Abstract
The serine-protease OMI/HTRA2, required for several cellular processes, including mitochondrial function, autophagy, chaperone activity, and apoptosis, has been implicated in the pathogenesis of both Alzheimer's disease (AD) and Parkinson's disease (PD). Western blot quantification of OMI/HTRA2 in frontal cortex of patients with AD (n=10) and control subjects (n=10) in two separate materials indicated reduced processed (active, 35 kDa) OMI/HTRA2 levels, whereas unprocessed (50 kDa) enzyme levels were not significantly different between the groups. Interestingly, the specific protease activity of OMI/HTRA2 was found to be significantly increased in patients with AD (n=10) compared to matched control subjects (n=10) in frontal cortex in two separate materials. Comparison of OMI/HTRA2 mRNA levels in frontal cortex and hippocampus, two brain areas particularly affected by AD, indicated similar levels in patients with AD (n=10) and matched control subjects (n=10). In addition, we analyzed the occurrence of the OMI/HTRA2 variants A141S and G399S in Swedish case-control materials for AD and PD and found a weak association of A141S with AD, but not with PD. In conclusion, our genetic, histological, and biochemical findings give further support to an involvement of OMI/HTRA2 in the pathology of AD; however, further studies are needed to clarify the role of this gene in neurodegeneration.—Westerlund, M., Behbahani, H., Gellhaar, S., Forsell, C., Carmine Belin, A., Anvret, A., Zettergren, A., Nissbrandt, H., Lind, C., Sydow, O., Graff, C., Olson, L., Ankarcrona, M., Galter, D. Altered enzymatic activity and allele frequency of OMI/HTRA2 in Alzheimer's disease.
Keywords: postmortem tissue, PRSS25, Western blot, in situ hybridization, protease assay
The serine-protease OMI, originally identified as a homologue of the bacterial chaperone high-temperature requirement A (HTRA), has been implicated in the pathogenesis of neurodegenerative disorders, such as Alzheimer's disease (AD) and Parkinson's disease (PD).
Involvement of OMI/HTRA2 in degenerative processes typical of AD pathology were first suggested when the enzyme was identified as presenilin-1 (PS1) interacting factor in a 2-hybrid system (1). Binding of PS1 increased the protease activity of OMI/HTRA2 (2), whereas the interaction between PS1 as part of the active γ-secretase complex and mouse Omi/HtrA2 in mitochondria resulted in reduction of the γ-secretase activity (3). The C-terminal region of OMI/HTRA2 was also identified as a binding site for amyloid β-peptide (Aβ) (4), and the protease has been implicated in the intracellular amyloid-precursor protein (APP) metabolism. In a cell-free in vitro system, OMI/HTRA2 delayed aggregation of Aβ, indicating a chaperone function of the enzyme (5). These findings are in line with an earlier study suggesting that a fraction of OMI/HTRA2 localizes to the endoplasmic reticulum, where it binds to immature APP and regulates APP degradation (6). A chaperone activity of OMI/HTRA2 has also been highlighted recently through the finding that its PDZ domain selectively binds and detoxifies neurotoxic oligomeric forms of Aβ (7).
Other lines of evidence linked OMI/HTRA2 to PD. The mouse strain mnd2 (motor neuron degeneration-2) displays striatal degeneration, microglia activation and Parkinson-like features, which are caused by a spontaneously occurring point mutation in Omi/HtrA2 (8, 9). More recently, OMI/HTRA2 was implicated in PD through a possible link to the PTEN-induced putative kinase-1 (PINK1) at the PARK6 locus (10). Phosphorylation of OMI/HTRA2 by PINK1 has been suggested to alter the protease activity, thereby increasing the resistance to mitochondria-induced stress. In agreement with these findings, decreased OMI/HTRA2 phosphorylation has been observed in brains of patients with PD carrying mutations in PINK1 (11). Finally, genetic studies have also implicated OMI/HTRA2 in PD (11, 12). Using a candidate gene approach, Strauss et al. (12)discovered two OMI/HTRA2 genetic variants in German PD patients. The two variants A141S (Ala141Ser; c.421G>T, rs72470544) and G399S (Gly399Ser; c.1195G>A, rs72470545), are located in exon 1 and 7, respectively, and result in defective OMI/HTRA2 protease activity, similar to the mouse mutation in the mnd-2 strain. On the basis of these data, it has been suggested that mutations abolishing OMI/HTRA2 protease activity may cause increased susceptibility to mitochondrial stress and neuronal death, thereby increasing the risk of developing PD.
The OMI/HTRA2 gene, at chromosome 2p12–13, codes for an inactive precursor protein (∼50 kDa), as is the case for most trypsin-like serine proteases. The precursor protein is mostly targeted to the mitochondrial intermembrane space due to an N-terminal signal and is processed to the proteolytically active form (∼35 kDa), most likely through autocleavage at amino acid residue 133, forming active noncovalent homotrimers (13). The first function described for OMI/HTRA2 was its role during apoptosis, when the active enzyme is released into the cytosol (14), binds to inhibitor of apoptosis proteins (IAPs), and relieves the inhibition of caspases (15–17). A caspase-independent proapoptotic role of OMI/HTRA2 has been described in B-lymphoid cells, following interleukin-3 withdrawal (18). Additional roles of OMI/HTRA2 are emerging, implicating the enzyme in modulation of mitochondrial activity and morphology through interaction with the fusion factor OPA1 (19), as well as a role in mitochondrial homeostasis and quality control of the mitochondrial network (20). A recent study demonstrated a further neuroprotective mechanism of OMI/HTRA2 by showing that the proteolytic activity of OMI/HTRA2 enhances autophagy in cell lines and increases degradation of mutant proteins involved in neurodegeneration such as α-synuclein and huntingtin (21).
