Abstract
Objectives
To examine the association between 25-hydroxyvitamin D (25[OH]D) and physical function in adults of advanced age.
Design
Cross-sectional and longitudinal analysis of physical function over 3 years of follow-up in the Cardiovascular Health Study All Stars.
Setting
Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Allegheny County, PA.
Participants
Community-dwelling adults aged 77–100 years (n=988).
Measurements
Serum 25(OH)D, short physical performance battery (SPPB) and grip and knee extensor strength assessed at baseline. Mobility disability (difficulty walking half a mile or up 10 steps) and activities of daily living (ADL) disability were assessed at baseline and every 6 months over 3 years of follow-up.
Results
30.8% of participants had deficient 25(OH)D (<20 ng/mL). SPPB scores were lower among those with deficient 25(OH)D compared to those with sufficient 25(OH)D (≥30 ng/mL) after adjusting for sociodemographic characteristics, season, health behaviors and chronic conditions (mean±SE: 6.53±0.24 vs. 7.15±0.25, p <0.01). Grip strength adjusted for body size was also lower among those with deficient versus sufficient 25(OH)D (mean±SE: 24.7±0.6 vs. 26.0±0.6 kg, p <0.05). Participants with deficient 25(OH)D were more likely to have prevalent mobility and ADL disability at baseline (OR (95% CI): 1.44 (0.96–2.14) and 1.51 (1.01–2.25), respectively) compared to those with sufficient 25(OH)D. Furthermore, participants with deficient 25(OH)D were at increased risk of incident mobility disability over 3 years of follow-up (HR (95% CI): 1.56 (1.06–2.30)).
Conclusion
Vitamin D deficiency was common and was associated with poorer physical performance, lower muscle strength, and prevalent mobility and ADL disability among community-dwelling adults of advanced age. Moreover, vitamin D deficiency predicted incident mobility disability.
Keywords: vitamin D, physical performance, muscle strength, mobility disability, ADL disability
INTRODUCTION
With the growth of the older population comes a parallel expectation of growth in the burden of age-related physical disability; therefore, identifying modifiable risk factors that prevent or delay the onset of physical disability is a public health priority. In the past two decades, the reported effects of 25-hydroxyvitamin D (25[OH]D) on health have extended beyond calcium homeostasis and musculoskeletal health to include cardiovascular disease, diabetes, hypertension, and osteoarthritis (1). Thus, low 25(OH)D levels may affect physical function through its direct role in muscle function as well as indirectly through the onset of chronic conditions, which are frequent causes of declines in physical function (2). Low 25(OH)D levels are common in older adults. Recent data from the National Health and Nutrition Examination Survey (NHANES) 2000–2004 show approximately one-third of adults aged 70 and older were vitamin D deficient (25[OH]D <20 ng/mL) and three-fourths were vitamin D insufficient (25[OH]D <30 ng/mL) (3). Older adults are at risk for low 25(OH)D levels because of reduced exposure to ultraviolet B radiation, reduced efficiency of pre-vitamin D synthesis in the skin, and inadequate vitamin D intake (4;5).
Cross-sectional studies show that that low 25(OH)D levels are associated with lower physical performance and muscle strength in older adults (6–12); however, the few longitudinal studies are inconsistent with some showing greater declines in physical performance and muscle strength while others show no association with low 25(OH)D levels (13–15). A limited number of cross-sectional studies have shown that low 25(OH)D levels are associated with disability in either mobility or activities of daily living (ADLs) (8;16–18). However, it is difficult to determine from cross-sectional studies whether low 25(OH)D levels precede the onset of disability or whether individuals have low 25(OH)D levels because they are more disabled and, therefore, less active resulting in less exposure to the sun and reduced endogenous vitamin D synthesis. Furthermore, the majority of previous studies have included older adults over a wide age range and not specifically focused on the oldest old (85+ years), proportionately the fastest growing segment of the U.S. population. Therefore, the objective of this study was to examine the association between 25(OH)D levels and physical performance, muscle strength and mobility and ADL disability in the oldest old as well as the association between 25(OH)D levels and incident mobility and ADL disability over three years of follow-up.
METHODS
Study Population
From April 2005 to May 2006, the Cardiovascular Health Study (CHS) cohort was recalled to re-evaluate physical and cognitive functioning as part of the CHS All Stars Study. CHS originally enrolled 5,888 men and women aged 65 and older for an ongoing study of the risk factors related to the onset and course of coronary heart disease and stroke (19). Initial CHS enrollment included 5,201 men and women from four field centers (Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Allegheny County, PA) recruited in 1989–90 and an additional 687 African-American men and women recruited in 1992–93. Of the original 5,888 CHS participants, 1,677 (28.5%) participated in the CHS All Stars Study examination (20). Of the original CHS participants who did not participate in the CHS All Stars Study examination, 85.6% (n=3,607) had died, 8.0% (n=339) were alive but did not give consent for the CHS All Stars Study examination, and 6.3% (n=265) participated in the CHS telephone follow-up but not the CHS All Stars Study functional assessment. For the 1,677 CHS All Stars Study participants, 1143 examinations (68.2%) were conducted in the clinic, home, or a combination of the clinic and home, and 534 examinations were conducted over the telephone (31.8%, including 144 telephone proxy interviews). Of those whose CHS All Stars Study examinations were conducted in the clinic or home, 1,042 (91.2%) participants had fasting blood samples for assessment of serum 25(OH)D. An additional 54 participants were excluded from the analysis sample due to missing covariates for a final sample size of 988.
