Abstract
Due to the transepidermal potential of 15-50 mV, inside positive, an injury current is driven out of all human skin wounds. The flow of this current generates a lateral electric field within the epidermis that is more negative at the wound edge than at regions more lateral from the wound edge1. Electric fields in this region could be as large as 40 mV/mm2, and electric fields of this magnitude have been shown to stimulate human keratinocyte migration toward the wounded region3. After flowing out of the wound, the current returns through the space between the epidermis and stratum corneum, generating a lateral field above the epidermis in the opposite direction. Here we report the results from the first clinical trial designed to measure this lateral electric field adjacent to human skin wounds non-invasively. Using a new instrument, the Dermacorder®, we found that the mean lateral electric field in the space between the epidermis and stratum corneum adjacent to a lancet wound in 18-25 year olds is 107-148 mV/mm, 48% larger on average than that in 65-80 year olds. We also conducted extensive measurements of the lateral electric field adjacent to mouse wounds as they healed and compared this field with histological sections through the wound to determine the correlation between the electric field and the rate of epithelial wound closure. Immediately after wounding the average lateral electric field was 122 ± 9 mV/mm. When the wound is filled in with a thick, disorganized epidermal layer, the mean field falls to 79 ± 4 mV/mm. Once this epidermis forms a compact structure with only three cell layers, the mean field is 59 ± 5 mV/mm. Thus, the peak-to-peak spatial variation in surface potential is largest in fresh wounds and slowly declines as the wound closes. The rate of wound healing is slightly greater when wounds are kept moist as expected but we could find no correlation between the amplitude of the electric field and the rate of wound healing.
Human epidermis is composed of seven polarized epithelial layers that each transports Na+ in an apical-basal direction by localizing Na+ channels to the apical side and K+ channels along with the Na+/K+ ATPase pumps to the basolateral membranes. This polarized distribution of ion transporters generates a transepidermal voltage difference of 15-50 mV, internally positive4. This “skin battery” will immediately drive current out of any low resistance pathway formed upon skin injury leading to the injury current that exits all wounds5. Dubois-Reymond first discovered this wound current 160 years ago using a galvanometer6. This was confirmed by Illingworth and Barker in 19807 using a more modern vibrating probe technique8. They measured up to 10 μA/cm2 exiting accidentally amputated fingertips in children during a two-week period following the injury when their fingers were placed in a conductive fluid. Most mammalian skin wounds are open to air rather than being submersed in a conducting liquid so there is no conductive path through which the injury current can flow other than back between the stratum corneum and epidermis. This flow of current between the epidermis and stratum corneum must generate a lateral electric field in that region bordering the wound. This electrical field has been measured in the skin of guinea pigs1 and newts9 but up to now there has been only one reported measurement of this electric field in humans2. The shortage of human studies is due, in part, to the difficulty of carrying out recordings in human wounds using the standard microelectrode technology. The Dermacorder® does not require any electrode contact at the wound site making possible the non-invasive measurement of electric fields near mammalian wounds. This electric field is the first signal generated upon wounding and it initiates the wound healing process by triggering the active migration of keratinocytes toward the wounded region by galvanotaxis3. We would predict that wounds exhibiting smaller electric fields will also exhibit slower healing rates. That indeed has been confirmed in the newt10 but it has not yet been investigated in humans.
We have published one previous paper describing the lateral electric field near mouse skin wounds2 and here we report results from the first clinical trial of the Dermacorder® on human skin wounds. We find that older individuals exhibit smaller wound fields than young individuals. We also include histological studies of mouse skin wounds that demonstrate a correlation between the magnitude of the wound field and the degree of wound healing in mouse skin.
MATERIAL AND METHODS
Dermacorder technique
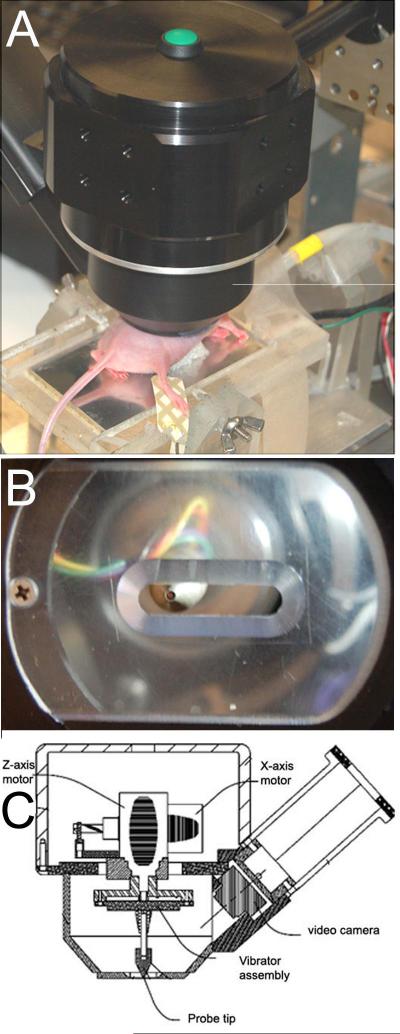
We have described this technique in detail previously2 so only a short explanation is provided here. The Dermacorder® (fig. 1) vibrates a small gold disk (0.5 mm in diameter) at approximately 900 Hz perpendicular to the skin surface about 200 μm above the surface. This disk forms a capacitor with the nearest conducting surface, the epidermis of the skin. Since we oscillate the distance between the disk and the skin, the charge on the capacitor also oscillates. This charge is proportional to the voltage difference between the gold disk (zero volts) and the unknown potential at the epidermal surface. By applying a known voltage to the skin surface, we can determine the voltage level for which the charge on the capacitor stops oscillating. Since that imposed voltage combines with the unknown epidermal potential to equal zero volts, it must be equal and opposite to the unknown skin surface potential in that local region. This is how the surface potential of the epidermis is determined. As we move the Dermacorder® probe to various positions and determine the imposed potential required to stop the oscillation in the charge, we obtain the surface potential in each position. In addition, the slope of the spatial voltage pattern at a given point can be used to determine the distance between the probe sensor and the skin at that point. We use this information to actively adjust the probe’s position to maintain a constant distance from the skin.
