Abstract
Neonatal exposure to antidepressants produces lasting impairments in male sexual behavior. Although perturbation of the serotonin system during neonatal life has been implicated in the long-term behavioral effects of neonatal antidepressant exposure, dose-response studies were necessary to confirm that inhibition of the serotonin transporter during the neonatal period is sufficient to produce impairments in sexual behavior. Therefore, the present study examined the dose-response effects of neonatal citalopram exposure on sexual behavior. In addition, the effects of exposure on anxiety-related behavior were examined since alterations in this behavioral measure could affect sexual behavior. Male Long-Evans rats were injected subcutaneously with citalopram (CTM) in one of three doses (5, 10 or 20 mg/kg/day), or saline (SAL) in a volume of 0.1 ml twice daily (07:00 and 14:00h) from PN8 to PN21. The rats were tested as adults (>PN90) for anxiety-like behavior and exploration in the elevated plus maze test and sexual behavior.
Neonatal citalopram exposure produced persistent reductions in male sexual behavior characterized by significant dose-dependent reductions in the percentage of male rats displaying mounting as well as dose-dependent reductions in the number of mounts and mount latency. Neonatal citalopram exposure also produced significant dose-dependent linear trends for reductions in intromission and ejaculation behavior. However, neonatal SSRI exposure was not found to produce any effects on exploration or anxiety-like behavior in the elevated plus maze test. The present findings support the hypothesis that inhibition of the serotonin transporter during neonatal life by an SSRI is directly responsible for the long-term effects on male sexual behavior.
Keywords: Neonatal exposure, Citalopram, Sexual behavior, Anxiety
1. Introduction
Selective serotonin reuptake inhibitors (SSRIs) are the most commonly prescribed medications for the treatment of major depression during pregnancy (Oberlander et al., 2006). However, a number of studies suggest that perinatal treatment of women with an SSRI may be harmful to the infant. For example, studies of the placental transfer of SSRIs and their metabolites in infants exposed during pregnancy have shown umbilical cord blood to maternal blood concentration ratios in the range of 0.12 to 0.86 at birth (Hendrick et al., 2003; Rampono et al., 2009). Multiple studies have also reported that a significant fraction of infants exposed to SSRIs in utero displayed adverse serotonergic symptoms during the first two weeks of life (Laine et al., 2003; Nordeng et al., 2001; Sanz et al., 2005). These data indicate that the fetus is being exposed to behaviorally active levels of the drugs in utero. Moreover, alterations in the behavioral response to painful stimuli as well as subtle delays in motor development in infants have been reported (Mulder et al., 2011; Casper et al., 2011; Oberlander et al., 2005; Casper et al., 2003; Costei et al., 2002; Nulman et al., 2002). Studies to date have not followed exposed children beyond early childhood (<72 months). Consequently, the long term effects of early SSRI exposure are not known. However, very recent studies by Croen et al. have linked fetal SSRI exposure to increased incidence of autistic spectrum disorder (Croen et al., 2011)
Serotonin is one of the first neurotransmitter systems to appear in the developing brain and has been proposed to play a trophic role in brain development through the regulation of processes such as cell proliferation, differentiation, and apoptosis (Lauder, 1990; Azmitia, 2001; Verney et al., 2002). Serotonergic activity in the developing brain is the highest in rats during the neonatal period up to postnatal day (PD) 21 and throughout the first two to five years in humans (Whitaker-Azmitia, 2005; Maciag et al; 2006a). Thus, disruption of the serotonergic system during early postnatal life would be expected to affect the development of neural circuits that control complex behaviors. In fact, neonatal exposure to serotonin reuptake inhibitors (citalopram, clomipramine, zimelidine, LU-10-134C) from postnatal day 8 to 21 has been shown to produce persistent behavioral alterations in adult animals that are evident long after drug withdrawal. These include impairments in sexual behavior, reductions in shock-induced aggression as well as, increased locomotor activity, rapid eye movement (REM) sleep, and ethanol consumption and increased immobility in the forced swim test (Mirmiran et al., 1981; Hilakivi et al., 1984; Vogel et al. 1988; Neil et al., 1990; Hansen et al., 1997; Maciag et al., 2006a; Maciag et al., 2006b). Moreover, our laboratory reported that in addition to the deficits in male sexual behavior produced as a result of neonatal exposure to the SSRI citalopram, exposure also resulted in significant reductions in the expression of the rate-limiting synthetic enzyme tryptophan hydroxylase in the midline subgroup of the dorsal raphe and the serotonin transporter in the frontal cortex in adult rats (Maciag et al., 2006a). In contrast, chronic exposure to an SSRI in adult rats was not found to produce any behavioral alterations or changes in serotonin circuitry proteins that persist beyond drug withdrawal and washout (Maciag et al., 2006a; Ansorge et al., 2008).
The dose-response curve is the basic currency of receptor pharmacology (Ross and Kenakin, 2001) and therefore our group recently extended our previous findings and reported that neonatal citalopram exposure resulted in dose-dependent reductions in the expression of the serotonin transporter in the hippocampus in adult rats (Weaver et al., 2010), indicating that selective inhibition of the serotonin transporter during neonatal life by an SSRI is directly responsible for the long-term effects of neonatal SSRI exposure on serotonin transporter expression. The aim of the present study was to determine whether inhibition of the serotonin transporter during neonatal life is also directly responsible for the long-term effects of neonatal SSRI exposure on adult behavior. Therefore, the dose-response effects of neonatal citalopram exposure on male sexual behavior as well as behaviors that might indirectly affect sexual behavior, such as exploration and anxiety-like behavior were examined. Moreover, we also sought to determine whether the effects of neonatal citalopram exposure on male sexual behavior are related to the effects on the expression of key enzymes within serotonergic neurons recently reported by our group (Maciag et al., 2006; Weaver et al., 2011).
