Abstract
The FASEB Journal is a pillar among biomedical publications, contributing greatly by disseminating the results of vision research during its lifetime. Progress over this period has been remarkable. George Wald (1) provided the first chemical understanding of the fundamental processes governing vision: the photoisomerization of 11-cis-retinal to all-trans-retinal and the enzymatic regeneration of this chromophore. Contributions of this extraordinary scientist (2, 3) set the stage for discoveries ranging from gross recording of various electrical responses to light to elucidation of signal transduction at a structural level, and from characterization of retinal diseases to successful treatments.
Keywords: G protein-coupled receptors, phototransduction, rhodopsin, membrane receptors, blindness, Ca2+ signaling
DETERMINING THE STRUCTURES OF VISUAL PIGMENTS
Amino acid sequencing of the membrane protein rhodopsin by the Hargrave and Ovchinnikov laboratories (4, 5) was a monumental step necessary to elucidate its membrane topology. This advance also constituted an early example of G protein signaling, a ubiquitous biological pathway just emerging at the time (6, 7). Shortly following this discovery was the brilliant contribution by a graduate student, Jeremy Nathans, who cleverly used novel techniques in molecular biology and inherited deficits in human color vision to clone all visual pigments of the human photoreceptors (8). This research helped later genetic investigations of inherited retinal diseases such as retinitis pigmentosa (9) and pathologies in color vision (10, 11). Subsequent biochemical and biophysical studies greatly improved our understanding of how rhodopsin functions, but full details of its molecular arrangements and three-dimensional structure determined by X-ray crystallography were not reported until 2000 (12). This breakthrough allowed for the generation of structural models for many other G protein-coupled receptors, and enhanced our understanding of how they bind ligands and interact with G proteins (13). Cloning and crystal structures of other phototransduction proteins occurred concurrently (summarized in refs. 14, 15).
NOT Ca2+, NOT cGMP, BUT cGMP AND Ca2+ AS THE “INTERNAL TRANSMITTER” OF PHOTOTRANSDUCTION
From light-activated visual pigments, the signal is transmitted by a chain of enzymatic steps that culminate in altered conductance of photoreceptor plasma membrane channels. Fesenko et al. (16) determined that these channels are regulated by cGMP and not directly by either Ca2+ or the more ubiquitously employed cAMP cascade. This observation was consistent with biochemical studies that revealed events upstream of such regulation (17). A flurry of activity then established the phototransduction mechanism in rods and cones, where an interplay of activation and regulation by Ca2+ and cGMP play the dominant roles. It was also determined that the signal is terminated (quenched) by numerous events including receptor phosphorylation and capping with arrestin (18, 19), enhancement of GTP-ase activity of G proteins by a regulator of G protein signaling (RGS; ref. 20), and increased rate of production of cGMP by guanylate cyclase along with its regulation by guanylate cyclase-activating proteins (21). The overall biochemical hypothesis was quantitatively verified by a combination of mouse genetics and physiology (reviewed in refs. 22–24). Today, G protein signal transduction is among the best- understood transduction systems in all of biology, and insights into other signaling systems are based on its clarification (25). These biochemical steps and their regulation comprise an adaptive mechanism that allows our vision to operate under a wide range of lighting conditions, ranging from a single photon to millions of photons per cell (reviewed in refs. 26, 27). Arguably, photoreceptors may be the most extensively studied cells in our body.