Figure 1.
Dermacorder® A. Photo of Dermacorder® mounted onto a manipulator and scanning a wound in mouse skin. B. View from below showing sensor and plastic window with slit for stabilizing skin during measurement. C. Sketch of the main components of the Dermacorder®
Clinical Trial
As registered with Clinicaltrials.gov (NCT00355823) using a protocol approved by the Eastern Virginia Medical School IRB, 10 males and 10 females 18-25 years old and 10 males and 10 females 65-80 years old were given three lancet wounds each at Virginia Clinical Research, Inc. in Norfolk, VA during 2006. The Dermacorder® using a round probe 0.5 mm in diameter first scanned a control region of skin on their volar forearm three times. A lancet wound was then made in the same region using the Ascensia Microlet Vaculance by Bayer Health Care with a 21 gauge lancet on the Vaculance’s deepest setting. This resulted in a dermal wound 0.5 mm wide that penetrated the epidermis and stopped within the dermis. A small drop of blood marked the lance site and was blotted dry prior to scanning the wound three times. A second wound was made within a few cm of the first one to determine variability within the same individual. For each wound, three scans were recorded and the instrument was repositioned between each scan. An image of the probe position for each voltage measurement was recorded. A third wound was made on the leg. If hair was present, it was removed with an electric shaver before wounding. Since the wound was similar in size to the probe, it was important to scan directly over the wound. Occasionally the probe position was not directly over the wound as evident by the stored video images and those data were not included in the averages presented in Table 1.
Table I.
Human clinical trial data
| Control | LA-1 | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | 18-29 yr | 65-80 yr | 18-29 yr | 65-80 yr | ||||
| M | F | M | F | M | F | M | F | |
| 1 | 28±41 | 39±14 | 28±1 | 39±2 | 45±0 | 195±43 | 55±18 | 71±0 |
| 2 | 0±16 | 30±22 | *100±8 | 46±2 | 146±80 | 202±84 | 57±5 | 56±4 |
| 3 | 13±31 | 28±24 | 33±6 | 55±3 | 56±12 | 123±71 | 59±1 | 53±1 |
| 4 | 13±5 | 79±44 | 36±8 | 32±1 | 146±0 | 172±19 | 41±14 | 99±1 |
| 5 | 3±27 | 39±20 | 27±5 | 54±2 | 79±34 | 81±26 | 34±1 | 52±10 |
| 6 | 7±8 | 11±18 | 27±4 | 42±4 | 163±63 | 88±20 | 49±7 | 26±7 |
| 7 | 7±4 | 26±48 | 42±5 | 27±3 | *28±10 | 76±53 | 71±13 | 52±11 |
| 8 | 14±60 | 22±6 | 53±7 | *148±0 | 101±59 | 79±12 | 71±8 | 55±18 |
| 9 | 33±12 | 9±5 | 62±7 | 37±9 | 98±12 | 158±49 | 51±10 | 74±15 |
| 10 | 10±5 | 3±3 | - | 56±12 | 131±35 | - | 76±27 | 65±3 |
| Mean | 13 | 29 | 39 | 43 | 107 | 130 | 56 | 60 |
| SEM | 3 | 13 | 4 | 3 | 13 | 17 | 5 | 6 |
| LA-2 | LL-1 | |||||||
|---|---|---|---|---|---|---|---|---|
| 18-29 yr | 65-80 yr | 18-29 yr | 65-80 yr | |||||
| M | F | M | F | M | F | M | F | |
| 1 | 160±59 | 208±33 | 62±3 | 73±4 | 127±31 | 84±25 | 71±3 | 111±4 |
| 2 | 178±73 | 112±34 | 82±0 | 32±1 | NA | 87±33 | 61±6 | 50±1 |
| 3 | 128±21 | 186±70 | 118±9 | 72±5 | 172±43 | 195±40 | 96±3 | 40±2 |
| 4 | - | 103±85 | 63±7 | NA | 81±14 | 101±52 | 55±16 | 103±21 |
| 5 | 193±5 | 106±28 | 69±17 | 42±7 | 67±15 | 101±44 | 32±1 | 111±55 |
| 6 | 94±10 | 174±42 | 51±14 | 65±8 | 147±32 | 217±42 | 52±4 | 38±7 |
| 7 | 107±20 | 176±19 | 42±1 | 59±5 | 81±12 | *353±35 | 45±1 | 71±8 |
| 8 | 169±40 | 115±25 | 43±1 | 106±41 | 169±37 | 140±49 | 69±2 | 82±28 |
| 9 | 101±23 | 176±113 | 62±11 | 152±0 | 142±38 | 168±54 | 110±25 | 81±5 |
| 10 | *512±112 | 128±32 | 45±5 | 42±5 | 156±43 | 124±40 | 75±16 | 69±2 |
| Mean | 141 | 148 | 64 | 71 | 127 | 135 | 68 | 76 |
| SEM | 14 | 13 | 8 | 12 | 13 | 16 | 8 | 9 |
All numbers indicate the lateral electric field associated with a lancet wound in units of mV/mm. Most represent an average of 3 Dermacorder® measurements on a lancet wound ± SEM. “M” indicates the males and “F” indicates the females.