Physical Performance
The Short Physical Performance Battery (SPPB) from the lower-extremity performance tests used in the Established Populations for the Epidemiologic Studies of the Elderly (EPESE) (21) was administered to assess lower extremity physical performance at the CHS All Stars Study examination. The SPPB consists of standing balance (side-by-side, semi- and full-tandem stands for 10 seconds each), time to complete 5 repeated chair stands, and a 3-m walk (without the use of assistive devices) to assess usual gait speed. Each of the three performance measures was assigned a score ranging from 0 to 4, with 0 representing inability to do the test and 4 representing the highest level of performance. For the standing balance, participants were assigned the following scores: 1 if they could only hold a side-by-side standing position for 10 seconds; 2 if they could hold a semi-tandem position for 10 seconds but were unable to hold a full-tandem position for more than 2 seconds; 3 if they could stand in a full-tandem position for 3 to 9 seconds; and 4 if they couldst and in a full-tandem position for 10 seconds. Scores of 1 to 4 for walking speed and chair stands were calculated according to cut-points based on quartiles of the time to perform each task established in EPESE (21). The three measures were summed to create an SPPB summary score ranging from 0 (worst) to 12 (best). Risk of future disability based on SPPB scores of less than 10, indicative of moderate to high disability risk, and 7 or less, indicative of high disability risk, were also calculated (22).
Muscle Strength
Grip and knee extensor strength were assessed at the CHS All Stars Study examination. Grip strength in kg was measured using an adjustable, hydraulic dynamometer (Jamar Hand Dynamometer, Fred Sammons, Inc.). The best result of three tries obtained with the dominant hand was used. Maximum strength of the knee extensor muscles was measured using an isometric dynamometer (Litek Isometric Chair, Bio Logic Engineering Inc., Dexter, MI). Knee extensor strength was assessed as the peak torque, expressed in Newton-meters (Nm), recorded during a 4-sec effort. Since body size is related to both muscle strength and 25(OH)D levels, we also examined the residuals of knee and grip strength by regressing absolute grip and knee strength on body weight and using the residuals as independent variables.
Mobility and ADL Disability
Self-reported physical function was assessed at the CHS All Stars Study examination and at 6-month intervals by telephone over 3 years of follow-up. Mobility disability was defined as two consecutive reports of having any difficulty walking up 10 steps or walking half a mile. Disability in activities of daily living (ADLs) was defined as two consecutive reports of having any difficulty with one or more of five ADLs: dressing, eating, bathing, toileting, or transferring from a bed or chair. For participants who reported any difficulty but died before the next scheduled participant contact, difficulty was assumed to have persisted until death.
Serum 25-hydroxyvitamin D
Serum 25(OH)D was measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) at the Mayo Clinic, Rochester, MN, in fasting blood samples taken at the CHS All Stars Study examination and the sum of 25(OH)D2 and 25(OH)D3 reported. Interassay coefficients of variation for 25(OH)D2 and 25(OH)D3 controls ranged from 6% to 14% and 6% to 13%, respectively.
Potential Confounders
Covariates included sociodemographic variables (age, gender, race, field center, education), smoking status (never, former, and current), and alcohol consumption (none, ≤7 drinks/week, >7 drinks/week). Body mass index (BMI) was calculated as weight in kg/height in meters squared and categorized as normal weight (BMI: <25.0 kg/m2), overweight (25.0–29.9 kg/m2), or obese (≥30 kg/m2). Physical activity was assessed using the modified Minnesota Leisure-Time Activities questionnaire which asked whether participants had engaged in any of 17 leisure time activities in the previous 2 weeks and the frequency (23). For these analyses, we used walking for physical activity and categorized participants as: none, <5 times/week, or ≥5 times/week. Cognition was assessed using the Modified Mini-Mental State Examination (3 MS) score (24). Depressive symptoms were assessed using a modified, 10-item Center for Epidemiologic Studies Depression (CES-D) scale (25). The diagnoses of cardiovascular disease (coronary heart disease, myocardial infarction, congestive heart failure, and stroke) were adjudicated by CHS committees based on standardized criteria. Hypertension, diabetes, arthritis of the knee, and osteoporosis were based on self-reported history with or without corroborating physical or laboratory findings. Season of the year (December – February, March – May, June –August, September – November) was included to account for seasonal effects on 25(OH)D. Because impaired kidney function can impair the conversion of 25(OH)D to 1,25(OH)2D, the active hormonal form, creatinine was included as measure of kidney function.
