Abstract
Poliovirus RNA polymerase requires a host factor to initiate RNA synthesis in vitro. The host factor was previously purified to near homogeneity from HeLa cells but was not assigned an enzymatic activity. This report describes the purification of a terminal uridylyltransferase that can act as host factor. By all criteria examined it is identical to the factor purified previously. It has the same molecular weight (68,000), chromatographic properties, and cellular localization. We present evidence that terminal uridylyltransferase can add uridine residues to the 3' poly(A) end of virion RNA and that these anneal back to the poly(A) and form a hairpin primer for polymerase.
Full text
PDF


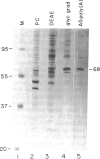
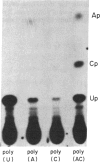
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambros V., Baltimore D. Protein is linked to the 5' end of poliovirus RNA by a phosphodiester linkage to tyrosine. J Biol Chem. 1978 Aug 10;253(15):5263–5266. [PubMed] [Google Scholar]
- Andrews N. C., Levin D., Baltimore D. Poliovirus replicase stimulation by terminal uridylyl transferase. J Biol Chem. 1985 Jun 25;260(12):7628–7635. [PubMed] [Google Scholar]
- BURDON R. H. The uptake of ribonucleotide 5'-trephosphates by nuclear ribosomes. Biochem Biophys Res Commun. 1963 Jun 20;11:472–476. doi: 10.1016/0006-291x(63)90095-0. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Smoler D. Primer requirement and template specificity of the DNA polymerase of RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1507–1511. doi: 10.1073/pnas.68.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron M. H., Baltimore D. Antibodies against the chemically synthesized genome-linked protein of poliovirus react with native virus-specific proteins. Cell. 1982 Feb;28(2):395–404. doi: 10.1016/0092-8674(82)90357-9. [DOI] [PubMed] [Google Scholar]
- Baron M. H., Baltimore D. In vitro copying of viral positive strand RNA by poliovirus replicase. Characterization of the reaction and its products. J Biol Chem. 1982 Oct 25;257(20):12359–12366. [PubMed] [Google Scholar]
- Baron M. H., Baltimore D. Purification and properties of a host cell protein required for poliovirus replication in vitro. J Biol Chem. 1982 Oct 25;257(20):12351–12358. [PubMed] [Google Scholar]
- Boege F. Simultaneous presence of terminal adenylyl, cytidylyl, guanylyl, and uridylyl transferase in healthy tomato leaf tissue: separation from RNA-dependent RNA polymerase and characterization of the terminal transferases. Biosci Rep. 1982 Jun;2(6):379–389. doi: 10.1007/BF01119300. [DOI] [PubMed] [Google Scholar]
- Brishammar S., Juntti N. A poly(U) polymerase in tobacco leaves. Biochim Biophys Acta. 1975 Apr 2;383(4):351–358. doi: 10.1016/0005-2787(75)90304-4. [DOI] [PubMed] [Google Scholar]
- Celma M. L., Pan J., Weissman S. M. Studies of low molecular weight RNA from cells infected with adenovirus 2. I. The sequences at the 3' end of VA-RNA I. J Biol Chem. 1977 Dec 25;252(24):9032–9042. [PubMed] [Google Scholar]
- Dasgupta A., Baron M. H., Baltimore D. Poliovirus replicase: a soluble enzyme able to initiate copying of poliovirus RNA. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2679–2683. doi: 10.1073/pnas.76.6.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A. Purification of host factor required for in vitro transcription of poliovirus RNA. Virology. 1983 Jul 15;128(1):245–251. doi: 10.1016/0042-6822(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Dasgupta A., Zabel P., Baltimore D. Dependence of the activity of the poliovirus replicase on the host cell protein. Cell. 1980 Feb;19(2):423–429. doi: 10.1016/0092-8674(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Dorsch-Häsler K., Yogo Y., Wimmer E. Replication of picornaviruses. I. Evidence from in vitro RNA synthesis that poly(A) of the poliovirus genome is genetically coded. J Virol. 1975 Dec;16(6):1512–1517. doi: 10.1128/jvi.16.6.1512-1517.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Baltimore D. Poliovirus-specific primer-dependent RNA polymerase able to copy poly(A). Proc Natl Acad Sci U S A. 1977 Sep;74(9):3677–3680. doi: 10.1073/pnas.74.9.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Petterson R. F., Ambros V., Hewlett N. J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francoeur A. M., Mathews M. B. Interaction between VA RNA and the lupus antigen La: formation of a ribonucleoprotein particle in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6772–6776. doi: 10.1073/pnas.79.22.