Abstract
The effect of in utero exposure to a maternal high-fat diet on the peripheral circadian system of the fetus is unknown. Using mRNA copy number analysis, we report that the components of the peripheral circadian machinery are transcribed in the nonhuman primate fetal liver in an intact phase-antiphase fashion and that Npas2, a paralog of the Clock transcription factor, serves as the rate-limiting transcript by virtue of its relative low abundance (10- to 1000-fold lower). We show that exposure to a maternal high-fat diet in utero significantly alters the expression of fetal hepatic Npas2 (up to 7.1-fold, P<0.001) compared with that in control diet-exposed animals and is reversible in fetal offspring from obese dams reversed to a control diet (1.3-fold, P>0.05). Although the Npas2 promoter remains largely unmethylated, differential Npas2 promoter occupancy of acetylation of fetal histone H3 at lysine 14 (H3K14ac) occurs in response to maternal high-fat diet exposure compared with control diet-exposed animals. Furthermore, we find that disruption of Npas2 is consistent with high-fat diet exposure in juvenile animals, regardless of in utero diet exposure. In summary, the data suggest that peripheral Npas2 expression is uniquely vulnerable to diet exposure.—Suter, M., Bocock, P., Showalter, L., Hu, M., Shope, C., McKnight, R., Grove, K., Lane, R., Aagaard-Tillery, K. Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates.
Keywords: Npas2, epigenetics, fetal origins of adult disease
The alarming escalation of childhood obesity rates in the United States may not be attributable to either environment or genetics alone (1–7). Rather, collective data from models of altered maternal nutrition and uteroplacental insufficiency suggest that the gestational milieu influences the postnatal phenotype to render a susceptibility to childhood obesity via in utero epigenetic alterations in both histone covalent modifications and DNA methylation (8–13). The rising level of pediatric obesity is associated with an increase in juvenile type II diabetes, nonalcoholic fatty liver disease, and premature death (14–17).
Our recent work in a nonhuman primate (NHP) model of maternal high-fat diet (HFD) exposure and obesity demonstrated that dietary exposure in utero results in a statistically significant hyperacetylation of fetal hepatic tissue at lysine 14 of histone H3 (H3K14ac) (9). A similar examination of histone modifications associated with gene repression, such as trimethylation of lysine 9 of histone H3 (H3K9me3), revealed that repressive modifications are minimally altered under conditions of a maternal HFD compared with a control diet (9). In an effort to identify genes that might undergo reprogramming in response to in utero exposure, chromatin immunoprecipitation (ChIP) with an antibody against H3K14ac followed by differential display PCR was performed. Using such an approach, we demonstrated that Npas2, a hepatic and forebrain paralog of the Clock transcription factor, was a candidate gene for fetal reprogramming via H3K14 acetylation.
The Clock family of circadian genes is proposed to help orchestrate the metabolic response, based on a number of lines of evidence. Glucose and lipid homeostasis are known to display circadian variation (18–20). Surgical ablation of the suprachiasmatic nucleus (SCN) impairs the control of glucose homeostasis and disruption of Clock or its heterodimeric partner BMAL1 results in suppression of diurnal variation in glucose and triglycerides (21). Although the SCN is the master regulator of circadian rhythms, studies of liver and adipose tissue demonstrate robust and coordinated expression of the circadian oscillation system as well as Clock-controlled downstream effectors (22, 23). Although the SCN is entrained by the light-dark cycle, a feeding schedule is able to entrain the peripheral circadian clock in the liver and consumption of an HFD can alter circadian rhythms (24–28).