Indicates outlier not included in the mean.
Control field measurements were made by dividing the Dermacorder® scan into three equal sections along the scan axis and drawing a line through each section that represented that section’s average surface potential. The difference between the center section’s average and the mean of the two outer section’s averages was taken as the control surface potential and that was divided by the distance between the outer and central section to obtain an electric field value.
Animals
21 SKH-1 female mice 1-4 months old were used for the skin wound closure study. The animals were maintained in an animal facility with a 12-hour light/dark cycle, and were housed in cages with food and water at a controlled temperature and humidity. All of the experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee, In-Vivo Technologies, Inc, Burlingame, CA. Before wounding, mice were anesthetized by isoflurane inhalation. The mice were kept warm on a platform heated to 38 °C. The back skin of each mouse was shaved and sterilized with 70% isopropyl alcohol. A 4-6mm long, full thickness linear wound was created by lifting a fold of skin away from the body and making a full thickness cut perpendicular to the fold using a pair of iridectomy scissors (Fine Science Tools # 15013-12, Foster City, CA). This same procedure was repeated three times on the same mouse to create a total of four uniform, bilateral wounds, two on each side. After scanning the wounds each day, they were covered with a 0.5 mm thick, elastic cotton mesh held in place by a 1 cm × 1 cm Bioclusive transparent dressing (Johnson & Johnson, New Jersey). The thin cotton mesh covering wounds on the left side of the mice was soaked with physiological saline (Hanks Buffered Salt Solution, HBSS) and the mesh covering wounds on the right side of the mice was composed of the same dressing without added saline. The dressings were changed daily.
Analysis of wound closure
Wound Area Measurement
Each wound site was photographed daily with a stereoscope and digital camera (Olympus DP71). Wound areas were determined by tracing the outer edge of the wounds with the aid of the software package, Image J (http://rsbweb.nih.gov/ij/download.html)
Electric Field Measurement of Mouse Wounds
While measuring the lateral electric field adjacent to each wound, the mice were kept warm on a heated stage under isoflurane anesthesia. The skin near the wound was first moisturized with distilled water to facilitate adhesion of a layer of thin plastic film applied to the wound area so that the Dermacorder probe would be opposed to a uniform surface. Liquids in the wound have a very different work function or electron affinity than the skin on either side of the wound; covering this liquid region with plastic film eliminates possible signals generated by the difference in electron affinity instead of actual surface potential differences. The mice were positioned so that the surfaces of the wound areas were horizontal. A 6-mm long scan was made perpendicular to the long axis of the wound using a 0.2 mm step size.
Histology
Mice were euthanized at various times after wounding. For wound morphology analysis, wound regions and the surrounding skin were excised, laid flat on a sheet of paper to prevent them from curling, and then fixed in 10% formalin solution. They were then embedded in paraffin and sectioned perpendicular to the long axis with 5 μm thick sections. At least four sections per wound were stained with hematoxylin & eosin (H&E) and analyzed.
RESULTS
Clinical Trial
Ten males and ten females in each of two age groups were recruited for an independently conducted clinical trial at Virginia Clinical Research in Norfolk, VA. Three Dermacorder® scans of a control region on the volar forearm were collected before making a lancet wound in the same region and scanning the wound (fig. 2). We recorded both surface potential (blue) and surface topography (red) with each scan and stored a photo of the probe over the tissue when each data point was collected. A scan of the skin prior to wounding usually indicated very little voltage variation over the scan (fig. 2 top). Once a lancet wound is made, the scan looks very different with the wounded region around 200 mV more negative than neighboring regions. The slope of this surface potential distribution at any given point represents the electric field at that point and this field is typically in the range of 100-200 mV/mm.
Figure 2.