2. Results
2.1. Body Weight
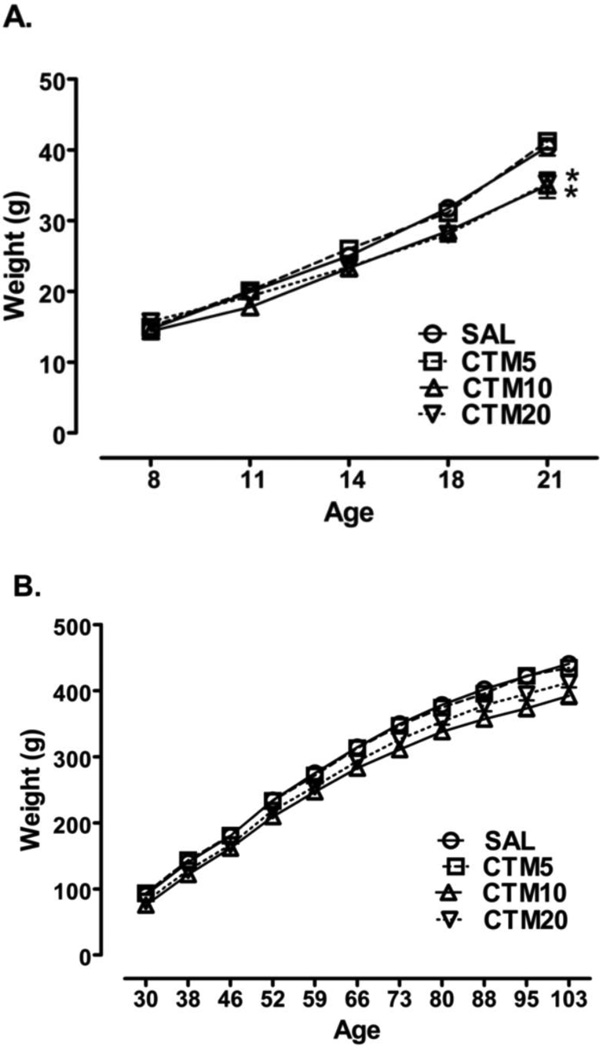
The dose-response effects of neonatal citalopram exposure on the body weight of rats following treatment were monitored daily throughout drug exposure (PD8-21) and subsequently monitored following drug exposure weekly into adulthood (PD30-103). Throughout the drug exposure period, there was a significant increase in body weight with age for all groups (F4, 188 = 828.2, P < 0.0001). In addition, an effect of drug exposure (F3, 188 = 3.403, P = 0.0252) and drug exposure by age interaction (F12, 188 = 4.504, P < 0.0001) was observed. Neonatal citalopram exposure produced a significant reduction in body weight at PD21 only during the PD8-21 exposure interval in the rats exposed to 10 mg/kg/d and 20 mg/kg/d of citalopram compared to saline treated rats (Figure 1A). During the post drug exposure period, there was also a significant increase in body weight with age for all the groups (F10, 370 = 4066, P < 0.0001). Again, an effect of drug exposure (F3, 370 = 4.992, P = 0.0052) as well as a drug exposure by age interaction (F30, 370 = 2.368, P = 0.0001) was found. A significant reduction in body weight was found from PD73-103 in the rats exposed to 10 mg/kg/d of citalopram only compared to the saline treated group (Figure 1B). However, no significant effect of exposure on body weight at any age throughout the PD30-103 period was found for the rats exposed to either 5 mg/kg/d or 20 mg/kg/d doses of citalopram.
Figure 1.
Effects of neonatal citalopram dose on body weight. The body weight of neonatal exposure to SAL (n = 9), CTM5 (n = 14), CTM10 (n = 9) and CTM20 (n = 9) was assessed (a.) during the treatment exposure between PD8-21. (b.) following treatment exposure between PD30-100. Data represents the mean ± SEM. Two-way analysis of variance (ANOVA) with repeated measures followed by Bonferroni corrected t-test. (* P < 0.05).
2.2. Elevated Plus Maze
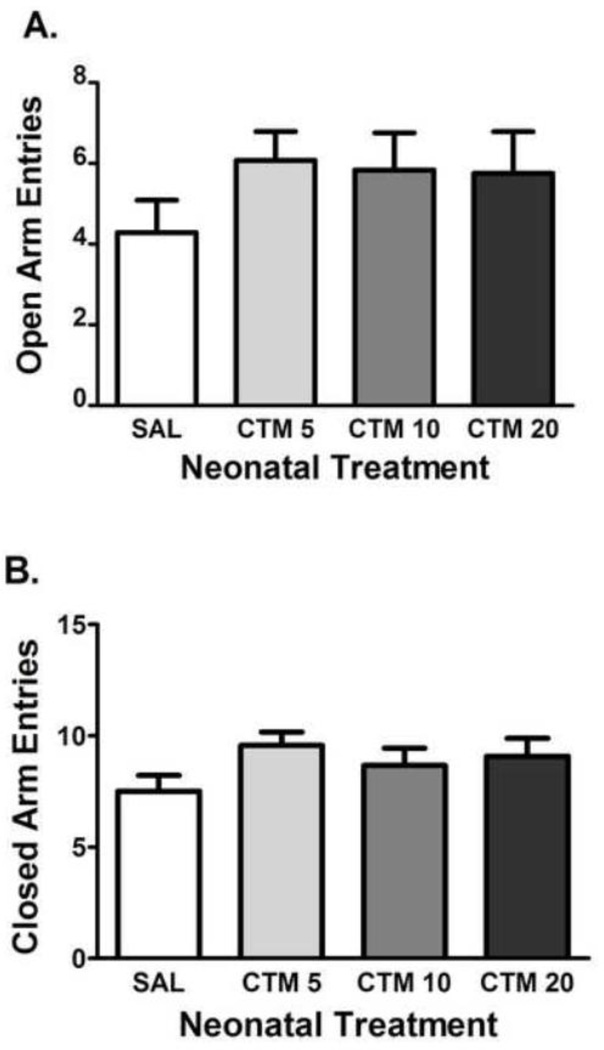
No effect of neonatal CTM exposure at any dose was observed on either measures of anxiety or exploration in the elevated plus maze. As for measures of anxiety-like behavior, there was no significant effect of drug exposure on the number of open arm entries (F3, 51 = 0.9352, P = 0.4310 – Figure 2A) or time spent in the open arms (F3,51 = 0.5591, P = 0.6446) (data not shown). Similarly, no significant effect of neonatal CTM exposure was observed on measures of exploration (number of closed arm entries: F3, 51 = 1.586, P = 0.2050 – Figure 2B; total arm entries: F3, 51 = 1.538, P = 0.2167 – data not shown). In addition, there was no effect of drug exposure on the time spent in the closed arms (data not shown).
Figure 2.
Effects of neonatal citalopram dose on measures of exploration in the elevated plus maze test. The effects of SAL (n = 14) CTM5 (n = 14), CTM10 (n = 12), and CTM20 (n = 12) on (a.) the number of closed arm entries. (b.) the number of total arm entries. Data represents the mean ± SEM.