DISCOVERY OF MELANOPSIN-EXPRESSING, INTRINSICALLY PHOTOSENSITIVE RETINAL GANGLION CELLS
In addition to the intense focus on the initial steps of vision, immense progress has been made concerning specific physiological functions associated with anatomically distinct classes of interneurons and general retinal synaptic circuitry. The technical advantage of controlled light that can be readily delivered, along with detailed analyses of rod and cone signaling pathways in the retina, helped to formulate a number of general rules for processing and computation of the initial light signal for the entire brain (28, 29). Key insights into the separation of “on” and “off” signaling by rod and cone pathways throughout the retina have evolved. Three other fundamental physiological activities are regulated by these interconnected networks, namely additional photoreceptor adaptation processes for scotopic and photopic vision (30); mechanisms of contrast enhancement and movement detection (31); and circadian rhythm, wherein a subset of intrinsically photosensitive ganglion cells plays an integral part. Melanopsin, an invertebrate homologue of rhodopsin discovered by homology cloning, is expressed in photosensitive frog dermal melanophores (32, 33). In mammals, orthologs of this visual pigment is present in a subset of vertebrate retinal ganglion cells. Additional studies proved that these retinal ganglion cells innervating the suprachiasmatic nucleus (SCN) of the hypothalamus are responsible for the photic entrainment mechanism (34, 35). Unraveling the complexity of this system continues to be a highly challenging aspect of visual research.
MOLECULAR INSIGHTS INTO CHEMISTRY AND ENZYMOLOGY OF THE RETINOID (VISUAL) CYCLE
In addition to interneurons, another cell layer, the retinal pigment epithelium (RPE), is essential for our vision. The RPE, along with photoreceptors, is responsible for the continuous renewal of spent chromophore, 11-cis-retinal. George Wald wrote in 1935: “Vitamin A is the precursor of visual purple as well as the product of its decomposition. The visual processes therefore constitute a cycle” (36). Branded the “visual cycle” or, perhaps more correctly, the “retinoid cycle,” it comprises a set of enzymatic reactions that converts the all-trans-form of the chromophore back to 11-cis-retinal. Numerous laboratories have contributed to our current molecular and structural understanding of this cycle, making it impossible to reference all of them here (reviewed in refs. 37, 38). Yet, the fundamental breakthroughs were the findings that a disabling mutation in the gene encoding the abundant RPE protein, RPE65, is associated with a congenital blindness called Leber congenital amaurosis (LCA; refs. 39, 40), resulting from the lack of 11-cis-retinal production (41). The crystal structure of RPE65 has been recently determined (42). The explosion of biochemical, physiological, pathological and clinical knowledge about the retinoid cycle is truly impressive, forming the basis of several therapies now under development to treat inherited and acquired human retinal diseases.
DATING THE BIRTH OF RETINAL NEURONS AND REGULATING ROD VS. CONE CELL FATE BY SPECIFIC TRANSCRIPTION FACTORS
The complexity of the visual system arises from time-dependent specification of cell differentiation governed by a cascade of sequentially expressed transcription factors. These transcription factors regulate the expression of the structural and functional proteins characteristic of terminally differentiated retinal cells. Mutations in the Pax-6 gene result in a complete lack of eyes in mice (43), humans (44), and Drosophila (45), providing a new perspective on eye evolution and development across vertebrates. Amazingly, when Pax-6 was expressed in various thoracic imaginal disc primordia of Drosophila, ectopic eye structures were induced on wings, legs, and antennae (46). Even more astounding, the ectopic eyes appeared morphologically normal and consisted of groups of fully differentiated ommatidia with a complete set of photoreceptor cells, suggesting that Pax-6 is the master control gene for eye morphogenesis. The list of involved transcription factors has grown over time as well as our knowledge of their complex roles in eye development. For example, discovery of the neural retina leucine zipper (NRL) gene (47) and thyroid hormone receptor β2 and subsequent reports that deletion of Nrl or Trb2 in mice resulted in the complete loss of rods or M-cones, respectively, accompanied by their transformation to S-cones (48, 49) shed light on the differential development of rod vs. cone photoreceptor cells. These contributions have had an impact far outside of retinal research, becoming classic examples in developmental neurobiology.
ADVANCEMENTS IN TREATING HUMAN DISEASE
In the past 25 yr, clinicians have been empowered with tools to recognize certain genetic defects in the visual system and a few therapies that allow for the prevention of visual deterioration or the restoration of vision in patients who a few decades ago were destined to develop blindness. More than 200 gene loci linked to human and animal eye (mostly retinal) diseases have been identified. This progress has resulted primarily from painstaking advances in visual research at a basic science level.