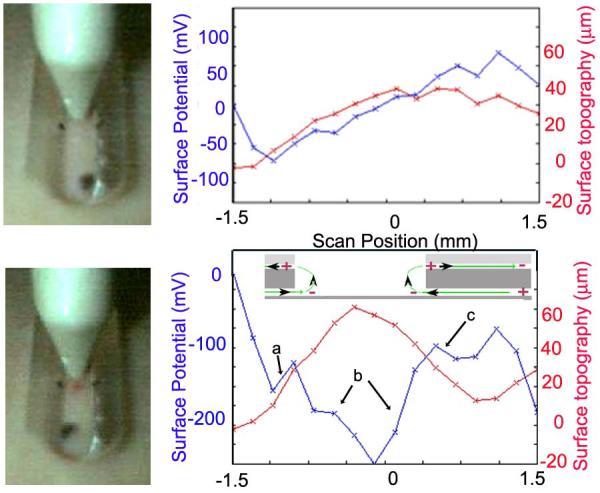
Typical Dermacorder® scan of a 3 mm long region of a 42 year-old human forearm before (top) and after (bottom) making a 0.5 mm wide lancet wound. Measurements were made in 0.2 mm increments. The electric field in a given region is the slope of the curve there: region “a” =180 mV/mm, region “b”=160 mV/mm, region “c”=90 mV/mm. The largest change in potential was always found in region “b” and that is where we usually measured the electric field. Inset in the lower figure shows the wound current pattern leading to the surface potential pattern detected. Current flows away from the wound in the space between the epidermis and stratum corneum while current flows toward the wound within and beneath the epidermis.
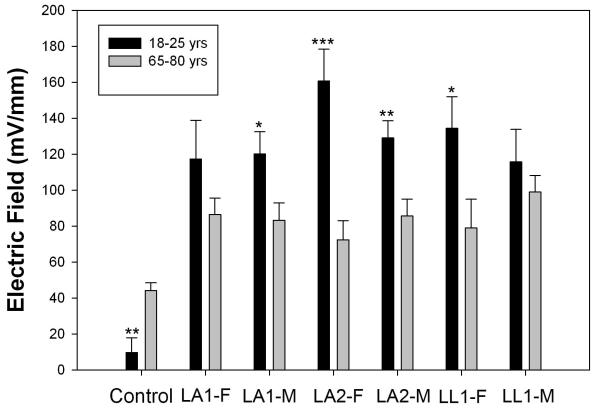
We compared the electric field near wounds in the arms and legs of the two age groups and found that the electric field near wounds in older individuals was always smaller than that in younger individuals (table 1 and fig. 3). The lateral electric field in the region between the epidermis and stratum corneum adjacent to a lancet wound in 18-25 year olds is 107-148 mV/mm, 48% larger on average than that in 65-80 year olds. We conducted a paired t-test with an alpha (type I error) of 0.05, and calculated the power of this test for concluding that there was a significant difference between the wound fields in the older and younger groups. We found the power of this test to be greater than 90% for all of the wounds except for the female LA-2 (second lancet wound made in the arm) where the power was 58% and for female leg wounds the power to detect the difference between the older and the younger ones was 25%. This wound field difference between age groups ranged from 44% to 55% with a mean of 50% and was significant in 4 of the 6 wound regions. In contrast, there was no significant difference between the wound fields in males and females.
Figure 3.
Mean electric field near fresh lancet wounds in 10 men and 10 women in each age group. Control was a scan of skin in the volar forearm prior to wounding. LA1 indicates the first lancet wound in the arm and LA2 indicates the second lancet wound a few inches away from the first. LL indicates a lancet wound in the leg. Significant differences between the mean wound fields in old and young volunteers are indicated by an asterisk above the bar. One asterisk indicates a p<0.05, two indicate p<0.01 and three indicate p<0.001. Bars represent S.E.M.
Another interesting difference was in the control scans of normal skin. Older individuals exhibited larger fluctuations in the unwounded skin’s electric fields than younger individuals. We generated these values by averaging the surface potential over the first and last millimeter of a control scan and subtracting that average from the central millimeter of the scan. For the younger group, the control scans indicated a mean field of 21 mV/mm, but the older group’s control scans indicated a field of 41 mV/mm. However the magnitudes of these fields in control skin were significantly smaller than that of the fields measured near skin wounds which averaged 131 mV/mm and 65 mV/mm in younger and older subjects, respectively.
One unexpected observation was that the field associated with the second lancet wound in the arm was always larger on average than that associated with the first wound a few cm away. This is most likely due to the inflammation generated by the first wound. Lancet wounds become inflamed very quickly and we have measured electric fields associated with inflammation alone (unpublished data). We suspect that cytokines released from white blood cells increase tight junction permeability resulting in local leakage currents that generate a lateral electric field.
