SUMMARY
The phenotypic attributes and molecular determinants for the regeneration of bone marrow (BM) sinusoidal endothelial cells (SECs) and their contribution to hematopoiesis are unknown. We show that after myelosuppression VEGFR2 activation promotes reassembly of regressed SECs, reconstituting hematopoietic stem and progenitor cells (HSPCs). VEGFR2 and VEGFR3 expression are restricted to BM vasculature, demarcating a continuous network of VEGFR2+VEGFR3+Sca1− SECs and VEGFR2+VEGFR3−Sca1+ arterioles. While chemotherapy (5FU) and sublethal irradiation (650 rad) induce minor SEC regression, lethal irradiation (950 rad) induces severe regression of SECs requiring BM transplantation (BMT) for regeneration. Conditional deletion of VEGFR2 in adult mice blocks regeneration of SECs in sublethally irradiated animals, preventing hematopoietic reconstitution. Inhibition of VEGFR2 signaling in lethally irradiated wild type mice rescued with BMT severely impairs SEC reconstruction, preventing engraftment and reconstitution of HSPCs. Therefore, activation of VEGFR2 is critical for regeneration of VEGFR3+Sca1− SECs that are essential for engraftment and restoration of HSPCs and hematopoiesis.
INTRODUCTION
The functional and structural basis of blood cell development lies within the bone marrow (BM), consisting of several microenvironments including the osteoblastic niche (OBN). The OBN is believed to maintain the quiescence of stem cells. Although the phenotypic and functional attributes of the OBN are well defined (Arai et al., 2004; Calvi et al., 2003; Hattori et al., 2002; Heissig et al., 2002; Kiel et al., 2005; Yoshihara et al., 2007; Zhang et al., 2003) the identity of the vascular compartments in BM contributing to hematopoietic stem and progenitor cell (HSPC) homeostasis is unknown. During hematopoietic recovery after myelosuppression, HSPCs might interact with BM vasculature (Avecilla et al., 2004; Kiel et al., 2005; Kopp et al., 2005b; Takakura et al., 2000), a process that could be important for engraftment of HSPCs and reconstitution of hematopoiesis. However, as the phenotypic signature and molecular pathways involved in maintenance and regeneration of the BM vasculature are unknown, the role of neoangiogenesis in the regulation of HSPCs remains to be elucidated.
Currently, the BM vasculature is loosely identified as a network of thin walled, fenestrated sinusoidal endothelial cells (SECs) and smooth muscle invested arterioles. SECs have a unique expression of adhesion molecules, allowing the trafficking and homing of HSPCs to the BM (Kataoka and Tavassoli, 1985; Naiyer et al., 1999; Rafii et al., 1995; Rafii et al., 1994; Sipkins et al., 2005, Kollet et al., 2006, Slayton et al., 2007). SECs consist solely of a basal lamina interposed between a single layer of endothelial cells (ECs) and are devoid of typical pericytic cells (Kopp et al., 2005b), but are believed to be surrounded by SDF1 expressing adventitial cells (Kiel and Morrison, 2006; Sugiyama et al., 2006) and/or F4/80+ clusters of myeloid cells (Sapoznikov et al., 2008). It is hypothesized that the integrity of SECs is supported by surrounding hematopoietic cells (Kopp et al., 2005a). However, this dependence is reciprocal in that SECs provide not only a conduit for mature hematopoietic cells to the circulation but also a cellular platform whereby HSPCs differentiate and set the stage for hematopoietic reconstitution (Avecilla et al., 2004; Mohle et al., 1997; Rafii et al., 1995; Rafii et al., 1994).
Studying the BM microvasculature has been hampered due to technical difficulties of working with calcified tissues. Furthermore, the angiogenic pathways regulating SEC maintenance and regeneration are unknown. Thrombospondins deployed from megakaryocytes were identified as negative regulators of BM SECs, however the positive regulators of SECs are unknown (Kopp et al., 2006). In particular, the contribution of VEGF receptors, VEGFR2 and VEGFR3, in supporting BM angiogenesis has not been investigated.
Herein, we established reproducible techniques for staining decalcified BM sections and for performing polyvariate flow cytometric analyses on BM, allowing the definition of an angiogenic profile of BM vasculature distinguishing between arterial vessels and SECs. We show that VEGFR3 is selectively expressed by SECs, but not arterioles, permitting the tracking and quantification of SECs. We demonstrate that, although at steady state VEGFR2 is dispensable for maintenance of SECs and HSPCs, after myelosuppressive insult lack of VEGFR2 activation results in impaired regeneration of VEGFR3+Sca1− SECs and HSPCs. Therefore, VEGFR2 is essential for orchestrating the engraftment and restoration of HSPC populations and reconstitution of hematopoiesis.
RESULTS
Bone marrow vasculature is demarcated by VEGFR2+VEGFR3+ SECs
For the first time, we were able to reproducibly immunophenotype BM vessels by modifying standard immunohistochemical (IHC) (Supp. Fig. 1) and immunofluorescence (IF) protocols and employing polyvariate flow cytometry. We identified angiogenic signatures and the precise geometric localization of BM arterioles and SECs using a panel of antibodies (Abs) against vascular specific markers, including but not limited to VE-cadherin, VEGFR2, VEGFR3, CD31, MECA32, and Sca1 (Fig. 1A, Supp. Table 1). Furthermore, we genetically tracked the expression of VEGFR2 and VEGFR1 in reporter mice, where the expression of gfp and lacZ are driven by the endogenous VEGFR2 promoter (VEGFR2-GFP mice) (Ema et al., 2006) (Fig. 1B–D) and VEGFR1 promoter (VEGFR1-lacZ mice) (Fong et al., 1999), respectively (Fig. 1Ac).
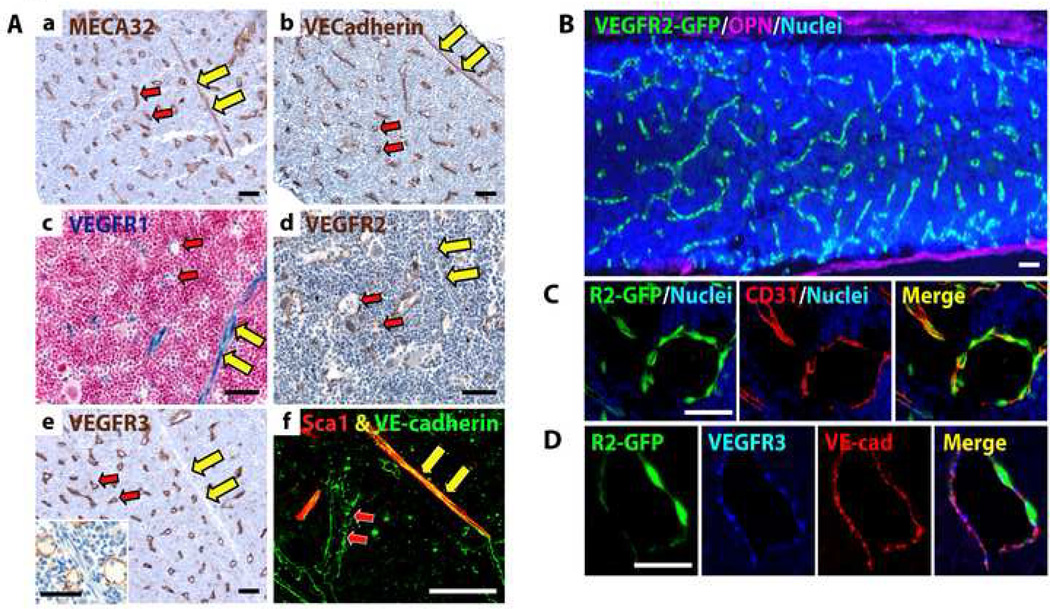
Figure 1. BM SECs are phenotypically marked as VEGFR2+VEGFR3+Sca1−, while arterioles are VEGFR2+VEGFR3−Sca1+.
The expression of VEGF receptors was determined by IHC of BM sections in WT mice and via genetic tracking in VEGFR1-lacZ and VEGFR2-GFP mice. A) Paraffin-embedded femoral sections from WT mice were stained with Abs for (a) MECA32, (b) VE-cadherin, (d) VEGFR2, (e) VEGFR3. High power image demonstrates the specificity of VEGFR3 expression to SECs and not arterioles (e, inset). (c) Femurs from VEGFR1-lacZ mice were processed for βgal activity. (f) Fixed-frozen BM sections were coimmunostained for Sca1 (red) and VE-cadherin (green). Arterioles are designated by yellow arrows, SECs by red arrows. B) Fluorescent microscopy of fixed-frozen femoral BM sections from VEGFR2-GFP mice stained for osteopontin (OPN, purple). Note VEGFR2-GFP+ vasculature (green). C–D) VEGFR2-GFP BM sections were coimmunostained for (C) CD31 (red) or (D) VEGFR3 (blue) and VE-cadherin (red), demonstrating selective expression of VEGFR2 on CD31+ and VE-cadherin+VEGFR3+ SECs. Nuclei are shown in blue. Micron bar = 50 µm.
