Abstract
Anomalous origin of left coronary artery from pulmonary artery (ALCAPA) is an infrequent, well described, but important anomaly of the coronary origin. Early diagnosis and prompt surgical treatment of the disease can be life saving. However, there are several potential sources of error in the seemingly simple stereotype diagnostic pattern. We report a case of ALCAPA and allude to some of the caveats in the diagnosis of this entity.
MeSH: Coronary artery, Heart defects, congenital
Case Report
A three month old female infant was referred to us with a diagnosis of dilated cardiomyopathy. The child was symptomatic for one month with complaints of fast breathing and feeding difficulties. Prior echo had revealed left ventricular (LV) dysfunction and mild mitral regurgitation. Tachycardia (rate 140 per minute), tachypnea were seen on examination. All peripheral pulses were equally felt. Blood pressure was 92/66 mm Hg in right upper limb. There was cardiomegaly, S3 gallop, and a pansystolic murmur in the apical area. Chest roentgenogram revealed cardiomegaly and pulmonary venous hypertension. An EKG showed increased left ventricular voltages, and Q waves in lead 1, 2, V5, V6 (fig. 1) that were less than 5 mm and less than 0.03 wide (similar to physiological Q waves).
Figure 1.
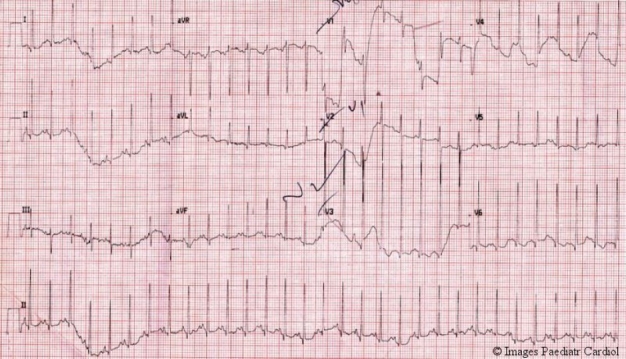
EKG showing left ventricular hypertrophy and sharp small q waves (almost physiological) in leads 1, 2, V5, V6.
Echocardiography showed a dilated LV with moderate mitral regurgitation. There was hyperechogenecity of the papillary muscles suggestive of scarring of the muscles. This increased the suspicion of ALCAPA, so the coronary origins were looked for.
On cursory examination (in PSAX view) there appeared a longitudinal structure arising from aorta which simulated the left coronary artery LCA (fig. 2), but the right coronary artery (RCA) appeared unusually dilated (fig. 3).
Figure 2.
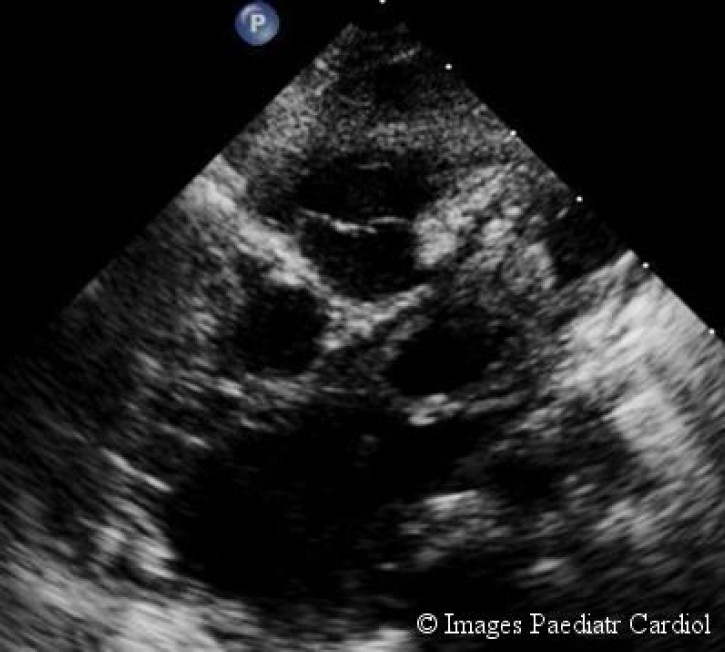
Left coronary artery appears to arise from aorta (may lead to misdiagnosis).
Figure 3.