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Hayashi T. T., MacFarlane K. Comparison of endogenous and exogenous RNA primers of poly(U) polymerase in rat hepatic ribosomes. Biochem J. 1979 Mar 1;177(3):895–902. doi: 10.1042/bj1770895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozumi N., Haruna I., Watanabe I., Mikoshiba K., Tsukada Y. Poly(U) polymerase in rat brain. Nature. 1975 Jul 24;256(5515):337–339. doi: 10.1038/256337a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Leung W. C., Leung M. F., Rawls W. E. Distinctive RNA transcriptase, polyadenylic acid polymerase, and polyuridylic acid polymerase activities associated with Pichinde virus. J Virol. 1979 Apr;30(1):98–107. doi: 10.1128/jvi.30.1.98-107.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milchev G. I., Hadjiolov A. A. Association of poly(A) and poly(U) polymerases with cytoplasmic ribosomes. Eur J Biochem. 1978 Mar;84(1):113–121. doi: 10.1111/j.1432-1033.1978.tb12147.x. [DOI] [PubMed] [Google Scholar]
- Morrow C. D., Gibbons G. F., Dasgupta A. The host protein required for in vitro replication of poliovirus is a protein kinase that phosphorylates eukaryotic initiation factor-2. Cell. 1985 Apr;40(4):913–921. doi: 10.1016/0092-8674(85)90351-4. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Detjen B., Pozzatti R., Wimmer E. The location of the polio genome protein in viral RNAs and its implication for RNA synthesis. Nature. 1977 Jul 21;268(5617):208–213. doi: 10.1038/268208a0. [DOI] [PubMed] [Google Scholar]
- Peters G., Harada F., Dahlberg J. E., Panet A., Haseltine W. A., Baltimore D. Low-molecular-weight RNAs of Moloney murine leukemia virus: identification of the primer for RNA-directed DNA synthesis. J Virol. 1977 Mar;21(3):1031–1041. doi: 10.1128/jvi.21.3.1031-1041.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson R. F., Ambros V., Baltimore D. Identification of a protein linked to nascent poliovirus RNA and to the polyuridylic acid of negative-strand RNA. J Virol. 1978 Aug;27(2):357–365. doi: 10.1128/jvi.27.2.357-365.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R. Compilation of small RNA sequences. Nucleic Acids Res. 1985;13 (Suppl):r155–r163. doi: 10.1093/nar/13.suppl.r155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Henning D., Tan E., Busch H. Identification of a La protein binding site in a RNA polymerase III transcript (4.5 I RNA). J Biol Chem. 1983 Jul 10;258(13):8352–8356. [PubMed] [Google Scholar]
- Rinke J., Steitz J. A. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982 May;29(1):149–159. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Requirement of 3'-terminal poly(adenylic acid) for the infectivity of poliovirus RNA. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2983–2987. doi: 10.1073/pnas.71.8.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano J. E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3' termini of RNA polymerase III transcripts. Cell. 1984 Jan;36(1):145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Wolin S. L., Rinke J., Pettersson I., Mount S. M., Lerner E. A., Hinterberger M., Gottlieb E. Small ribonucleoproteins from eukaryotes: structures and roles in RNA biogenesis. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):893–900. doi: 10.1101/sqb.1983.047.01.103. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Smellie R. M. Chain extension of ribonucleic acid by enzymes from rat liver cytoplasm. Biochem J. 1968 Oct;109(4):485–494. doi: 10.1042/bj1090485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M., Smellie R. M. Polyribonucleotide synthesis by subfractions of microsomes from rat liver. Biochem J. 1968 Sep;109(2):229–238. doi: 10.1042/bj1090229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes J. R., Smellie R. M. The synthesis of polyribonucleotides by cytoplasmic enzymes. Biochem J. 1966 May;99(2):347–355. doi: 10.1042/bj0990347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Polyadenylic acid at the 3'-terminus of poliovirus RNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1877–1882. doi: 10.1073/pnas.69.7.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P., Dorssers L., Wernars K., Van Kammen A. Terminal uridylyl transferase of Vigna unguiculata: purification and characterization of an enzyme catalyzing the addition of a single UMP residue to the 3'-end of an RNA primer. Nucleic Acids Res. 1981 Jun 11;9(11):2433–2453. doi: 10.1093/nar/9.11.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]