Using this approach, we found that in steady state conditions the vasculature of femurs from C57BL/6J wild type (WT) mice consists of small arterioles and capillaries that span the BM and supply radially and regularly distributed SECs (Fig. 1A, B). SECs are an interconnected network of vessels arising from cortical capillaries and encompass the major BM vascular compartment that interacts with hematopoietic cells (Supp. Movie 1). At steady state, both arterioles and SECs were immunopositive for VE-cadherin, MECA32, VEGFR2 and CD31 (Fig. 1A–D, Supp. Table 1). β-galactosidase (βGal) staining of BM from VEGFR1-lacZ mice indicated that VEGFR1 is expressed in both arterioles and SECs (Fig. 1Ac, Supp. Table 1). IHC of BM from WT mice (Fig. 1Ae, f) and confocal IF of BM from VEGFR2-GFP mice (Fig. 1D) revealed that BM SECs are specifically VEGFR3+ and Sca1−, while arterioles are VEGFR3− and Sca1+. In order to exclude the possibility that VEGFR3+ vessels are of lymphatic origin, we stained femurs for the lymphatic marker LYVE1 and found that the BM is devoid of LYVE1+ lymphatics (data not shown).
Based on these studies, we designated an immunophenotypic signature for steady state BM SECs as VE-cadherin+MECA32+CD31+VEGFR2+VEGFR3+Sca1− while BM arterioles were identified as VE-cadherin+ MECA32+CD31+VEGFR2+VEGFR3−Sca1+ (Fig. 1, Supp. Table 1). Therefore, differential expression of Sca1 and VEGFR3 can be used to identify BM arterioles and SECs.
VEGFR2 and VEGFR3 expression in BM is restricted to SECs
Previous uncorroborated studies have suggested that non-EC populations might express VEGFR2 and VEGFR3. To resolve this issue, we used IF and polyvariate flow cytometry. Osteopontin (OPN) IF on BM from VEGFR2-GFP mice demonstrated that there is no overlap between VEGFR2-GFP+ cells and OPN+ osteoblasts (OBs) (Fig. 1B). Co-IF on BM from VEGFR2-GFP mice shows that VEGFR2-GFP+ cells, but not OPN+ OBs, are CD31+ and VE-cadherin+VEGFR3+ (Fig. 1B–D). To confirm that VEGFR2 expression is restricted to SECs, we performed polyvariate flow cytometry on BM mononuclear cells from crushed and enzymatically processed femurs. We show that VEGFR2-GFP+ cells are comprised of CD31+VEGFR2+CD45−CD11b−TER119− SECs, lacking expression of hematopoietic markers (Fig. 2A and data not shown). In order to prove that VEGFR3 is expressed exclusively by functional SECs, we injected WT mice with fluorophore conjugated Isolectin GS-IB4 to identify functional and patent SECs. Using a similar polyvariate flow cytometric approach, we demonstrated that VEGFR3 is expressed on functional CD45−VE-cadherin+Isolectin GS-IB4+ SECs (Fig. 2B). In addition, cKit+Lineage−Sca1+ HSPCs (KLS) were VEGFR3−, suggesting that VEGFR3 expression within the BM is restricted to SECs (Fig. 2C).
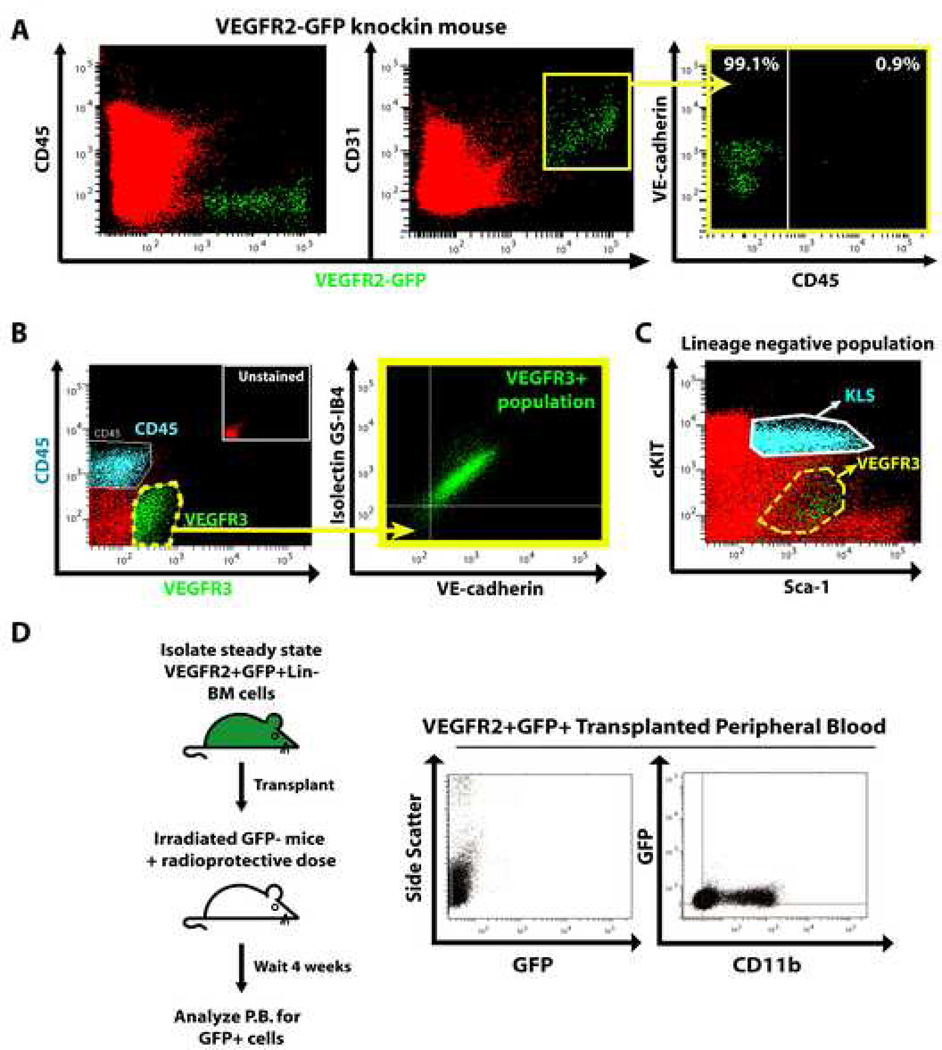
Figure 2. VEGFR2 and VEGFR3 are primarily expressed on functional SECs.
Whole BM from femurs/tibias of VEGFR2-GFP mice was prepared by disruption and enzymatic digestion. Mononuclear cells, excluding TER119+ cells, were analyzed by polyvariate flow cytometry. A) BM VEGFR2-GFP+ cells are CD45− and co-express CD31. CD31+VEGFR2-GFP+ cells express varying levels of VE-cadherin and are CD45 negative (99.1%). B) Prior to BM isolation, WT mice were injected i.v. with Isolectin GS-IB4 to identify patent/functional vessels. Harvested BM cells were stained with for CD45, VEGFR3 and VE-cadherin. CD45 and VEGFR3 staining are mutually exclusive. Virtually all VEGFR3+ cells co-express VE-cadherin and bound Isolectin GS-IB4. C) Polyvariate analysis of BM from WT mice demonstrating that the majority of KLS population (HSPCs, blue) lacks VEGFR3 (highlighted in green within the dashed yellow line). D) Five thousand VEGFR2+GFP+Lin−BM cells harvested from β-actin transgenic mice were transplanted into groups of lethally irradiated mice (n=9) with a radioprotective dose of WT GFP−BM. After 4 weeks PB was assessed for the presence of GFP+ cells. No GFP+ cells were detected in the PB from any of the transplanted mice.
Nonetheless, it is possible that subsets of hematopoietic repopulating cells may be VEGFR2+. To rule this out, we purified VEGFR2+GFP+lineage− cells from β-actin-GFP mice, where GFP is universally expressed, and transplanted these cells into lethally irradiated mice with a radioprotective dose of GFP− cells. Consistent with the previously published report (Haruta et al., 2001), VEGFR2+GFP+lineage− cells failed to repopulate lethally irradiated mice (Fig. 2D), whereas VEGFR2−GFP+ cells reconstituted host hematopoiesis (data not shown). Moreover, we demonstrate that in Col2.3GFP reporter mice, where expression of GFP is driven by the 2.3kb fragment of the collagen alpha 1 type I promoter and is restricted to OBs (Dacic et al., 2001), VEGFR2 and VEGFR3 were not expressed by GFP+ OBs (Fig. 3D). Collectively, these data suggest that the expression of VEGFR2 and VEGFR3 is restricted to SECs, and even if VEGFR2 is expressed on a small population of non-SECs it does not serve any important hematopoietic function. Therefore, interference with VEGFR2 or VEGFR3 signaling has no direct effect on the reconstitution of OBs or hematopoietic cells.