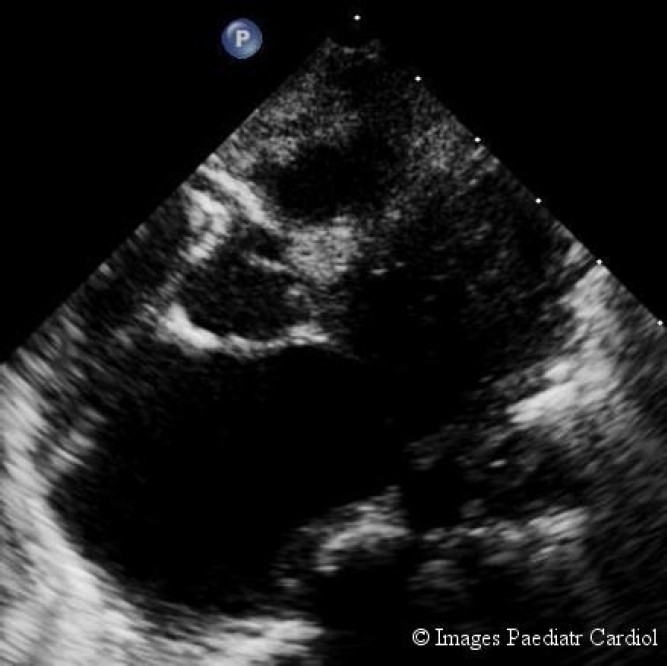
Dilated right coronary artery is seen.
On further probing with color Doppler, the LCA was clearly identified to originate from pulmonary artery (PA) with retrograde flow towards the great vessels (blue) (fig. 4), and the origin from PA was seen with red flow in the PA (fig. 5). This clinched the diagnosis of ALCAPA. The patient underwent successful surgical correction.
Figure 4.
Moderate mitral regurgitation, hypokinetic posterior wall. Note the hyperechogenic papillary muscles.
Figure 5.
The left coronary carries blood towards the great vessels (blue, contrary to the normal red), and the origin of left coronary artery from PA is seen as red flow into the pulmonary artery.
Discussion
ALCAPA is the abnormal origin of the LCA from one of the pulmonary sinuses. Though well tolerated in fetal life, the reduction in the pulmonary artery pressure after birth leads to myocardial ischemia in the LCA territory. Ischaemic chest pain in infants is often misdiagnosed and patients generally present with a picture of ‘dilated cardiomyopathy’ and some mitral regurgitation after significant myocardial injury. A variable degree of collaterals between the RCA and the LCA develop and there may be significant left to right shunt in the survivors. Differences in the collateralization and the severity of pulmonary hypertension are responsible for the differences in the clinical presentation.
Electrocardiographic features
Although the ECG is usually abnormal, a normal ECG does not rule out ALCAPA.1 Abnormal q waves in the lateral leads (I, aVL and V4-V6) are commonly seen in ALCAPA, unlike normal infants in whom this is extremely rare. Q waves in inferior leads are uncommon in ALCAPA. Small q wave may be occasionally seen in lead II, but q waves in lead III are not seen in ALCAPA. The morphology of the q wave is also unique in that it is much deeper but narrower when compared to adult ischemic heart disease. A typical example of ALCAPA ECG from another patient is shown in fig. 6. The ECG, especially in older children, may show left ventricular hypertrophy with left axis deviation of QRS (due to the hypertrophy of the posterior basal part of left ventricle). Other features include poor R wave progression with loss of R wave height and ST-T changes in left precordial leads.
Figure 6.
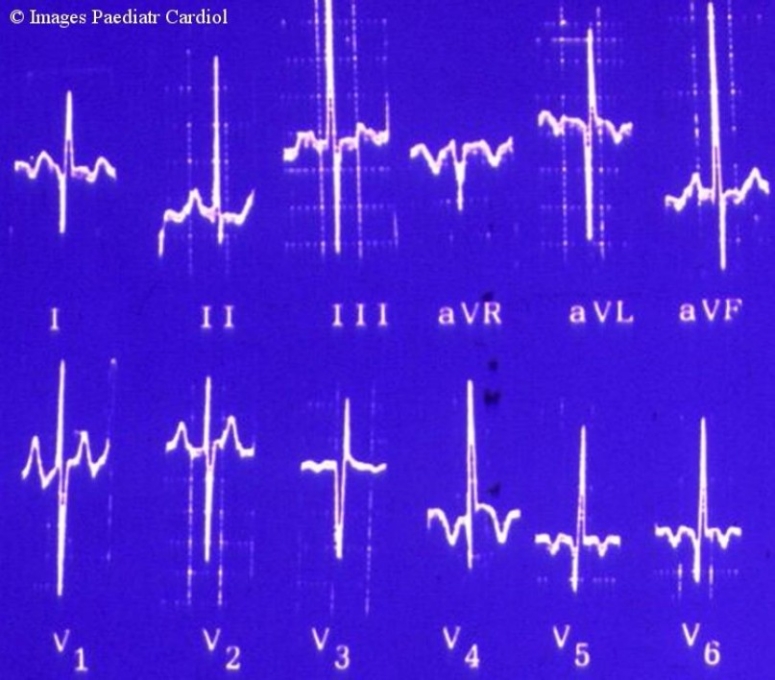
Typical ALCAPA EKG, from another patient showing typical findings of pathological Q waves in I, V3-V6.
