Variables | |||||
| Women (%) | 68 | 64 | 59 | 57 | 0.0001 |
| HTN | Referent | 1.35(0.9-2.1) | 1.30(0.8-2.2) | 1.41(0.8-2.5) | 0.38 |
| DM | Referent | 1.89(1.1-3.2) | 0.92(0.5-1.8) | 2.21(1.2-4.0) | 0.008 |
| Impaired | |||||
| Glu | Referent | 1.77(1.3-2.3) | 1.80(1.3-2.4) | 1.42(0.9-2.0) | 0.0001 |
| High TRG | Referent | 2.42(1.7-3.4) | 2.92(2.0-4.1) | 4.36(3.0-6.3) | 0.0001 |
| Low HDL | Referent | 1.37(1.1-1.7) | 1.35(1.1-1.7) | 2.11(1.6-2.8) | 0.0001 |
| MetS | Referent | 1.55(1.1-2.1) | 2.56(1.8-3.7) | 3.02(1.9-4.7) | 0.0001 |
Multivariable-adjusted for age, gender, BMI, smoking, alcohol and medications for hypertension, diabetes and dyslipidemia, respectively
VAT: visceral adipose tissue; LA(HU): liver attenuation in Hounsfield Units; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FPG: Fasting plasma glucose; hemoglobin A1C; TRG: Triglyceride; HTN: Hypertension; DM: Diabetes mellitus; MetS: Metabolic syndrome.
presented as median (25%, 75% percentile)
Among continuous variables, higher levels of fasting glucose, HbA1C, triglycerides, and lower levels of HDL-C were observed in the Low-LA/High-VAT group, the Low-LA/Low-VAT group and the High-LA/High-VAT group after adjustment for age, gender, smoking, alcohol consumption, BMI and medications for hypertension, diabetes and dyslipidemia as compared to the High-LA/Low-VAT group (p range 0.002 - 0.0001). Similar patterns were also observed for dichotomous variables. The risk factor prevalence was higher with greater levels of VAT (High-LA/High-VAT; p<0.0001), lower levels of log LA (Low-LA/Low-VAT; p range 0.001 - 0.0001) or both (Low-LA/High-VAT; p<0.0001) as compared to group with a reference group (High-LA/Low-VAT) (Figure 1).
Figure 1.
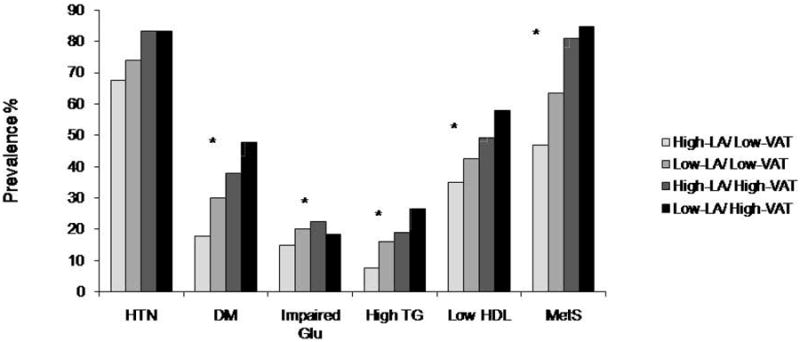
Prevalence of hypertension (HTN), diabetes (DM), impaired fasting glucose, high triglyceride, low HDL-C and Metabolic syndrome (MetS) among four group patterns (High-LA/Low-VAT, n=1704; Low-LA/Low-VAT, n=422; High-LA/High-VAT, n=436; Low-LA/High-VAT, n=320).
* represent probability (p<0.0001) for linear trend across four groups.
VAT: visceral adipose tissue; LA: liver attenuation in Hounsfield Units; Glu: glucose; TG: triglyceride; HDL: high-density lipoprotein.
Discussion
Principal Findings
Both low LA (i.e. high level of fatty liver) and VAT are independently associated with cardiometabolic risk factors including fasting glucose, HbA1c, triglycerides, HDL-C, diabetes and metabolic syndrome. The significant associations persisted after additional adjustment for each other. However, the magnitude of the effect size of VAT was larger than those of LA in the association with cardiometabolic risk factors, with the exception of blood pressure, fasting glucose and HbA1C.
In the Context of the Current Literature
Studies have consistently documented the association of fatty liver with VAT and its predominant role in the regulation of glucose and lipid metabolism.3, 8, 9, 13 Elevated fatty liver has been found to be associated with cardiometabolic risk factors independent of VAT.3, 10, 25, 26 Thus, several studies suggest that commonly observed associations of cardiometabolic abnormalities with VAT is primarily due to elevated fatty liver associated with obesity.10, 25, 26 Indeed, fatty liver has been shown in our study to be significantly associated with cardiometabolic risk factors independent of VAT. However, our study also found that the associations with triglycerides and HDL-C were stronger for VAT as compared to fatty liver, suggesting that VAT may be more important for metabolic abnormalities than fatty liver. This is in contrast to the finding from the prior literature in which insulin action was impaired and hepatic very-low-density lipoprotein-triglyceride secretion rate was increased in subjects with high liver fat content but matched on VAT volume as compared to subjects with high VAT but matched on liver fat content, demonstrating that fatty liver, not VAT, is linked with metabolic complications of obesity.10 The discrepancy between two studies may be due to the matching scheme in their study design, which results in a non-generalizable and obscure subset of the entire dataset, or to the African American samples in our study.