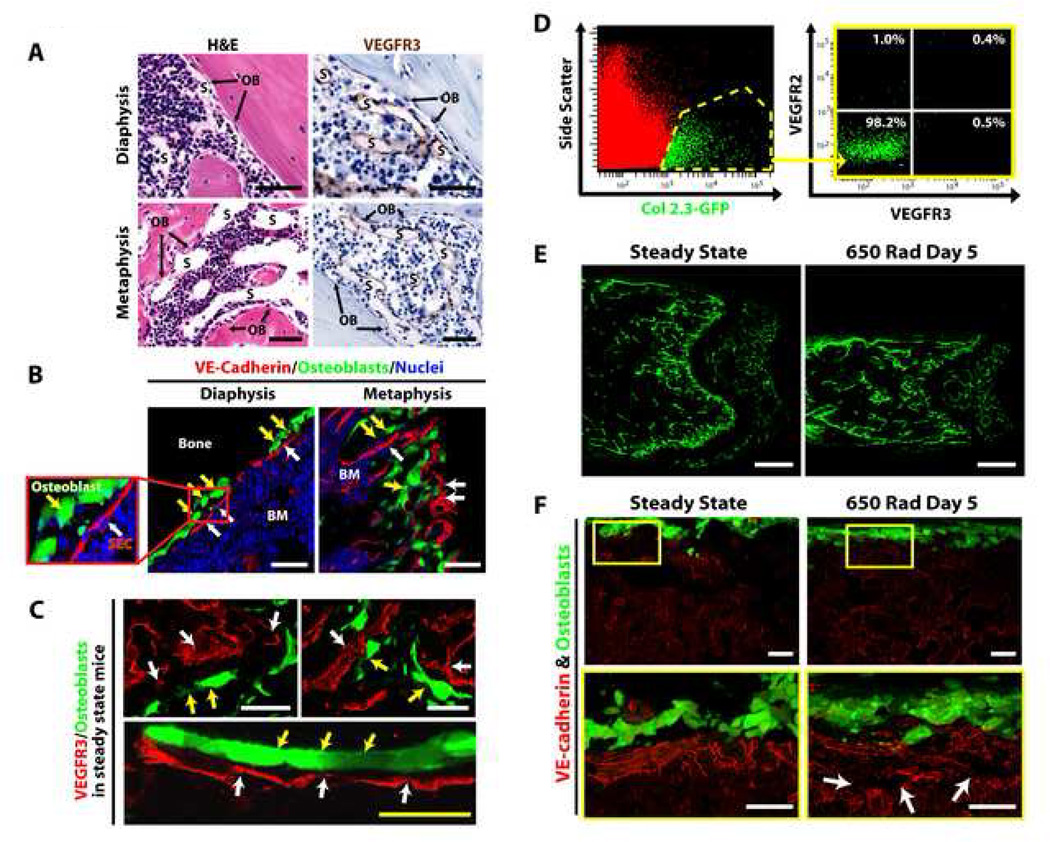
Figure 3. VEGFR2−VEGFR3− OBs are preserved within the BM of irradiated Col2.3GFP mice.
A) H&E and anti-VEGFR3 IHC of femurs from WT mice demonstrating the localization of VEGFR3+ SECs (S) in close apposition to osteoblasts (OBs) in both the diaphyseal and metaphyseal marrow. B–C) Co-IF for VE-cadherin (red) (B) and VEGFR3 (red) (C) in steady state Col2.3GFP mice BM showing the proximity of the SECs (white arrows) to GFP+ OBs (yellow arrows). D) Polyvariate flow cytometry of BM from steady state Col2.3GFP mice indicating that GFP+ OBs lack the expression of VEGFR2 and VEGFR3. E) Low power confocal micrographs of femurs from Col2.3GFP mice at steady state and at day 5 after 650 rad demonstrates persistence of GFP+ OBs after irradiation. F) IF for VE-cadherin (red) demonstrating alteration of the BM vasculature (white arrows) after irradiation, while the structural integrity of GFP+ OBs is maintained. White micron bar = 50 µm, yellow micron bar = 20 µm.
Irradiation remodels SECs but not OBs
It has been speculated, but not corroborated, that the OBN and BM SECs are spatially separate and functionally distinct entities. To resolve this controversy, we employed VEGFR3 and VE-cadherin as specific markers of BM vasculature, to determine the physical location of BM SECs in relation to OBs. Hematoxylin and eosin (H&E) and anti-VEGFR3 IHC demonstrated that BM SECs are always in close proximity or directly apposed to OBs (Fig. 3A). Consistent with these results, in Col2.3GFP mice, both in the diaphysis and metaphysis, GFP+ OBs reside in direct apposition to VE-cadherin+VEGFR3+ SECs (Fig 3B–C). Three-dimensional imaging confirms that OBs are invested by VEGFR3+ SECs (Supp. Movie 2). After treating Col2.3GFP mice with 650 rad, there is a disruption of VE-cadherin+ vessels while the physical integrity of GFP+ OBs is maintained (Fig. 3E–F). These data suggest that VEGFR3−VEGFR2− OBs (Fig. 3D) and VE-cadherin+VEGFR3+ BM SECs form a continuous compound cellular niche and there is no major regression of GFP+ OBs after irradiation.
Degree of injury to SECs is dictated by the extent of myelosuppressive injury
As shown in Figure 3F, irradiation induces a significant alteration in the BM vasculature. Thus, to define molecular pathways involved in BM SEC regeneration, we employed three models of myelosuppression. This allowed examination of the consequences of mild, moderate and severe regression of SECs on reconstitution of hematopoiesis. First, we used the 5-fluorouracil (5FU, 250 mg/kg) model of myelosuppression, which induces mild regression of SECs (Fig. 4A–C). To induce moderate and severe vascular regression in the BM we treated mice with sublethal (650 rad) or lethal (950 rad) irradiation, respectively. We observed an incremental loss of SECs in WT mice treated with 950 rad > 650 rad > 5FU as quantified by costaining with EC specific markers (Fig. 4A–C). The severe regression of SECs in mice irradiated with lethal 950 rad dose was rescued with BM transplantation (BMT) with 5×105 whole BM cells (950 rad + BMT) (Fig. 4C), providing an ideal model to test the role of regeneration of SECs in reconstitution of hematopoiesis.
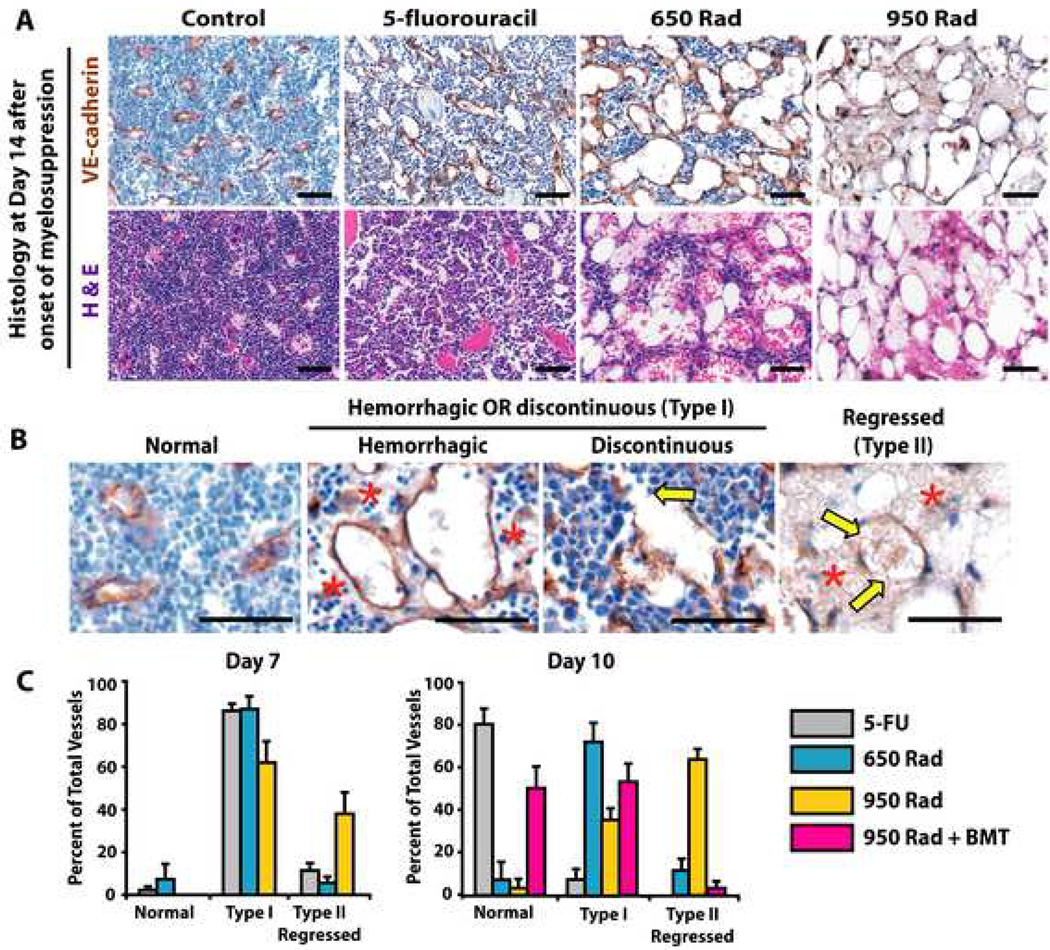
Figure 4. Magnitude of myelosuppression correlates with the extent of damaged Type I and Type II SECs.