Circadian gene expression is maintained through the coordinated action of Clock (or its paralog Npas2), BMAL1, cryptochrome, and period genes (29). CLOCK and BMAL1 form a heterodimer that directly induces the expression of genes encoding negative regulators, including the crytochrome (Cry) and the Period (Per) genes (see Fig. 1A; ref. 30). It has been proposed that the absolute level of CLOCK protein in Drosophila is the limiting factor regulating circadian transcription (31). The central Clock of the SCN orchestrates peripheral synchronicity, including hepatic entrainment, via the regulated expression of the peripheral clocks. Npas2 is a Clock paralog, expressed in the forebrain cortices, basal ganglia, and liver (32, 33). Like Clock, Npas2 shares BMAL1 as an obligate heterodimeric partner (34). NPAS2:BMAL1 heterodimers positively regulate Per and Cry gene expression in a circadian transcriptional oscillation pattern. After accumulating in the cytoplasm, PER and CRY proteins translocate to the nucleus and regulate the activity of CLOCK:BMAL1 to complete a transcriptional/translational feedback loop. Ergo, the peak expression of these two distinct sets of genes occurs in antiphase of one another. A secondary regulatory loop consists of Rev-erb-α and ROR-α and is similarly activated by NPAS2/CLOCK:BMAL1 dimers to represses BMAL1 transcription (35).
Figure 1.
Expression of circadian genes in fetal liver. A) Schematic diagram showing the feedback loop of the circadian genes. Expression of circadian-regulated genes is driven by the binding of an NPAS2:BMAL1 dimer to the E-box within the circadian gene promoters. The dimer drives transcription of the Per and Cry genes as well as Rev-erb-α and RORα. Increased levels of Per, Cry, and Rev-erb-α inhibit expression of Bmal1 and Npas2, providing a feedback mechanism of transcriptional regulation. B) Relative mRNA copy number in fetal liver from control diet-fed mothers shows that low copy number of Npas2 is associated with increased Per2 and Rev-erb-α. C) mRNA copy number in fetal liver from HFD-fed mothers does not follow the tight pattern of expression as seen in the control diet animals. D) Although Npas2 has a very low copy number in fetal liver, it is readily amplified by PCR.
Although little is known about fetal circadian rhythms in relation to developmental physiology, it is proposed that circadian rhythms are established in the fetus by virtue of maternal cues, probably to prepare the fetus for the postnatal environment (reviewed in refs. 36, 37). In primates, diurnal changes in levels of hormones in the fetal bloodstream, such as progesterone, dehydroepiandrosterone sulfate, and thyroid-stimulating hormone, are seen at middle to late gestation (38–40). In rats, the fetal SCN shows rhythmic changes in circadian genes at embryonic day 20, and these rhythms can be disrupted through changing the maternal light-dark cycle (41, 42). Disruption of fetal circadian rhythm development during gestation may influence adult rhythms and can have effects on illnesses associated with depression and metabolic syndrome (reviewed in ref. 43). In a mouse model of restricted maternal feeding, it has been determined that the maternal feeding schedule is able to entrain the fetal SCN and liver independently of the maternal SCN or light-dark cycle (44).
In sum, our focus on Npas2 as a candidate gene for regulating peripheral circadian gene expression comes from three lines of evidence. First, the paralog of Npas2, Clock, has been reported to be a limiting factoring regulating expression in the SCN (31). Second, in humans, specific single nucleotide polymorphisms of Npas2 have been linked to risk factors for the development of metabolic syndrome (45). Third, our previous work revealed Npas2 as a gene potentially reprogrammed by H3K14ac with exposure to a maternal HFD in utero (9).
In this study, we report that in utero exposure to a maternal HFD disrupts fetal hepatic circadian gene expression in an NHP model of maternal obesity. Reversion to a control diet, even with persistent maternal obesity, returns the fetal circadian expression to that of control diet-exposed animals. In an effort to decipher the molecular mechanisms underscoring our observations, we observe that Npas2 is a limiting factor for transcriptional regulation through absolute copy number analysis of peripheral circadian genes. Extensive epigenetic analysis of the functional promoter Npas2 demonstrates that the reprogramming of this peripheral circadian regulator does not occur through DNA methylation, but rather via H3K14-specific enrichment surrounding the critical promoter elements [e.g., ROR response element (RORE) binding sites] driving Npas2 transcription. Finally, we report in 1-yr-old animals exposed to either an HFD or control diet in utero that the postweaning diet, and not the diet exposure in utero, influences Npas2 expression. Juvenile animals fed the HFD after weaning have significantly disrupted Npas2 expression.