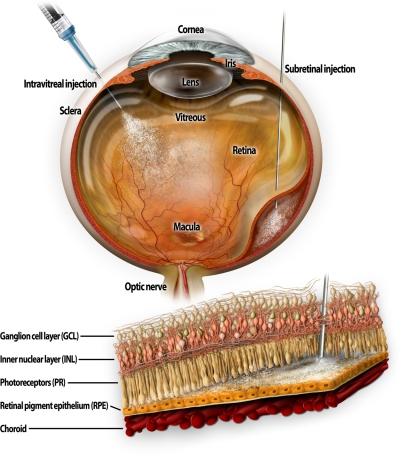
Transgenic mouse models of retinal degeneration not only provide information about the function of ocular genes but also lead to strategies that can be tested for treatment of inherited and acquired human retinal diseases. For example, such studies led to recognition of the centrosome-cilia-outer segment connection as a major defect not only in inherited retinal/macular diseases, but also in multiple syndromic diseases affecting cochlea, olfaction, cerebellum and kidney. An important “proof-of concept” example was the rescue of the visual defect in LCA animal models with disabled Rpe65 or another gene called Lrat by either gene transfer or artificial retinoid replacement therapy (50, 51). These studies have now moved to clinical trials with the potential of restoring vision to humans affected by this devastating disease. Encouraging results of the first human trials were recently reported (52–54). Other promising new advances should follow in areas such as transplantation of RPE, photoreceptors and possibly stem cells into degenerating retina. Such progress will be gradual as more basic science knowledge is required before such procedures become specific treatments for blinding disorders. Additional gene therapy vectors need to be developed to target gene expression to specific classes of retinal cells to achieve a better understanding of the involved pathology and fundamental biology. There is hope that new-generation vectors will be effective when delivered intravitreally and will not involve detaching the fragile and diseased retina as is now required (Fig. 1).
Figure 1.
Structure of the human eye. The cross-section of the human eye illustrates injection of a drug into the vitreous cavity and in the subretinal space that leads to temporary retinal detachment.
Another basic observation that befit ophthalmology was the discovery that elevated VEGF correlated with neovascularization, ultimately causing blindness in diabetic retinopathy (55, 56), retinopathy of prematurity (57), and the wet form of age-related macular degeneration (AMD; ref. 58). Introduction of a neutralizing Fab fragment of an antibody with its activity against VEGF in a drug called ranibizumab, introduced by Genenetech Inc., has improved the treatment of wet AMD in particular (59–61). So, now the wet form of AMD may actually respond to treatment.
AND NOW WE ARE READY FOR THE NEXT 25 YEARS!
I am very confident that progress and discovery in vision research from many laboratories will continue at an even greater pace than before. The visual system that converts a physical stimulus into biochemical/chemical language has fascinated scientists for decades. Many secrets of this system remain to be revealed, and, no doubt, their solution will pave the way for intellectual and technical advances in other areas of biology. For example, elucidation of proteins and genes involved in ciliogenesis, disc morphogenesis, and vectorial transport is likely to be accomplished within a few years. The primary foci will then be on disease mechanisms and the structural biology aspects related to vision. Progress will involve combining genetics and genomics to high- and low-resolution cell biology methods to take us from atoms to whole neurons, thereby facilitating structural understanding of molecular events underlying vision.
Advances are definite, especially in genetics, because of established predispositions to complex retinal diseases such as AMD combined with the development of improved gene- and cell-based therapies. As molecular targets are identified, pharmacology should yield novel treatments for vision adversely affected by a single gene defect or multifactorial diseases such as AMD or glaucoma. Immunological studies have clarified our understanding of the progression of AMD and other retinal diseases, and we now have the potential to reveal triggers of this pathology, and reveal the opportunity for additional treatments. Is AMD primarily a chronic inflammatory disease or just a secondary manifestation of other pathological triggers? Much more must be learned. Still in its infancy is our understanding of the development and maintenance of retinal cells and gene regulatory networks controlled by diverse signaling pathways, transcriptional regulatory proteins and noncoding RNAs (such as microRNAs), or continuous renewal of photoreceptor outer segments after phagocytosis. Transgenic animals and their use for basic science and preclinical applications, gene profiling, high throughput sequencing, embryonic and induced pluripotent stem cell technology, and other methodologies will provide us with truly remarkable advancements in vision research.