Despite the biochemical studies implicating OMI/HTRA2 in AD pathology, few data are available on possible changes in expression levels or enzyme activity in AD brain tissue. A recent report showed that in AD brain tissue, OMI/HTRA2 immunoreactivity localizes to senile plaques and neurofibrillary tangles (22). We investigated proteolytic activity and expression of OMI/HTRA2 in human postmortem brain tissues and analyzed the occurrence of the protease-deficient gene variants A141S and G399S in Swedish AD and PD case-control samples.
MATERIALS AND METHODS
Human brain tissue
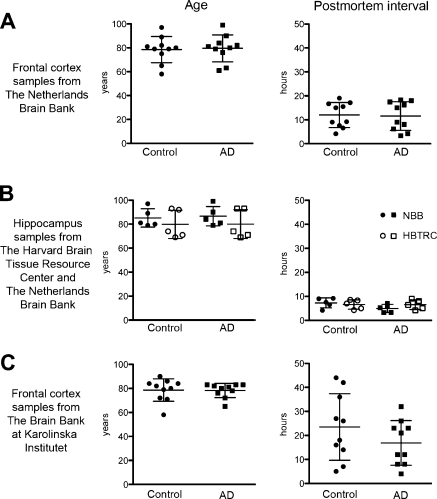
Human postmortem brain samples were provided by 3 different brain banks. A comparison of age and postmortem interval (PMI) of the 3 brain sample materials is shown in Fig. 1. Frontal cortex (AD, n=10; control, n=10) and hippocampus samples (AD, n=5; control, n=5) were provided by the Netherlands Brain Bank (NBB; Amsterdam, The Netherlands; Fig. 1A). Hippocampus samples were also provided by the Harvard Brain Tissue Resource Center (HBTRC; Belmont, MA, USA; AD, n=5; control, n=5; Fig. 1B). Cryosections of all brain samples were mounted on Superfrost Plus microscope slides (Mezel-Gläser, Braunschweig, Germany) and used for in situ hybridization studies. Additional sections from the frontal cortex samples from NBB were also used for protein quantification and enzyme activity assays. Further tissue samples from frontal cortex were provided by the Brain Bank at Karolinska Institutet (BBKI; Stockholm, Sweden; AD, n=10; control, n=10; Fig. 1C) and used for protein quantification and enzyme activity assay. AD and control subjects were matched for age, sex (not shown), and PMI in each individual material, while there were substantial differences between the materials, particularly with regard to PMI range.
Figure 1.
Age and postmortem interval (PMI) matching of controls and AD cases from 3 different sources. A) The Netherlands Brain Bank (NBB) provided frontal cortex samples from 10 pairs of age-, sex-, and PMI-matched AD and control brains, which were used for both in situ hybridization and protein assays. B) Hippocampus samples from the Harvard Brain Tissue Resource Center (HBTRC; AD, n=5; control, n=5) were analyzed by in situ hybridization in combination with hippocampus samples from the NBB (AD, n=5; control, n=5), all with a short PMI. C) The Brain Bank at Karolinska Institutet (BBKI) provided frontal cortex samples from 10 additional age- and sex-matched brains with a longer PMI, which were used for protein analysis.
S100 extract preparation and Western blot analysis
Frozen cortical brain tissue from NBB and BBKI was homogenized in cold (4°C) buffer (0.23 M mannitol, 0.07 M sucrose, 20 mM HEPES, 0.5 mM EDTA, 0.1% BSA, and 2% n-dodecyl-β-d-maltoside, pH 7.2), and centrifuged at 20,000 g at 4°C. The supernatants were further centrifuged at 100,000 g, resulting in S100 extracts. Protein concentrations were determined using a protein assay kit (Pierce BCA; VWR International AB, Stockholm, Sweden). Samples were dissolved in Laemmli buffer, and equal amounts (20 μg) were resolved on a gradient Tris-glycine SDS-PAGE gel (Bio-Rad, Hercules, CA, USA) and transferred to a nitrocellulose membrane. Nonspecific binding was blocked with 5% nonfat milk, and membranes were incubated overnight with an anti-OMI/HTRA2 antibody specific for both processed (35 kDa) and unprocessed (50 kDa) enzyme (R&D Systems, Minneapolis, MN, USA; 1:2000, rabbit polyclonal) or an anti-β-actin antibody (A2066; Sigma, St Louis, MO, USA; 1:700, rabbit polyclonal). Following incubation with appropriate secondary antibodies (horseradish peroxidase-linked antibody; Amersham Bioscience; GE Healthcare AB, Stockholm, Sweden) signals were developed using a chemoluminescence substrate (Pierce Super Signal West Pico kit; Pierce, Thermo Fisher Scientific, Lowell, MA, USA). Western blot signals were analyzed and quantified using a CCD digital imaging camera with QuantityOne software (Bio-Rad, Hercules CA, USA). After appropriate exposure time, the signal intensity of each particular lane from 2 separate experiments was quantified. The quantified average values of OMI/HTRA2 was normalized to the average β-actin for each brain sample and grouped according to disease status. Group levels were compared with Student's t test using GraphPad Prism 5 (GraphPad, San Diego, CA, USA) and expressed as means ± sd.
OMI/HTRA2 enzyme activity assay
The proteolytic activity was analyzed in S100 extracts from frontal cortex samples provided by NBB and BBKI. The assay is based on immunoprecipitation of OMI/HTRA2, and the use of a fluorescent peptide and has been adapted for tissue samples from a previously described assay for cell extracts (23). Briefly, S100 extracts were incubated with OMI/HTRA2 antibody (R&D Systems) for 24 h. The OMI-antibody complexes were bound to the preimmune serum precoupled to protein A- and G-Sepharose beads at 4°C for 1 h. Beads were then washed 3 times with PBS and resuspended in protease assay buffer (50 mM Tris-HCl, pH 8.0; 0.5 mM EDTA; and 1 mM dithiothreitol) containing 10 μM H2-Opt fluorescent substrate [H2-optimal substrate; Mca-IRRVSYSF (Dnp) KK, where Mca is 7-methoxycoumarin-4-acetic acid and Dnp is N-dinitrophenyldiaminopropionic acid, as described in detail in ref. 23] for activity assays and incubated for 1 h at 30°C. Fluorescence was monitored on a multiwell plate reader, (Tecan, Grödig, Austria) using the filter pair Ex (360/40), Em (395/25). To control for background fluorescence, we used either buffer only (B) or buffer with fluorescent peptide (H2-Opt). As specificity control of the OMI/HTRA2 proteolytic activity, we used purified inactive Omi/HtrA2; S276C, a missense mutation in the Omi/HtrA2 gene, which is located in the protease domain and results in a loss of protease function (kind gift from L. Miguel Martins, Cell Death Regulation Laboratory, MRC Toxicology Unit, Leicester, UK).