MATERIALS AND METHODS
Experimental design
All animal procedures were done in accordance with approval from the Institutional Animal Care and Use Committees of both Baylor College of Medicine and the Oregon National Primate Research Center (ONPRC), as described previously (3). At 5–6 yr of age, young adult female Japanese macaques were bred and socially housed in indoor/outdoor enclosures in groups of 5 to 9 with 1 to 3 males/group. Animals in the control diet group were fed standard monkey chow, which provides 14% total calories from fat (Monkey Diet 5052; Lab Diet, Richmond, IN, USA), whereas those in the HFD group were fed a diet with 32% calories from fat (Custom Diet 5A1F; Test Diet, Richmond, IN, USA) and supplemented with calorically dense treats. Before initiation into either the control diet or HFD protocol, all study animals were fed a control diet. In the diet-reversal cohort, during the 5th year of the HFD study protocol, formerly HFD-fed, obese dams were reversed to consume the control diet (14% total calories from fat; Monkey Diet 5052). This reversal was initiated at the start of the breeding season (1–4 mo before approximate conception) and maintained throughout gestation and the postnatal weaning interval. The mean time to conception was 42 ± 19 d, with 1 animal conceiving ∼5 d after diet reversal. Time to conception was ∼40 d for most animals.
Animals were allowed to breed naturally and were tested for pregnancy by palpation; results were then confirmed, and the pregnancy was gestationally dated (fetal biometry) by sonogram. Pregnancies were terminated by cesarean section at gestational age 130 by trained ONPRC veterinarians. With respect to the preoperative protocol, dams were deprived of food overnight (∼16 h) before induction of anesthesia for cesarean delivery. Per a priori defined study protocol, cesarean deliveries were initiated on each occasion within a 30-min period (10:00–10:30 AM) to ensure uniformity with respect to both interval of food deprivation, as well as time of day. For induction of anesthesia, dams were sedated with ketamine hydrochloride (100 mg/ml) at a dose of 10–15 mg/kg. Once sedated, dams were brought to the surgical suite and anesthetized under isoflurane gas with induction at 3% and maintenance at 1.0–1.5%. Cesarean delivery (uterine hysterotomy) was performed by trained veterinarians and their designated surgical staff and followed standard operative procedures. After delivery, fetuses were anesthetized with sodium pentobarbital and thereafter exsanguinated. During subsequent necropsy, fetal livers were harvested, flash-frozen in liquid nitrogen, and stored at −80°C until use.
Pre- and postoperative care of the dams with respect to analgesia for pain control was provided by the veterinary staff; animals received an intravenous dose of hydromorphone (0.5 mg if weighing <10 kg and 1.0 mg if weighing >10 kg) preoperatively (time of induction of anesthesia) and postoperatively (completion of procedure). Subsequent postoperative hydromorphone was administered within 6 h with long-acting buprenorphine (0.3 mg i.m.) 4 h thereafter for overnight analgesia. On postoperative day 1, hydromorphone was administered at 8:00 AM, 12:00, and 4:00 PM, buprenorphine being again administered at 10:00 PM. All study protocols were approved by the ONPRC Institutional Animal Care and Use Committee and were performed in a protocol-compliant and uniform fashion.
For the juvenile animals (aged to 1 yr postterm) used in this study, pregnancies were allowed to progress to term (gestational day 167), with delivery occurring with spontaneous onset of labor and vaginal birth. Offspring were kept with birth mothers and either consumed the same diet postnatally as the mother [control diet of both dam and offspring (CTR/CTR) and HFD of both dam and offspring (HF/HF)] or postweaning were fed the alternate diet than the mother [control diet dam with HFD to the offspring (CTR/HF) or HFD dam with control diet to the offspring (HF/CTR)].