2.3. Sexual behavior
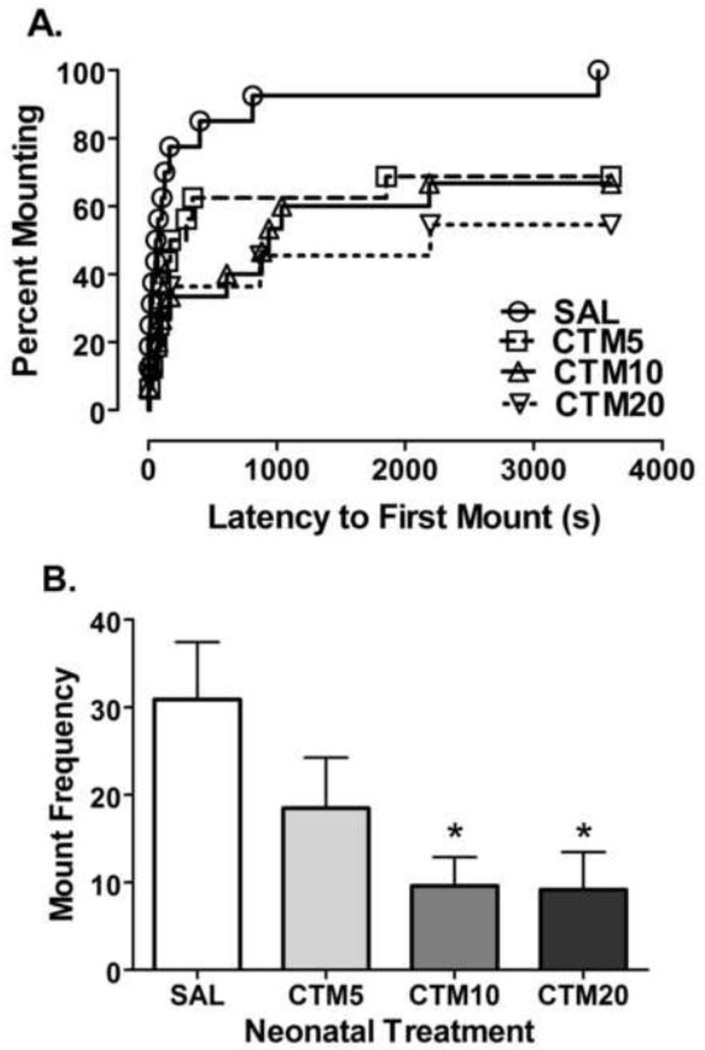
Data from two cohorts were analyzed to assess differences in measures of sexual behavior. Two-factor analysis of variance did not reveal a significant effect of cohort or a significant treatment by cohort interaction for any of the sexual behavior measures analyzed. Therefore, the data from the two cohorts were pooled. Kaplan-Meier analysis of pooled experimental data for mounting behavior showed a significant dose-dependent reduction in the percentage of rats displaying mounting behavior for the citalopram treated groups compared to saline (Log-rank (Mantel-Cox) Test = 12.94, df = 3, P = 0.0048) as well as a significant linear trend in the reduction in the percentage of rats mounting as the dose of citalopram increased (Log-rank test for linear trend = 8.906, df = 1, P = 0.0028) (Figure 3A). Individual comparisons to saline showed a significant reduction in the percentage rats displaying mounting behavior for each treatment group (SAL vs CTM5: Log-rank (Mantel-Cox) Test = 5.442, df = 1, P = 0.0197; SAL vs CTM10: Log-rank (Mantel-Cox) Test = 8.782, df = 1, P = 0.0030; SAL vs CTM20: Log-rank (Mantel-Cox) Test = 8.580, df = 1, P = 0.0034). Moreover, there was a significant effect of treatment on mount number (F3, 57 = 3.658, P = 0.0179) with a significant reduction in mount number for the rats exposed to both the 10 and 20 mg/kg/d doses of citalopram compared to saline (SAL vs CTM10: Bonferroni Multiple Comparison Test, t = 2.901, P < 0.05; SAL vs CTM20: t = 2.715, P < 0.05). Post test for linear trend also revealed a significant reduction in mount number as the dose of citalopram increased (Post-test for linear trend: slope = −3.699, r2 = 0.1340, P = 0.0047 – Figure 3B). As for mount latency, there was a significant effect of treatment revealed (F3, 57 = 3.196, P = 0.0306) with a significant increase in mount latency found in the rats exposed to 20 mg/kg/d of citalopram compared to saline (Bonferroni Multiple Comparison test, t = 2.802, P < 0.05) (data not shown). There was also a significant linear trend for an increase in mount latency as the dose of citalopram increased (Pos-test for linear trend: slope = 254.1, r2 = 0.1264, P = 0.0064).
Figure 3.
Effects of neonatal citalopram dose on mounting behavior. The effects of SAL (n = 16), CTM5 (n = 16), CTM10 (n = 15) and CTM20 (n = 11) on (a.) the percentage of rats displaying mounting behavior. Kaplan-Meier survival analysis of mounting behavior using Log-Rank (Mantel-Cox) statistic showed an overall impairment in mounting (Log-Rank = 12.94, df = 3, P = 0.0048) with a significant reduction in each treatment group compared to SAL group (SAL vs. CTM5 = 5.442, df=1, P = 0.0197; SAL vs. CTM10 = 8.782, df=1, P = 0.0030; SAL vs. CTM20 = 8.580, df=1, P = 0.0034) (b) the number of mounts. Data represents the mean ± SEM. Single-factor ANOVA followed by Bonferroni corrected t-test and Post-test for linear trend (* P < 0.05). A significant dose-dependent trend for a reduction in mount number was found.