Statistical Analyses
Analyses of physical performance, muscle strength, and physical function by 25(OH)D status were conducted using SAS statistical software version 9.1 (SAS Institute, Inc., Cary, NC). Serum 25(OH)D status was categorized as <20 ng/mL, 20–<30 ng/mL, and ≥30 ng/mL, common cut-points used to define 25(OH)D deficiency, insufficiency and sufficiency, respectively (1). Differences in the frequencies and means of covariates by 25(OH)D status were examined using chi-square and analysis of variance (ANOVA). Two-way interactions between gender and 25(OH)D were tested but were not significant (p > 0.10). Thus, all analyses are presented in the total population. For cross-sectional analyses, multiple linear regression models for continuous physical performance and muscle strength outcomes (SPPB, gait speed, and grip and knee extensor strength) and logistic regression models for dichotomous physical performance (SPPB scores <10 and ≤7) and prevalent functional outcomes (mobility and ADL disability) were used to examine the effect of 25(OH)D status on physical function at the CHS All Stars Study examination. For incident mobility and ADL disability, Cox proportional hazards regression models were used after excluding participants with prevalent mobility and ADL disability, respectively, at baseline. For participants who reported incident mobility or ADL disability, follow-up time was calculated from the date of the CHS All Stars visit until the date of the first of the two consecutive reports of disability. Participants who survived with no evidence of incident mobility or ADL disability were censored at their next to the last 6-month contact. Participants who died with no evidence of incident mobility or ADL disability were censored at their time of death; and those who were lost to follow-up were censored at their last visit. Models were adjusted for potential confounding variables including sociodemographic characteristics (age, gender, race, education, field center), health behaviors (physical activity, smoking, alcohol consumption), season of year, BMI, cognition, depression, kidney function, and prevalent chronic conditions (cardiovascular disease, diabetes, and osteoporosis) if they were known to be associated with 25(OH)D and/or physical function or were associated with 25(OH)D at p <0.10 in univariate analyses. Tests for linear trends across categories of 25(OH)D were conducted using the median value in each 25(OH)D category as a continuous variable in the linear regression models.
RESULTS
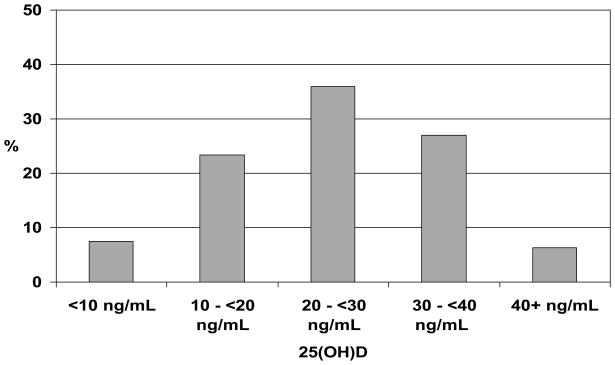
The mean age of the study population was 85.2 years (range: 77–100), 64.5% were women, and 16.7% were black. CHS All Stars participants excluded from the present analysis (n = 689, 41.1%) were more likely to be female, older, and have less than a high school education (p <0.05). Thirty-three percent of participants had sufficient 25(OH)D levels (≥30 ng/mL) while 30.8% had deficient 25(OH)D levels (<20 ng/mL; see Figure 1). The descriptive characteristics of the study population by 25(OH)D status are shown in Table 1. Blacks, participants with less than a high school education, participants who did not report walking for physical activity in the past two weeks, and participants with diabetes were more likely to have deficient 25(OH)D levels. Participants with deficient 25(OH)D levels were more likely to have had their 25(OH)D levels measured in the winter, abstain from alcohol consumption, have a higher BMI, report more depressive symptoms, and have lower cognitive function scores. However, participants with osteoporosis were more likely to have sufficient 25(OH)D levels.
Figure 1.
Distribution of 25-Hyroxyvitamin D (25[OH]D) Levels: CHS All Stars.
Table 1.
Baseline Participant Characteristics by 25-Hydroxyvitamin D (25[OH]D) Status: CHS All Stars*
| Total sample | 25(OH)D | ||||
|---|---|---|---|---|---|
| <20 ng/mL | 20–<30 ng/mL | ≥30 ng/mL | P-value | ||
| N (%) | 988 | 304 (30.8) | 355 (35.9) | 329 (33.3) | |
| Age (yrs), mean ± SD | 85.2 ± 3.6 | 85.2 ± 3.7 | 85.3 ± 3.7 | 85.1 ± 3.4 | 0.68 |
| Female gender, % | 64.5 | 66.1 | 60.8 | 66.9 | 0.20 |
| Black race, % | 16.7 | 30.3 | 13.0 | 8.2 | <0.001 |
| Field center, % | |||||
| Forsyth Co., NC | 20.8 | 19.7 | 20.8 | 21.9 | 0.65 |