In situ hybridization
The in situ hybridization method is based on the protocol described earlier (24). In brief, target mRNA-specific oligonucleotide probes were labeled with 33P-dATP (Perkin Elmer, Boston, MA, USA) using terminal deoxynucleotidyl transferase (TdT) (Amersham Biosciences, Little Chalfont, UK). Cryosections were hybridized overnight with the radiolabeled oligonucleotide, washed, dehydrated and exposed to autoradiographic films (BioMax; Eastman Kodak, Rochester, NY, USA). After appropriate exposure time, films were developed and digitalized, and signal intensity was quantified using the Image J program. The 10 samples from NBB for which both hippocampus and frontal cortex samples were provided (5 AD and 5 control) had a short PMI, comparable to the HBTRC samples (Fig. 1B), and results from mRNA quantification were, therefore, combined. Expression levels were compared with Student's t test using appropriate software (GraphPad Prism 5).
The OMI/HTRA2-specific oligonucleotide sequences corresponded to exons 3–5 and 8, respectively (NM_013247, nt 1501–1547 and 1862–1911). The glial fibrillary acidic protein (GFAP)-specific sequence corresponded to exons 3–4 (NM_002055, nt 616–666). The oligonucleotide sequence specific for Ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) has been described before (25).
AD and PD case-control DNA materials
The AD and PD case-control materials were obtained after informed consent and approval of the local ethics committee, and DNA samples were prepared from peripheral blood according to standard protocols. The AD sample consisted of 351 patients (Karolinska University Hospital Huddinge; mean age of onset 78.9 yr), and 348 nondemented, age and gender-matched control participants from the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K; mean age 79.0; control 1). The material has been discussed in more detail earlier (26).
The PD sample consisted of 281 patients (Karolinska University Hospital, Stockholm and Sahlgrenska Hospital, Göteborg, Sweden; mean age of onset 60.3 yr) and 308 neurologically healthy control participants (213 from the Karolinska University Hospital, Stockholm, and Sahlgrenska Hospital, Göteborg; mean age 63.3 yr; and 95 blood donors of unknown age from the Karolinska University Hospital, Stockholm, Sweden; control 2).
Genotyping
Pyrosequencing was used to genotype the variants A141S (rs72470544, 421G>T) and G399S (rs72470545, 1195G>A). In brief, Taq polymerase, a forward and a reverse primer (one labeled with biotin in the 5′-end) were used to amplify an ∼150-bp fragment of genomic DNA containing the variable region of interest (27). For A141S, we used the following primers: a biotinylated forward primer, 5′-GGCAGTGCTGTTGTTGTTGT-3′; a reverse primer, 5′-CGATATAGACCACGGCAGGT-3′; and a sequencing primer, 5′-GACTCCGGGGAGAAG-3′. For G399S, we used as biotinylated primer 5′-TTGGCTAATAGGGTGATCTGTG-3′, reverse primer 5′-TATCACACTGCAGCCTCTCC-3′, and sequencing primer 5′-GGTGTGCAGGGGAG-3′. Biotinylated DNA sequences were immobilized onto streptavidin-coated Sepharose beads. Using a Vacuum Prep Tool (Biotage, Uppsala, Sweden), we separated the DNA fragment from the PCR reaction, washed in 70% ethanol, denatured in 0.2 M NaOH, and annealed to a sequencing primer (∼15 bases) in the opposite direction as the biotinylated primer. Mutation analysis was performed using a SNP reagent kit and pyrosequencing (PSQ 96MA system; Biotage AB, Uppsala, Sweden). Differences in genotype distributions were analyzed using a χ2 test and 2-sided P values. Statistical significance was defined as P < 0.05. Distributions of genotypes were tested for Hardy-Weinberg equilibrium consistency in patients and control subjects. A141S (TT) and G399S (AA) were not detected in the material; hence, the genotypes were excluded from the statistical analysis.
RESULTS
Processed OMI/HTRA2 levels are decreased in frontal cortex from AD brains
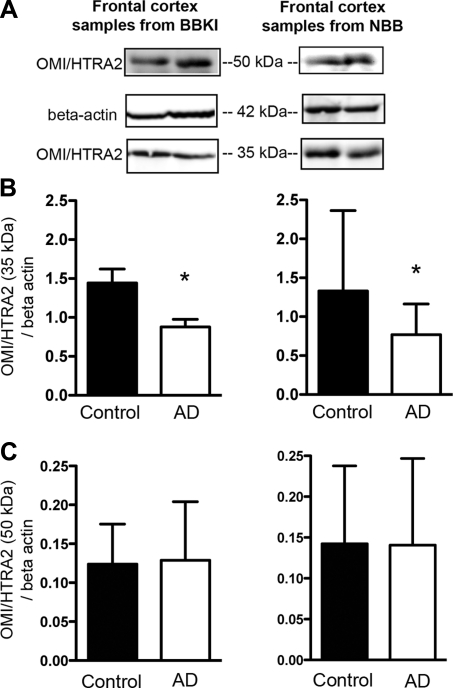
To examine OMI/HTRA2 protein levels, S100 extracts were prepared from postmortem frontal cortex, followed by Western blot analysis with an anti-OMI/HTRA2-specific antibody, and the signal densities were quantified (Fig. 2A). Densitometry evaluations of processed OMI/HTRA2 (35 kDa) levels normalized to β-actin revealed a significant decrease in AD cases compared to controls in both tissue samples from BBKI (AD, n=10; control, n=10) and from NBB (AD, n=10; control, n=10; Fig. 2B). On the other hand, levels of unprocessed OMI/HTRA2 (50 kDa) normalized to β-actin did not differ markedly between AD and control brains regardless of the source of brain tissue (BBKI and NBB).