The finding that African Americans have lower levels of abdominal VAT and fatty liver6, 12, 14, 27 yet experience higher levels of cardiometabolic risk compared to other ethnic groups15 remains a paradox. However, the associations of cardiometabolic risk factors with fatty liver and abdominal VAT in African Americans are not fully explored. Results from our current study demonstrate that both fatty liver and VAT are independent risk factors for cardiometabolic abnormalities, which are consistent with prior studies.3, 8, 9 More importantly, this association with cardiometabolic abnormalities in our study is stronger for VAT than for fatty liver. These observations not only support a consistent and particular role for fatty liver and VAT in association with cardiometabolic risk factors but also reinforce the importance of abdominal VAT in pathogenesis of lipid-related metabolic risk in African American populations.
Potential Mechanisms
It has been hypothesized that the adverse effects of fatty liver is related to metabolic connection with VAT.8, 9 However, it is uncertain whether a relationship between VAT and fatty liver plays a joint role in the development of cardiometabolic abnormalities. In fact, only ≈20% of total nonesterified fatty acid derived from lipolysis of VAT are delivered into the liver in obese individuals.28 Even though VAT is the strongest correlate of fatty liver, the correlation reported in the present study (-0.30) and in the Framingham Heart Study (-0.34) is relatively modest.3 These observations suggest that although VAT and fatty liver are metabolically connected, this connection may not be mediated via fatty acid delivery and uptake alone.
Different pathways including patterns of proteins and adipokines associated with cardiometabolic abnormalities could explain the differential influence of VAT and fatty liver on metabolic profiles. For example, C-reactive protein, leptin, interleukin-6 and adiponectin are associated with visceral adiposity and is closely associated with cardiometabolic abnormalities7, 29 whereas α2-Heremans-Schmid glycoprotein/fetuin-A (AHSG) and circulating retinol-binding protein 4 (RBP4) are produced in the liver and highly associated with insulin resistance and fat accumulation in the liver.30, 31 Indeed, participants with high VAT and high fatty liver in our study have high rates of adverse cardiometabolic phenotypes, particularly compared to those with high VAT or high fatty liver alone. Our results support that VAT and fatty liver differentially but interactively associate with cardiometabolic abnormalities.
Implications
The epidemic of obesity is particularly pronounced in African Americans. Paradoxically, African Americans also have lower levels of fatty liver and abdominal fat. The results of the present study demonstrate that both fatty liver and abdominal fat are independently associated with cardiometabolic abnormalities, but the association is stronger for VAT than for fatty liver. Whether attempts to reduce VAT and liver fat in African Americans can help lower cardiovascular outcomes requires further study.
Strengths and Limitations
The strength of the present study is the large, well-characterized African American cohort with a wealth of metabolic traits and covariates measured. Some limitations warrant mention. The findings are cross-sectional and derived from an observational study; thus, neither temporality nor causality can be inferred. The study cannot directly take insulin resistance and physical activity into account because these two variables were not measured at the contemporaneous Jackson Heart Study exam. CT is a relatively insensitive measure of fatty liver compared to hepatic triglyceride content measured by proton magnetic resonance spectroscope,4, 12 which may bias our results toward the null and underestimate the relative strength of the association between fatty liver and risk factors.
Conclusions
Both fatty liver and VAT are independently associated with cardiometabolic abnormalities, but the associations with triglyceride and HDL-C are stronger for VAT than for fatty liver.
Acknowledgments
The authors thank the staff, interns and participants in Jackson Heart Study for their long-term commitment and important contributions to the study. We thank Drs. Caroline S. Fox, DeMarc Hickson, Aurelian Bidulescu, J Jeffery. Carr, Herman A. Taylor for their significant contribution to this manuscript.
Sources of Funding The Jackson Heart Study is supported by the National Heart, Lung, and Blood Institute and the National Center on Minority Health and Health Disparities. Funding for Dr. Herman A. Taylor was provided under contracts N01-HC-95170, N01-HC-95171 and N01-C-95172 from the National Heart, Lung and Blood Institute and the National Center on Minority Health and Health Disparities.
Footnotes
Disclosure The authors declared no conflict of interest.