WT mice were treated with 250 mg/kg 5FU i.v. 650 rad, 950 rad and allowed to recover. A cohort of 950 rad treated mice were transplanted with 5×105 whole BM cells (950 rad + BMT). Femurs were harvested at day 7, 10 and 14 and were processed in paraffin for IHC with BM EC markers, including VE-cadherin & VEGFR3. A) Day 14 H&E and anti-VE-cadherin IHC of the models of myelosuppression. B) Prototypical examples of day 14 VE-cadherin+ normal, discontinuous or hemorrhagic (Type I), and hemorrhagic/discontinuous and EC denuded (Type II/regressed) SECs. The yellow arrows represent the discontinuity in the vessel wall of the injured and regressed SECs, while the red star highlights the areas of profuse hemorrhage detected adjacent to damaged SECs. C) Quantitation of Type I and Type II vessels in femoral BM of 5FU, 650 rad, 950 rad and 950 rad + BMT mice analyzed at day 7 and 10 post myelosuppressive insult. Y-axis represents the percent of normal, Type I, and Type II vessels quantitated in three 200X fields. Micron bar = 50 µm.
To categorize and quantify the severity and degree of damage to regenerating SECs in each model of myelosuppression, we devised a staging system (Fig. 4B, C). At days 7 and 10 after treatment of mice with 5FU, 650 rad or 950 rad with or without BMT, two types of abnormal SECs were evident. During the early recovery after 5FU and early and late phases of recovery after 650 rad, there was a predominance of Type I vessels presenting as discontinuous or hemorrhagic vessels (Fig. 4B, C). Although by day 10 the SECs in 5FU treated mice were primarily non-hemorrhagic and non-discontinuous, in mice treated with 650 rad and more prominently with 950 rad (without BMT rescue) SECs were discontinuous and hemorrhagic with denuded ECs from vessel walls, designated as Type II regressed vessels (Fig 4 B, C). There were no normal vessels present in the femurs of mice irradiated with 950 rad without BMT and by day 10 there was no evidence of vascular regeneration of the regressed vessels (Fig. 4C). However, in 950 rad + BMT treated mice, there was significant vascular recovery with emergence of normal and Type I vessels and significant reduction of Type II regressed vessels (Fig. 4C). These data suggest that SECs are malleable and dynamic vessels with potential to regenerate and remodel into functional SECs. However, the molecular pathways driving the regeneration and assembly of regressed SECs are unknown. We hypothesized they may involve the activation of VEGF receptors, given their significant expression by BM SECs (Figs. 1–3).
Regeneration of SECs is dependent on activation of VEGF/VEGFR2 pathway
Using IHC and mean fluorescent intensity (MFI) measurements of whole BM cells, we show that VEGFR2 and VEGFR3 are variably expressed by remodeling SECs in 5FU treated mice (Supp. Fig. 2A, Supp. Table 1) and regressing BM SECs in mice treated with 950 rad at day 5 (Supp. Fig. 2B, Supp. Table 1). Although there is a slight, yet significant, down regulation of VEGFR3 expression during early stages of hematopoietic recovery, there is a robust upregulation of VEGFR2 expression on day 5 post irradiation, as determined by MFI of VEGFR2 and VEGFR3 expression on CD45−VE-cadherin+ CD31+Sca1− SECs (Supp. Fig. 2Ba,b, Supp. Table 1). Furthermore, qPCR on BM from 5FU treated mice demonstrated that VEGF-C and VEGF-A are significantly upregulated at varying times after myelosuppression (Supp. Fig. 2Ca, Cb), guaranteeing activation of VEGFR2 during hematopoietic recovery. VEGF-A protein was upregulated in BM plasma from 650 rad treated mice as compared to untreated animals (Supp. Fig. 2D). These data suggest that VEGFR2 and VEGFR3 and their cognate ligands are present and variably expressed during hematopoietic recovery, and may play a role in the reconstruction of regressed SECs without influencing the function of OBs.
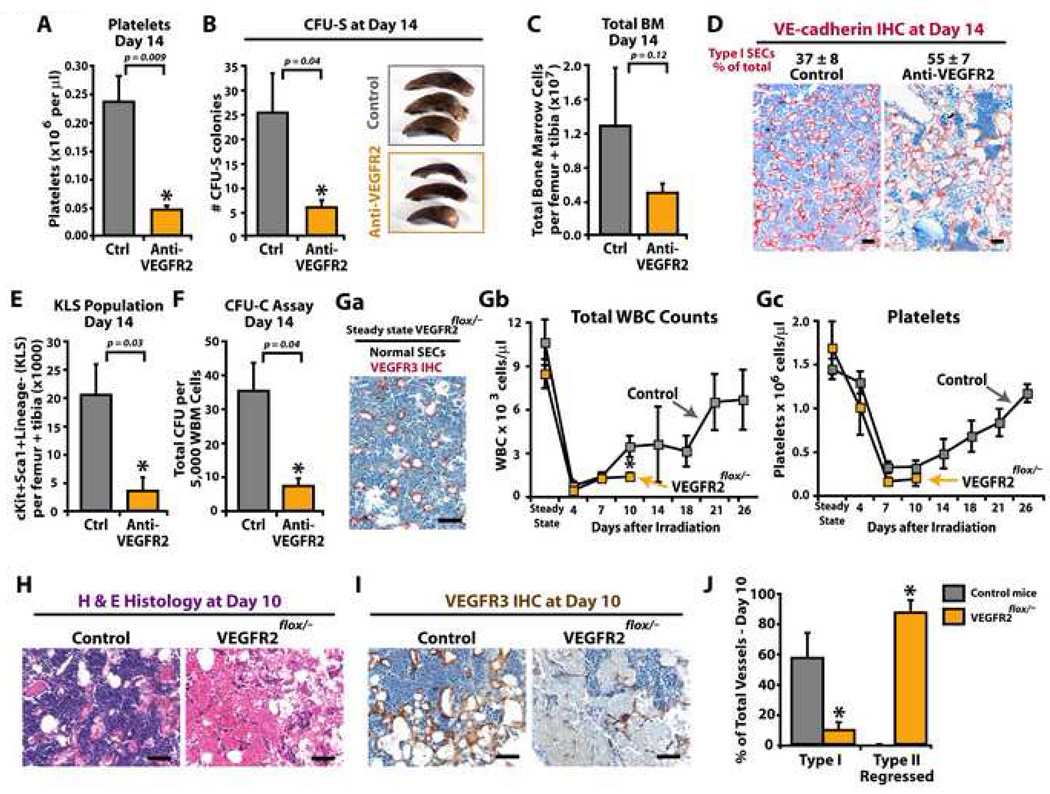
To test this hypothesis, WT mice, myelosuppressed with 5FU, 650 rad or 950 rad + BMT, were treated with neutralizing monoclonal antibodies (mAb) against VEGFR2 (DC101) or VEGFR3 (mF4-31C1) (Prewett et al., 1999; Pytowski et al., 2005; Tammela et al., 2008). Treatment of 5FU myelosuppressed mice with mAb to either VEGFR2 or VEGFR3 had only marginal effect on delaying vascular recovery within the BM (data not shown). However, when 650 rad treated mice were injected with mAb to VEGFR2, there was a significant reduction in HSPC reconstitution. On day 14 after irradiation, mice treated with mAb to VEGFR2, but not the control IgG group, showed significant thrombocytopenia (Fig. 5A), reduced number of spleen colony forming units (CFU-S) (Fig. 5B) and BM cell counts (Fig. 5C). Defects in BM cytoarchitecture with a predominance of disorganized, leaky and hemorrhagic (Type I vessels) VE-cadherin+ SECs was observed in BM from VEGFR2 mAb treated mice even at 14 days post-treatment (Fig. 5D). Additionally, there was a profound decrease in KLS population (Fig. 5E) and CFU-Cs (Fig. 5F). These data suggest that blocking VEGFR2 signaling during BM regeneration after myelosuppression interferes with proper assembly of SECs, impairing hematopoiesis.
Figure 5. Impaired regeneration of SECs after 650 rad irradiation results in failure of hematopoietic reconstitution.