| Sacramento Co., CA | 35.3 | 33.6 | 36.1 | 36.2 | |
| Washington Co., MD | 16.8 | 15.5 | 17.5 | 17.3 | |
| Pittsburgh, PA | 27.0 | 31.2 | 25.6 | 24.6 | |
| Education, % | |||||
| < High school | 18.3 | 27.6 | 15.8 | 12.5 | <0.001 |
| High school-some college | 55.0 | 52.0 | 54.6 | 58.0 | |
| ≥ College degree | 26.7 | 20.4 | 29.6 | 29.5 | |
| Season, % | |||||
| Sep–Nov | 31.0 | 25.7 | 30.7 | 36.2 | 0.05 |
| Dec–Feb | 29.0 | 35.2 | 27.0 | 25.5 | |
| Mar–May | 14.5 | 15.1 | 15.2 | 13.1 | |
| Jun–Aug | 25.5 | 24.0 | 27.0 | 25.2 | |
| Smoking, % | |||||
| Never smoker | 50.8 | 46.0 | 52.4 | 53.5 | 0.39 |
| Former smoker | 45.8 | 50.0 | 44.2 | 43.5 | |
| Current smoker | 3.4 | 4.0 | 3.4 | 3.0 | |
| Weekly alcohol intake, % | |||||
| None | 50.1 | 59.2 | 48.4 | 43.5 | 0.001 |
| 1–7/wk | 40.7 | 35.2 | 40.8 | 45.6 | |
| >7/wk | 9.2 | 5.6 | 10.7 | 10.9 | |
| Walking for physical activity in previous 2 weeks, % | |||||
| None | 49.8 | 54.6 | 51.6 | 43.5 | 0.05 |
| <5 times/week | 29.4 | 26.3 | 27.3 | 34.4 | |
| ≥5 times/week | 20.8 | 19.1 | 21.1 | 22.2 | |
| BMI status, % | |||||
| Normal weight (<25.0 kg/m2) | 37.8 | 29.3 | 37.5 | 45.9 | <0.001 |
| Overweight (25.0–29.9 kg/m2) | 40.5 | 43.1 | 38.0 | 33.5 | |
| Obese (≥30 kg/m2) | 21.8 | 27.6 | 24.5 | 20.5 | |
| Creatinine (mg/dL), mean ± SD | 1.09 ± 0.53 | 1.12 ± 0.68 | 1.04 ± 0.41 | 1.11 ± 0.48 | 0.08 |
| Depression (CES-D score), mean ± SD | 6.1 ± 4.7 | 6.8 ± 5.0 | 6.0 ± 4.6 | 5.6 ± 4.4 | 0.003 |
| Cognition (3MSE score), mean ± SD | 88.6 ± 10.7 | 86.4 ± 10.8 | 89.3 ± 9.8 | 89.7 ± 11.4 | <0.001 |
| Prevalent disease, % | |||||
| Diabetes | 14.5 | 21.0 | 13.2 | 9.7 | <0.001 |
| Hypertension | 64.0 | 66.1 | 61.7 | 64.4 | 0.49 |
| Osteoporosis | 23.7 | 14.8 | 25.6 | 29.8 | <0.001 |
| Knee arthritis | 27.7 | 29.0 | 28.4 | 25.8 | 0.64 |
| CVD† | 22.9 | 26.0 | 23.9 | 18.8 | 0.08 |
Means ± SD or frequencies with chi-square or ANOVA to evaluate the distribution across categories of 25(OH)D.
CVD: myocardial infarction, congestive heart failure, and stroke.
Physical performance by 25(OH)D status is shown in Table 2. SPPB scores were significantly lower among those with deficient 25(OH)D levels compared to those with sufficient 25(OH)D levels even after adjusting for sociodemographic characteristics, season, health behaviors and chronic conditions (p <0.01). Of the individual SPPB components, standing balance, repeated chair stand, and gait speed scores were all significantly associated with 25(OH)D status after adjusting for sociodemographic characteristics and season (p for trend, <0.05). However, only the standing balance score was significantly associated with 25(OH)D status after further adjustment for health behaviors and chronic conditions (p for trend, 0.003). Although 25(OH)D status was associated with 3-m gait speed among those who completed the walk after adjusting for sociodemographic characteristics and season; the association was attenuated and no longer significant after adjustment for health behaviors and chronic conditions.
Table 2.
25-Hydroxyvitamin D (25[OH]D) Status and Physical Performance (LS Means ± SE): CHS All Stars
| 25(OH)D | ||||
|---|---|---|---|---|
| <20 ng/mL | 20–<30 ng/mL | ≥30 ng/mL | P for trend | |
| SPPB score (range, 0–12) | ||||
| N | 293 | 339 | 322 | |
| Model 1 | 6.12 ± 0.18 ‡ | 6.62 ± 0.19 * | 7.09 ± 0.20 | <0.001 |
| Model 2 | 6.53 ± 0.24 † | 6.87 ± 0.24 | 7.15 ± 0.25 | 0.006 |
| 3 m/15 ft gait speed score (range, 0–4) | ||||
| N | 296 | 345 | 323 | |
| Model 1 | 2.61 ± 0.08 † | 2.71 ± 0.08 * | 2.89 ± 0.09 | 0.006 |
| Model 2 | 2.80 ± 0.10 | 2.81 ± 0.10 | 2.91 ± 0.11 | 0.24 |
| Standing balance score (range, 0–4) | ||||
| N | 302 | 349 | 328 | |
| Model 1 | 2.37 ± 0.08 ‡ | 2.67 ± 0.08 | 2.82 ± 0.09 | <0.001 |
| Model 2 | 2.49 ± 0.12 † | 2.73 ± 0.11 | 2.81 ± 0.12 | 0.003 |
| Chair stand score (range, 0–4) | ||||
| N | 303 | 353 | 329 | |
| Model 1 | 1.09 ± 0.07 * | 1.15 ± 0.07 * | 1.32 ± 0.08 | 0.01 |
| Model 2 | 1.19 ± 0.10 | 1.23 ± 0.10 | 1.36 ± 0.10 | 0.08 |
| 3 m/15 ft gait speed (m/sec) | ||||
| N | 263 | 315 | 302 | |
| Model 1 | 0.73 ± 0.01 † | 0.75 ± 0.01 * | 0.78 ± 0.02 | 0.003 |
| Model 2 | 0.75 ± 0.02 | 0.75 ± 0.02 | 0.77 ± 0.02 | 0.18 |
Model 1 adjusted for age, gender, race, education, field center, and season.