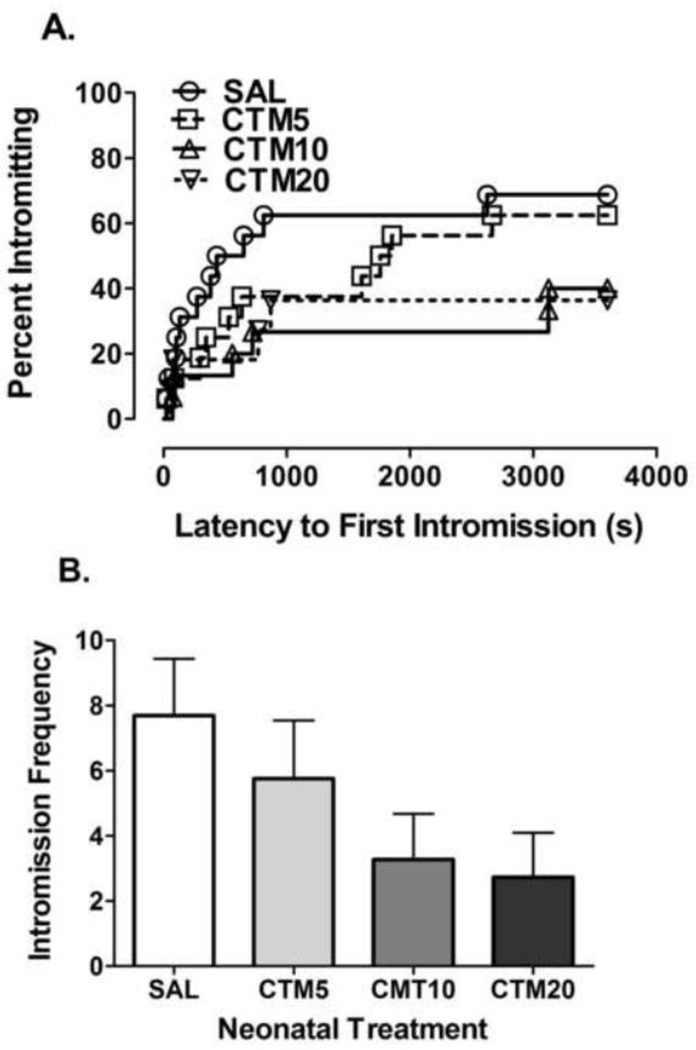
Kaplan-Meier analysis of the pooled data for intromission behavior showed that neonatal CTM exposure did not produce a significant effect on the percentage of rats displaying intromissions (Log-rank (Mantel-Cox) test = 4.983, df = 3, P = 0.1730 – Figure 4A). However, a significant linear trend for a reduction in the percentage of rats displaying intromissions as the dose of citalopram increased was revealed (Log-rank test for trend = 4.495, df = 1, P = 0.0340). As for the intromission number and intromission latency, no significant effect of treatment was found (Intromission Number: F3, 57 = 1.923, P = 0.1369; Intromission Latency: F3, 57 = 1.818, P = 0.1549), although a significant linear trend for a decrease in intromission frequency as the dose of citalopram increased was observed (Post-test for linear trend, slope = −0.8682, r2 = 0.08520, P = 0.0281 – Figure 4B).
Figure 4.
Effects of neonatal citalopram dose on intromission behavior. The effects of SAL (n = 16), CTM5 (n = 16), CTM10 (n = 15) and CTM20 (n = 11) on (a.) The percentage of rats displaying intromission behavior. Kaplan-Meier survival analysis of intromission behavior using Log-Rank (Mantel-Cox) statistic did not reveal a significant effect of treatment on intromission behavior (Log-Rank = 4.495, df = 3, P = 1730). A significant linear trend for a dose-dependent reduction in the percentage of rats intromitting was found (Log-rank test for trend = 4.495, df =1, P = 0.0340). (b.) number of intromissions. Single-factor ANOVA followed by Bonferroni corrected t-test and Post-test for linear trend. A significant dose-dependent trend for a reduction in intromission number was found.
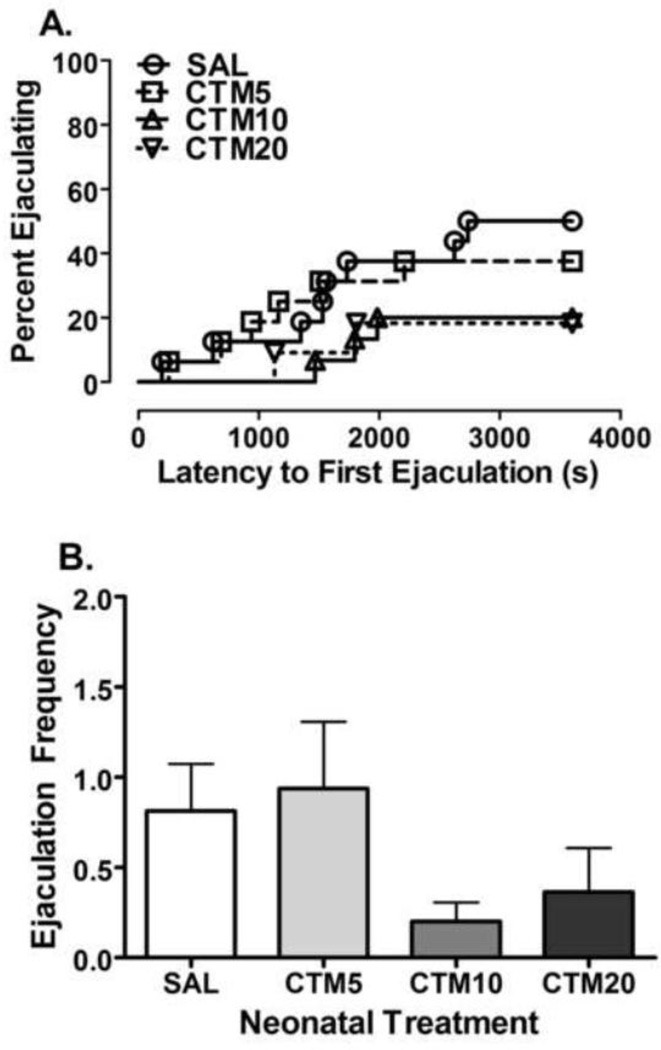
As for ejaculation behavior, Kaplan-Meier analysis of pooled data did not reveal a significant effect of treatment on the percentage of rats displaying ejaculations (Log-rank (Mantel-Cox) Test = 4.502, df = 3, P = 0.2122 – Figure 5A). However, a linear trend for a reduction in the percentage of rats ejaculating as the dose of citalopram increased was evident (Log-rank test for trend = 4.102, df = 1, P = 0.0428). No significant effect of treatment on the number of ejaculations or ejaculation latency was found (Ejaculation number: F3, 57 = 1.716, P = 0.1747; Ejaculation Latency: F3, 57 = 1.490, P = 0.2275) (data not shown).
Figure 5.
Effects of neonatal citalopram dose on ejaculation behavior. The effects of SAL (n = 16), CTM5 (n = 16), CTM10 (n = 15) and CTM20 (n = 11) on (a.) The percentage of rats displaying ejaculation behavior. Kaplan-Meier survival analysis of ejaculation behavior using Log-Rank (Mantel-Cox) statistic did not reveal a significant effect of treatment on ejaculation behavior (Log-rank = 4.502, df = 3, P = 0.2122). A significant linear trend for a dose-dependent reduction in the percentage of rats ejaculating was found (Log-rank test for trend = 4.102, df = 1, P = 0.0428). (b.) number of ejaculations. Data represents the mean ± SEM.