The consequence of 650 rad in the reconstitution of hematopoiesis was evaluated in WT (A–F) and VEGFR2 conditional knockdown (VEGFR2flox/−) mice (G–J). A–F) WT mice were treated with 650 rad with and without neutralizing mAb to VEGFR2 (DC101, 800 µg/dose thrice weekly). At day 14, PB, spleens and BM were harvested for evaluation of (A) platelets, (B) CFU-spleen forming units (CFU-S), (C) total BM mononuclear cells, and (D) IHC quantification of Type I VE-cadherin+ vessels. E–F) BM mononuclear cells were evaluated for KLS population (E), and colony forming units (CFU-C) (F). G–J) Groups of ROSA-CreERT2VEGFR2e3loxP/− mice and control WT and ROSA-CreERT2VEGFR2e3loxP/+ mice were treated with tamoxifen and allowed to recover. Mice were given 650 rad, and hematopoietic recovery and vessel integrity were monitored. Ga) Steady state untreated femurs from VEGFR2flox/− mice were immunostained with anti-VEGFR3 Abs and vascular integrity of SECs (normal versus Type I/II vessels) was assessed. Virtually all vessels in VEGFR2flox/− mice prior to irradiation are normal SECs. Gb-c) WBC (Gb) and platelets (Gc) were counted at designated time points. At steady state conditions after tamoxifen treatment, there is no difference between the WBC and platelets counts as compared to non irradiated controls. H–J) At day 10, femurs were harvested and SEC status was evaluated using H&E (H) and anti-VEGFR3 IHC (I). J) VEGFR3 stained BM sections were assessed for Type I and Type II SECs. Although the control animals consistently show signs of SEC recovery, VEGFR2 knockdown animals have a profound persistence in Type II regressed SECs. Micron bar = 50 µm.
Conditional deletion of VEGFR2 gene in adult mice does not affect steady state hematopoiesis
Neutralizing mAb to VEGFR2 blocks only the paracrine/extrakine activation of VEGFR2. As VEGFR2 can be activated by both extrakine and intrakine loops in ECs (Lee et al., 2007), we generated mice with floxed VEGFR2 alleles to interrogate the role of complete VEGFR2 deficiency on the reconstitution of HSPCs in steady state conditions and after myelosuppression in adult mice. We targeted exon 3 of vegfr2 gene, which does not contain any known regulatory elements, to generate VEGFR2e3loxP/e3loxP mice (Supp. Fig. 3A,B). VEGFR2e3loxP/e3loxP mice were crossed with VEGFR2+/− mice to generate VEGFR2e3loxP/− mice, which were crossed with ROSA-CreERT2 transgenic mice, to establish the ROSA-CreERT2 VEGFR2e3loxP/− mice and ROSA-CreERT2VEGFR2e3loxP/+ controls (Supp. Fig. 3C). Tamoxifen treatment of these mice yielded VEGFR2flox/− and control VEGFR2flox/+ mice. VEGFR2flox/− mice have complete knockdown of VEGFR2 in BM as determined by real time PCR of genomic DNA for VEGFR2 gene excision (Supp. Fig. 3D) and IHC for VEGFR2 protein (Supp. Fig. 3E). There were no defects in steady state hematopoiesis in VEGFR2flox/− mice, with white blood cells (WBCs) and platelets remaining at normal levels several weeks after tamoxifen treatment (Fig. 5Gb,c). Furthermore, at steady state VEGFR3+ SECs in VEGFR2flox/− mice were phenotypically normal (Fig. 5Ga). This is consistent with results in which WT mice at steady state but chronically treated with mAb to VEGFR2 (or VEGFR3), did not show any hematological defects during and after treatment (Supp. Fig. 4). These data suggest that, although during embryonic development deletion of VEGFR2 (Ema et al., 2006; Shalaby et al., 1995) results in angiogenic defects, at steady state conditional deletion of VEGFR2 in adult mice does not affect hematopoiesis.
Reconstitution of SECs and hematopoiesis is impaired in irradiated VEGFR2-deficient mice
To determine whether inhibition of both paracrine and intrakine VEGFR2 signaling is more effective in blocking regeneration of SECs and imparting a more severe hematopoietic impairment, VEGFR2flox/− mice were sublethally challenged with 650 rad. As compared to control mice, there was a significant delay in WBC (Fig. 5Gb) and platelet (Fig. 5Gc) recovery in VEGFR2flox/− mice, with persistent life-threatening pancytopenia on day 10 post irradiation. Furthermore, there was a severe defect in recovery of VEGFR3+ SECs resulting in the appearance of Type II regressed vessels (Fig. 5H–J). These data suggest that loss of both VEGFR2 intrakine and extrakine signaling results in a considerable impairment in SEC regeneration and defects in hematopoietic reconstitution. Therefore, the extent of damage to SECs and the degree of VEGFR2 inhibition dictate the rate of reconstruction of SECs and hematopoietic recovery.
VEGFR2 is essential for engraftment and reconstitution of HSPCs after severe myelosuppression
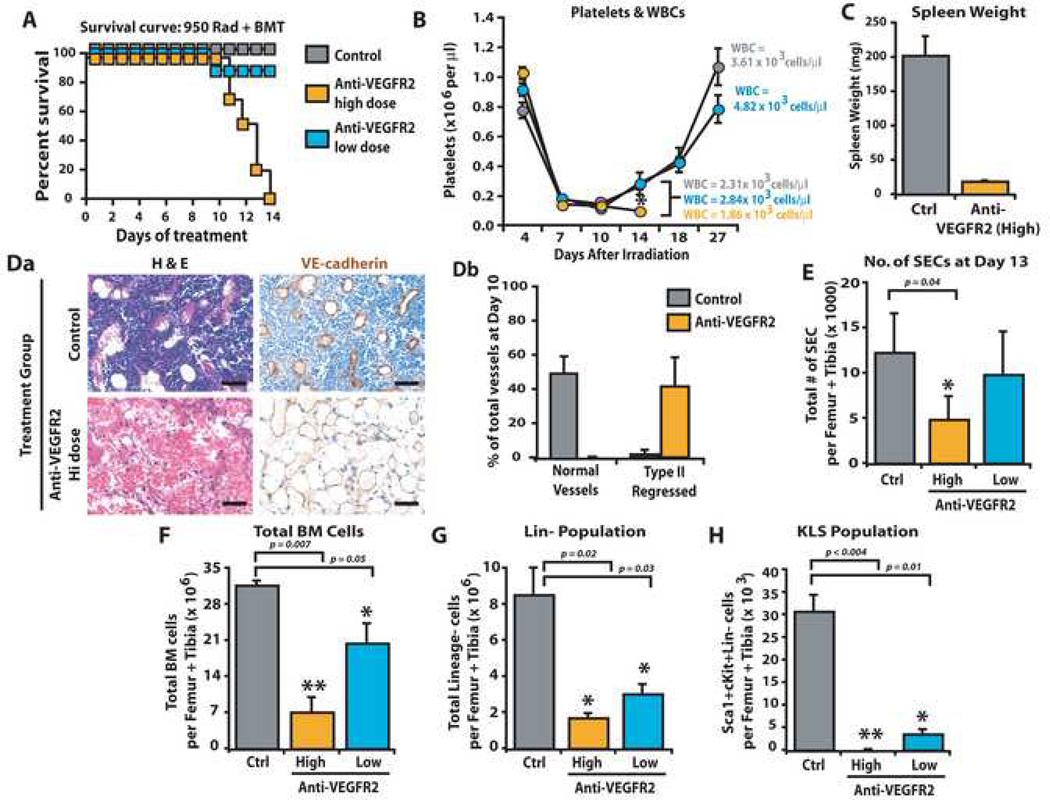
To evaluate the significance of VEGFR2 activation on regeneration of SECs and restoration of HSPC function after myelosuppression, we studied the effect of varying VEGFR2 inhibition in a HSPC transplantation model. We employed the 950 rad + BMT treated mouse model since in this model transplantation of HSPCs induces rapid regeneration of the Type II regressed SECs (Fig. 4C) allowing interrogation of the role of VEGFR2 inhibition in mediating SEC regeneration and HSPC engraftment. WT mice were lethally irradiated with 950 rad followed by transplantation with HSPCs and were treated with low dose (400 µg/dose, thrice weekly) and high dose (800 µg/dose, thrice weekly) mAb to VEGFR2. In mice treated with 950 rad + BMT in combination with high dose mAb to VEGFR2 there was a significant inhibition in hematopoietic recovery, resulting in demise of the mice (n=10, P<0.05) (Fig. 6A). The majority of mice treated with high dose, but not low dose, mAb to VEGFR2 succumbed to BM suppression due to persistent thrombocytopenia and leukopenia (Fig. 6B). Treatment of mice with high dose mAb to VEGFR2 prevented rebound splenomegaly (Fig. 6C) and abrogated the regeneration of normal VE-cadherin+ vessels as the BM was primarily populated with discontinuous, hemorrhagic, denuded, regressed Type II SECs (Fig. 6Da, Db).
Figure 6. Engraftment and replenishment of HSPCs require VEGFR2 dependent regeneration of regressed SECs.