Model 2 adjusted for variables in model 1 plus BMI status, walking for physical activity, smoking, alcohol use, creatinine, depression, cognition, diabetes, osteoporosis, and cardiovascular disease.
= p<0.05,
= p<0.01,
= p<0.001 from 25(OH)D ≥30 ng/mL.
The association between 25(OH)D status and disability risk based on SPPB scores was also examined. Participants with deficient 25(OH)D levels had more than twice the odds of having an SPPB score of less than 10, indicative of moderate to high disability risk, compared to those with sufficient 25(OH)D levels after adjusting for sociodemographic characteristics, season, health behaviors, and chronic conditions (OR (95% CI): 2.32 (1.37–3.94)). Participants with deficient 25(OH)D levels also had significantly higher odds of having an SPPB score of 7 or less, indicative of high disability risk, compared to those with sufficient 25(OH)D levels (OR (95% CI): 1.50 (1.01–2.22)). There were no significant differences in disability risk based on SPPB scores between those with insufficient 25(OH)D levels and those with sufficient 25(OH)D levels (OR (95% CI): 1.38 (0.90–2.12) for SPPB score of less than 10; 1.27 (0.89–1.81) for SPPB score of 7 or less).
Muscle strength by 25(OH)D status is shown in Table 3. There were no significant associations between 25(OH)D status and absolute grip or knee extensor strength. However, both grip and knee extensor strength adjusted for body weight were significantly lower among those with deficient 25(OH)D levels compared to those with sufficient 25(OH)D levels after adjusting for sociodemographic characteristics and season (p <0.05) suggesting poorer muscle quality. The association remained significant for grip strength adjusted for body weight after further adjustment for health behaviors and chronic conditions (p for trend, 0.02).
Table 3.
25-Hydroxyvitamin D (25[OH]D) Status and Muscle Strength (LS Means ± SE): CHS All Stars
| 25(OH)D | ||||
|---|---|---|---|---|
| <20 ng/mL | 20–<30 ng/mL | ≥30 ng/mL | P for trend | |
| Absolute grip strength (Kg) | ||||
| N | 295 | 348 | 324 | |
| Model 1 | 24.8 ± 0.4 | 25.0 ± 0.4 | 25.2 ± 0.4 | 0.46 |
| Model 2 | 25.4 ± 0.6 | 25.4 ± 0.6 | 25.6 ± 0.6 | 0.62 |
| Grip strength adjusted for body size (Kg) | ||||
| N | 295 | 348 | 324 | |
| Model 1 | 23.2 ± 0.4 † | 23.9 ± 0.4 * | 25.0 ± 0.5 | <0.001 |
| Model 2 | 24.7 ± 0.6 * | 25.1 ± 0.6 | 26.0 ± 0.6 | 0.02 |
| Absolute knee extensor strength (Nm) | ||||
| N | 262 | 308 | 292 | |
| Model 1 | 71.9 ± 1.9 | 73.9 ± 1.9 | 72.8 ± 2.0 | 0.73 |
| Model 2 | 72.3 ± 2.6 | 74.2 ± 2.5 | 73.0 ± 2.6 | 0.77 |
| Knee extensor strength adjusted for body size (Nm) | ||||
| N | 262 | 308 | 292 | |
| Model 1 | 67.0 ± 1.9 * | 70.7 ± 1.9 | 72.9 ± 2.0 | 0.02 |
| Model 2 | 70.2 ± 2.6 | 72.9 ± 2.5 | 73.9 ± 2.6 | 0.12 |
Model 1 adjusted for age, gender, race, education, field center, and season.
Model 2 adjusted for variables in model 1 plus BMI status (for absolute strength only), walking for physical activity, smoking, alcohol use, creatinine, depression, cognition, diabetes, osteoporosis, and cardiovascular disease.
= p<0.05,
= p<0.001 from 25(OH)D ≥30 ng/mL.
The prevalence of mobility disability at baseline was 31.1% and of ADL disability was 29.6%. Participants with deficient and insufficient 25(OH)D levels had approximately 50% higher odds of having prevalent mobility and ADL disability at baseline compared to those with sufficient 25(OH)D levels after adjusting for sociodemographic characteristics, season, health behaviors, and chronic conditions (see Table 4). Among participants free of mobility disability at baseline with at least two follow-up contacts (n = 653), 196 participants (30.0%) developed mobility disability over the 3-year follow-up (median follow-up, 2.5 years). Participants with deficient 25(OH)D levels were at significantly increased risk of developing mobility disability compared to those with sufficient 25(OH)D levels after adjusting for sociodemographic characteristics, season, health behaviors, and chronic conditions (see Table 4). Among participants free of ADL disability at baseline with at least two follow-up contacts (n = 665), 126 participants (18.9%) developed ADL disability over the 3-year follow-up (median follow-up, 2.6 years). Neither deficient nor insufficient 25(OH)D levels were significantly associated with increased risk of developing ADL disability.
