In looking at the past, present and then to the future of this field of inflammation research, as in many facets of history and human experience, is it possible to accurately project the future of a scientific area? This is a particular challenge since many of the new and exciting discoveries in the biomedical sciences are, for the most part, serendipitous (1–3). As a result, our knowledge of pathophysiologic processes is nonlinear with time, entering as rather sporadic leaps in our appreciation of the complexities of human health and a given disease process. Certainly, from the relatively recent change in the concept of resolution as a passive means to complete an acute inflammatory response to the discovery and role of novel autacoid biosynthetic pathways for potent active mediators and mechanisms of resolution that can be used to stimulate the return to homeostasis is a 180° change in concepts and potential for new treatments in the past 25 yr (4–6). The prevailing notion in treating inflammation likely began with Hippocrates and the use of salicylates present in willow bark to knowledge of the cellular events in the acute inflammatory response and the ability of aspirin to inhibit key mediators of inflammation (7). Aspirin (a derivative of salicylates) proved to be one of the more commercially successful drugs. The total organic synthesis of aspirin was accomplished in the 1850s, and the drug was introduced into the market in the early 1900s. Therefore, many therapeutic agents were introduced with the goal of mimicking aspirin's mechanism of action, namely, inhibiting cyclooxygenases and prostaglandin biosynthesis (8, 9). The notion of blocking proinflammatory mediators, enzymes, and pathways, whether by small-molecule inhibitors, receptor antagonists, or neutralizing antibodies, is a concept that prevailed in the past century and is still in wide use today. Nonetheless, new evidence has emerged that points to an unobvious and new direction, namely, stimulating resolution and the return of the tissue to homeostasis with endogenous chemical mediators of resolution.
For this Silver Anniversary edition of The FASEB Journal, I shall take a brief tour of our understanding and the changes in concepts that have evolved around the process of resolution of acute inflammation and its implications for human disease as well as the opportunities that now open in terms of novel therapeutics and treatments.
It is appropriate to start with two definitions from Taber's Cyclopedic Medical Dictionary (10):
resolution 1. Decomposition; absorption or breaking down of the products of inflammation. 2. Cessation of inflammation without suppuration. The return to normal.
resolvent 1. Promoting disappearance of inflammation. 2. That which causes dispersion of inflammation.
These two definitions evolved since ancient times in our appreciation of disease and injury at the gross and tissue levels and, most important, the effect of phagocytic cells in inflamed tissues as viewed from tissue histology by pathologists (Table 1). As early as the ancient medical encyclopedias, as recorded in several chapters of the Canon of Medicine in the 11th century CE and translated to Europe around the 12th century (11), the notion of “resolvent mollificants” for the treatment of inflammation was clearly evident. However, what a resolvent constituted was not clear, nor was its molecular form known. As indicated by the definition in Taber's, a resolvent promotes or causes the dispersion of inflammation. In the present day, the first notions of an agonist stimulating processes that can return the inflamed tissues to homeostasis arise from the identification and structural elucidation of potent mediators termed resolvins, or resolution-phase interaction products (12–14).
Table 1.
Timeline for resolution of inflammation and regeneration link to ω-3 PUFAs
| Year | Discovery | Reference |
|---|---|---|
| 11th century; translated to the West in the 12th century | Resolvent mollificants for treating “inflammatory foci” | 11 |
| 1979 | Inflammatory exudate resolved vs. exudate organized | 15 |
| 1988 | Cellular events in resolution of inflammation; macrophage clearing of dead PMN cells and debris | 19–21 |
| 1994 | Complete resolution as an outcome of acute inflammation; chronic inflammation as a failure to resolve | 22, 26 |
| 1996 | Differential signals for chemical mediators | 32 |
| Lipoxins are a stop signal for PMN recruitment and nonphlogistic stimuli for monocytes | ||
| 1999 | COX-2 may have anti-inflammatory properties | 40 |
| 1999 | Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes | 75 |
| 2000 | Lipoxins stimulate macrophage phagocytosis of apoptotic PMN | 35, 52, 53, 76 |
| 2000 | Discovery of resolvin E1 and the link to ω-3 PUFAs; resolution is a biosynthetically active process | 12 |
| 2001 | Mediator eicosanoid class switching from prostaglandins and leukotrienes to lipoxins in resolution | 36 |
| 2002 | Discovery of the resolvins, protectins, and their aspirin-triggered forms | 13 |