WT mice were treated with 950 rad + BMT in combination with mAb to VEGFR2 (DC101) at high dose (800 µg/dose) or low dose (400 µg/dose), or control IgG, thrice weekly. At day 13, PB, BM and spleen were processed for analyses. A) Survival plot for mice given 950 rad + BMT in combination with anti-VEGFR2 mAb treatment (low and high, blue and orange, respectively) vs. control (grey) demonstrating that 100% of mice treated with high dose mAb succumbed to hematopoietic failure even after BMT (n=10, P<0.001). B) Platelet and WBC counts are decreased in mice treated with high dose (n=6, P<0.006 as compared to low dose, orange circles), but not low dose, mAb to VEGFR2 (blue circles) or IgG control (gray circles). C) As compared to IgG control mice, irradiated 950 rad + BMT treated mice injected with high dose, but not low dose, anti-VEGFR2 mAb showed a marked decrease in splenic mass (n=6, P<0.001). D) IHC for BM vessels using VE-cadherin (Da) reveals a predominance of Type II VE-cadherin+ regressed BM vessels (Db) (n=5, P<0.04). E) Total number of SECs was quantified by polyvariate flow cytometry. Calculating the total number of VEGFR3+VEGR2+VE-cadherin+CD31+CD45−CD11b−Sca1− vessels demonstrated a reduction in SECs in high dose mAb treated mice vs. control (n=3 P<0.04). F–H) Treatment of 950 rad + BMT irradiated mice with high dose or low dose mAb to VEGFR2, but not control IgG, resulted in a statistically significant decrease in total BM mononuclear cells (F, P<0.007 for high dose, P<0.05 for low dose), decrease in Lin− cells (G, P<0.02 for high dose, P<0.03 for low) and virtually absent KLS (H, P<0.004) in high dose and diminished KLS (P<0.01) in low dose treated mice. Micron bar = 50 µm.
Using polyvariate flow cytometry, we quantitatively assessed the number of regressed VEGFR3+ SECs in the 950 rad + BMT mice treated with anti-VEGFR2 mAb. On day 13 after 950 rad + BMT treatment the absolute number of VEGFR3+Sca1− SECs, quantified by the number of VEGFR2+VEGFR3+VE-cadherin+CD31+Sca1−CD45−CD11b−Ter119− SECs, was diminished in mice treated with high dose mAb to VEGFR2 but not low dose mAb treatment (Fig. 6E) or controls. The failure of hematopoietic recovery in mice treated with high dose mAb to VEGFR2 was due to a profound decrease in total hematopoietic cells and lineage− (Lin−) cells (Fig. 6F, G). In particular, the KLS population was reduced to a virtually undetectable level (Fig. 6H). Although control mice had 30,744 KLS cells, high dose anti-VEGFR2-treated mice had only 135 KLS and low dose anti-VEGFR2 treated mice had 3,568 KLS cells (Fig. 6H). These data suggest that inhibition of VEGFR2, selectively expressed by SECs but not OBs, prevents regeneration of damaged SECs thereby interfering with engraftment of HSPCs and replenishment of KLS population leading to hematopoietic failure in an OB-independent manner (Schema Fig. 7).
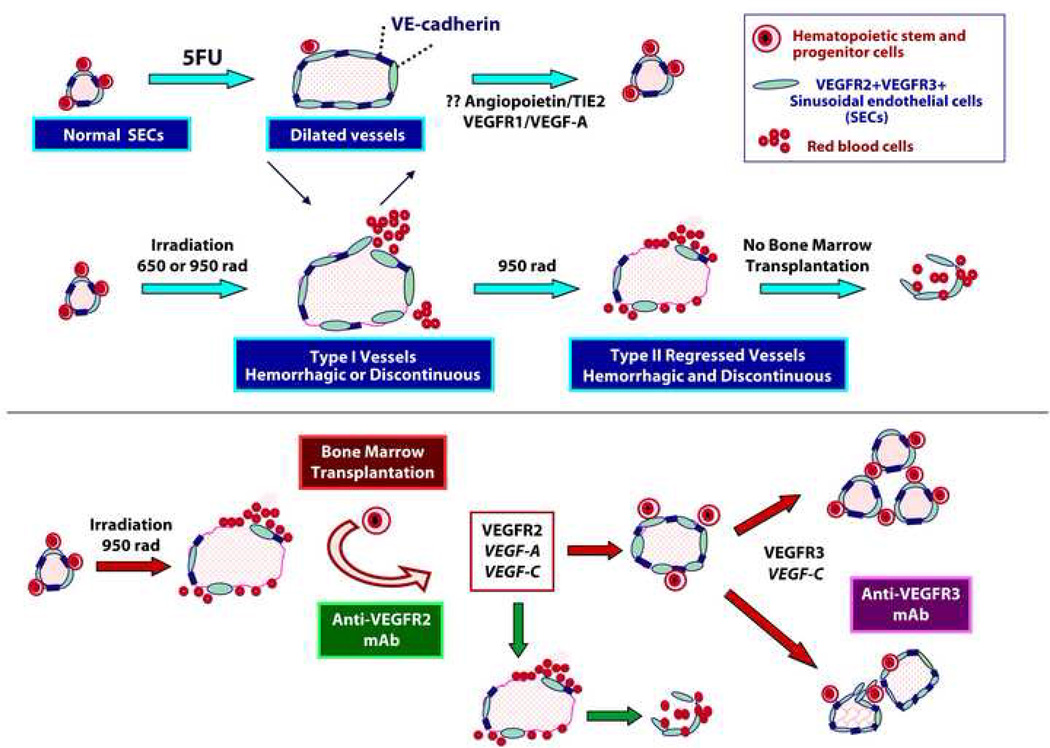
Figure 7. Schema depicting the effect of VEGFR2 and VEGFR3 inhibition in the generation of Type I and Type II vessels and reconstitution of HSPCs.
Interfering with VEGFR2 signaling in mice treated with potent myelosuppressive insults, such as 650 to 950 rad results in significant impairment in the reconstruction of regressed SECs, while inhibition of VEGFR3 results in a defect in patterning of SECs. VEGFR2 inhibition impairs BMT induced regeneration of SECs resulting in the failure of engraftment and reconstitution of HSPCs. Regeneration of SECs after 5FU may be driven by VEGFR2 independent but VEGFR1 and Tie2 dependent regeneration of hemangiogenic cells (Hattori et al., 2002; Kopp et al., 2005).
Blocking VEGFR3 in mice treated with 950 rad + BMT does not affect regeneration of BM vasculature but interferes with the proper remodeling of neovessels leading to the formation abnormal predominantly Type I vessels (Supp. Fig. 5E) along with a significant decrease in the Lin− and CFU-C populations (Supp. Fig. 5B,D). In 950 rad + BMT mice treated with mAb to VEGFR3, there is a trend in reduction of KLS population at day 10 (Supp. Fig. 5C). Thus, after severe myelosuppressive insult extrakine activation of the VEGF-C/VEGFR3 signaling pathway is not essential for regeneration of Type II regressed vessels, but instead regulates proper patterning of regenerating VEGFR2+ SECs, thereby, accelerating progenitor cell (CFU) expansion thereby fine tuning hematopoietic reconstitution.
DISCUSSION
The first evidence illustrating a critical role for SECs in the regulation of hematopoiesis was the finding by our group that revascularization of the BM after myelosuppression fully restored thrombopoiesis in thrombopoietin-deficient mice (Avecilla et al., 2004). These data demonstrated that regeneration of functional SECs is sufficient for lineage-specific differentiation of hematopoietic progenitors. However, uncertainties in spatial location, angiogenic determinants and phenotypic attributes of BM vasculature have evaded functional definition due to technical barriers of working with calcified tissues and the non static nature of the hematopoietic system (Kopp et al., 2005b; Kopp et al., 2006). Using technical advances in BM preparation, we provide comprehensive phenotypic and functional data establishing a molecular signature of BM SECs at steady state and during hemangiogenic regeneration. We demonstrate that at steady state BM SECs, representing the predominant vascular surface area in BM, are a continuous network of interconnected VE-cadherin+ VEGFR2+VEGFR3+Sca1− vessels, while smooth muscle invested arterioles are VE-cadherin+ VEGFR2+VEGFR3−Sca1+. We show that the extent and rate of SEC regeneration after myelosuppression dictate the degree of hematopoietic recovery. Severe myeloablation induced by 650 or 950 rad irradiation does not significantly disrupt OBs, but leads to regression of VEGFR2+VEGFR3+Sca1− SECs and the magnitude of the regression correlates with the severity in hematopoietic failure. Delay in VEGFR2 dependent recovery of SECs results in failure of HSPC expansion leading to life threatening pancytopenia. These data indicate that timely VEGFR2 dependent reassembly of SECs after severe myeloablative regression is critical for reconstitution of HSPCs and hematopoiesis.
We demonstrate that virtually every OB is in close apposition to SECs establishing a compound OB-SEC niche. The close association of SECs with OBs suggests that targeting VEGFR2 and VEGFR3 may interfere with hematopoiesis through a non-SEC mediated mechanism. Indeed, the expression of VEGFR2 and VEGFR3 might not be limited to vascular cells within BM (Gerber et al., 2002; Haruta et al., 2001). To address this issue, we tracked VEGFR2+ SECs in VEGFR2-GFP mice and OBs in Col2.3GFP mice to show that VEGFR2 is restricted to SECs, and not OBs or other non-EC compartments in BM. In agreement with Haruta et al (Haruta et al., 2001) we demonstrate that even if a subset of VEGFR2+ cells contains an unrecognized hemangiogenic cell type, it does not serve a hematopoietic function as transplantation of VEGFR2+ cells fails to engraft lethally irradiated mice. Therefore, targeting VEGFR2 will only interfere with the regeneration of the SECs but not OBs or other non-ECs in BM.