Table 4.
25-Hydroxyvitamin D (25[OH]D) Status and Prevalent (OR (95% CI)) and Incident (HR (95% CI)) Mobility and ADL Disability: CHS All Stars
| 25(OH)D | |||
|---|---|---|---|
| <20 ng/mL | 20–<30 ng/mL | ≥30 ng/mL | |
| Prevalent disability | |||
| Mobility disability | |||
| N | 303 | 355 | 329 |
| Prevalent disability, N (%) | 111 (36.6) | 117 (33.0) | 79 (24.0) |
| OR (95% CI): Model 1 | 1.85 (1.28–2.66) | 1.66 (1.17–2.34) | 1.00 |
| OR (95% CI): Model 2 | 1.44 (0.96–2.14) | 1.53 (1.05–2.22) | 1.00 |
| ADL disability | |||
| N | 304 | 355 | 329 |
| Prevalent disability, N (%) | 106 (34.9) | 109 (30.7) | 78 (23.7) |
| OR (95% CI): Model 1 | 1.81 (1.26–2.61) | 1.50 (1.06–2.12) | 1.00 |
| OR (95% CI): Model 2 | 1.51 (1.01–2.25) | 1.37 (0.94–2.00) | 1.00 |
| Incident disability* | |||
| Mobility disability | |||
| N | 183 | 227 | 243 |
| Disability events, N (%) | 81 (44.3) | 54 (23.8) | 61 (25.1) |
| HR (95% CI): Model 1 | 1.82 (1.27–2.60) | 0.90 (0.63–1.31) | 1.00 |
| HR (95% CI): Model 2 | 1.56 (1.06–2.30) | 0.83 (0.56–1.21) | 1.00 |
| ADL disability | |||
| N | 188 | 234 | 243 |
| Disability events, N (%) | 44 (23.4) | 37 (15.8) | 45 (18.5) |
| HR (95% CI): Model 1 | 1.18 (0.75–1.84) | 0.79 (0.51–1.22) | 1.00 |
| HR (95% CI): Model 2 | 0.98 (0.61–1.56) | 0.75 (0.48–1.18) | 1.00 |
Model 1 adjusted for age, gender, race, education, field center, and season.
Model 2 adjusted for variables in model 1 plus BMI status, walking for physical activity, smoking, alcohol use, creatinine, depression, cognition, diabetes, osteoporosis, and cardiovascular disease.
Among participants free of mobility or ADL disability at baseline with at least two follow-up contacts over 3 years.
In secondary analyses, we examined the association between 25(OH)D levels and physical function stratified by race. In whites (n = 823), the results were similar (data not shown). However, while the trends were similar in blacks (n = 165), the associations were no longer significant. Because osteoporosis has been associated with frailty in older adults and vitamin D supplements, along with calcium, are typically recommended to patients with osteoporosis, we also examined the association between 25(OH)D levels and physical function restricted to participants who did not report having osteoporosis (n = 754). The associations between 25(OH)D and physical performance, muscle strength, and mobility and ADL disability among participants without osteoporosis were similar to those found in the entire cohort at baseline (data not shown). Among participants who did not report having osteoporosis or mobility disability at baseline (n = 512), participants with deficient 25(OH)D levels were at significantly increased risk of developing mobility disability compared to those with sufficient 25(OH)D levels after adjusting for sociodemographic characteristics and season (HR (95% CI): 1.71 (1.12–2.62)); however, after further adjustment for health behaviors and chronic conditions, the association was attenuated and no longer significant (1.29 (0.82–2.03)).
DISCUSSION
In the CHS All Stars, 25(OH)D deficiency was common among community dwelling adults of advanced age and was associated with poorer physical performance, lower muscle strength, and both prevalent and incident mobility disability after adjusting for sociodemographic characteristics, season, health behaviors, and chronic conditions. Older adults with deficient 25(OH)D levels had significantly lower SPPB scores than those with sufficient 25(OH)D levels. Older adults with deficient 25(OH)D levels were also approximately 50% more likely to report prevalent mobility and ADL disability. Furthermore, because of its longitudinal nature, the current study is the first to show that older adults with deficient 25(OH)D levels were at increased risk of developing mobility disability over 3 years of follow-up.
Similar to previous cross-sectional studies in older adults (6–12), we found that adults of advanced age with deficient 25(OH)D levels had significantly poorer physical performance compared to those with sufficient 25(OH)D levels based on the SPPB. The mean SPPB scores for individuals with deficient 25(OH)D levels were approximately 0.6 points lower than that of those with sufficient 25(OH)D after adjusting for multiple confounders. Perera and colleagues have previously reported that a difference of 0.5 points on the SPPB represents a small albeit clinically meaningful difference (26). Of the individual SPPB components, older adults with deficient 25(OH)D levels had significantly lower standing balance scores compared to those with sufficient 25(OH)D levels after adjusting for sociodemographic characteristics, season, health behaviors, and chronic conditions. Physical performance, and balance in particular, may be one of the reasons why individuals with low 25(OH)D levels are at greater risk for falls (27).