| 2002 | Peptide mediators in resolution; annexin activation of ALX/FPR-2 receptor and enhanced macrophage phagocytosis | 52, 53, 76 |
| 2006 | Efferocytosis of apoptotic PMN cells; removal of macrophages; failure to chronicity | 49 |
| 2007 | Consensus report on resolution of inflammation: definitions and terms | 6 |
| 2009 | Report of the first patients treated with a resolvin, an agonist to stimulate and treat inflammation | 47 |
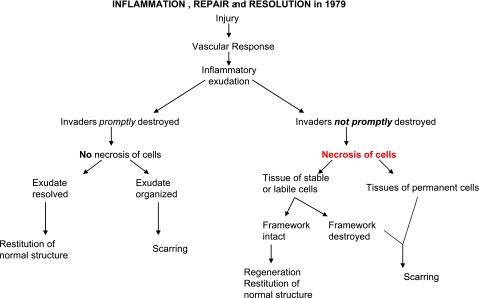
In the timeline of literature that gives rise to our appreciation of resolution of self-limited acute inflammation as an active biochemical process (Table 1), by 1979, Robbins and Cotran (15), in the second edition of their famous textbook of pathology, provided a schematic illustration (redrawn in Fig. 1) that depicts inflammatory exudate resolved vs. exudate reorganization. They also introduced the notion of precise cellular events involved in resolution vs. the path to chronic inflammation. Yet, at the time, these events were considered to be passive, in that the initial leukocyte stimulus, the chemoattractant, was thought to dissipate, simply burn out, or simply dilute the signal that would then lead to decreased infiltration or stopping of the leukocyte recruitment to the infected or injured tissues (16). Professor Ramzi Cotran (17) was first to recognize that the cells of the vessel wall, the endothelium, were activated, a process, along with neutrophil [polymorphonuclear (PMN) cell] activation (18), critical to mounting a well-integrated acute inflammatory response. The cellular events in the resolution of inflammation were further advanced from the studies of Savill, Henson, and Haslett in 1989 (19, 20), with the recognition that macrophages (the big eaters), which were already known to clear up tissue and cellular debris (21), advanced to uptake dead apoptotic PMN cells and cellular debris as part of the resolution sequence of events. Complete resolution as an ideal outcome of acute inflammation and the notion that chronic inflammation can evolve as a failure to resolve recurring bouts of acute inflammation emerged in 1994 in the fifth edition of the Cotran et al. (22) textbook. This set the stage for these cellular pictures and events in the resolution of inflammation when considering the context of an acute inflammatory response.
Figure 1.
Inflammation, repair, and resolution in 1979.
A little more than 25 yr ago, Sune Bergström, Bengt Samuelsson, and Sir John Vane each shook the hand of the Swedish king as they received their Nobel Prize medals in December 1982, for their work on the discovery and biosynthesis of the prostaglandins and on the action of aspirin. Prostaglandins play important roles in many physiological responses, e.g., renal function, hemodynamics, reproduction, and, notably, inflammation, which was already recognized at the time (9, 23). The notion of chemical mediators was also already brewing in the air from the studies of von Euler on histamine (24). Considering inflammation, it was clear at that time that many chemical signals are involved in the acute inflammatory response and the usual associated collateral tissue damage: for example, the leakage of reactive oxygen species from the primary phagocytic vacuole to cause tissue destruction, the release of lysosomal enzymes, etc. (18), and the potent actions of the lipid-derived platelet-activating factor (PAF), arachidonic acid and its conversion to prostaglandins and leukotrienes, as well as complement, and the cytokines (25). The structure-function and biosynthesis of the eicosanoids, as carried out by Professor Bengt Samuelsson and colleagues (23), in many respects led our current understanding and appreciation of structure-activity relationships and what we today call chemical biology, as well as metabolomics, much of which emerged from the study of these very potent short-lived arachidonate-derived local-acting autacoids like prostacyclin and thromboxane (23).
Following the award ceremony during the week of the Nobel Fest in December, there is a splendid Nobel dinner at which special utensils and glassware are used, including special Nobel schnapps flasks by Orrefors. The design was beautifully sculpted by Gunnar Cyrén and introduced by the official glass artisans of the King of Sweden. The base of these flasks, pictured in Fig. 2, portrays the devil; hence, at the end of this momentous celebration, there is a thoughtful reminder of the alcohol and its power of intoxication, and the devil at the bottom of the glass serves as a caution. This image also reminds us that the devil is in the details in studying inflammation and its complex resolution mechanisms. The key to controlling inflammation, as needed to treat inflammatory diseases, lies in appreciating the molecules and mechanisms in resolution toward the structure-function or the molecular details in resolution of acute inflammation–the termination of this protective response of the host.
Figure 2.