So, keep your eyes open for advances in vision research! There's more to come that will transform bench research into successful therapy for human disease conditions. As George Wald wrote, “A scientist should be the happiest of men!” because one can accomplish seemingly unattainable aims in just a decade or so.
Acknowledgments
The author thanks Dr. Leslie T. Webster, Jr. (Case Western Reserve University) and members of his laboratory for valuable comments on the manuscript.
This work was supported in part by U.S. National Institutes of Health grant R01-EY0008061. The author has also solicited the opinions of his colleagues in the field, including Drs. Anthony Adamis, Anand Swaroop, Maurine Neitz, Jack Saari, Vadim Arshavsky, King-Wai Yau, Robert Molday, Thomas Sakmar, Gordon Fain, Sam Wu, Wolfgang Baehr and Janet Sparrow. While the author is grateful for their advice and their views have shaped his opinion, they are not responsible for the final selection of the topics presented.
The opinions expressed in editorials, essays, letters to the editor, and other articles comprising the Up Front section are those of the authors and do not necessarily reflect the opinions of FASEB or its constituent societies. The FASEB Journal welcomes all points of view and many voices. We look forward to hearing these in the form of op-ed pieces and/or letters from its readers addressed to journals@faseb.org.
REFERENCES
- 1. Wald G. (1968) Molecular basis of visual excitation. Science 162, 230–239 [DOI] [PubMed] [Google Scholar]
- 2. Hubbard R., Wald E. (1999) George Wald memorial talk. Novartis Found Symp. 224, 5–18, discussion 18–20 [DOI] [PubMed] [Google Scholar]
- 3. Dowling J. E. (1997) George Wald (1906–97). Nature 387, 356. [DOI] [PubMed] [Google Scholar]
- 4. Hargrave P. A., McDowell J. H., Curtis D. R., Wang J. K., Juszczak E., Fong S. L., Rao J. K., Argos P. (1983) The structure of bovine rhodopsin. Biophys. Struct. Mech. 9, 235–244 [DOI] [PubMed] [Google Scholar]
- 5. Ovchinnikov Iu A., Abdulaev N. G., Feigina M., Artamonov I. D., Bogachuk A. S. (1983) [Visual rhodopsin. III. Complete amino acid sequence and topography in a membrane]. Bioorg. Khim. 9, 1331–1340 [PubMed] [Google Scholar]
- 6. Bitensky M. W., Yamazaki A., Wheeler M. A., George J. S., Rasenick M. M. (1984) The mechanism of activation of light-activated phosphodiesterase and evidence for homology with hormone-activated adenylate cyclase. Adv. Cyclic Nucleotide Protein Phosphorylation Res. 17, 227–237 [PubMed] [Google Scholar]
- 7. Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E., Mumford R. A., Slater E. E., Sigal I. S., Caron M. G., Lefkowitz R. J., Strader C. D. (1986) Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature 321, 75–79 [DOI] [PubMed] [Google Scholar]
- 8. Nathans J., Hogness D. S. (1983) Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell 34, 807–814 [DOI] [PubMed] [Google Scholar]
- 9. Dryja T. P., McGee T. L., Reichel E., Hahn L. B., Cowley G. S., Yandell D. W., Sandberg M. A., Berson E. L. (1990) A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature 343, 364–366 [DOI] [PubMed] [Google Scholar]
- 10. Nathans J., Piantanida T. P., Eddy R. L., Shows T. B., Hogness D. S. (1986) Molecular genetics of inherited variation in human color vision. Science 232, 203–210 [DOI] [PubMed] [Google Scholar]
- 11. Nathans J., Thomas D., Hogness D. S. (1986) Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science 232, 193–202 [DOI] [PubMed] [Google Scholar]