Correlation between the state of wound healing and the wound’s electric field
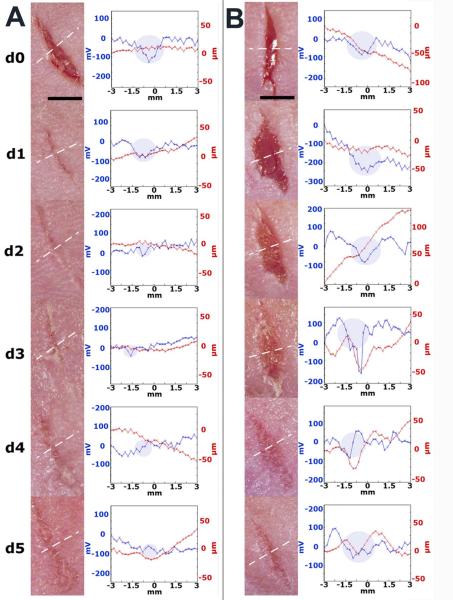
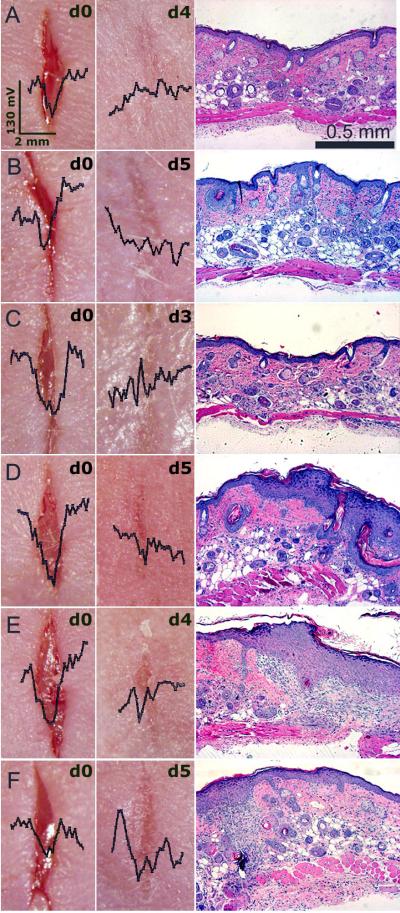
In order to study the correlation between the wound’s lateral electric field and the stage of wound healing, we made 6-8 mm long, linear, full-thickness wounds in mouse skin. These wounds were scanned daily with the Dermacorder® along a line perpendicular to the wound axis and mice were sacrificed on various days following wounding to allow removal of the wound region and generation of histological sections perpendicular to the plane of the skin. SKH-1 mice exhibited a range of wound healing rates. Some wounds healed in as little as 3 days, while others took more than 5 days to heal. A typical time course of two wounds exhibiting very different healing rates is presented in fig. 4. The wound in fig. 4A healed very quickly and did not exhibit any significant electric field by day 3 while the wound in fig. 4B still exhibited a field after 5 days of healing. By watching the captured video image associated with each scan point, we determined the scan region corresponding to the wound, indicated with a shaded disk on the graph. The red line normally indicates the surface topography. However, if the contact pressure on the skin is not uniform along the slit, it is possible to have the skin protrude into the slit more on one end than on the other. This will result in a sloped red line that is not truly indicative of the surface topography.
Figure 4.
Dermacorder® scans of two mouse skin wounds over a 5-day period. The blue line in each scan represents the surface potential distribution and the red line represents the surface topology. The shaded disks represent the region of the scan at which the Dermacorder® was over the wound. The white dotted line on the photos indicates the path of the probe as it scanned the wound. Both of these wounds had moist dressings but exhibited very different healing rates. A. Fast healing wound in which the electric field could not be detected on days 4 or 5. B. Slower healing wound in which the electric field was easily detected all five days after wounding.
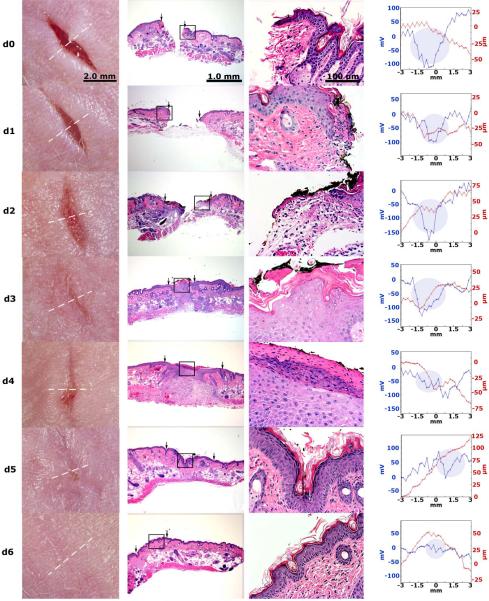
In order to provide a more detailed correlation between the wound’s electric field and wound healing, we generated histologic sections of wounds at different times after wounding to compare with the electric field measurement made just before sacrificing the mouse to remove the wounded skin for histology (fig. 5). Fifty-seven wounds were sectioned and stained with H&E at different time points in order to observe the wound morphology. On the day the wounds were created (day 0) a large gap was present between the two ends of the wound and the average lateral electric field was 122 ± 9 mV/mm (SEM, N=14). The size of this gap gradually decreased on days 1–2. Within three days after generating a full-thickness wound, the skin had closed the wound region but the degree of epidermal reorganization varied greatly. In some cases the cell layers of epidermis looked well organized and compact (fig. 6 A-C) and the mean lateral electric field was much smaller (59 ± 5 mV/mm (SEM, N=23)). In other cases, the epidermis was much less organized and much thicker (fig. 6D-F) and the mean lateral electric field was 79 ± 4 mV/mm (SEM, N=20). These less organized epithelia usually continue to exhibit a significant electric field as shown superimposed on the photos of the wounds in fig. 6. We conclude that the mean surface potential difference between the center of the wound and the two edges provides an indication of the state of wound healing. Only when the epidermis forms a tight, three-layered structure does the electric field approach control levels.
Figure 5.