Devil in the flask: 3 devil flasks from Orrefors, designed and sculpted by Gunnar Cyrén. These schnapps flasks are used at the Nobel dinner in Stockholm during the Nobel Festival each year.
CHEMICAL MEDIATORS OF RESOLUTION
In the early 1990s, it became clear that differential formation, activation, and inactivation of local autacoids, such as the lipoxins (LXs; refs. 4, 26) act on both human neutrophils and monocytes but show differential cell-type-specific actions on these cells and are inactivated locally by monocytes (27). Although the LXs were born in Stockholm in the early 1980s in the Samuelsson laboratory (28), it took some time to uncover that their functions are anti-inflammatory and to demonstrate their potent endogenous actions, stopping neutrophil infiltration and hence local stop signals (29–31). In these findings from many laboratories around the world (31), it became clear that the LXs are potent stimuli for monocytes and their nonphlogistic recruitment, i.e., they do not stimulate the release of reactive oxygen species or proinflammatory signals. This differential action on neutrophils vs. monocytes paved the way for LXs to transition their actions from inflammation to repair (32, 33).
Introduction of stable analogs also permitted critical experiments that demonstrated for the first time that LXs could block PMN cell recruitment, regulate inflammation, reduce fibrosis (4, 29, 30, 34), and also stimulate phagocytosis of apoptotic PMN cells by macrophages (35). These findings further fortified the notion that LXs could play an important role in resolving mechanisms (26). Notably, LXs clearly display unique actions and do not function like PGE2 in the inflammatory response and its resolution, in that LXs accelerate resolution, as when and where in their biosynthesis proved critical in identifying their functions, since they are produced locally in the resolution phase in exudates (29, 36).
Also, Adriano Rossi and colleagues (37) established that the widely used anti-inflammatories, glucocorticoids, also stimulate macrophages to clear apoptotic leukocytes. Much of the community interested in controlling inflammation was focused at the time on making better aspirins, superaspirins that could inhibit the newly uncovered inducible cyclooxygenase denoted COX-2 (8). Unfortunately, this also proved to be a challenging target (38, 39), giving rise to complications apparent with prolonged use of selective COX-2 inhibitors. More important, it became evident that COX-2 (the “bad COX” responsible for the proinflammatory eicosanoids; ref. 8) also plays an important role in endogenous anti-inflammation (40).
These findings created a constellation of data sets from several laboratories that could be stitched together in a network connection, given the clarity of hindsight. Together, they set the stage for the discovery of the first resolvin, RvE1, and its link to precursor polyunsaturated fatty acids (PUFAs) using an unbiased LC-MS/MS approach (12). This observation also led to the notion that resolution is a biosynthetically active process and that mediators regulate the cellular traffic of resolution. In parallel investigations, leukocyte trafficking and lipid mediator profiling using LC-MS/MS-based lipidomics led to identification of mediator class switching (36), from the initiation phase evoked by prostaglandins and leukotrienes, to LXs in the resolution phase, to the discovery of potent mediators that stop neutrophil activation and are proresolving, in that they stimulate macrophage uptake of apoptotic cells and neutrophils as well as microbes and enhance their destruction (13, 41). In addition to the E series of resolvins, DHA, the other major n-3 PUFA in marine oils, is a precursor to D-series resolvins and, in neural systems, to neuroprotectins, e.g., neuroprotectin D1 (NPD1; refs. 42, 43). This was accompanied by the structural elucidation and identification of aspirin-triggered epimeric forms of the protectins and resolvins (13, 44) and introduction of stable analogs of these compounds that share the very unique mechanism of resolvins and proresolving mediators (45): namely, dampening neutrophilic infiltration without being immunosuppressive, actually enhancing microbial killing (46), and stimulating macrophages to remove apoptotic cells and microbes (44). Most recently, the agonist resolvin was introduced to stimulate resolution of inflammation in humans for the treatment of ocular inflammation in a clinical trial designed by Dr. Per Gjorstrup(47). Hopefully, this first study of the effect of resolvins in humans will open the door to future studies of agents that can stimulate the resolution mechanisms and resolve acute inflammation as well as disperse chronic inflammation to alleviate human diseases where uncontrolled inflammation underlies part of the pathophysiology.
The birth of aspirin-triggered lipid mediators (12, 13, 48), the understanding of catabasis as the return from the battle of disease to tissue homeostasis (14), and the recognition of the important role of macrophages in efferocytosis (49–51) at present provide many new opportunities to appreciate the cellular and molecular events in resolution and the identification of the first chemical mediators or signals of resolution (4). Treatment with glucocorticosteroid proved to activate the annexin system with the production of endogenous anti-inflammatory peptide ligands that activate the LX receptor (ALX/FPR2) on leukocytes to stop neutrophil recruitment and neutrophil-mediated tissue damage (52). Next, Prof. Perretti and colleagues demonstrated that annexin-derived peptide also enhances macrophage uptake of apoptotic neutrophils (53) as do the LXs and their aspirin-triggered endogenous epimers (52). Hence, peptides can also be proresolving mediators (Table 1).