Liver tissue for the juvenile animals was obtained by biopsy, performed via laparoscopic assisted approaches with attainment of core biopsy. For this procedure, offspring were deprived of food for 3 h before induction of anesthesia and were sedated with Telazol at 5 mg/kg i.m. to obtain deep anesthesia. Trained veterinary surgical staff made an ∼5-mm keyhole port incision, and 2 core liver biopsy specimens were obtained using alligator forceps.
mRNA copy number
Copy number of fetal hepatic Npas2, Per2, and Rev-erb-α for control diet (n=5) and HFD (n=9) in utero exposed fetuses was determined using quantitative PCR (qPCR) with TaqMan probes (Applied Biosystems, Carlsbad, CA, USA). A standard curve of known copy number was created using plasmids of the cloned genes, with high correspondence among plasmid replicates (r>0.95). Results from the qPCR of each animal were extrapolated from the standard curve to determine the absolute copy number of each gene.
qPCR
For gene expression analysis, the following animals were used: fetal control diet (n=9), fetal HFD (n=10), fetal reversed diet (n=7), juvenile CTR/CTR (n=5), juvenile CTR/HF (n=4), juvenile HF/CTR (n=4), and juvenile HF/HF (n=5). Each gene analyzed in this study was first cloned from Japanese macaque total mRNA using the Strataclone system (catalog no. 240205; Stratagene, LA Jolla, CA, USA) and subsequently sequenced. Sequences were used to design custom TaqMan primers and probes for gene expression analysis (Table 1). RNA was then extracted from each liver sample using the NucleoSpin Kit (740955; Machery Nagel, Bethlehem, PA, USA). RNA was converted to cDNA using the High Capacity cDNA Reverse Transcription Kit (2368814; Applied Biosystems). Real-time PCR was performed for each sample in quadruplicate and repeated three times.
Table 1.
TaqMan primers and probes used for qPCR
| Gene | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| Npas2 | GTCTCAGCGCAAACGGAAA | TTACTGAGGAATGAAGGCTTCCA | CTGTGACATTCAGCAGGA |
| Bmal | GTACCAACATGCAACGCAATG | TGAACAGCCATCCTTAGCACAGT | CCAGGAAATTAGATAAAC |
| Cry1 | GCGAAGGTGTCGGCTATGA | GTCCTCCACGGAGAAATTCAAG | CCGGAGCCTCCTT |
| Cry2 | CAGCCAGCCGACGTGTTC | CAAAGGTCAAGCGGGTCACT | AAGGCTGTTCAAGGAA |
| Per1 | GGACTCATGCCCGTTGGA | CACCGATGCCAACAGCAAT | TCATTGCCACTTGAAC |
| Per2 | GGTGACCCTCGCTGGACAT | GCAGGTGAAAGCCAACGAA | AGCAGCTGGTAATACT |
| Rev-erb-a | TTGCCTCGGGCTTCCA | GACGGAAAAAGCCCTTGCA | CGTGCACGCCTGTG |
| ROR-α | GGCTGGCATACTTCCCATCA | AATGTGCCGTGCCTTTGACT | AGTACACGGTGTTGTTC |
| GAPDH | GGGAAGGTGAAGGTCGGAGT | AAGCAGCCCTGGTGACCA | ACGGATTTGGTCGTATTGGGCGC |
Gene expression analysis
For data presented as a fold change in expression due to maternal diet exposure, the ΔΔCT method was used for analysis (46). Fold change was calculated for each individual animal to show the range of variability between samples in the HFD-exposed animals.