Figure 2.
Comparison of OMI/HTRA2 protein levels in frontal cortex of AD and controls from two separate sources: BBKI and NBB. S100 extracts were prepared from postmortem frontal cortex and probed with an OMI/HTRA2 antibody. A) Representative OMI/HTRA2 and β-actin immunoblots for AD and control brains. B, C) Quantification of 35 kDa (processed; B) and 50 kDa (unprocessed; C) OMI/HTRA2 normalized to β-actin. Values are means ± sd. *P < 0.05; Student's t test.
OMI/HTRA2 enzyme activity was enhanced in cortex from patients with AD compared to control subjects
In addition to the protein levels, we compared the enzymatic activity of OMI/HTRA2 in frontal cortex samples and compared it between AD and control subjects.
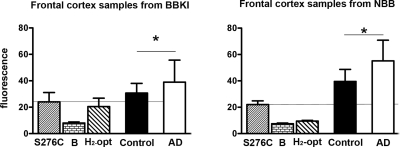
In contrast to protein levels, OMI/HTRA2-specific protease activity was found to be significantly higher in frontal cortex from patients with AD compared to control subjects (Fig. 3). The increased enzymatic activity in patients with AD was observed in both materials (BBKI and NBB), although the overall activity was higher in brain samples from NBB, possibly reflecting the much lower PMI (see Fig. 1).
Figure 3.
Analysis of OMI/HTRA2 proteolytic activity in cortical tissue of patients with AD and age-matched control individuals from the BBKI and the NBB (AD, n=10+10; control, n=10+10). OMI/HTRA2 protease activity was significantly higher in AD brains as compared to control brains in both groups. S276C denotes fluorescence induced by purified, mutated, inactive OMI/HTRA2; B denotes background fluorescence of the buffer only; H2-Opt denotes fluorescent substrate added to the buffer. *P < 0.05; Student's t test.
No significant difference in OMI/HTRA2 mRNA expression levels between AD and control brains in frontal cortex and hippocampus
OMI/HTRA2 mRNA expression was analyzed and quantified together with other neuronal and glial markers in two brain regions affected by AD, frontal cortex and hippocampus.
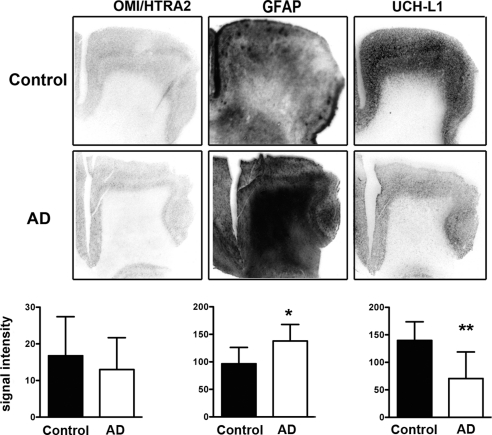
We analyzed gene expression levels in frontal cortex samples provided by NBB (AD, n=10; matched control, n=10; Fig. 4). Quantification of OMI/HTRA2 mRNA levels, analyzed with a probe targeting exons 3–5, indicated a slight reduction in cortical layers of AD brains; however, the reduction was not significantly different from control levels. Analysis with a second OMI/HTRA2-specific probe targeting exon 8, common to both splice variants described for this gene, generated similar results (data not shown). By contrast, levels of GFAP mRNA were significantly increased in the cortical gray matter of AD frontal cortex, and, conversely, levels of UCH-L1 mRNA were significantly decreased in cortical layers of AD brains compared to matched controls. Furthermore, OMI/HTRA2 mRNA levels were much lower compared to both GFAP and UCH-L1 in all samples.
Figure 4.
OMI/HTRA2, GFAP, and UCH-L1 mRNA expression in frontal cortex of patients with AD and control subjects. mRNA expression levels for all 3 genes were quantified in gray matter and compared between control subjects (n=10) and patients with AD (n=10). Values are means ± sd. *P < 0.05, **P < 0.01; Student's t test.
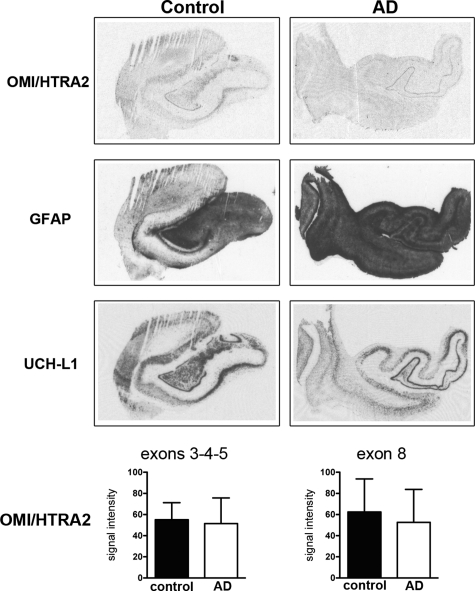
For quantification analysis of OMI/HTRA2 mRNA levels in hippocampus (Fig. 5), brain samples provided by NBB (AD, n=5; control, n=5) and HBTRC (AD, n=5; control, n=5) were pooled, since mean age and PMI were comparable (see Fig. 1B). We detected relatively high expression of OMI/HTRA2 mRNA in the densely packed layer of granular neurons in the dentate gyrus, but no significant difference between AD and control brains. In situ hybridization with a second oligonucleotide probe targeting exon 8 of OMI/HTRA2 gave again very similar results (Fig. 5).