Comparison of the wound’s electric field with the wound histology at different stages of wound healing. Each row represents a different wound scanned on the day after wounding indicated on the far left. Histological sections taken perpendicular to the wound axis are shown at two magnifications. The Dermacorder® scan (far right) locations are indicated by the dotted white line in the photograph and were made just before fixing the skin for histology. The shaded disk indicates the region of the scan at which the probe was over the wound. Dermacorder® scans are all on the same scale and indicate that the spatial size of the wound field declines with time along with the maximum variation in surface potential. The magnitude of the electric field (given by the slope of the potential curve) is relatively constant at 122 mV/mm until day 6 when the wound is healed.
Figure 6.
Dermacorder® scans of 6 wounds just after creating them and 3-5 days later. Histological section on the right of each wound was prepared from the excised skin wound pictured to the left of it. A-C: Wounds that formed a compact epidermal layer exhibited little to no electric field. D-F: Wounds that were more slowly healing and exhibited diffuse epidermal layers still exhibited an electric field 3-5 days after wounding. The scale bar on the wound photo in “A” applies to all of the wound photos and the scale bar in the histological section in A applies to all sections beneath it.
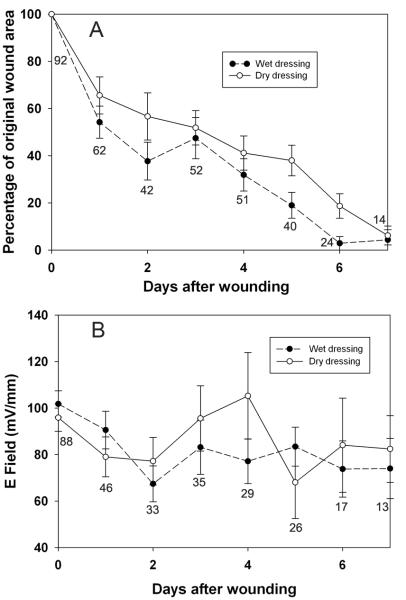
Rate of skin wound healing
After wound creation, wound areas were measured daily until the mice were sacrificed. The kinetics of wound healing for each wound varies. Scabs appeared on wounds by day 1 (N=15), day 2 (N=15) or day 3 (N=16) after wounding. By day 5, the scabs usually fell off. Most of wounds were marked with scars after wounds had completely sealed. By day 6, the wounded areas were hardly visible and often neither scars nor scabs could be observed (fig. 5). The wound area steadily decreased over time with those wounds covered in wet dressings healing significantly faster than those covered with dry dressings (fig. 7). The largest reduction in wound area was observed on day 1 after the wounds were made (59±5% of the original, n = 58). Wounds then gradually healed with an approximately 10% reduction each day on day 2 to day 7. On days 2, 5 and 6, wounds covered by wet dressings exhibited a significantly faster wound healing rate than wounds covered by dry dressings (p = 0.004) (fig. 7A). When the electric field measured near wet and dry wounds was analyzed, no consistent significant difference could be detected between the two treatments (fig. 7B).
Figure 7.
Normalized mean wound area and mean electric field during 6 days after wounding using either wet (solid circles) or dry (open circles) dressings. Each point represents the mean of the number of measurements listed below each point. Bars indicate the SEM.
Magnitude of the Dermacorder® signal can be Influenced by the liquid on the skin
Toward the end of this study we discovered that the magnitude of the Dermacorder® signal near skin wounds is sensitive to the nature of the liquid applied before placing the plastic film to the skin. We normally moisten the skin with distilled water to facilitate the adherence of the film. However, when olive oil is used instead, the Dermacorder® records a 2- to 5-fold larger surface potential difference between the wound center and the edge (fig. 8). The use of oil enhances the sensitivity of the Dermacorder® by amplifying the surface potential gradient over the wound
Figure 8.
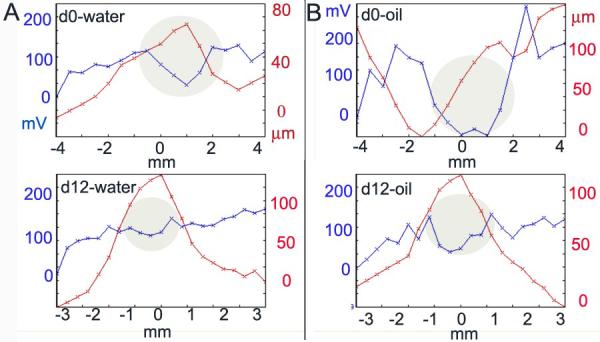
The magnitude of the Dermacorder® signal can be influenced by the dielectric constant of the fluid placed on the wound. A. Dermacorder® scan of a wound on day 0 and day 12 using water (dielectric constant = 80) under the Saran layer. B. Scan of the same wound as in “A” on the same two days using olive oil (dielectric constant = 3) under the Saran. This was repeated 5 times with similar results.
DISCUSSION
New Findings
This relatively new approach to measuring the electric field in mammalian skin provides a non-invasive indicator of the organizational state of the epidermis during wound healing. There is an excellent correlation between the magnitude of the wound’s electric field and the organization of the healing epidermis. We also find that the magnitude of the field near a skin wound falls with age in both men and women. Wounds that are kept moist heal faster than dry wounds but the electric fields associated with the two are not significantly different. Finally, the application of oil on the skin under the plastic film enhances the magnitude of the signal detected by 2- to 5-fold.