Reference List
- 1.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, Sr, O’Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, Taylor HA. Impact of Abdominal Visceral and Subcutaneous Adipose Tissue on Cardiometabolic Risk Factors: The Jackson Heart Study. J Clin Endocrinol Metab. 2010;95:5419–26. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, Hirschhorn JN, O’Donnell CJ, Fox CS. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979–87. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thamer C, Machann J, Stefan N, Haap M, Schafer S, Brenner S, Kantartzis K, Claussen C, Schick F, Haring H, Fritsche A. High visceral fat mass and high liver fat are associated with resistance to lifestyle intervention. Obesity (Silver Spring) 2007;15:531–8. doi: 10.1038/oby.2007.568. [DOI] [PubMed] [Google Scholar]
- 5.Goran MI, Bergman RN, Gower BA. Influence of total vs. visceral fat on insulin action and secretion in African American and white children. Obes Res. 2001;9:423–31. doi: 10.1038/oby.2001.56. [DOI] [PubMed] [Google Scholar]
- 6.Despres JP, Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol. 2000;20:1932–8. doi: 10.1161/01.atv.20.8.1932. [DOI] [PubMed] [Google Scholar]
- 7.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 8.Koda M, Kawakami M, Murawaki Y, Senda M. The impact of visceral fat in nonalcoholic fatty liver disease: cross-sectional and longitudinal studies. J Gastroenterol. 2007;42:897–903. doi: 10.1007/s00535-007-2107-z. [DOI] [PubMed] [Google Scholar]
- 9.van der PD, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, London R, Peduto T, Chisholm DJ, George J. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449–57. doi: 10.1002/hep.22350. [DOI] [PubMed] [Google Scholar]
- 10.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–5. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Haring HU. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–16. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 12.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49:791–801. doi: 10.1002/hep.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakobsen MU, Berentzen T, Sorensen TI, Overvad K. Abdominal obesity and fatty liver. Epidemiol Rev. 2007;29:77–87. doi: 10.1093/epirev/mxm002. [DOI] [PubMed] [Google Scholar]
- 14.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, Ravussin E, Ryan DH, Smith SR, Bouchard C. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91:7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferdinand KC. Coronary artery disease in minority racial and ethnic groups in the United States. Am J Cardiol. 2006;97:12A–9A. doi: 10.1016/j.amjcard.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA., Jr Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15:S6–29. [PubMed] [Google Scholar]
- 17.Wilson JG, Rotimi CN, Ekunwe L, Royal CD, Crump ME, Wyatt SB, Steffes MW, Adeyemo A, Zhou J, Taylor HA, Jr, Jaquish C. Study design for genetic analysis in the Jackson Heart Study. Ethn Dis. 2005;15:S6–37. [PubMed] [Google Scholar]
- 18.Piekarski J, Goldberg HI, Royal SA, Axel L, Moss AA. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology. 1980;137:727–9. doi: 10.1148/radiology.137.3.6934563. [DOI] [PubMed] [Google Scholar]
- 19.Davidson LE, Kuk JL, Church TS, Ross R. Protocol for measurement of liver fat by computed tomography. J Appl Physiol. 2006;100:864–8. doi: 10.1152/japplphysiol.00986.2005. [DOI] [PubMed] [Google Scholar]
- 20.Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, Vauthey JN, Charnsangavej C. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307–12. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 21.Carithers T, Dubbert PM, Crook E, Davy B, Wyatt SB, Bogle ML, Taylor HA, Jr, Tucker KL. Dietary assessment in African Americans: methods used in the Jackson Heart Study. Ethn Dis. 2005;15:S6–55. [PubMed] [Google Scholar]
- 22.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24:e13–e18. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 23.Pou KM, Massaro JM, Hoffmann U, Lieb K, Vasan RS, O’Donnell CJ, Fox CS. Patterns of abdominal fat distribution: the Framingham Heart Study. Diabetes Care. 2009;32:481–5. doi: 10.2337/dc08-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, Robins SJ, O’Donnell CJ, Hoffmann U, Jacques PF, Booth SL, Vasan RS, Wolf M, Wang TJ. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59:242–8. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen-Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab. 2003;284:E1065–E1071. doi: 10.1152/ajpendo.00442.2002. [DOI] [PubMed] [Google Scholar]
- 26.Magkos F, Fabbrini E, Mohammed BS, Patterson BW, Klein S. Increased whole-body adiposity without a concomitant increase in liver fat is not associated with augmented metabolic dysfunction. Obesity (Silver Spring) 2010;18:1510–5. doi: 10.1038/oby.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–8. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taksali SE, Caprio S, Dziura J, Dufour S, Cali AM, Goodman TR, Papademetris X, Burgert TS, Pierpont BM, Savoye M, Shaw M, Seyal AA, Weiss R. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008;57:367–71. doi: 10.2337/db07-0932. [DOI] [PubMed] [Google Scholar]
- 30.Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Krober SM, Machicao F, Fritsche A, Haring HU. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 2006;29:853–7. doi: 10.2337/diacare.29.04.06.dc05-1938. [DOI] [PubMed] [Google Scholar]
- 31.Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Schleicher E, Fritsche A, Haring HU. High circulating retinol-binding protein 4 is associated with elevated liver fat but not with total, subcutaneous, visceral, or intramyocellular fat in humans. Diabetes Care. 2007;30:1173–8. doi: 10.2337/dc06-2342. [DOI] [PubMed] [Google Scholar]