Figure 5.
OMI/HTRA2, GFAP, and UCH-L1 mRNA expression in the hippocampus of Alzheimer's patients and controls. OMI/HTRA2 expression levels were quantified in the granular layer of the dentate gyrus of AD samples (n=10) and control samples (n=10) using 2 oligonucleotide probes targeting different exons of the gene (exons 3–5 or exon 8, respectively). OMI/HTRA2 examples shown were hybridized with the probe targeting exon 8. Values are means ± sd; significance was tested with Student's t test.
Association analysis of A141S and G399S in AD and PD case-control materials
We genotyped Swedish AD and PD case-control materials for the occurrence of the two missense variants A141S and G399S in OMI/HTRA2 using pyrosequencing (Table 1). The selection of the two genetic variants was based on findings from a previous study performed in a German PD material (12). Comparing genotype frequencies between cases and controls using the χ2 test revealed a weak association between the A141S variant and AD (uncorrected value of P=0.0453), with the heterozygote GT genotype being more frequent among patients. Comparing genotype frequencies of the A141S variant in the PD case-control material showed no statistically significant difference between the groups. Moreover, the G399S mutation was absent in the AD case-control material, and its distribution was not significantly different between PD cases and controls. We also compared allele frequencies of the A141S and G399S variants in the AD and PD case-control materials and report a weak association between A141S and AD, with the T-allele being more common in patients than control subjects (P=0.049; data not shown).
Table 1.
Genotype frequencies of the OMI/HTRA2 missense variants A141S and G399S in Swedish AD and PD patients compared to controls
| Variant | Genotype frequency [% (n)] | Patients (n) | χ2 [OR (95% CI)] | P value | ||
|---|---|---|---|---|---|---|
| AD | ||||||
| A141S | GG | GT | TT | Total | χ2 | P |
| Control 1 | 95.6 (305) | 4.4 (14) | 0 (0) | 319 | 1.96 | 0.0453 |
| AD | 91.7 (289) | 8.3 (26) | 0 (0) | 315 | (1.003 to 3.828) | |
| G399S | GG | GA | AA | Total | χ2 | P |
| Control 1 | 100 (318) | 0 (0) | 0 (0) | 318 | NA | NA |
| AD | 100 (304) | 0 (0) | 0 (0) | 304 | ||
| PD | ||||||
| A141S | GG | GT | TT | Total | χ2 | P |
| Control 2 | 94.3 (280) | 5.7 (17) | 0 (0) | 297 | 0.8111 | 0.5793 |
| PD | 95.3 (264) | 4.7 (13) | 0 (0) | 277 | (0.3863 to 1.703) | |
| G399S | GG | GA | AA | Total | χ2 | P |
| Control 2 | 99.0 (298) | 1.0 (3) | 0 (0) | 301 | 0.3625 | 0.3625 |
| PD | 99.6 (274) | 0.4 (1) | 0 (0) | 275 | (0.0375 to 3.508) | |
The two control materials, control 1 and control 2, were age-matched to the AD and PD materials, respectively. A141S (TT) and G399S (AA) were not detected in the materials, and hence, they were excluded from the statistical analysis. OR, odds ratio; CI, confidence interval; NA, not applicable.
DISCUSSION
OMI/HTRA2 has been suggested to be implicated in the pathogenesis of both AD and PD due to its important roles in mitochondrial maintenance and programmed cell death (13), authophagy (21), and proteolytic stress (7). Until now, only one study has analyzed OMI/HTRA2 in human brain material, although biochemical studies using cell culture conditions or isolated proteins have reported an interaction of OMI/HTRA2 with key factors of the AD pathology: Aβ (4, 28), APP (6), PS1 (1), and γ-secretase (3). Recently, Kawamoto et al. (22) reported for the first time abnormal accumulation of OMI/HTRA2 immunoreactivity in senile plaques and neurofibrillary tangles, the neuropathological hallmarks of AD, but the researchers caution that the finding might be nonspecific and that further analyses of human brain tissue are necessary to clarify the role of OMI/HTRA2 in AD.
In this study, we investigated OMI/HTRA2 expression levels and enzymatic activity in postmortem brain tissue from patients diagnosed with AD and control individuals matched for age, sex, and PMI. Interestingly, we detected a significant reduction of processed OMI/HTRA2 protein levels normalized to β-actin in patients with AD. The finding was observed in 2 separate materials of 10 control subjects and 10 patients with AD each, one collected in Sweden and another sampled in the Netherlands. Levels of unprocessed OMI/HTRA2 (50 kDa) normalized to β-actin were 10 times lower than those of processed OMI/HTRA2, and they were not significantly different from control levels. Our results from mRNA quantification of OMI/HTRA2 were in line with the finding of unperturbed levels of unprocessed enzyme. As expected, we did not find differences between the mRNA expression levels quantified with two different oligonucleotide probes targeting exon 8 and exons 3–5, respectively. Both target sequences are common to the splice variants described for OMI/HTRA2 (1). In both brain regions analyzed, frontal cortex, and the dentate gyrus, the mRNA levels revealed no significant differences between patients and control subjects. One possible explanation could be that pathological changes occur mostly at the post-translational level and induce increased degradation of processed OMI/HTRA2 in patients with AD. Furthermore, we showed significantly reduced UCH-L1 mRNA levels, indicating neuronal loss or reduced cellular levels. However, the cortical neuronal loss typical of AD is not reflecting significant reduction of OMI/HTRA2, possibly because of the low expression level. We also found significantly increased levels of the astroglial marker GFAP mRNA in the gray matter of frontal cortex, in line with the often-reported gliosis in patients with AD. Both results are expected in view of earlier analysis, and they validate the suitability of the tissue and methods applied.