3. Discussion
This study is the first to our knowledge to report on the dose-response effects of neonatal SSRI exposure on male sexual behavior. We demonstrate that inhibition of the reuptake of serotonin by the highly selective SSRI citalopram during the neonatal period is directly related to the impairments in male sexual behavior as evidenced specifically by dose-dependent impairments in mounting behavior. Although neonatal citalopram exposure resulted in a significant reduction in long-term body weight at the end of the treatment interval as well as during the post-treatment interval in the adult rats, it is unlikely that this effect is related to the effects of exposure on sexual behavior given that the long-term effects of citalopram on body weight in the adult rats were only evident for the rats exposed to 10 mg/kg/d dose and not the highest dose given. Similarly, the effects of neonatal citalopram exposure on sexual behavior are not likely related to an increase in anxiety-like behavior or exploration because there was no change in either of these behavioral indices in the rats tested in the elevated plus maze. Consequently, alterations in anxiety-like behavior or general motor activity are not thought to contribute to the effects of neonatal citalopram exposure on adult sexual behavior.
Neonatal citalopram exposure resulted in deficits in sexual behavior with significant reductions in mounting behavior characterized by dose-dependent reductions in the percentage of rats displaying mounting behavior, mount number and mount latency. However, maximal effects were achieved for mount number at the intermediate dose with no further decrement observed at the highest dose given. There were no significant effects of neonatal citalopram exposure on intromission and ejaculation behavior, although there was a significant dose-dependent linear trend for a reduction in the percentage of rats displaying both intromission behavior and ejaculation behavior. Although not statistically significant, the effects on these measures were also found to be maximal at the intermediate dose. It is important to note that, among the control group, only 70% of the rats displayed intromissive behavior and 50% displayed ejaculatory behavior and it is likely that the lack of statistical effect found was due to low performance by the control group on these measures. It is possible that the use of naïve male rats contributed to this effect. However, a recent study by our laboratory found that sexual behavior examined on the third sexual exposure resulted in a similar percentage of rats engaging in intromissive and ejaculatory behavior in the control group (i.e. 70% displaying intromissive behavior and 55% displaying ejaculatory behavior) (Rodriquez-Porcel et al., in press). Moreover, earlier reports on the effects of neonatal antidepressant exposure on male sexual behavior have also reported less than 100% of the rats displaying intromissive and ejaculatory behavior even after two previous sexual exposures (DeBoer et al., 1987; Neil et al., 1990; Velaquez-Moctezuma et al., 1993). A recent report on sexual performance in male rats stated that even after nine previous sexual exposures up to 40% of the rats showed low or no sexual behavior, with the previous exposure contributing to a reduction in ejaculation latency in rats with optimal levels of sexual behavior (Alvarenga et al., 2010). These data suggest that the low performance of intromissive and ejaculatory behavior found in the present study are unlikely to have been solely due to the use of naïve male rats. However, the sexual behavior data from the present study are in line with our previous studies (Maciag et al., 2006a, 2006b, 2006c) as well as others using the non-selective antidepressant clomipramine (Velazquez-Moctezuma et al., 1993; Vogel et al., 1996; Neill et al., 1990, Feng et al., 2001, 2003).
Given that citalopram is a racemate consisting of the S(+)-enatiomer (escitalopram) and the R(−)-enantiomer and the S(+) entantiomer is responsible for the antidepressant effects of citalopram and the R(−)-enantiomer has been shown to disrupt the effects of the S(+)-entantiomer on the serotonin transporter (Sanchez, 2006), it is possible that the effects of neonatal citalopram on male sexual behavior were limited due to an increase in the concentration of R(−)-enantiomer with increasing dose that opposed the impairments in male sexual behavior thought to be produced by the S(+)-enantiomer. Previous studies have in fact shown a small dose-dependent increase in the ratio of the R(−)-entantiomer compared to the S(+)-enantiomer in adult rats chronically administered citalopram within the dosing range examined in the present study (i.e. 10–20 mg/kg/d) (Kugelberg et al., 2001). However, examination of the effects of these doses on locomotor activity in the open field did not reveal a clear effect of an increase in dose on the parameters measured (Kugelberg et al., 2002). Similarly, the effects of neonatal citalopram exposure on male sexual behavior revealed maximal effects at the intermediate dose for some parameters (mount number) but not others (percentage of rats mounting and mount latency). Therefore, it is not likely that differences in the ratio of the R(−)-entantiomer versus the S(+)-enantiomer were involved in the dose effects produced on male sexual behavior.
The data from the present study provide evidence that neonatal citalopram exposure is directly responsible for the long-term deficits in male sexual behavior as demonstrated by the dose-dependent impairments in mounting behavior. Overall, the data from this study indicate that neonatal exposure to citalopram primarily produces an impairment in the initiation of sexual behavior or more specifically, the motivational aspect of sexual behavior (i.e. reductions in mount latency and the percentage of rats mounting), with lesser effects on the consummatory aspects of sexual behavior (i.e. number of mounts, intromission and ejaculation behavior) in naïve male rats (Everitt, 1990; Meisel and Sachs, 1994). In fact, exposure to fluoxetine throughout the gestational and neonatal period in mice was found to impair sexual motivation as assessed using the sexual incentive test, although no effects were found on male sexual behavior (Gouvea et al., 2008). The lack of effects on male sexual behavior in this study are likely due to the low baseline level of sexual activity observed among both the control and treated rats.
Sexual behavior is a complex behavior that involves neural and hormonal regulation. It is possible that alterations in the sex organs and/or gonadal hormones may have led to the deficits in the present study. However, previous studies have not shown any long-term effects of early antidepressant exposure in rodents on plasma testosterone levels or alterations in the weight of the male sex organs (i.e. testes, epididymis, seminal vesicle) (Mirmiran et al., 1981; Bonilla-Jaime, 2003; Gouvea et al., 2008). On the other hand, given that serotonin is known to play a role in brain development, being transiently present during early postnatal life in neuronal populations that control the processing of sensory information (i.e. tactile, visual, auditory) (Lebrand et al., 1996) it is possible that the effects of neonatal exposure on sexual behavior are due to more global deficits in sensory perception. SSRI exposure during the neonatal period in rodents and throughout the third trimester in humans has been shown to alter behavioral reactivity to tactile stimuli (Oberlander, 2002; Ansorge et al., 2008; Lee 2009). A recent study from our laboratory has provided further evidence that neonatal SSRI exposure results in abnormalities in the behavioral response to sensory stimuli in rats (Rodriguez-Porcel et al., 2011). Consequently, additional testing will be necessary to elucidate the specific sensory alterations that play a role in the long-term impairments in male sexual behavior as a result of neonatal SSRI exposure.