At steady state conditional deletion of VEGFR2 in adult mice has no observable effect on the integrity of BM SECs, while after irradiation there is a failure in BM SEC regeneration when VEGFR2 signaling is impaired. These data compliment the report of Lee et al that demonstrates the constitutive deletion of VEGF-A autocrine signaling in surviving adult mice manifested no apparent hematological defects (Lee et al., 2007). As Lee et al did not evaluate the role of VEGF-A/VEGFR2 autocrine loop after myelosuppression, it remains to be determined whether the intrakine VEGF-A pathway plays a dominant role in the regulation of hematopoietic recovery. Nonetheless, we show that a mAb specific for the extracellular domain of VEGFR2 blocks engraftment and abrogates reconstitution of HSPCs after irradiation, indicating that VEGFR2 extrakine activation contributes to the engraftment and restoration of hematopoiesis. Collectively, our data propose that both paracrine and autocrine activation of VEGFR2 plays a seminal role in the reassembly of functional SECs after myelosuppression.
How does myelosuppression switch quiescent VEGFR2 independent SECs to initiate a VEGFR2 dependent neoangiogenic program? The release of pro- and anti- angiogenic factors by hematopoietic cells might dictate not only the quiescent state but also the regeneration potential of SECs. For example, myelosuppression may induce OBs and megakaryocytes (Mohle et al., 1997) to secrete VEGF-A. Once hematopoietic recovery is complete mature cells, such as polyploid megakaryocytes, release anti-angiogenic factors including thrombospondins (Kopp et al., 2006), which suppress angiogenesis temporizing any further increase in hematopoietic activity.
In this study, we distinguished SECs from BM arterioles. Given the low numbers of VEGFR3−Sca1+ arterioles, it is difficult to assess the contribution of arterioles to hematopoiesis. However, as arterioles may participate in regulating homing of transplanted HSPCs identifying the pathways involved in sustaining their stability may shed light on homing receptors that capture circulating HSPCs. The angiogenic factors required for maintenance and regeneration of BM arterioles are not known and require further investigation.
Until now, BM vasculature has been defined by its morphological attributes and there are no consistent molecular definitions of the vascular compartments within BM. Arterioles and SECs have been interchangeably referred to as the BM vascular niche without a unified approach to define their functional contribution to hematopoiesis. As such, our data will not only resolve the confusion pervading the BM angiogenesis but also sets forth a mechanistic language to define and identify the relevant BM vasculature compartments. Quantification of VEGFR2+VEGFR3+Sca1− SECs will provide predictive surrogate biomarkers to assess responses to anti-angiogenic therapy and evaluate the degree of HSPC and hematopoietic reconstitution after myelosuppression.
Accumulating evidence suggests that the vasculature within each organ is heterogeneous and is tailored to serve the physiological requirements of that organ. Here, we show that BM SECs represent a network of malleable vessels requiring reciprocal interaction with hematopoietic cells for rapid VEGFR2 dependent regeneration, orchestrating engraftment and replenishment of HSPCs. We also set forth the concept that selective targeting of SECs via VEGFR2 inhibition, which does not affect the physical integrity of the OBs, is sufficient to block hematopoietic recovery suggesting that OBs most likely do not play a significant role in hematopoietic reconstitution after myeloablation. Therefore, therapeutic administration of angiogenic factors may provide a novel means to accelerate reconstitution of stabilized functional VEGFR2+VEGFR3+Sca1− SECs thereby avoiding fatal complications associated with chemotherapy and irradiation induced pancytopenia.
MATERIALS AND METHODS
Animals
C57BL/6J (WT) and C57BL/6J-Tg (ACTbEGFP)10sb/J mice were obtained from Jackson Laboratories (Bar Harbor, ME). VEGFR1-lacZ heterozygous mice were a gift from Dr. Guo-Hua Fong (Fong et al., 1999). Floxed VEGFR2 (VEGFR2e3loxP/e3loxP) mice were generated by Dr. Thomas Sato. Col2.3GFP mice were obtained from Dr. David Rowe (Dacic et al., 2001). VEGFR2-GFP mice were from Dr. Janet Rossant (Ema et al., 2006; Shalaby et al., 1995). Animal experiments were performed with authorization from the Institutional Animal Care and Use Committee of Weill Cornell Medical College using age and sex matched animals.
BM and organ histology and IHC
Tissues were fixed in 2–4% paraformaldehyde (PFA). Femurs were decalcified using Decalcifying Solution (Richard-Allan Scientific, MI) and frozen in OCT Compound (Sakura Finetek, CA) or embedded in paraffin. Paraffin sections were stained with hematoxylin and eosin (H&E, Histoserv, MD). For detection of VE-cadherin, MECA32, VEGFR2 and VEGFR3, paraffin sections were antigen retrieved using Target Retrieval Solution (DAKO, CA). In some cases, IHC for VEGFR2 was performed on frozen sections. After endogenous peroxidase and non specific protein block (5% BSA, 10% donkey serum, 0.02% Tween-20) primary Abs were incubated overnight at 4°C as follows: anti-VEGFR3 mAb (AFL4, 2 µg/ml, BD, CA), anti-pan EC antigen mAb (MECA32, 10 µg/ml, BD), anti-VE-cadherin polyclonal Ab (pAb, 1 µg/ml, R&D Systems, MN), anti-VEGFR2 pAb (T014, 10 µg/ml, gift from Dr. Phil Thorpe), anti-VEGFR2 mAb (Avas12α1, 10 µg/ml, BD). After secondary pAb and streptavidin horseradish peroxidase incubations (Jackson IR, PA) staining was developed with DAB+ or AEC+ (DAKO) and briefly counterstained in Mayer’s hematoxylin (DAKO).
VEGFR1-lacZ staining
Adult VEGFR1-lacZ mice were perfused with 0.2% glutaraldehyde. Femurs were post fixed for 4 hours (h) at 4°C, decalcified in 10% EDTA, cryoprotected and sectioned in OCT at 12 µm. Sections were incubated in X-Gal staining solution at 37°C for 12–16 h and counterstained with Nuclear Fast Red (Biomeda, CA).
BM IF and detection of GFP
WT, VEGFR2-GFP and Col2.3GFP mice were perfused with 4% PFA. Femurs were post fixed, decalcified in 10% EDTA, cryoprotected and snap frozen in OCT. 12–30 µm sections were blocked (5% donkey serum/0.3% Triton X-100) and incubated in primary Ab: anti-VEGFR3 mAb (mF4-31C1, 10µg/ml, ImClone Systems, NY, or AFL4, 4 µg/ml), anti-VE-cadherin pAb (2 µg/ml), anti-Sca1/Ly-6A/E mAb (D7, 10 µg/ml, BD). After incubation in fluorophore conjugated secondary antibodies (1 µg/ml, Jackson IR) sections were counterstained with TOPRO3, DAPI or Propidium Iodide (Molecular Probes/Invitrogen, CA).
Flow cytometric analyses, identification and quantification of BM SECs and arterioles
Purified mAbs were conjugated to Alexa Fluor dyes or Qdots per manufacturer's protocols (Molecular Probes/Invitrogen). Whole BM cells from WT, VEGFR2-GFP and Col2.3GFP animals were mechanically denuded of muscle/connective tissue, crushed in a sterile mortar and digested in collagenase, while agitating at 37°C for 45 min. Stained cells were analyzed on LSRII-SORP (BD). Data was processed with FACSDiva 6.1 software (BD). Doublets were excluded by FSC-W × FSC-H and SSC-W × SSC-H analysis, single stained channels were used for compensation, and fluorophore minus one (FMO) controls were used for gating. Fluorescent IgG controls were used to exclude non specific Ab binding. mAbs were purchased from BD except where noted: VE-cadherin (BV13, ImClone); VEGFR3 (mF4-31C1); VEGFR2 (DC101, ImClone, and Avas12α1); Sca1 (E13-161.7), CD45 (30-F11), CD11b (M1/70); TER119 (TER119); CD31 (MEC13.3); cKit (ACK45); Gr-1 (RB6-8C5); CD41 (MWREG30); CD3 (17A2); CD45 B220 (RA3-6B2).
For quantification of SECs, bones were mechanically prepared as above and the number of SECs was quantified by costaining with conjugated antibodies to VEGFR2, VEGFR3, VE-cadherin, CD31, CD45, CD11b, Ter119, and Sca1. Number of SECs equals the number of VEGFR2+VEGFR3+VE-cadherin+ CD31+Sca1−CD45−CD11b−Ter119− cells. VEGFR2+VEGFR3−VE-cadherin+ CD31+Sca1+CD45−CD11b−Ter119− cells were scored as arterioles and were not counted in the total number of SECs.
Intensity of VEGFR2 and VEGFR3 expression was determined by calculating the Mean Fluorescent Intensity (MFI) with FACSDiva software.
Isolation and transplant of VEGFR2+Lin− and VEGFR2−Lin− population
Total BM from C57BL/6J-Tg (ACTbEGFP)10sb/J mice was magnetically depleted of mature cells (Miltenyi Biotech, CA). The VEGFR2+ fraction from the Lin− population was sorted using FACS Aria flow sorter (BD). Five thousand VEGFR2+Lin− cells were transplanted into lethally irradiated (950 rad) WT mice with a radioprotective dose of 1×106 WT total BM cells. Control VEGFR2−Lin−GFP+ cells were also transplanted. After 4 weeks the number of circulating GFP+ cells in PB from transplanted mice was evaluated by flow cytometry.