Low 25(OH)D levels were associated with weaker muscle strength in previous studies (6–9;12;15). While absolute grip and knee extensor strength were not associated with 25(OH)D levels in the current study, older adults with deficient 25(OH)D levels had significantly lower muscle strength after adjusting strength for body weight, potentially indicating poorer muscle quality. The lack of an association with absolute muscle strength is likely due to negative confounding of body size as larger individuals tend to be stronger but have lower 25(OH)D levels.
Older adults with deficient 25(OH)D levels were more than twice as likely to be at moderate to high disability risk and 50% more likely to be at high disability risk, based on SPPB scores of less than 10 or 7 or less (22), respectively, compared to those with sufficient 25(OH)D levels. Similarly, older adults with deficient 25(OH)D levels were approximately 50% more likely to report prevalent mobility and ADL disability. Previous studies have also shown that low 25(OH)D levels are more prevalent among older adults with mobility and/or ADL disability (8;16–18). However, because previous studies have been cross-sectional in design, it is difficult to determine whether low 25(OH)D levels preceded the onset of reported disability or whether individuals had low 25(OH)D levels since they were more disabled and, because of limited outdoor activity, had less exposure to the sun and reduced endogenous vitamin D synthesis.
Because of the longitudinal nature of the current study, we were able to examine whether low 25(OH)D levels predict incident disability. Older adults with deficient 25(OH)D levels were at increased risk of developing mobility disability over 3 years of follow-up. However, low 25(OH)D levels were not associated with incident ADL disability. Thus, low 25(OH)D levels may affect earlier stages of the disablement process (i.e., mobility disability) because of vitamin D’s role in the musculoskeletal system but have less of an effect on later stages of the disablement process (i.e., ADL disability) as other factors such as cognitive function and psychological state may be more relevant.
Whether or not improving 25(OH)D levels, which can be done inexpensively and safely with vitamin D supplementation, will improve physical performance and delay the onset of disability in older adults has not been determined. Randomized controlled trials of vitamin D supplementation on physical performance and muscle strength among older adults have been inconclusive (28;29). However, supplemental vitamin D doses of 800–1000 IU/d have been suggested to prevent falls and fractures as well as other adverse health outcomes in older adults (1;27;30). In the current study, participants with osteoporosis were less likely to have deficient 25(OH)D levels. A possible explanation is that participants diagnosed with osteoporosis have received recommendations to take a vitamin D-containing supplement from their physician; however, information on vitamin D supplement use was not obtained at the CHS All Stars visit. The associations between 25(OH)D levels and physical function were similar when analyses were restricted to participants who did not report having osteoporosis.
Important characteristics of the CHS All Stars cohort limit the generalization of these findings. Only individuals who attended the All Stars Study clinic visit or had an in-home visit (68% of the All Stars cohort) were included in these analyses. Thus, it is possible that the association between low 25(OH)D and worse physical function may have been underestimated since participants who were excluded are more likely to have had worse physical function and lower 25(OH)D levels. Mobility and ADL disability were both based on self-report. However, previous studies have shown that self-reported physical function is valid and has clinical significance (31). Furthermore, the use of two consecutive reports of mobility and ADL disability reduces the influence of transient disability. The observational design of our study does not allow us to evaluate a causal association between 25(OH)D and physical function. However, through its influence on calcium transport, uptake of inorganic phosphate for the production of energy-rich phosphate compounds, and protein synthesis in the muscle (32), it is biologically plausible that low 25(OH)D levels may result in poorer physical performance, lower muscle strength and reduced physical function. Major strengths of the current study are the advanced age of the study participants and the longitudinal nature of the study with information on physical function collected over 3 years of follow-up allowing an examination of the association between 25(OH)D levels and incident disability risk.
In conclusion, vitamin D deficiency was common and was associated with poorer physical performance, lower muscle strength, and prevalent mobility and ADL disability among community-dwelling adults of advanced age. Moreover, vitamin D deficiency predicted incident mobility disability over three years of follow-up. These results, along with other recent findings showing the importance of 25(OH)D on multiple health outcomes, underscore the need for definitive trials of vitamin D supplementation on physical performance and disability and other health outcomes among older adults.
Acknowledgments
The authors express their gratitude to the CHS and All Stars participants and staff.
Funding/Support: This work was supported by grants from the National Institute on Aging (R01 AG023629; K01 AG030506 to DKH) and by a research grant from the Investigator-Initiated Studies Program of Merck & Co., Inc. CHS was supported by contracts N01-HC85079 through N01-HC85086, N01-HC35129, N01 HC15103, N01 HC55222, N01-HC75150, N01-HC45133, and U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through R01 AG15928, R01 AG20098, and R01 AG027058 from the National Institute on Aging, R01 HL075366 from the National Heart, Lung and Blood Institute, and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center P30 AG024827. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Sponsor’s Role:
The funding institutes had no role in the design, methods, subject recruitment, data collection, analysis, or manuscript preparation or in the decision to submit the manuscript for publication.
Footnotes
Portions of this work were presented in abstract form at the Gerontological Association of America Annual Meeting, Atlanta, GA, November 21, 2009 and the American Geriatrics Society Annual Meeting, Orlando, FL, May 15, 2010.