As previously mentioned, our group recently reported that neonatal citalopram exposure results in dose-dependent reductions in the expression of the serotonin transporter in the hippocampus of adult male rats (Weaver et al., 2010). The results of this study showed clear dose-dependent reductions in the expression of the serotonin transporter in both the dorsal and ventral hippocampus with significant reductions in the rats treated with 20 mg/kg/d of citalopram. On the other hand, although the results of the present study showed dose-dependent reductions in male sexual behavior, specifically mounting behavior, maximal effects were achieved at the intermediate dose of 10 mg/kg/d with no further reductions in behavior shown at the 20 mg/kg/d dose. This suggests that the effects of neonatal citalopram exposure on expression of the serotonin transporter in the hippocampus and male sexual behavior are not likely to be directly related. However, a recent study showed deficits in the sexual behavior of serotonin transporter knockout rats characterized by a reduction in ejaculation number compared to control rat and rats with a partial knockout of the serotonin transporter (Chan et al., 2010). The present study also showed reductions in the number of ejaculations achieved with increasing doses of neonatal citalopram exposure, although this effect was not statistically significant. Our results in conjunction with the data reported by Chan et al., (2010) suggest that functional inactivation of the serotonin transporter either by pharmacological or genetic means during a critical period of brain development sensitive to disruption of serotonergic system function leads to long-term alterations in male sexual behavior. Further studies are needed to determine the specific mechanism(s) underlying the long-term effects of neonatal citalopram exposure on male sexual behavior.
We did not find any changes in behavior in the rat tested the elevated plus maze test as adults (PD90). Although only a handful of studies have examined the effects of neonatal antidepressant exposure on behavior in the elevated plus maze test, the majority of the studies, like our study, did not report any changes in anxiety-like behavior in rats or mice (Ansorge et al., 2008; Popa et al., 2008; Ribas et al., 2008). Our data suggests that neonatal citalopram exposure does not have any long-term effects on anxiety-like behavior or exploration in adult rats. Although the effects of neonatal citalopram exposure on depression-related behaviors were not assessed in the present study, we previously reported that neonatal exposure to the 10 mg/kg/d dose of citalopram did not result in alterations in either immobility in the forced swim test or saccharin-sweetened fluid consumption (Maciag et al., 2006c). Consequently, the effects of neonatal citalopram exposure on male sexual behavior are unlikely to be related to alterations in measures of depression.
Although it is difficult to definitively compare brain development between humans and rodents, studies suggest that in terms of brain maturation, the neonatal exposure period of PD8-21 used in the present study is approximately equivalent to exposure in humans during the third trimester and throughout early childhood (Bayer et al., 1993; Clancy et al., 2001; Ansorge et al., 2008). Therefore, the long-term impairments in an adult-specific behavior (i.e. sexual behavior) in rodents as a result of neonatal SSRI exposure in the present study suggest that studies on the long-term effects of SSRI exposure in utero or through breastfeeding are warranted. Given the widespread use of SSRIs during pregnancy and the lack of studies to date on the effects of in utero SSRI exposure beyond early childhood, it is possible that there may be unforeseen effects in humans that are not manifested until adolescence or adulthood.
4. Material and Methods
4.1. Animals
The male offspring of 12 timed-pregnant Long-Evans rats (Harlan, Illinois, USA) were used in this experiment. All procedures were approved by the UMMC Animal Care and Use Committee and complied with AAALAC and NIH standards. The dams were allowed to deliver undisturbed and the sex of the pups was determined on PD3. Both female and male pups were allowed to remain with their dam throughout the treatment period. Litter sizes were not adjusted and ranged from 6–12 pups. The male pups were tattooed for identification on PD6. Beginning on PD8, the male pups with atleast one pup/treatment group represented in each litter were assigned to one of four treatment groups and subcutaneously injected with citalopram (CTM) at a dose of 2.5 mg/kg (CTM5 = 5 mg/kg/d), 5 mg/kg (CTM10 = 10 mg/kg/d), 10 mg/kg (CTM20 = 20 mg/kg/d) (Tocris, Ellisville, MO), or saline (SAL) (Sigma-Aldrich, St Louis, MO) in a volume of 0.1 ml twice daily (0700 and 1400 hours) from PD8-21. We previously reported that neonatal exposure to citalopram at the dose of 10 mg/kg/d resulted in a serum concentration of 58±13 ng/ml which was well within the human therapeutic range of 28–247 ng/ml found in the literature (Maciag et al., 2006a). Therefore the doses used were designed to still be within the therapeutic range while allowing us to assess dose-response effects of treatment. During treatment, the pups were handled only for daily weighing and drug injections. After PD28, the pups were weaned from their dams and same-sex housed in groups of 2–3/cage. The animals were housed under standard laboratory conditions on a reverse light/dark cycle with the lights out from 12 pm-12 am and given access to food and water ad lib. The animals were left undisturbed with the exception of weekly weighing until the start of behavioral testing. All behavioral testing was conducted during the dark phase of the light/dark cycle.
4.2. Elevated Plus Maze
The elevated plus maze test was performed on PD90 as detailed by File (2004). The maze consisted of four arms formed in the shape of a plus sign made of black Plexiglas with two open arms and two closed arms, both of equal length and width (50 × 10 cm). The height of the enclosed walls of the closed arms was 40 cm. The four arms were arranged around a central platform that was 10 × 10 cm in diameter with each of the closed or open arms opposite of each other. The entire unit was built on a platform that was 50 cm high. The maze was located on a table in a sound attenuated room and the camera for video-recording each test was hung from the ceiling directly above the maze. The test began after the animal was placed in the center of the maze facing an open arm. Each test animal was allowed to explore the maze freely for 5 min. The number of entries and time spent in the open arms was used to determine measures of anxiety-like behavior and the number of closed arm entries and the total number of arm entries were used to determined measures of exploration.