Myelosuppression models
Mice received one of three different myelosuppression strategies: 1) a single intravenous (i.v.) injection of 5-fluorouracil (5FU, 250 mg/kg body weight); 2) 650 rad irradiation; or 3) 950 rad irradiation, either alone or followed by i.v. administration of 5×105 whole BM cells (BMT). Where indicated, cohorts of mice were treated with 400 µg/dose or 800 µg/dose of neutralizing mAb to VEGFR2 (DC101), or 800 µg/dose of neutralizing mAb to VEGFR3 (mF4-31C1), or control IgG (Jackson IR) intraperitoneally (i.p.) thrice weekly until the time points indicated in the text or until moribund.
Image acquisition and image analysis
Histological and IHC images of BM sections were captured with AxioCam and AxioVision software (Zeiss, NY) mounted on Olympus BX51 microscope (Olympus America, NY). IF images were captured on AxioVert LSM510Meta confocal microscope (Zeiss). Selected images were rendered with Volocity 4 3D imaging software (Improvision, MA). Digital images were analyzed for SEC (VE-cadherin+ and/or VEGFR3+) integrity as described in figure legends using ImageJ (NIH, MD) and Adobe Photoshop (Adobe, San Jose, CA). Type I and Type II vessels were assessed and quantified by visual examination of three 200X images per femur, spanning the BM cavity. Discontinuous or hemorrhagic SECs were scored as Type I abnormal SECs. Discontinuous, hemorrhagic and denuded vessels were scored as Type II regressed vessels. VEGFR3−Sca1+ vessels or vessels that were phenotypically apparent arterioles due to smooth muscle investment were not included as normal, Type I or Type II SECs.
Peripheral blood analysis
Retro-orbital peripheral blood (PB) was collected using micro-hematocrit capillary tubes (Fisher Scientific, PA). WBC counts with differential, hemoglobin, calculated hematocrit and platelets were obtained using Bayer Advia 120 Multi-Species Hematology Analyzer (Bayer HealthCare, NY) with multi-species software (Bayer).
Isolation and analysis of cKit+lineage−Sca1+/KLS population
Total BM cells were isolated from myelosuppressed WT mice treated with anti-VEGFR2 mAb, anti-VEGFR3 mAb or control IgG and were enriched using Lineage Cell Depletion Kit (Miltenyi), yielding >95% purity. Lin− cells were stained with anti-Sca1 (D7, BD) and anti-cKit (2B8, BD) and analysis was performed on LSRII SORP using FACSDiva 6.1 software for data processing. Doublets were excluded by FSC-W × FSC-H and SSC-W × SSC-H analysis and single stained channels were used for compensation.
CFU-S assay
Spleens from mice treated with anti-VEGFR2 or control were removed at day 12–14 after myelosuppression, fixed in Bouin’s solution (Sigma Aldrich, MO), briefly washed in PBS, weighed and CFU-S were counted and/or imaged on a Zeiss Discovery stereo microscope (Zeiss).
Generation of inducible VEGFR2 knockdown mice
Exon 3 of vegfr2 was targeted to generate the VEGFR2e3loxP/e3loxP mice (Supp. Fig. 3). VEGFR2e3loxP/e3loxP mice were bred with VEGFR2+/−mice to generate VEGFR2e3loxP− mice. VEGFR2e3loxP/− were bred with ROSA-CreERT2 transgenic mice to establish the ROSA-CreERT2 VEGFR2e3loxP/− line (and controls ROSA-CreERT2VEGFR2e3loxP/+). These mice were treated with tamoxifen at a dose of 5 mg/150 ml sunflower oil (Sigma) i.p. for 6 days interrupted for 3 days after the 3rd dose. After 3 days of respite, the 4th dose was re-instituted for an additional 3 days. Mice were allowed to recover a minimum of 15 days before experiments commenced.
Statistical Analyses
Results were analyzed using Student’s t-test. P less than 0.05 was considered significant. Results are expressed as mean value ± SEM.
Supplementary Material
ACKNOWLEDGEMENTS
SR and TNS are funded by NIH. SR is supported by Howard Hughes Medical Institute and Ansary Stem Cell Institute. A.K. was supported in part by Deutsche Forschungsgemeinschaft (KR 2154/2-1). We thank Mollie Goetz, Keith Kobylarz and Allison Spencer for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Dacic S, Kalajzic I, Visnjic D, Lichtler AC, Rowe DW. Col1a1-driven transgenic markers of osteoblast lineage progression. J Bone Miner Res. 2001;16:1228–1236. doi: 10.1359/jbmr.2001.16.7.1228. [DOI] [PubMed] [Google Scholar]
- Ema M, Takahashi S, Rossant J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood. 2006;107:111–117. doi: 10.1182/blood-2005-05-1970. [DOI] [PubMed] [Google Scholar]
- Fong GH, Zhang L, Bryce DM, Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development. 1999;126:3015–3025. doi: 10.1242/dev.126.13.3015. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- Haruta H, Nagata Y, Todokoro K. Role of Flk-1 in mouse hematopoietic stem cells. FEBS Lett. 2001;507:45–48. doi: 10.1016/s0014-5793(01)02921-0. [DOI] [PubMed] [Google Scholar]
- Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, Hicklin DJ, Zhu Z, Bohlen P, Witte L, et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med. 2002;8:841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires mmp-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka M, Tavassoli M. Identification of lectin-like substances recognizing galactosyl residues of glycoconjugates on the plasma membrane of marrow sinus endothelium. Blood. 1985;65:1163, 1171. [PubMed] [Google Scholar]
- Kiel MJ, Morrison SJ. Maintaining hematopoietic stem cells in the vascular niche. Immunity. 2006;25:862–864. doi: 10.1016/j.immuni.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM Family Receptors Distinguish Hematopoietic Stem and Progenitor Cells and Reveal Endothelial Niches for Stem Cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005a;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- Kopp HG, Avecilla ST, Hooper AT, Shmelkov SV, Ramos CA, Zhang F, Rafii S. Tie-2 activation contributes to hemangiogenic regeneration after myelosuppression. Blood. 2005b doi: 10.1182/blood-2004-11-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp HG, Hooper AT, Broekman MJ, Avecilla ST, Petit I, Luo M, Milde T, Ramos CA, Zhang F, Kopp T, et al. Thrombospondins deployed by thrombopoietic cells determine angiogenic switch and extent of revascularization. J Clin Invest. 2006;116:3277–3291. doi: 10.1172/JCI29314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XM, Hu Z, Jorgenson ML, Wingard JR, Slayton WB. Bone marrow sinusoidal endothelial cells undergo nonapoptotic cell death and are replaced by proliferating sinusoidal cells in situ to maintain the vascular niche following lethal irradiation. Exp Hematol. 2008;36:1143–1156. doi: 10.1016/j.exphem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Mohle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci U S A. 1997;94:663–668. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiyer AJ, Jo DY, Ahn J, Mohle R, Peichev M, Lam G, Silverstein RL, Moore MA, Rafii S. Stromal derived factor-1-induced chemokinesis of cord blood CD34(+) cells (long-term culture-initiating cells) through endothelial cells is mediated by E-selectin. Blood. 1999;94:4011–4019. [PubMed] [Google Scholar]
- Prewett M, Huber J, Li Y, Santiago A, O'Connor W, King K, Overholser J, Hooper A, Pytowski B, Witte L, et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, Skobe M, Boardman KC, Swartz MA. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97:14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- Rafii S, Shapiro F, Pettengell R, Ferris B, Nachman RL, Moore MA, Asch AS. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood. 1995;86:3353–3363. [PubMed] [Google Scholar]
- Rafii S, Shapiro F, Rimarachin J, Nachman RL, Ferris B, Weksler B, Moore MA, Asch AS. Isolation and characterization of human bone marrow microvascular endothelial cells: hematopoietic progenitor cell adhesion. Blood. 1994;84:10–19. [PubMed] [Google Scholar]
- Sapoznikov A, Pewzner-Jung Y, Kalchenko V, Krauthgamer R, Shachar I, Jung S. Perivascular clusters of dendritic cells provide critical survival signals to B cells in bone marrow niches. Nat Immunol. 2008;9:388–395. doi: 10.1038/ni1571. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Sipkins DA, Wei X, Wu JW, Runnels JM, Cote D, Means TK, Luster AD, Scadden DT, Lin CP. In vivo imaging of specialized bone marrow endothelial microdomains for tumor engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayton WB, Li XM, Butler J, Guthrie SM, Jorgensen ML, Wingard JR, Scott EW. The Role of the Donor in the Repair of the Marrow Vascular Niche Following Hematopoietic Stem Cell Transplant. Stem Cells. 2007 doi: 10.1634/stemcells.2007-0158. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, Satake M, Suda T. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 2000;102:199–209. doi: 10.1016/s0092-8674(00)00025-8. [DOI] [PubMed] [Google Scholar]
- Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.