Author Contributions:
DKH contributed to the conceptualization of the manuscript idea, analysis and interpretation of the data, and writing of the first draft of the manuscript. ABN and SBK contributed to the conceptualization and design of the CHS All Stars, acquisition of subjects and data, interpretation of the data, and critical revisions of the manuscript. JAT, CCD, and AMA contributed to the analysis and interpretation of the data and critical revisions of the manuscript. PHMC and JAR contributed to the acquisition of subjects and data and critical revisions of the manuscript. CHH contributed to the critical revisions of the manuscript. All authors approved the last version of the manuscript.
Conflict of Interest
DKH, JAT, and CCD received funding from a research grant (PI: DKH) from the Investigator-Initiated Studies Program of Merck & Co., Inc., to conduct the analyses in the CHS All Stars. Merck & Co., Inc., had no role in data collection, data analysis or manuscript preparation or in the decision to submit the manuscript for publication.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 3.Looker AC, Pfeiffer CM, Lacher DA, et al. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88:1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 5.Bailey RL, Dodd KW, Goldman JA, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140:817–822. doi: 10.3945/jn.109.118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mowe M, Haug E, Bohmer T. Low serum calcidiol concentration in older adults with reduced muscular function. J Am Geriatr Soc. 1999;47:220–226. doi: 10.1111/j.1532-5415.1999.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff HA, Stahelin HB, Urscheler N, et al. Muscle strength in the elderly: Its relation to vitamin D metabolites. Arch Phys Med Rehabil. 1999;80:54–58. doi: 10.1016/s0003-9993(99)90307-6. [DOI] [PubMed] [Google Scholar]
- 8.Zamboni M, Zoico E, Tosoni P, et al. Relation between vitamin D, physical performance, and disability in elderly persons. J Gerontol A Biol Sci Med Sci. 2002;57:M7–11. doi: 10.1093/gerona/57.1.m7. [DOI] [PubMed] [Google Scholar]
- 9.Dhesi JK, Bearne LM, Moniz C, et al. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res. 2002;17:891–897. doi: 10.1359/jbmr.2002.17.5.891. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 11.Greenspan SL, Resnick NM, Parker RA. Vitamin D supplementation in older women. J Gerontol A Biol Sci Med Sci. 2005;60:754–759. doi: 10.1093/gerona/60.6.754. [DOI] [PubMed] [Google Scholar]
- 12.Houston DK, Cesari M, Ferrucci L, et al. Association between vitamin D status and physical performance: The InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62:440–446. doi: 10.1093/gerona/62.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 14.Wicherts IS, van Schoor NM, Boeke AJ, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 15.Dam TT, von MD, Barrett-Connor EL. Sex-specific association of serum vitamin D levels with physical function in older adults. Osteoporos Int. 2009;20:751–760. doi: 10.1007/s00198-008-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semba RD, Garrett E, Johnson BA, et al. Vitamin D deficiency among older women with and without disability. Am J Clin Nutr. 2000;72:1529–1534. doi: 10.1093/ajcn/72.6.1529. [DOI] [PubMed] [Google Scholar]
- 17.Isaia G, Giorgino R, Rini GB, et al. Prevalence of hypovitaminosis D in elderly women in Italy: Clinical consequences and risk factors. Osteoporos Int. 2003;14:577–582. doi: 10.1007/s00198-003-1390-7. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K, Nishiwaki T, Ueno K, et al. Serum 25-hydroxyvitamin D levels and activities of daily living in noninstitutionalized elderly Japanese requiring care. J Bone Miner Metab. 2005;23:488–494. doi: 10.1007/s00774-005-0633-4. [DOI] [PubMed] [Google Scholar]
- 19.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 20.Newman AB, Arnold AM, Sachs MC, et al. Long-term function in an older cohort-the cardiovascular health study all stars study. J Am Geriatr Soc. 2009;57:432–440. doi: 10.1111/j.1532-5415.2008.02152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55A:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 23.Siscovick DS, Fried L, Mittelmark M, et al. Exercise intensity and subclinical cardiovascular disease in the elderly. The Cardiovascular Health Study. Am J Epidemiol. 1997;145:977–986. doi: 10.1093/oxfordjournals.aje.a009066. [DOI] [PubMed] [Google Scholar]
- 24.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 25.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 26.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 27.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: A meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latham NK, Anderson CS, Reid IR. Effects of vitamin D supplementation on strength, physical performance, and falls in older persons: A systematic review. J Am Geriatr Soc. 2003;51:1219–26. doi: 10.1046/j.1532-5415.2003.51405.x. [DOI] [PubMed] [Google Scholar]
- 29.Annweiler C, Schott AM, Berrut G, et al. Vitamin D-related changes in physical performance: A systematic review. J Nutr Health Aging. 2009;13:893–898. doi: 10.1007/s12603-009-0248-x. [DOI] [PubMed] [Google Scholar]
- 30.Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: A meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169:551–561. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 31.Fried LP, Young Y, Rubin G, et al. Self-reported preclinical disability identifies older women with early declines in performance and early disease. J Clin Epidemiol. 2001;54:889–901. doi: 10.1016/s0895-4356(01)00357-2. [DOI] [PubMed] [Google Scholar]
- 32.Ceglia L. Vitamin D and its role in skeletal muscle. Curr Opin Clin Nutr Metab Care. 2009;12:628–633. doi: 10.1097/MCO.0b013e328331c707. [DOI] [PMC free article] [PubMed] [Google Scholar]