- 12. Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., Yamamoto M., Miyano M. (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739–745 [DOI] [PubMed] [Google Scholar]
- 13. Filipek S., Teller D. C., Palczewski K., Stenkamp R. (2003) The crystallographic model of rhodopsin and its use in studies of other G protein-coupled receptors. Annu. Rev. Biophys. Biomol. Struct. 32, 375–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Polans A., Baehr W., Palczewski K. (1996) Turned on by Ca2+! The physiology and pathology of Ca(2+)-binding proteins in the retina. Trends Neurosci. 19, 547–554 [DOI] [PubMed] [Google Scholar]
- 15. Ridge K. D., Abdulaev N. G., Sousa M., Palczewski K. (2003) Phototransduction: crystal clear. Trends Biochem. Sci. 28, 479–487 [DOI] [PubMed] [Google Scholar]
- 16. Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. (1985) Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 313, 310–313 [DOI] [PubMed] [Google Scholar]
- 17. Fung B. K., Hurley J. B., Stryer L. (1981) Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc. Natl. Acad. Sci. U. S. A. 78, 152–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuhn H. (1974) Light-dependent phosphorylation of rhodopsin in living frogs. Nature 250, 588–590 [DOI] [PubMed] [Google Scholar]
- 19. Kuhn H., Dreyer W. J. (1972) Light dependent phosphorylation of rhodopsin by ATP. FEBS Lett. 20, 1–6 [DOI] [PubMed] [Google Scholar]
- 20. Angleson J. K., Wensel T. G. (1993) A GTPase-accelerating factor for transducin, distinct from its effector cGMP phosphodiesterase, in rod outer segment membranes. Neuron 11, 939–949 [DOI] [PubMed] [Google Scholar]
- 21. Palczewski K., Subbaraya I., Gorczyca W. A., Helekar B. S., Ruiz C. C., Ohguro H., Huang J., Zhao X., Crabb J. W., Johnson R. S., Walsh K. A., Gray-Keller M. P., Detwiler P. B., Baehr W. (1994) Molecular cloning and characterization of retinal photoreceptor guanylyl cyclase-activating protein. Neuron 13, 395–404 [DOI] [PubMed] [Google Scholar]
- 22. Arshavsky V. Y., Lamb T. D., Pugh E. N., Jr. (2002) G proteins and phototransduction. Annu. Rev. Physiol. 64, 153–187 [DOI] [PubMed] [Google Scholar]
- 23. Yau K. W., Hardie R. C. (2009) Phototransduction motifs and variations. Cell 139, 246–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo D. G., Xue T., Yau K. W. (2008) How vision begins: an odyssey. Proc. Natl. Acad. Sci. U. S. A. 105, 9855–9862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pierce K. L., Premont R. T., Lefkowitz R. J. (2002) Seven-transmembrane receptors. Nat. Rev. Mol. Cell. Biol. 3, 639–650 [DOI] [PubMed] [Google Scholar]
- 26. Fain G. L., Matthews H. R., Cornwall M. C., Koutalos Y. (2001) Adaptation in vertebrate photoreceptors. Physiol. Rev. 81, 117–151 [DOI] [PubMed] [Google Scholar]
- 27. McBee J. K., Palczewski K., Baehr W., Pepperberg D. R. (2001) Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog. Retin. Eye Res. 20, 469–529 [DOI] [PubMed] [Google Scholar]
- 28. Werblin F. S., Dowling J. E. (1969) Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J. Neurophysiol. 32, 339–355 [DOI] [PubMed] [Google Scholar]
- 29. Zhang A. J., Wu S. M. (2009) Receptive fields of retinal bipolar cells are mediated by heterogeneous synaptic circuitry. J. Neurosci. 29, 789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Green D. G., Dowling J. E., Siegel I. M., Ripps H. (1975) Retinal mechanisms of visual adaptation in the skate. J. Gen. Physiol. 65, 483–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nishiguchi K. M., Sandberg M. A., Kooijman A. C., Martemyanov K. A., Pott J. W., Hagstrom S. A., Arshavsky V. Y., Berson E. L., Dryja T. P. (2004) Defects in RGS9 or its anchor protein R9AP in patients with slow photoreceptor deactivation. Nature 427, 75–78 [DOI] [PubMed] [Google Scholar]