Clinical trial
The first clinical trial of the Dermacorder® on human lancet wounds revealed two interesting age-dependent differences in the electrical properties of skin. The first difference is that the scans of control skin regions in older subjects indicated significantly larger fields present than the scans of controls in young subjects. The second difference is that the field lateral to the lancet wound in young skin is 48% larger than the wound field in older skin.
Several factors could contribute to these two age-related differences in the electrical characteristics of human skin. It has been known for thirty years that both the number of cells in the epidermis and their height decreases with age11-14. This thinning of the epidermis will make it more susceptible to mechanical damage that could explain the enhanced fields in control skin measurements. Perhaps the most striking change in aging skin is the number of dermal papillae15 that decreases from 41 per mm2 to 14 per mm2. These papillae are supplying the epidermis with water and nutrients via the dermal vasculature. This reduction in nutrient supply, combined with the 62% lower blood flow16 to the ageing epidermis would result in a reduction in the ATP availability that is critical to energize the Na+/K+-ATPase at the basal end of the epidermal cells. This reduced ATPase activity would result in a lower transepidermal potential4, which would result in a reduced injury current.
The wound’s electric field as an indicator of wound closure
The TEP of 20-50 mV across all regions of our skin will drive positive current out of any skin wound immediately upon wounding. That means the wound current is the earliest signal that a wound has occurred. If the wounded region is not immersed in a conductive liquid, the only return current path available is between the stratum corneum and epidermis all around the wound. It is this return current flow beneath the stratum corneum that generates the lateral electric field detected by the Dermacorder®. This signal will remain until the healing process restores tight junctions in the epidermal layers to increase the transepidermal resistance to current flow there. We find that simply closing the wound is not sufficient to eliminate the lateral electric field (fig. 4B, 5 and 6D-F). During the maturation and remodeling phase of wound healing the epidermis must reestablish its compact structure and high resistance. Once that occurs, the wound current and consequent electric field will approach control levels (fig. 6A-C). This supports our hypothesis that when the wound’s electric field is no longer detectable, the wound healing process is complete.
The rate of wound healing is independent of the electric field strength
Our previous galvanotaxis studies with human keratinocytes indicated that the mean translocation velocity of those cells was not proportional to the imposed electric field and was fairly constant for field strengths between 50 and 400 mV/mm3. Therefore, we are not surprised that the rate of wound healing observed here in mouse wounds is not correlated with the electric field strength. However, the directionality of keratinocyte translocation in an imposed field is highly dependent on the field strength and can be quickly reversed by reversing the field direction3,17. Kucerova, et al.18 have studied wound healing in mouse corneal epithelia and disputes this claim, finding no correlation between the healing rate and the direction of wound-induced current. However, that epithelium does not exhibit the same electrical properties found in skin. Microelectrode measurements detected an electric field in the tear layer but not along the inner surface of the corneal epithelium19. Moreover, the Kucerova, et al.18 result contradicts several other studies from the same group that have reported a strong influence of electric fields on directional migration17 in vitro.
Oil versus water
We discovered recently that the Dermacorder® signal could be enhanced by coating the skin with oil instead of water prior to applying the Saran layer. This may be due to the oil diffusing into the region between the epidermis and the stratum corneum, increasing the resistance in that space which will in turn increase the lateral voltage gradient generated by the wound current in that region alone. With oil under the Saran, even a wound that had been healing for 12 days generated a detectable field. It is important to note that this increase in the electric field strength outside the epidermis does not reflect an increase in the wound current but rather reflects an increase in the resistance through which the current is flowing in that region. Therefore, it will not necessarily lead to an increase of the field within the epidermis because the resistance in that region is independent of the resistance between the epidermis and stratum corneum.
We also confirmed the well-known property of moist wounds to heal faster than dry ones20-22. We were interested in determining if the electric field near moist wounds would be larger than that near dry wounds; however, we were unable to detect a consistent difference between the wet and dry wound fields. While the fields near dry or moist wounds are similar, the current density could be significantly larger in moist wounds due to the higher conductivity. It is possible that it is this higher current density that is the critical factor for faster wound healing.
It is worth mentioning the conclusion from our earlier paper2 that we would expect the electric field within the multilayered epidermis to be smaller than that measured here between the epidermis and stratum corneum. Due to the larger area through which the current can flow within the three cell layers comprising the epidermis, we estimate that the maximum electric field within that layer is about one-fourth of the field measured by the Dermacorder®.
In summary, we conclude that the Dermacorder® can be used to non-invasively determine the degree of wound healing that has occurred in a given wound. By monitoring either the peak-to-peak surface potential or the electric field at the wound edge, one can tell if the wound has formed a compact epidermal layer with high resistance characteristic of a fully healed wound.
ACKNOWLEDGMENTS
We thank Dr. Antoinette Hood and David Vendt for their help with the clinical trial design and data collection, respectively. This study was supported by NIH R44 GM069194 to RN.