The unperturbed OMI/HTRA2 mRNA levels and reduced levels of processed OMI/HTRA2 protein in AD frontal cortex are in contrast to the significantly increased enzymatic activity observed in the AD brain samples. This result is particularly interesting since the OMI/HTRA2 enzymatic activity is essential for its normal function in autophagy (21), as well as caspase-dependent (14–17) and caspase-independent cell death (18). It can be speculated that the increased OMI/HTRA2 enzyme activity in AD brains is part of a compensatory mechanism at the cellular level, in order to reduce the proteolytic stress of neurotoxic Aβ through activation of autophagy, a mechanism described recently in cell culture and in a mouse model of AD.
The increased enzyme activity in AD brain tissue is unexpected in view of the association of the A141S variant with AD. A previous study, which demonstrated a reduced proteolytic activity of S141 OMI/HTRA2 compared to the A141 variant, was performed in vitro, with purified recombinant enzyme. The OMI/HTRA2 proteolytic activity in tissues from S141 heterozygous carrier has not yet been reported, but it is possible that the expected mixture of S141 and A141 protein forms could influence the enzyme activity differently. It should be noted that only one of the brain samples analyzed in this study was carrying the A141S variant (data not shown). Neither the OMI/HTRA2 expression levels nor the protease activity from this brain sample deviated from the other AD cases. We therefore concluded that the elevated proteolytic activity detected in the 20 AD cases analyzed is determined by multiple factors possibly including genetic variants other than the ones analyzed in the present study.
Our finding might reflect several different pathological processes in the AD brain. On the one hand, the increased OMI/HTRA2 activity might enhance autophagy and be neuroprotective. On the other hand, proteolytic stress causes autocleavage of OMI/HTRA2 at A133, a process that facilitates the release into the cytosol and the binding to the antiapoptotic factor XIAP (29). It is possible that cells initially respond to stress conditions induced in the AD brain by increasing OMI/HTRA2 activity; however, severe stress may trigger apoptosis, leading to OMI/HTRA2 release from mitochondria and suppression of the inhibitor of apoptosis proteins (IAPs). In our study, we have measured total OMI/HTRA2 activity (mitochondrial and cytosolic activity) and cannot conclude whether the increased activity in AD brains is a protective response aiming at retaining mitochondrial function and homeostasis, or whether it reflects an activation of cell death processes.
Interestingly, a similar pattern of increased enzyme activity has recently been reported for neprilysin and insulin-degrading enzyme in cortical tissue of patients with AD (30), although enzyme levels had previously been reported unchanged or even decreased compared to matched control subjects (31, 32).
To find a genetic link between OMI/HTRA2 and AD, we investigated possible differences in genotype frequencies in an AD case-control material. On the basis of previous genetic studies, indicating a possible association with PD, we selected two missense variants A141S and G399S for analysis. Both variants cause defective activation of OMI/HTRA2 protease activity in vitro (12). Our results indicate for the first time a weak association of the nonsynonymous variant A141S with increased risk of AD. The significance of the association was weak (P<0.05), and applying the Bonferroni correction for multiple testing increases the P value (corrected P=0.0885). Therefore, further genetic studies in independent and larger AD case-control materials are needed in order to clarify the potential genetic link between OMI/HTRA2 variants and risk of AD.
In addition, we studied the occurrence of OMI/HTRA2 variants among Swedish patients with PD. Recent reports (33, 34), including a large-scale association study in 20 case-control materials (35) have not been able to replicate previous findings (12, 36) of an association between genetic variants of OMI/HTRA2 and risk for PD. In agreement with the lack of association previously reported, we did not find an association between OMI/HTRA2 variants and the risk of PD.
In summary, we report altered enzymatic activity and protein levels of OMI/HTRA2 in brain samples of patients with AD as compared to control subjects together with a weak association of a genetic variant of OMI/HTRA2 and risk for AD. Our data support recent in vitro studies showing a potential involvement of OMI/HTRA2 in AD pathology.
Acknowledgments
The authors thank Karin Lundströmer, Karin Pernold, Eva Lindqvist, and Ann-Christin Thelander for excellent technical assistance and Prof. Laura Fratiglioni (Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden) for providing control samples from the SNAC-K project. The NBB (Amsterdam, the Netherlands) and the BBKI (Stockholm, Sweden) provided human brain samples from patients and control subjects. Brain tissue was also provided by the HBTRC (Belmont, MA, USA), which is supported in part by U.S. Public Health Service grant PHS/R 24-MH 068855. The authors also thank Dr. Miguel L. Martins (Cell Death Regulation Laboratory, MRC Toxicology Unit, Leicester, UK) for the kind gift of the S276C mutant Omi/HtrA2 protein.
This study was supported by research grants from Swedish Brain Power, the Swedish Research Council, the Swedish Parkinson Foundation, the Swedish Brain Foundation, Dainippon Sumitomo Pharma (Osaka, Japan), and Karolinska Institutet Funds.