The present and ever-expanding universe of proinflammatory mediators includes the many cytokines and chemokines present and the inflammasome (for a recent review, see refs. 54, 55). The importance of pattern-recognition receptors (56) and “eat-me” signals, e.g., apoptotic phosphatidylserine (57), inform us that there are likely to be as many players and local mediators in resolution to homeostasis as there are within the initiation process. This would not be surprising. But what is of special interest is that activation of resolution cascades enhances the resolution response and appears to evoke a classic feed-forward mechanism (58).
The universe of lipids encompasses more than PUFAs, and the advances in software and computers enabling rapid mass spectrometry without requiring the derivatization of samples open the possibility to categorize many known and new lipids, an activity currently taken by the Lipid Metabolites and Pathways Strategy (MAPS) program, which links sphingolipids, PUFAs, lipid mediators, cholesterol, sterols and many others (59). Studying self-limited inflammation-resolution and structural elucidation of novel lipid mediators and their structure-activity relationships presently helped to define the novel biological property, namely, a proresolving mediator and process. Using unbiased LC-MS/MS techniques with self-limited inflammatory exudates and a systems approach also affirmed that resolvins are biosynthesized from endogenous ω-3 essential fatty acids (44). They are enhanced in transgenic fat-1-overexpressing mice (60) and exert potent actions that stimulate resolution and limit further recruitment of inflammatory cells, such as neutrophils. These opened an even wider landscape with links to nutrition, where we had already known that there are beneficial actions of ω-3 essential fatty acids (61–63); however, the mechanism and effect of these at the cellular and molecular levels in inflammatory diseases were not clear and were subject to debate (62) because of a lack of structure-activity relationship from the ω-3 fatty acids. The new relationship between the ω-3 fatty acids and resolution of inflammation suggests that micronutrition via local edema feeds the resolution process and has a direct effect on the time course, duration and evolution of inflammation and likely on its failure, in the case of malnutrition of the resolving exudate (64).
The late Professor Guido Majno, a founding father of molecular pathology (65), in his textbook (16), forged the future for LXs and resolvins when he wrote:
Overall, the mediators at work in the various phases of wound healing overlap with those of acute and chronic inflammation and fibrosis. As regards the antiinflammatory eicosanoids found in the resolving phase of inflammation, lipoxins, and resolvins, they have not yet been studied in wounds, but it would be surprising if they did not take part in the noble enterprise of wound healing. (16)
His insight and prophecy proved to be the case in that LXs, protectin D1, and D-series resolvins are shown to reduce tissue injury (66, 67) and prevent fibrosis (68). These are exciting possibilities and open up many opportunities for considering novel therapeutic approaches for treating inflammation and inflammation-associated diseases (see consensus report, ref. 6, and ref. 37).
FUTURE RESOLUTION AT 25 yr AND BEYOND
It is a certain bet that, in the 25 yr ahead, more cellular and molecular details in self-limited inflammation and its resolution will be discovered. The devil is in the details of resolution of inflammation and the return of inflamed tissues to homeostasis; the structure-function of resolution mechanisms will permit how we can use these new molecules and relations to maintain health and prevent and treat diseases.
Rigorous attention to cellular-molecular definitions, precise structure-activity relationships, and the use of tissue resolution indices (6, 69) can help to propel this area forward and open up a new appreciation of inflammation-associated disease mechanisms and potentially opportunities for designer therapeutics; for example, recent results from Prof. Derek Gilroy and colleagues (70) that identified those human subjects who are “early resolvers” and those who are “delayed resolvers.” This landmark study defines the resolving dynamics of the human phenotypes in healthy subjects (70); in the 25 yr ahead, they will surely be interrogated at the molecular levels. There is also the glimmer of emerging evidence from clinical trials that designer resolvins and mimetics of this natural process (47, 71) will be useful in making analogs (72) of these potent mediators, which also extend from inflammation into direct action in resolving pain, both peripherally and centrally (73).