DNA methylation analysis
Genomic DNA was isolated from each liver sample (n=4/group) using a DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA). Sodium bisulfite treatment of each sample was performed using an E-Z DNA Methylation Gold Kit (Zymo Research, Orange, CA, USA). Three fragments of the Npas2 proximal promoter region, region I (−1699 to −170), region II (−170 to +110), and region III (+110 to +575), were amplified by PCR. Region I contains the RORE of Npas2. Four additional liver specimens per group were used to further probe the region surrounding and including the RORE. PCR products were cloned using the Strataclone PCR Cloning Kit. A minimum of 6 colonies for each PCR product was selected. Plasmids were purified from each individual colony using a MiniPrep Kit (27106; Qiagen). Samples were sequenced by Lone Star Labs (Houston, TX, USA). Figure 3A, which depicts each individual CpG site, was created using ABI Methyl Prime software (Applied Biosystems).
Figure 3.
Changes in fetal Npas2 expression due to maternal HFD exposure are not associated with changes in methylation of the proximal promoter region. A) Schematic diagram of the Npas2 proximal promoter region analyzed for differential methylation. Pink lines indicate location of individual CpG sites analyzed. RORE lies within region I. B) Methylation status of each CpG analyzed. ○, unmethylated cytosines; ● methylated cytosine. Additional animals were used to analyze the region around the RORE. Each horizontal line represents 1 clone.
ChIP and analysis
ChIP was performed as described previously (9) with the following modifications. Approximately 100 mg of frozen liver tissue from each animal (control diet, n=6; HFD, n=7; reversed diet, n=7) was fixed in 1% formaldehyde for 15 min at room temperature and quenched with 125 mM glycine. Cells were lysed using a Dounce homogenizer. Chromatin was completely digested into mononucleosomes using 2 U of micrococcal nuclease (Sigma-Aldrich, St. Louis, MO, USA) per sample, with incubation at 37°C for 10 min.
Approximately 100 μg of chromatin was used for each immunoprecipitation (IP). Ten percent of the reaction was saved before the addition of antibody as the input. Each IP was performed side by side with a no-antibody control. Three microliters of antibody was used per IP and incubated overnight with rotation at 4°C (H3K14ac, 04-1044, and H3K9me3, 05-1242; Millipore, Billerica, MA, USA). Preblocked protein A beads (20334; Pierce, Rockford, IL, USA) were added and incubated with rotation for 3 h at room temperature. Beads were washed, resuspended in buffer containing proteinase K (19131; Qiagen), and incubated overnight with shaking at 37°C. Concomitantly, proteinase K was added to the input samples and incubated overnight at 37°C. Cross-links were reversed by incubation at 65°C for 5 h. DNA was purified using phenol-chloroform extraction followed by ethanol precipitation.
Enrichment of promoter occupancy by virtue of histone modifications was determined using qPCR with the following primers: Npas2 RORE (5′-GTTTTCACCGGCTCTGTCTTG-3′ and 5′-GAGGCAGAGGTACAGGAATAATCC-3′) and Npas2 intron (5′-GCCGTTTGCTTCTTGGAAA-3′ and 5′-AACCGATTCTACATTTACGCACAGT-3′). DNA from each sample was diluted 1:4, and 4 μl of diluted DNA was added to each reaction. PCR was performed using SYBR Green (4309115; Applied Biosystems) in the iCycler (Bio-Rad Laboratories, Hercules, CA, USA). To determine percentage IP, samples were first normalized to the input for each sample (ΔΔCT). Percentage IP was calculated as 2−ΔΔCT.
Statistical analysis
Real-time PCR was analyzed using the 2−ΔΔCT method, as described previously (46). The fold change for each individual animal compared with those for the control diet samples is displayed. An independent-sample t test was performed for each gene, comparing the HFD with the control diet and diet reversal with the control diet. An independent-sample 1-tailed t test was performed to analyze the differential methylation in the RORE of Npas2 between the control diet- and HFD-exposed animals. An independent-sample 2-tailed t test was performed for the ChIP analysis, comparing the HFD with the control diet and diet reversal with the control diet.