Echocardiographic diagnosis
The diagnosis of ALCAPA on echocardiography is often easy, but occasionally missed even by an experienced echocardiographer. In most cases children are referred to the echo lab to identify the etiology of congestive heart failure. ALCAPA should be suspected in all young infants with ventricular dysfunction and regional wall motion abnormality akin to that seen in myocardial infarction. Classically there is scarred and thinned out myocardium at the anterolateral LV while the postrobasal part may show hypertrophy and hyperfunction. Features such as hyperechogenecity (scarring) of the papillary muscles, endocardial fibroelastosis of LV and mitral regurgitation are almost always present, but often subtle and may be missed if not looked for.
Dilated right coronary system including posterior descending artery and the presence of myocardial sinusoids especially in the ventricular septum also suggest ALCAPA.2,3 It is important to note that these features are often not prominent, especially in patients with poor collaterals (who have severe ventricular dysfunction).
Once ALCAPA is suspected because of clinical and indirect echocardiographic clues, direct imaging of the coronary origin should be attempted. Usually it is possible to identify LCA originating from PA, but in difficult cases a high parasternal position in parasagittal long axis view of main PA is often useful. Absence of aortic origin of LCA in a minimum of three views was proposed as a diagnostic criterion by some authors.4 But sometimes, spurious aortic origin (thus missing the diagnosis) can be ‘created’ due to presence of fluid filled transverse sinus of pericardium which can artificially mimic the origin of the left coronary artery from aorta.5 Color Doppler showing the flow in coronary artery towards the aorta (blue) is abnormal and confirms the diagnosis of ALCAPA. Yet again, this is not always present either because of angulation of the transducer, or due to the presence of pulmonary artery hypertension.6
Conclusion
ALCAPA is an important cause of dilated cardiomyopathy like picture in infants. A high index of suspicion and awareness of various pitfalls in the diagnosis of ALCAPA can help in achieving the right diagnosis.
References
- 1.Flaherty JT, Spach MS, Boineau JP, Canent RV, Jr, Barr RC, Sabiston DC., Jr Cardiac Potentials on Body Surface of Infants with Anomalous Left Coronary Artery (Myocardial Infarction) Circulation. 1967;36:345–58. doi: 10.1161/01.cir.36.3.345. [DOI] [PubMed] [Google Scholar]
- 2.Myers KA, Pitts MT, Sandor GG. Abnormal flow in the posterior descending artery: an echo doppler clue to the anomalous origin of left anterior descending coronary artery from the pulmonary trunk. Pediatr Cardiol. 2009;30:546–8. doi: 10.1007/s00246-009-9388-3. [DOI] [PubMed] [Google Scholar]
- 3.Hildreth B, Junkel P, Allada V, Sintek C, Sapin S. An uncommon echocardiographic marker for anomalous origin of the left coronary artery from the pulmonary artery: Visualization of intercoronary collaterals within the ventricular septum. Pediatr Cardiol. 2001;22:406–8. doi: 10.1007/s002460010263. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell RL, Hurwitz RA, Girod DA, Weyman AE, Feigenbaum H. Two-dimentional echocardiographic differentiation of anomalous left coronary artery from congestive cardiomyopathy. Am Heart J. 1983;106:710–6. doi: 10.1016/0002-8703(83)90092-3. [DOI] [PubMed] [Google Scholar]
- 5.Robinson PJ, Sullivan ID, Kumpeng V, Anderson RH, Macartney FJ. Anomalous origin of the left coronary artery from the pulmonary trunk. Potential for false negative diagnosis with cross sectional echocardiography. Br Heart J. 1984;52:272–7. doi: 10.1136/hrt.52.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta A, Shah S, Misri A, Suresh PV, Maheshwari S. Severe mitral regurgitation: a misleading presentation. Ind Heart J. 2009;61:303–5. [PubMed] [Google Scholar]