- 32. Provencio I., Jiang G., De Grip W. J., Hayes W. P., Rollag M. D. (1998) Melanopsin: an opsin in melanophores, brain, and eye. Proc. Natl. Acad. Sci. U. S. A. 95, 340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Provencio I., Rodriguez I. R., Jiang G., Hayes W. P., Moreira E. F., Rollag M. D. (2000) A novel human opsin in the inner retina. J. Neurosci. 20, 600–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berson D. M., Dunn F. A., Takao M. (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073 [DOI] [PubMed] [Google Scholar]
- 35. Hattar S., Liao H. W., Takao M., Berson D. M., Yau K. W. (2002) Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wald G. (1935) Carotenoids and the visual cycle. J. Gen. Physiol. 19, 351–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Travis G. H., Golczak M., Moise A. R., Palczewski K. (2007) Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 47, 469–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Von Lintig J., Kiser P. D., Golczak M., Palczewski K. (2010) The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem. Sci. 35, 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marlhens F., Bareil C., Griffoin J. M., Zrenner E., Amalric P., Eliaou C., Liu S. Y., Harris E., Redmond T. M., Arnaud B., Claustres M., Hamel C. P. (1997) Mutations in RPE65 cause Leber's congenital amaurosis. Nat. Genet. 17, 139–141 [DOI] [PubMed] [Google Scholar]
- 40. Gu S. M., Thompson D. A., Srikumari C. R., Lorenz B., Finckh U., Nicoletti A., Murthy K. R., Rathmann M., Kumaramanickavel G., Denton M. J., Gal A. (1997) Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat. Genet. 17, 194–197 [DOI] [PubMed] [Google Scholar]
- 41. Redmond T. M., Yu S., Lee E., Bok D., Hamasaki D., Chen N., Goletz P., Ma J. X., Crouch R. K., Pfeifer K. (1998) Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat. Genet. 20, 344–351 [DOI] [PubMed] [Google Scholar]
- 42. Kiser P. D., Golczak M., Lodowski D. T., Chance M. R., Palczewski K. (2009) Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc. Natl. Acad. Sci. U. S. A. 106, 17325–17330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hill R. E., Favor J., Hogan B. L., Ton C. C., Saunders G. F., Hanson I. M., Prosser J., Jordan T., Hastie N. D., van Heyningen V. (1991) Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 354, 522–525 [DOI] [PubMed] [Google Scholar]
- 44. Glaser T., Walton D. S., Maas R. L. (1992) Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat. Genet. 2, 232–239 [DOI] [PubMed] [Google Scholar]
- 45. Quiring R., Walldorf U., Kloter U., Gehring W. J. (1994) Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science 265, 785–789 [DOI] [PubMed] [Google Scholar]
- 46. Halder G., Callaerts P., Gehring W. J. (1995) Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 267, 1788–1792 [DOI] [PubMed] [Google Scholar]
- 47. Swaroop A., Xu J. Z., Pawar H., Jackson A., Skolnick C., Agarwal N. (1992) A conserved retina-specific gene encodes a basic motif/leucine zipper domain. Proc. Natl. Acad. Sci. U. S. A. 89, 266–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mears A. J., Kondo M., Swain P. K., Takada Y., Bush R. A., Saunders T. L., Sieving P. A., Swaroop A. (2001) Nrl is required for rod photoreceptor development. Nat. Genet. 29, 447–452 [DOI] [PubMed] [Google Scholar]