4.3. Sexual behavior
Sexual behavior was tested in adult rats after PD100 as previously described (Maciag et al., 2006a). Naïve male rats were currently used to assess male sexual behavior in keeping with what was previously done to allow us to compare and build on our reported findings. Moreover, naïve male rats depend heavily on the display of appropriate behavior by the female rat for the initiation of sexual behavior whereas the initiation of sexual behavior in experienced males is largely independent of the female’s behavior and is influenced by associative learning to a degree that previous sexual experience can buffer the effects of physiological and pharmacological treatments (Agmo and Picker, 1990; Pfaus et al., 2001). Therefore, naïve male rats were preferentially used in the previous and present studies. The ovariectomized females were brought into behavioral estrus by subcutaneous injections of estradiol benzoate (15 µg, 48 and 24 hours prior to testing) (Sigma Aldrich, St. Louis, MO) and progesterone (1 mg, 4–6 hours prior to testing) (Sigma Aldrich, St. Louis, MO) both dissolved in mineral oil. On the day of testing, each male rat was allowed to habituate to the testing chamber for 10 minutes prior to introduction of the female rat. Each test lasted 60 minutes and was analyzed for number of and latency to the first mount, intromission and ejaculation. The male rats were tested with the female rats in a clean cage with fresh bedding. The sexual encounters were videotaped and analyzed for number of mounts, intromissions, ejaculations, latency (time) to first mount, intromission and ejaculation as previously described (Maciag et al., 2006b). An experienced rater blind to treatment observed and scored sexual behavior. Rats from two separate cohorts were tested for sexual behavior. Behavioral analysis of the first cohort of rats showed that the females did not consistently display the characteristic proceptive and receptive behaviors necessary to ensure initiation of sexual behavior by untreated male rats (Ferraz et al., 2001). Therefore, the tests where there was an inadequate display of proceptive and receptive behavior by the female rats were eliminated from analysis and a total of 20 sexual behavior tests from the first cohort were analyzed. A total of 38 tests from a second cohort of rats were analyzed for sexual behavior and the proceptive and receptive behavior of the female rats was pretested prior to the start of testing in this cohort to ensure the display of a high level of these behaviors in the females used.
4.4. Statistical Analysis
The data were analyzed using Graphpad Prism statistical software (version 5.02 for Windows, GraphPad software, San Diego, CA). Single factor analysis of variance (ANOVA) was used to analyze the data from the elevated plus maze test. For sexual behavior, Sexual behavior data was analyzed for percentage of rats exhibiting mounting, intromission and ejaculation behavior using Kaplan-Meier survival analysis with Log-Rank (Mantel-Cox) statistic as well as Log-rank test for linear trend. Parametric measures of sexual behavior (number and latency to first mount, intromission and ejaculation) were analyzed using Single factor ANOVA followed by Bonferroni correct t-test and Post-test for linear trend. For sexual behavior, the data from two cohorts of rats were analyzed by two-way ANOVA to determine if there were any significant effects of cohort or cohort by treatment interactions. Given that no significant effects of cohort or cohort by treatment interactions were observed, the data from the two cohorts were pooled and analyzed as previously described.
Highlights.
>We examined the dose-response of early life SSRI exposure on adult behavior in rats.
>We found that citalopram exposure dose-dependently impaired male sexual behavior.
>In contrast citalopram exposure had no effect on measures of anxiety or activity.
>Inhibiting the serotonin transporter in early life disrupts adult male behavior in rats.
>Similar exposure may have behavioral effects in humans that persist to adulthood.
Acknowledgments
The authors would like to thank Dr. K. Weaver for technical support. This manuscript was supported by NIH Grant Number MH084194 to Rick CS Lin, NIH Grant Number RR017701 to Ian A Paul and Kimberly L Simpson and the Alliance for Graduate Education in Mississippi (AGEM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing interests relevant to this manuscript.
References
- Agmo A, Picker Z. Catecholamines and the initiation of sexual behavior in male rats without sexual experience. Pharmacol Biochem Behav. 1990;35:327–334. doi: 10.1016/0091-3057(90)90164-d. [DOI] [PubMed] [Google Scholar]
- Alvarenga TA, et al. Influence of progesterone on sexual performance in male rats. J Sex Med. 2010;7:2435–2444. doi: 10.1111/j.1743-6109.2010.01851.x. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, et al. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56:413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- Bayer SA, et al. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicol. 1993;14:83–144. [PubMed] [Google Scholar]
- Casper RC, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142:402–408. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- Capser RC, et al. Length of prenatal exposure to selective serotonin reuptake inhibitor (SSRI) antidepressants: Effects on neonatal adaptation and psychomotor development. 2011 doi: 10.1007/s00213-011-2270-z. [DOI] [PubMed] [Google Scholar]
- Chan JSW, et al. The serotonin transporter plays an important role in male sexual behavior: a study in serotonin transporter knockout rats. J Sex Med. 2010;8:97–108. doi: 10.1111/j.1743-6109.2010.01961.x. [DOI] [PubMed] [Google Scholar]
- Clancy B, et al. Translating developmental time across mammalian species. Neurosci. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Costei AM, et al. Perinatal outcome following third trimester exposure to paroxetine. Arch Pediatr Adolesc Med. 2002;156:1129–1132. doi: 10.1001/archpedi.156.11.1129. [DOI] [PubMed] [Google Scholar]
- Croen LA, et al. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch. Gen. Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.73. (Published online 7/04/11) [DOI] [PubMed] [Google Scholar]
- De Boer S, et al. Neurobehavioral teratogenic effects of clomipramine and alpha-methyldopa. Neurotoxicol Teratol. 1989;11:77–84. doi: 10.1016/0892-0362(89)90089-5. [DOI] [PubMed] [Google Scholar]
- Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Feng P, et al. The critical window of brain development from susceptive to insusceptive. Effects of clomipramine neonatal treatment on sexual behavior. Brain Res Dev Brain Res. 2001;129:107–110. doi: 10.1016/s0165-3806(01)00158-4. [DOI] [PubMed] [Google Scholar]
- Feng P, et al. Impairments of ERK signal transduction in the brain in a rat model of depression induced by neonatal exposure of clomipramine. Brain Research. 2003;991:195–205. doi: 10.1016/j.brainres.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Ferraz MR, et al. How REM sleep deprivation and amantadine affects male rat sexual behavior. Pharmacol Biochem Behav. 2001;69:325–332. doi: 10.1016/s0091-3057(01)00508-1. [DOI] [PubMed] [Google Scholar]