REFERENCE LIST
- 1.Barker AT, Jaffe LF, Vanable JW., Jr. The glabrous epidermis of cavies contains a powerful battery. Am J Physiol. 1982;242:R358–R366. doi: 10.1152/ajpregu.1982.242.3.R358. [DOI] [PubMed] [Google Scholar]
- 2.Nuccitelli R, Nuccitelli P, Ramlatchan S, Sanger R, Smith PJ. Imaging the electric field associated with mouse and human skin wounds. Wound Repair Regen. 2008;16(3):432–41. doi: 10.1111/j.1524-475X.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimura KY, Isseroff RR, Nuccitelli R. Human keratinocytes migrate to the negative pole in direct current electric fields comparable to those measured near mammalian wounds. J Cell Sci. 1995;109:199–207. doi: 10.1242/jcs.109.1.199. [DOI] [PubMed] [Google Scholar]
- 4.Foulds IS, Barker AT. Human skin battery potentials and their possible role in wound healing. Br J Dermatol. 1983;109:515–22. doi: 10.1111/j.1365-2133.1983.tb07673.x. [DOI] [PubMed] [Google Scholar]
- 5.Nuccitelli R. A role for endogenous electric fields in wound healing. Curr Top Dev Biol. 2003;58:1–26. doi: 10.1016/s0070-2153(03)58001-2. [DOI] [PubMed] [Google Scholar]
- 6.DuBois-Reymond E. Vorlaufiger abrifs einer untersuchung uber den sogenannten froschstrom und die electomotorischen fische. Ann Phys U Chem. 1843;58:1. [Google Scholar]
- 7.Illingworth CM, Barker AT. Measurement of electrical currents emerging during the regeneration of amputated fingertips in children. Clin Phys Physiol Meas. 1980;1:87–9. [Google Scholar]
- 8.Jaffe LF, Nuccitelli R. An ultrasensitive vibrating probe for measuring extracellular currents. J Cell Biol. 1974;63:614–28. doi: 10.1083/jcb.63.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGinnis ME, Vanable JW., Jr. Electrical fields in Notophthalmus viridescens limb stumps. Dev Biol. 1986;116:184–93. [Google Scholar]
- 10.Iglesia DDS, Cragoe EJ, Jr., Vanable JW., Jr. Electric field strength and epithelization in the newt (Notophthalmus viridescens) J Exp Zool. 1996;274:56–62. doi: 10.1002/(SICI)1097-010X(19960101)274:1<56::AID-JEZ6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 11.Marks R. Measurement of biological ageing in human epidermis. Br J Dermatol. 1981;104(6):627–33. doi: 10.1111/j.1365-2133.1981.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 12.Diridollou S, Vabre V, Berson M, Vaillant L, Black D, Lagarde JM, Gregoire JM, Gall Y, Patat F. Skin ageing: changes of physical properties of human skin in vivo. Int J Cosmet Sci. 2001;23(6):353–62. doi: 10.1046/j.0412-5463.2001.00105.x. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharyya TK, Thomas JR. Histomorphologic changes in aging skin: observations in the CBA mouse model. Arch Facial Plast Surg. 2004;6(1):21–5. doi: 10.1001/archfaci.6.1.21. [DOI] [PubMed] [Google Scholar]
- 14.Neerken S, Lucassen GW, Bisschop MA, Lenderink E, Nuijs TA. Characterization of age-related effects in human skin: A comparative study that applies confocal laser scanning microscopy and optical coherence tomography. J Biomed Opt. 2004;9(2):274–81. doi: 10.1117/1.1645795. [DOI] [PubMed] [Google Scholar]
- 15.Sauermann K, Clemann S, Jaspers S, Gambichler T, Altmeyer P, Hoffmann K, Ennen J. Age related changes of human skin investigated with histometric measurements by confocal laser scanning microscopy in vivo. Skin Res Technol. 2002;8(1):52–6. doi: 10.1046/j.0909-752x.2001.10297.x. [DOI] [PubMed] [Google Scholar]
- 16.Petrofsky JS, McLellan K, Bains GS, Prowse M, Ethiraju G, Lee S, Gunda S, Lohman E, III, Schwab E. The influence of ageing on the ability of the skin to dissipate heat. Med Sci Monit. 2009;15(6):CR261–CR268. [PubMed] [Google Scholar]
- 17.Zhao M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin Cell Dev Biol. 2009;20(6):674–82. doi: 10.1016/j.semcdb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Kucerova R, Walczysko P, Reid B, Ou J, Leiper LJ, Rajnicek AM, McCaig CD, Zhao M, Collinson JM. The role of electrical signals in murine corneal wound re-epithelialization. J Cell Physiol. 2011;226(6):1544–53. doi: 10.1002/jcp.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiang M, Robinson KR, Vanable JW., Jr. Electrical fields in the vicinity of epithelial wounds in the isolated bovine eye. Exp Eye Res. 1992;54:999–1003. doi: 10.1016/0014-4835(92)90164-n. [DOI] [PubMed] [Google Scholar]
- 20.Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293–4. doi: 10.1038/193293a0. [DOI] [PubMed] [Google Scholar]
- 21.Hinman CD, Maibach H. Effect of air exposure and occlusion on experimental human skin wounds. Nature. 1963;200:377–8. doi: 10.1038/200377a0. 377-8. [DOI] [PubMed] [Google Scholar]
- 22.Field FK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167(1A):2S–6S. doi: 10.1016/0002-9610(94)90002-7. [DOI] [PubMed] [Google Scholar]