Some steps need to be taken that will surely lead to discovery, particularly those that will enable evidence-based practices for individualized optimal maintenance of the immune system, the neuronal system, and the related axis. These will come into play when this field addresses the question of the minimum daily amount of PUFAs (both n-6 and n-3) required for timely resolution of inflammatory insults. Clinicians will be able to routinely profile the precursor ω-3 fatty acids and PUFAs in the blood and tissues of individuals to get a “dipstick” of these essential nutrients. The transformation of these PUFAs, including the ω-3- and arachidonate-derived mediators (e.g., eicosanoids, D-series resolvins, and maresins) and their metabolomics might be detailed on an individual basis for blood chemistries in the near future as the clinical pathology/wellness report. Only widescale implementation is needed, for the methods are already in the hands of researchers. We should then be able to personalize the levels of essential PUFAs to the precise amounts needed by each individual and each site of utilization in men and women, old and young. We may then prescribe, with the knowledge of evidence-based medicine, the needed proresolving precursors and agents tailored to direct use in vivo.
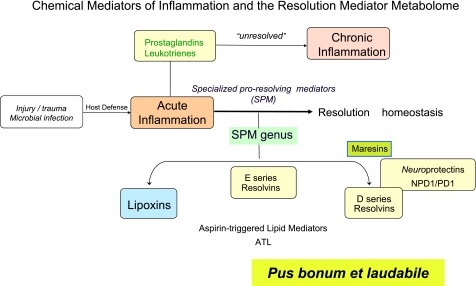
In the 19th and 20th centuries, inhibiting production of inflammatory mediators prevailed as a mode of treatment. In the next 25 yr, perhaps the field will grow to use agonists of resolution, and in this way return to the concept from the 11th century Canon of Medicine, which appears to be a “back to the future” scenario for stimulating resolution of inflammation as a treatment modality (Table 1) and pus bonum et laudabile (good and laudable pus; ref. 17). Hence, in 2010, we appreciate the good molecules also present in pus and evolving exudates that together constitute a new genus of specialized proresolving mediators (Fig. 3). It is difficult to quickly turn around a battleship that has momentum. Nonetheless, along these lines, nutraceuticals, such as PUFA derivatives and/or related mimetics of resolution autacoids, could be prescribed either alone or with specific resolvins and/or combined with other classic anti-inflammatories, e.g., NSAIDs, steroids, etc. Designer resolvins might be used alone or together with other anti-inflammatory or proresolving therapies.
Figure 3.
Chemical mediators of inflammation and the resolution mediator metabolome in 2010.
It is equally likely that the mechanisms of resolution can also be targets of impairment, genetic losses, or markers of aberrant resolvin production. Such conditions or defects in resolution might be observed in the future as molecular defects leading to many chronic inflammatory diseases. The loss of a resolvin receptor on leukocytes, for example, may lead to uncontrolled inflammation, as we have reported that overexpression of a receptor for a proresolving mediator enhances resolution, shortening the resolution interval and the time required to return to homeostasis (74). These are wishful thoughts, looking 25 yr past and further, as to where the resolution trajectory starts, and may take us in the future, for resolution mechanisms in terms of the molecules, mediators, and cellular and molecular mechanisms and their likely effects in resolution pharmacology and the treatment of human diseases. That cool devil!
Acknowledgments
The author is an inventor on patents assigned to Brigham and Women's Hospital and Partners HealthCare (Boston, MA, USA) on the composition of matter, uses, and clinical development of anti-inflammatory and proresolving lipid mediators. These are licensed for clinical development. The author retains founder stock in Resolvyx Pharmaceuticals.
The author's laboratory is supported by NIH grant R01 GM038765.