REFERENCES
- 1. Gray C. W., Ward R. V., Karran E., Turconi S., Rowles A., Viglienghi D., Southan C., Barton A., Fantom K. G., West A., Savopoulos J., Hassan N. J., Clinkenbeard H., Hanning C., Amegadzie B., Davis J. B., Dingwall C., Livi G. P., Creasy C. L. (2000) Characterization of human HtrA2, a novel serine protease involved in the mammalian cellular stress response. Eur. J. Biochem. 267, 5699–5710 [DOI] [PubMed] [Google Scholar]
- 2. Gupta S., Singh R., Datta P., Zhang Z., Orr C., Lu Z., Dubois G., Zervos A. S., Meisler M. H., Srinivasula S. M., Fernandes-Alnemri T., Alnemri E. S. (2004) The C-terminal tail of presenilin regulates Omi/HtrA2 protease activity. J. Biol. Chem. 279, 45844–45854 [DOI] [PubMed] [Google Scholar]
- 3. Behbahani H., Pavlov P. F., Wiehager B., Nishimura T., Winblad B., Ankarcrona M. (2010) Association of Omi/HtrA2 with gamma-secretase in mitochondria. Neurochem. Int. 57, 668–675 [DOI] [PubMed] [Google Scholar]
- 4. Park H. J., Seong Y. M., Choi J. Y., Kang S., Rhim H. (2004) Alzheimer's disease-associated amyloid beta interacts with the human serine protease HtrA2/Omi. Neurosci. Lett. 357, 63–67 [DOI] [PubMed] [Google Scholar]
- 5. Kooistra J., Milojevic J., Melacini G., Ortega J. (2009) A new function of human HtrA2 as an amyloid-beta oligomerization inhibitor. J. Alzheimers Dis. 17, 281–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huttunen H. J., Guenette S. Y., Peach C., Greco C., Xia W., Kim D. Y., Barren C., Tanzi R. E., Kovacs D. M. (2007) HtrA2 regulates beta-amyloid precursor protein (APP) metabolism through endoplasmic reticulum-associated degradation. J. Biol. Chem. 282, 28285–28295 [DOI] [PubMed] [Google Scholar]
- 7. Liu M. L., Liu M. J., Shen Y. F., Ryu H., Kim H. J., Klupsch K., Downward J., Hong S. T. (2009) Omi is a mammalian heat-shock protein that selectively binds and detoxifies oligomeric amyloid-beta. J. Cell Sci. 122, 1917–1926 [DOI] [PubMed] [Google Scholar]
- 8. Jones J. M., Albin R. L., Feldman E. L., Simin K., Schuster T. G., Dunnick W. A., Collins J. T., Chrisp C. E., Taylor B. A., Meisler M. H. (1993) mnd2: a new mouse model of inherited motor neuron disease. Genomics 16, 669–677 [DOI] [PubMed] [Google Scholar]
- 9. Jones J. M., Datta P., Srinivasula S. M., Ji W., Gupta S., Zhang Z., Davies E., Hajnoczky G., Saunders T. L., Van Keuren M. L., Fernandes-Alnemri T., Meisler M. H., Alnemri E. S. (2003) Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature 425, 721–727 [DOI] [PubMed] [Google Scholar]
- 10. Alnemri E. S. (2007) HtrA2 and Parkinson's disease: think PINK? Nat. Cell Biol. 9, 1227–1229 [DOI] [PubMed] [Google Scholar]
- 11. Plun-Favreau H., Klupsch K., Moisoi N., Gandhi S., Kjaer S., Frith D., Harvey K., Deas E., Harvey R. J., McDonald N., Wood N. W., Martins L. M., Downward J. (2007) The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nat. Cell Biol. 9, 1243–1252 [DOI] [PubMed] [Google Scholar]
- 12. Strauss K. M., Martins L. M., Plun-Favreau H., Marx F. P., Kautzmann S., Berg D., Gasser T., Wszolek Z., Muller T., Bornemann A., Wolburg H., Downward J., Riess O., Schulz J. B., Kruger R. (2005) Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson's disease. Hum. Mol. Genet. 14, 2099–2111 [DOI] [PubMed] [Google Scholar]
- 13. Vande Walle L., Lamkanfi M., Vandenabeele P. (2008) The mitochondrial serine protease HtrA2/Omi: an overview. Cell Death Differ. 15, 453–460 [DOI] [PubMed] [Google Scholar]
- 14. Suzuki Y., Imai Y., Nakayama H., Takahashi K., Takio K., Takahashi R. (2001) A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol. Cell 8, 613–621 [DOI] [PubMed] [Google Scholar]
- 15. Hegde R., Srinivasula S. M., Zhang Z., Wassell R., Mukattash R., Cilenti L., DuBois G., Lazebnik Y., Zervos A. S., Fernandes-Alnemri T., Alnemri E. S. (2002) Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J. Biol. Chem. 277, 432–438 [DOI] [PubMed] [Google Scholar]
- 16. Martins L. M., Iaccarino I., Tenev T., Gschmeissner S., Totty N. F., Lemoine N. R., Savopoulos J., Gray C. W., Creasy C. L., Dingwall C., Downward J. (2002) The serine protease Omi/HtrA2 regulates apoptosis by binding XIAP through a reaper-like motif. J. Biol. Chem. 277, 439–444 [DOI] [PubMed] [Google Scholar]
- 17. Verhagen A. M., Silke J., Ekert P. G., Pakusch M., Kaufmann H., Connolly L. M., Day C. L., Tikoo A., Burke R., Wrobel C., Moritz R. L., Simpson R. J., Vaux D. L. (2002) HtrA2 promotes cell death through its serine protease activity and its ability to antagonize inhibitor of apoptosis proteins. J. Biol. Chem. 277, 445–454 [DOI] [PubMed] [Google Scholar]
- 18. Vande Walle L., Wirawan E., Lamkanfi M., Festjens N., Verspurten J., Saelens X., Vanden Berghe T., Vandenabeele P. (2010) The mitochondrial serine protease HtrA2/Omi cleaves RIP1 during apoptosis of Ba/F3 cells induced by growth factor withdrawal. Cell Res. 20, 421–433 [DOI] [PubMed] [Google Scholar]