- File SE, et al. Animal Tests of Anxiety. Current Protocols in Neuroscience. 2004:8.3.6–8.3.8. doi: 10.1002/0471142301.ns0803s26. [DOI] [PubMed] [Google Scholar]
- Gouvea TS, et al. Maternal exposure to the antidepressant fluoxetine impairs sexual motivation in adult male mice. Pharmacol Biochem Behav. 2008;90:416–419. doi: 10.1016/j.pbb.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Hansen HH, et al. Neonatal administration of the selective reuptake inhibitor Lu 10-134-C increases forced swimming-induced immobility in adult rats: a putative animal model of depression. J Pharmacol Exp Ther. 1997;283:1333–1341. [PubMed] [Google Scholar]
- Hendrick V, et al. Placental passage of antidepressant medications. Am J Psychiatry. 2003;160:993–996. doi: 10.1176/appi.ajp.160.5.993. [DOI] [PubMed] [Google Scholar]
- Hilakivi LA, et al. Effects of neonatal treatment with clomipramine on adult ethanol related behavior in the rat. Brain Res. 1984;317:129–132. doi: 10.1016/0165-3806(84)90148-2. [DOI] [PubMed] [Google Scholar]
- Kugelberg FC, et al. In vivo steady-state pharmacokinetic outcome following clinical and toxic doses of racemic citalopram to rats. Br J Pharmacol. 2001;132:1683–1690. doi: 10.1038/sj.bjp.0704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelberg FC, et al. Effects of chronic citalopram treatment on central and peripheral spontaneous open-field behaviours in rats. Pharmacol and Toxicol. 2002;90:303–310. doi: 10.1034/j.1600-0773.2002.900603.x. [DOI] [PubMed] [Google Scholar]
- Laine K, et al. Effects of exposure to selective serotonin inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch Gen Psychiatry. 2003;60:720–726. doi: 10.1001/archpsyc.60.7.720. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann N Y Acad Sci. 1990;600:297–313. doi: 10.1111/j.1749-6632.1990.tb16891.x. discussion 314. [DOI] [PubMed] [Google Scholar]
- Maciag D, et al. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006a;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag D, et al. Neonatal Citalopram Exposure Produces Lasting Changes in Behavior which are Reversed by Adult Imipramine Treatment. European Journal of Pharmacology. 2006b;532:265–269. doi: 10.1016/j.ejphar.2005.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag D, et al. Evidence that the deficit in sexual behavior in adult rats neonatally exposed to citalopram is a consequence of 5-HT1 receptor stimulation during development. Brain Res. 2006c;1125:171–175. doi: 10.1016/j.brainres.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel RL, Sachs BD. The physiology of male sexual behavior. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2nd ed. vol. 2. New York: Raven Press; 1994. pp. 3–105. [Google Scholar]
- Mirmiran M, et al. Suppression of active sleep by chronic treatment with chlorimipramine during early postnatal development: effects upon adult sleep and behavior in the rat. Brain Res. 1981;204:129–146. doi: 10.1016/0006-8993(81)90657-0. [DOI] [PubMed] [Google Scholar]
- Mulder EJH, et al. Selective serotonin reuptake inhibitors affect neurobehavioral development in the human fetus. Neuropsychopharmacology. 2011;36:1961–1971. 2011. doi: 10.1038/npp.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill D, et al. Diminished sexual activity in a new animal model of endogenous depression. Neuroscience and Biobehavioral Reviews. 1990;14:73–76. doi: 10.1016/s0149-7634(05)80162-9. [DOI] [PubMed] [Google Scholar]
- Nordeng H, et al. Neonatal withdrawal syndrome after in utero exposure to selective serotonin reuptake inhibitors. Acta Paediatr. 2001;90:288–291. [PubMed] [Google Scholar]
- Nulman I, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry. 2002;159:1889–1895. doi: 10.1176/appi.ajp.159.11.1889. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, et al. Pain reactivity in 2-month-old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics. 2005;115:411–425. doi: 10.1542/peds.2004-0420. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, et al. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63:898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, et al. Conditioning and sexual behavior: a review. Horm Behav. 2001;40:291–321. doi: 10.1006/hbeh.2001.1686. [DOI] [PubMed] [Google Scholar]
- Popa D, et al. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior. J Neurosci. 2008;28:3546–3554. doi: 10.1523/JNEUROSCI.4006-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampono J, et al. Placental transfer of SSRI and SNRI antidepressants and effects on the neonate. Pharmacopsychiatry. 2009;42:95–100. doi: 10.1055/s-0028-1103296. [DOI] [PubMed] [Google Scholar]
- Ribas VR, et al. Neonatal administration of fluoxetine did not alter the anxiety indicators, but decreased the locomotor activity in adult rats in the elevated plus-maze. Arq Neuropsiquiatr. 2008;66:844–847. doi: 10.1590/s0004-282x2008000600013. [DOI] [PubMed] [Google Scholar]
- Ross EM, Kenakin TP. Pharmacodynamics: Mechanisms of drug action and the relationship between drug concentration and effect. In: Hardman JG, Limbird LE, editors. Goodman and Gillman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill Co.; 2001. p. 39. [Google Scholar]
- Sanchez C. The pharmacology of citalopram enantiomers: the antagonism by R-citalopram on the effect of S-citalopram. Basic Clin Pharmacol and Toxicol. 2006;99:91–95. doi: 10.1111/j.1742-7843.2006.pto_295.x. [DOI] [PubMed] [Google Scholar]
- Sanz EJ, et al. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: A database analysis. Lancet. 2005;365:482–487. doi: 10.1016/S0140-6736(05)17865-9. [DOI] [PubMed] [Google Scholar]
- Velazquez-Moctezuma J, et al. Behavioral effects of neonatal treatment with clomipramine, scopolamine, and idazoxan in male rats. Pharmacol Biochem Behav. 1993;46:215–217. doi: 10.1016/0091-3057(93)90343-r. [DOI] [PubMed] [Google Scholar]
- Verney C, et al. Changing distribution of monoaminergic markers in the developing human cerebral cortex with special emphasis on the serotonin transporter. Anat Rec. 2002;267:87–93. doi: 10.1002/ar.10089. [DOI] [PubMed] [Google Scholar]
- Vogel G, et al. Animal depression model by neonatal clomipramine: reduction of shock induced aggression. Pharmacol Biochem Behav. 1988;31:103–106. doi: 10.1016/0091-3057(88)90319-x. [DOI] [PubMed] [Google Scholar]
- Vogel G, et al. Dose-Dependent Decrements in Adult Male Rat Sexual Behavior After Neonatal Clorimipramine Treatment. Pharmacology Biochemistry and Behavior. 1996;54:605–609. doi: 10.1016/0091-3057(95)02276-7. [DOI] [PubMed] [Google Scholar]
- Weaver KJ, et al. Neonatal Exposure to Citalopram Selectively Alters the Expression of the Serotonin Transporter in the Hippocampus: Dose-Dependent Effects. Anat Rec. 2010;293:1920–1932. doi: 10.1002/ar.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? Int J Dev Neurosci. 2005;23:75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]