REFERENCES
- 1. Friedman M., Friedland G. W. (1998) Medicine's 10 Greatest Discoveries, Yale University Press, New Haven, CT, USA [Google Scholar]
- 2. Beveridge W. I. B. (1950) The Art of Scientific Investigation, Random House, New York [Google Scholar]
- 3. Bernard C. (1957) An Introduction to the Study of Experimental Medicine (Greene H. C. trans), Dover, New York: Originally published in 1865; first published in English in 1927 [Google Scholar]
- 4. Serhan C. N. (2002) Endogenous chemical mediators in anti-inflammation and pro-resolution. Curr. Med. Chem. Anti-Inflamm. Anti-Allergy Agents 1, 177–192 [Google Scholar]
- 5. Gilroy D. W., Lawrence T., Perretti M., Rossi A. G. (2004) Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug. Discov. 3, 401–416 [DOI] [PubMed] [Google Scholar]
- 6. Serhan C. N., Brain S. D., Buckley C. D., Gilroy D. W., Haslett C., O'Neill L. A. J., Perretti M., Rossi A. G., Wallace J. L. (2007) Resolution of inflammation: state of the art, definitions and terms. FASEB J. 21, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flower R. J. (2006) Prostaglandins, bioassay and inflammation. Br. J. Pharmacol. 147, S182–S192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vane J. R., Botting R. M. eds. (2001) Therapeutic Roles of Selective COX-2 Inhibitors, William Harvey Press, London [Google Scholar]
- 9. Vane J. R. (1982) Adventures and excursions in bioassay: the stepping stones to prostacyclin. In Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures pp. 181–206, Almqvist & Wiksell, Stockholm [Google Scholar]
- 10. Taber C. W. (1970) Taber's Cyclopedic Medical Dictionary, 11th Ed., F. A. Davis, Philadelphia [Google Scholar]
- 11. Avicenna (Abu cAli Sina) (1999) The Canon of Medicine (al-Qanun fi'l-tibb) (adapted by Bahktiar, L.), Great Books of the Islamic World, Chicago [Google Scholar]
- 12. Serhan C. N., Clish C. B., Brannon J., Colgan S. P., Chiang N., Gronert K. (2000) Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 192, 1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Serhan C. N., Hong S., Gronert K., Colgan S. P., Devchand P. R., Mirick G., Moussignac R.-L. (2002) Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J. Exp. Med. 196, 1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Serhan C. N., Savill J. (2005) Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197 [DOI] [PubMed] [Google Scholar]
- 15. Robbins S. L., Cotran R. (1979) Pathologic Basis of Disease, 2nd Ed., W. B. Saunders, Philadelphia [Google Scholar]
- 16. Majno G., Joris I. (2004) Cells, Tissues, and Disease: Principles of General Pathology, 2nd Ed., Oxford University Press, New York [Google Scholar]
- 17. Majno G., Cotran R. S., Kaufman N. eds. (1982) Current Topics in Inflammation and Infection, Williams & Wilkins, Baltimore [Google Scholar]
- 18. Weissmann G., Smolen J. E., Korchak H. M. (1980) Release of inflammatory mediators from stimulated neutrophils. N. Engl. J. Med. 303, 27–34 [DOI] [PubMed] [Google Scholar]
- 19. Savill J. S., Henson P. M., Haslett C. (1989) Phagocytosis of aged human neutrophils by macrophages is mediated by a novel “charge-sensitive” recognition mechanism. J. Clin. Invest. 84, 1518–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. (1989) Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 83, 865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haslett C., Henson P. M. (1988) In The Molecular and Cellular Biology of Wound Repair (Clark R., Henson P. M. eds), Plenum Press, New York [Google Scholar]
- 22. Cotran R. S., Kumar V., Robbins S. L., Schoen F. J. (1994) Robbins Pathologic Basis of Disease, 5th Ed., W. B. Saunders, Philadelphia [Google Scholar]
- 23. Samuelsson B. (1982) From studies of biochemical mechanisms to novel biological mediators: prostaglandin endoperoxides, thromboxanes and leukotrienes. In Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures pp. 153–174, Almqvist & Wiksell, Stockholm: [DOI] [PubMed] [Google Scholar]
- 24. Von Euler U. S. (1978) Pieces in the puzzle. In The Excitement and Fascination of Science: Reflections by Eminent Scientists, Vol. 2 (Gibson W. C. ed) pp. 675–686, Annual Reviews, Palo Alto, CA, USA [Google Scholar]
- 25. Houck J. C. ed. (1979) Chemical Messengers of the Inflammatory Process, Elsevier/North-Holland Biomedical Press, Amsterdam [Google Scholar]
- 26. Serhan C. N. (1994) Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim. Biophys. Acta 1212, 1–25 [DOI] [PubMed] [Google Scholar]
- 27. Serhan C. N., Fiore S., Brezinski D. A., Lynch S. (1993) Lipoxin A4 metabolism by differentiated HL-60 cells and human monocytes: conversion to novel 15-oxo and dihydro products. Biochemistry 32, 6313–6319 [DOI] [PubMed] [Google Scholar]