- 49. Ng L., Hurley J. B., Dierks B., Srinivas M., Salto C., Vennstrom B., Reh T. A., Forrest D. (2001) A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat. Genet. 27, 94–98 [DOI] [PubMed] [Google Scholar]
- 50. Acland G. M., Aguirre G. D., Ray J., Zhang Q., Aleman T. S., Cideciyan A. V., Pearce-Kelling S. E., Anand V., Zeng Y., Maguire A. M., Jacobson S. G., Hauswirth W. W., Bennett J. (2001) Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 28, 92–95 [DOI] [PubMed] [Google Scholar]
- 51. Batten M. L., Imanishi Y., Tu D. C., Doan T., Zhu L., Pang J., Glushakova L., Moise A. R., Baehr W., Van Gelder R. N., Hauswirth W. W., Rieke F., Palczewski K. (2005) Pharmacological and rAAV gene therapy rescue of visual functions in a blind mouse model of Leber congenital amaurosis. PLoS Med. 2, e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cideciyan A. V., Aleman T. S., Boye S. L., Schwartz S. B., Kaushal S., Roman A. J., Pang J. J., Sumaroka A., Windsor E. A., Wilson J. M., Flotte T. R., Fishman G. A., Heon E., Stone E. M., Byrne B. J., Jacobson S. G., Hauswirth W. W. (2008) Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. U. S. A. 105, 15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maguire A. M., Simonelli F., Pierce E. A., Pugh E. N., Jr., Mingozzi F., Bennicelli J., Banfi S., Marshall K. A., Testa F., Surace E. M., Rossi S., Lyubarsky A., Arruda V. R., Konkle B., Stone E., Sun J., Jacobs J., Dell'Osso L., Hertle R., Ma J. X., Redmond T. M., Zhu X., Hauck B., Zelenaia O., Shindler K. S., Maguire M. G., Wright J. F., Volpe N. J., McDonnell J. W., Auricchio A., High K. A., Bennett J. (2008) Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bainbridge J. W., Smith A. J., Barker S. S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G. E., Stockman A., Tyler N., Petersen-Jones S., Bhattacharya S. S., Thrasher A. J., Fitzke F. W., Carter B. J., Rubin G. S., Moore A. T., Ali R. R. (2008) Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 358, 2231–2239 [DOI] [PubMed] [Google Scholar]
- 55. Adamis A. P., Miller J. W., Bernal M. T., D'Amico D. J., Folkman J., Yeo T. K., Yeo K. T. (1994) Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am. J. Ophthalmol. 118, 445–450 [DOI] [PubMed] [Google Scholar]
- 56. Aiello L. P., Avery R. L., Arrigg P. G., Keyt B. A., Jampel H. D., Shah S. T., Pasquale L. R., Thieme H., Iwamoto M. A., Park J. E., Nguyen H. V., Aiello L. M., Ferrara N., King G. L. (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 331, 1480–1487 [DOI] [PubMed] [Google Scholar]
- 57. Pierce E. A., Avery R. L., Foley E. D., Aiello L. P., Smith L. E. (1995) Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc. Natl. Acad. Sci. U. S. A. 92, 905–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lopez P. F., Sippy B. D., Lambert H. M., Thach A. B., Hinton D. R. (1996) Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest. Ophthalmol. Vis. Sci. 37, 855–868 [PubMed] [Google Scholar]
- 59. Brown D. M., Kaiser P. K., Michels M., Soubrane G., Heier J. S., Kim R. Y., Sy J. P., Schneider S., Group A. S. (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 355, 1432–1444 [DOI] [PubMed] [Google Scholar]
- 60. Gillies M. C., Wong T. Y. (2007) Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 356, 748–749, author reply 749–750 [PubMed] [Google Scholar]
- 61. Rosenfeld P. J., Brown D. M., Heier J. S., Boyer D. S., Kaiser P. K., Chung C. Y., Kim R. Y., Group M. S. (2006) Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 355, 1419–1431 [DOI] [PubMed] [Google Scholar]