- 19. Kieper N., Holmstrom K. M., Ciceri D., Fiesel F. C., Wolburg H., Ziviani E., Whitworth A. J., Martins L. M., Kahle P. J., Kruger R. (2010) Modulation of mitochondrial function and morphology by interaction of Omi/HtrA2 with the mitochondrial fusion factor OPA1. Exp. Cell Res. 316, 1213–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dagda R. K., Chu C. T. (2009) Mitochondrial quality control: insights on how Parkinson's disease related genes PINK1, parkin, and Omi/HtrA2 interact to maintain mitochondrial homeostasis. J. Bioenerg. Biomembr. 41, 473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li B., Hu Q., Wang H., Man N., Ren H., Wen L., Nukina N., Fei E., Wang G. (2010) Omi/HtrA2 is a positive regulator of autophagy that facilitates the degradation of mutant proteins involved in neurodegenerative diseases. Cell Death Differ. 17, 1773–1784 [DOI] [PubMed] [Google Scholar]
- 22. Kawamoto Y., Ito H., Kobayashi Y., Suzuki Y., Takahashi R. (2010) Localization of HtrA2/Omi immunoreactivity in brains affected by Alzheimer's disease. Neuroreport 21, 1121–1125 [DOI] [PubMed] [Google Scholar]
- 23. Martins L. M., Turk B. E., Cowling V., Borg A., Jarrell E. T., Cantley L. C., Downward J. (2003) Binding specificity and regulation of the serine protease and PDZ domains of HtrA2/Omi. J. Biol. Chem. 278, 49417–49427 [DOI] [PubMed] [Google Scholar]
- 24. Galter D., Buervenich S., Carmine A., Anvret M., Olson L. (2003) ALDH1 mRNA: presence in human dopamine neurons and decreases in substantia nigra in Parkinson's disease and in the ventral tegmental area in schizophrenia. Neurobiol. Dis. 14, 637–647 [DOI] [PubMed] [Google Scholar]
- 25. Galter D., Westerlund M., Belin A. C., Olson L. (2007) DJ-1 and UCH-L1 gene activity patterns in the brains of controls, Parkinson and schizophrenia patients and in rodents. Physiol. Behav. 92, 46–53 [DOI] [PubMed] [Google Scholar]
- 26. Belin A. C., Bjork B. F., Westerlund M., Galter D., Sydow O., Lind C., Pernold K., Rosvall L., Hakansson A., Winblad B., Nissbrandt H., Graff C., Olson L. (2007) Association study of two genetic variants in mitochondrial transcription factor A (TFAM) in Alzheimer's and Parkinson's disease. Neurosci. Lett. 420, 257–262 [DOI] [PubMed] [Google Scholar]
- 27. Ronaghi M., Uhlen M., Nyren P. (1998) A sequencing method based on real-time pyrophosphate. Science 281, 363, 365 [DOI] [PubMed] [Google Scholar]
- 28. Liu M. L., Liu M. J., Kim J. M., Kim H. J., Kim J. H., Hong S. T. (2005) HtrA2 interacts with A beta peptide but does not directly alter its production or degradation. Mol. Cells 20, 83–89 [PubMed] [Google Scholar]
- 29. Seong Y. M., Choi J. Y., Park H. J., Kim K. J., Ahn S. G., Seong G. H., Kim I. K., Kang S., Rhim H. (2004) Autocatalytic processing of HtrA2/Omi is essential for induction of caspase-dependent cell death through antagonizing XIAP. J. Biol. Chem. 279, 37588–37596 [DOI] [PubMed] [Google Scholar]
- 30. Miners J. S., Baig S., Tayler H., Kehoe P. G., Love S. (2009) Neprilysin and insulin-degrading enzyme levels are increased in Alzheimer disease in relation to disease severity. J. Neuropathol. Exp. Neurol. 68, 902–914 [DOI] [PubMed] [Google Scholar]
- 31. Miners J. S., Van Helmond Z., Chalmers K., Wilcock G., Love S., Kehoe P. G. (2006) Decreased expression and activity of neprilysin in Alzheimer disease are associated with cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 65, 1012–1021 [DOI] [PubMed] [Google Scholar]
- 32. Carpentier M., Robitaille Y., DesGroseillers L., Boileau G., Marcinkiewicz M. (2002) Declining expression of neprilysin in Alzheimer disease vasculature: possible involvement in cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 61, 849–856 [DOI] [PubMed] [Google Scholar]
- 33. Ross O. A., Soto A. I., Vilarino-Guell C., Heckman M. G., Diehl N. N., Hulihan M. M., Aasly J. O., Sando S., Gibson J. M., Lynch T., Krygowska-Wajs A., Opala G., Barcikowska M., Czyzewski K., Uitti R. J., Wszolek Z. K., Farrer M. J. (2008) Genetic variation of Omi/HtrA2 and Parkinson's disease. Parkinsonism Relat. Disord. 14, 539–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simon-Sanchez J., Singleton A. B. (2008) Sequencing analysis of OMI/HTRA2 shows previously reported pathogenic mutations in neurologically normal controls. Hum. Mol. Genet. 17, 1988–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kruger R., Sharma M., Riess O., Gasser T., Van Broeckhoven C., Theuns J., Aasly J., Annesi G., Bentivoglio A. R., Brice A., Djarmati A., Elbaz A., Farrer M., Ferrarese C., Gibson J. M., Hadjigeorgiou G. M., Hattori N., Ioannidis J. P., Jasinska-Myga B., Klein C., Lambert J. C., Lesage S., Lin J. J., Lynch T., Mellick G. D., de Nigris F., Opala G., Prigione A., Quattrone A., Ross O. A., Satake W., Silburn P. A., Tan E. K., Toda T., Tomiyama H., Wirdefeldt K., Wszolek Z., Xiromerisiou G., Maraganore D. M. (2009) A large-scale genetic association study to evaluate the contribution of Omi/HtrA2 (PARK13) to Parkinson's disease. [E-pub ahead of print] Neurobiol Aging doi: 10.1016/j.neurobiolaging.2009.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bogaerts V., Nuytemans K., Reumers J., Pals P., Engelborghs S., Pickut B., Corsmit E., Peeters K., Schymkowitz J., De Deyn P. P., Cras P., Rousseau F., Theuns J., Van Broeckhoven C. (2008) Genetic variability in the mitochondrial serine protease HTRA2 contributes to risk for Parkinson disease. Hum. Mutat. 29, 832–840 [DOI] [PubMed] [Google Scholar]