- 28. Serhan C. N., Hamberg M., Samuelsson B. (1984) Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl. Acad. Sci. U. S. A. 81, 5335–5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takano T., Clish C. B., Gronert K., Petasis N., Serhan C. N. (1998) Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J. Clin. Invest. 101, 819–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takano T., Fiore S., Maddox J. F., Brady H. R., Petasis N. A., Serhan C. N. (1997) Aspirin-triggered 15-epi-lipoxin A4 and LXA4 stable analogs are potent inhibitors of acute inflammation: Evidence for anti-inflammatory receptors. J. Exp. Med. 185, 1693–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serhan C. N. guest ed. (2005) Special issue on lipoxins and aspirin-triggered lipoxins. Prostaglandins Leukot. Essent. Fatty Acids 73, 139–321 [DOI] [PubMed] [Google Scholar]
- 32. Maddox J. F., Serhan C. N. (1996) Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J. Exp. Med. 183, 137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Serhan C. N. (1997) Lipoxins and novel aspirin-triggered 15-epi-lipoxins (ATL): a jungle of cell-cell interactions or a therapeutic opportunity? Prostaglandins. 53, 107–137 [DOI] [PubMed] [Google Scholar]
- 34. Bandeira-Melo C., Serra M. F., Diaz B. L., Cordeiro R. S. B., Silva P. M. R., Lenzi H. L., Bakhle Y. S., Serhan C. N., Martins M. A. (2000) Cyclooxygenase-2-derived prostaglandin E2 and lipoxin A4 accelerate resolution of allergic edema in Angiostrongylus costaricensis-infected rats: relationship with concurrent eosinophilia. J. Immunol. 164, 1029–1036 [DOI] [PubMed] [Google Scholar]
- 35. Godson C., Mitchell S., Harvey K., Petasis N. A., Hogg N., Brady H. R. (2000) Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 164, 1663–1667 [DOI] [PubMed] [Google Scholar]
- 36. Levy B. D., Clish C. B., Schmidt B., Gronert K., Serhan C. N. (2001) Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2, 612–619 [DOI] [PubMed] [Google Scholar]
- 37. Rossi A. G., Sawatzky D. A. eds. (2008) The Resolution of Inflammation, Birkhäuser Verlag AG, Basel [Google Scholar]
- 38. Catella-Lawson F., Reilly M. P., Kapoor S. C., Cucchiara A. J., DeMarco S., Tournier B., Vyas S. N., FitzGerald G. A. (2001) Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N. Engl. J. Med. 345, 1809–1817 [DOI] [PubMed] [Google Scholar]
- 39. FitzGerald G. A., Patrono C. (2001) The coxibs, selective inhibitors of cyclooxygenase-2. N. Engl. J. Med. 345, 433–442 [DOI] [PubMed] [Google Scholar]
- 40. Gilroy D. W., Colville-Nash P. R., Willis D., Chivers J., Paul-Clark M. J., Willoughby D. A. (1999) Inducible cycloxygenase may have anti-inflammatory properties. Nat. Med. 5, 698–701 [DOI] [PubMed] [Google Scholar]
- 41. Serhan C. N., Chiang N., Van Dyke T. E. (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8, 249–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mukherjee P. K., Marcheselli V. L., Barreiro S., Hu J., Bok D., Bazan N. G. (2007) Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc. Natl. Acad. Sci. U. S. A. 104, 13152–13157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bazan N. G., Calandria J. M., Serhan C. N. (2010) Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J. Lipid Res. 51, 2018–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Serhan C. N. (2007) Resolution phases of inflammation: novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Annu. Rev. Immunol. 25, 101–137 [DOI] [PubMed] [Google Scholar]
- 45. Serhan C. N., Chiang N. (2008) Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br. J. Pharmacol. 153(Suppl. 1), S200–S215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spite M., Norling L. V., Summers L., Yang R., Cooper D., Petasis N. A., Flower R. J., Perretti M., Serhan C. N. (2009) Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461, 1287–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brooks D. W. (2009) Resolvyx announced positive data from trial of resolvin RX-10045 for dry eye. [Online] Eye Doc News, http://eyedocnews.com/002059-resolvyx-announces-positive-data-from-trial-of-resolvin-rx-10045-for-dry-eye/
- 48. Clària J., Serhan C. N. (1995) Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc. Natl. Acad. Sci. U. S. A. 92, 9475–9479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vandivier R. W., Henson P. M., Douglas I. S. (2006) Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 129, 1673–1682 [DOI] [PubMed] [Google Scholar]
- 50. Tabas I. (2010) Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10, 36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Soehnlein O., Lindbom L. (2010) Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 10, 427–439 [DOI] [PubMed] [Google Scholar]
- 52. Perretti M., Chiang N., La M., Fierro I. M., Marullo S., Getting S. J., Solito E., Serhan C. N. (2002) Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat. Med. 8, 1296–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maderna P., Yona S., Perretti M., Godson C. (2005) Modulation of phagocytosis of apoptotic neutrophils by supernatant from dexamethasone-treated macrophages and annexin-derived peptide Ac(2–26). J. Immunol. 174, 3727–3733 [DOI] [PubMed] [Google Scholar]
- 54. Dinarello C. A. (2010) Anti-inflammatory agents: present and future. Cell 140, 935–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schroder K., Tschopp J. (2010) The inflammasomes. Cell 140, 821–832 [DOI] [PubMed] [Google Scholar]
- 56. Medzhitov R. (2010) Inflammation 2010: new adventures of an old flame. Cell 140, 771–776 [DOI] [PubMed] [Google Scholar]
- 57. Savill J., Gregory C. (2007) Apoptotic PS to phagocyte TIM-4: eat me. Immunity 27, 830–832 [DOI] [PubMed] [Google Scholar]
- 58. Serhan C. N. (2010) Novel resolution mechanisms in acute inflammation: to resolve or not? Am. J. Pathol. 177, 1576–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schmelzer K., Fahy E., Subramaniam S., Dennis E. A. (2007) The lipid maps initiative in lipidomics. Methods Enzymol. 432, 247–273 [DOI] [PubMed] [Google Scholar]
- 60. Hudert C. A., Weylandt K. H., Wang J., Lu Y., Hong S., Dignass A., Serhan C. N., Kang J. X. (2006) Transgenic mice rich in endogenous n-3 fatty acids are protected from colitis. Proc. Natl. Acad. Sci. U. S. A. 103, 11276–11281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. GISSI-Prevenzione Investigators (1999) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet 354, 447–455 [PubMed] [Google Scholar]
- 62. Lands W. E. M. (2005) Fish, Omega-3 and Human Health, 2nd Ed., AOCS Press, Champaign, IL, USA [Google Scholar]
- 63. Simopoulos A. P., Leaf A., Salem N., Jr. (1999) Workshop on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. J. Am. Coll. Nutr. 18, 487–489 [DOI] [PubMed] [Google Scholar]
- 64. Kasuga K., Yang R., Porter T. F., Agrawal N., Petasis N. A., Irimia D., Toner M., Serhan C. N. (2008) Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J. Immunol. 181, 8677–8687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Damjanov I. (2010) In memoriam. Guido Majno M.D., 1922–2010. Am. J. Pathol. 177, 2150–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gronert K., Maheshwari N., Khan N., Hassan I. R., Dunn M., Schwartzman M. L. (2005) A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J. Biol. Chem. 280, 15267–15278 [DOI] [PubMed] [Google Scholar]
- 67. Hassan I. R., Gronert K. (2009) Acute changes in dietary omega-3 and omega-6 polyunsaturated fatty acids have a pronounced impact on survival following ischemic renal injury and formation of renoprotective docosahexaenoic acid-derived protectin D1. J. Immunol. 182, 3223–3232 [DOI] [PubMed] [Google Scholar]
- 68. Duffield J. S., Hong S., Vaidya V., Lu Y., Fredman G., Serhan C. N., Bonventre J. V. (2006) Resolvin D series and protectin D1 mitigate acute kidney injury. J. Immunol. 177, 5902–5911 [DOI] [PubMed] [Google Scholar]
- 69. Bannenberg G. L., Chiang N., Ariel A., Arita M., Tjonahen E., Gotlinger K. H., Hong S., Serhan C. N. (2005) Molecular circuits of resolution: Formation and actions of resolvins and protectins. J. Immunol. 174, 4345–4355 [DOI] [PubMed] [Google Scholar]
- 70. Morris T., Stables M., Colville-Nash P., Newson J., Bellingan G., de Souza P. M., Gilroy D. W. (2010) Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc. Natl. Acad. Sci. U. S. A. 107, 8842–8847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Arita M., Oh S., Chonan T., Hong S., Elangovan S., Sun Y.-P., Uddin J., Petasis N. A., Serhan C. N. (2006) Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J. Biol. Chem. 281, 22847–22854 [DOI] [PubMed] [Google Scholar]
- 72. Serhan C. N., Petasis N. A. (2011) Resolvins and protectins in inflammation-resolution. Chem. Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xu Z.-Z., Zhang L., Liu T., Park J.-Y., Berta T., Yang R., Serhan C. N., Ji R.-R. (2010) Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 16, 592–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Krishnamoorthy S., Recchiuti A., Chiang N., Yacoubian S., Lee C.-H., Yang R., Petasis N. A., Serhan C. N. (2010) Resolvin D1 binds human phagocytes with evidence for pro-resolving receptors. Proc. Natl. Acad. Sci. U. S. A. 107, 1660–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu Y., Cousin J. M., Hughes J., Van Damme J., Seckl J. R., Haslett C., Dransfield I., Savill J., Rossi A. G. (1999) Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J. Immunol. 162, 3639–3646 [PubMed] [Google Scholar]
- 76. Maderna P., Cottell D. C., Toivonen T., Dufton N., Dalli J., Perretti M., Godson C. (2010) FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J. 24, 4240–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]