RESULTS
mRNA copy number of circadian genes from fetal liver shows tight regulation in control but not HFD-exposed animals
To study the alterations in circadian gene expression in our nonhuman primate model, the relative mRNA levels were specifically quantified by virtue of mRNA copy number for 3 circadian-related genes: Npas2, Per2, and Rev-erb-α. As introduced above, it is well established that the peak levels of Npas2 show a different phase than those of Per2 and Rev-erb-α. Specifically, when Npas2 expression is low, Per2 and Rev-erb-α expression will be high because of the feedback inhibitory mechanism of circadian gene expression. Traditionally, in most animal models of circadian gene expression, animals will be sacrificed at specific time points to determine the phase of gene expression levels over a 24-h period (47–50). However, in our primate model, this is not technically feasible. Thus, in an effort to determine the circadian phase of gene expression levels, we alternately quantified overall mRNA copy number of each gene as measured by extrapolation from a standard curve of known copy number. As shown in Fig. 1, copy number for Npas2, Per2 and Rev-erb-α can thereafter be plotted for each animal and connected by a line to determine the difference between each gene in each animal. In fetal livers of control diet-fed dams (Fig. 1B), levels of Npas2, Per2, and Rev-erb-α demonstrate little variation between each individual animal, and a low copy number of Npas2 is associated with increased Per2 and Rev-erb-α. This result is demonstrative of intact phase-antiphase transcriptional oscillation circadian biology in the developing fetus. However, in the HFD-exposed offspring, the overall mRNA copy number varies greatly among individual animals, and the overall phase difference between genes is not as substantial as that seen in the control diet animals (Fig. 1C). This result suggests that the circadian expression levels are dampened due to in utero HFD exposure, and the normal integrative mechanisms have been disrupted in the developing fetus by virtue of the maternal diet.
Npas2 copy number was substantially lower than that of Per2 and Rev-erb-α, thereby suggesting that Npas2 may be the rate-limiting step in regulating transcriptional oscillation. The difference in copy number was also significant between control diet- and HFD-exposed animals (P=0.033). Therefore, RT-PCR was performed to validate Npas2 amplification under our experimental conditions. All three genes were distinctly visible after stringent PCR, and there was no nonspecific amplification (Fig. 1D). Thus, intact peripheral circadian transcriptional oscillation is occurring in the developing fetal primate liver, and Npas2 is uniquely present in the fetal liver at a low copy number. Based on these initial observations and the literature, we surmised that it is probable that Npas2 expression serves as the gatekeeper to maintaining circadian transcription regulation rhythms, especially in the developing fetus.
Expression of hepatic Npas2 and downstream circadian effectors is significantly disrupted in offspring exposed to a maternal HFD
Our previous studies identified Npas2 as a fetal gene that is reprogrammable in utero under conditions of a maternal HFD (9). To extend these analyses to our larger study cohort, fetal hepatic Npas2 gene expression from control diet, HFD, and reversed diet dams (i.e., persistent maternal obesity in dams reversed to the control diet in year 5) was quantified using qPCR (Fig. 2). When we compared means of our entire fetal cohort (n=26), there was a nonsignificant change (2.9-fold, P=0.220) in the HFD-exposed offspring, whereas the animals exposed to the reversed diet showed levels of expression similar to those with the control diet (1.3-fold, P=0.306; Fig. 2A). Of note and consistent with our mRNA copy number data presented in Fig. 1, a tight correlation of the expression within the control and diet reversed groups was observed, suggesting maintenance of intact phase-antiphase circadian transcriptional oscillation. However, significant divergent variance in expression levels from the HFD-exposed offspring was observed (range of 0.5- and 10.5-fold change). Because feeding can drive circadian amplitude and oscillation of the mammalian hepatic transcriptome (51), it was possible that our observations could be attributable to disruption in either phase or amplitude of the circadian cycle.
Figure 2.
Circadian gene expression is disrupted in fetal liver exposed to a maternal HFD. A) Npas2 expression level for each individual fetal animal in the control diet (n=9), HFD (n=10), and reversed diet (n=7) groups. Although the overall difference in average fold change does not differ among groups, the range of expression fold change is much greater in the HFD-exposed group. B) Npas2 expression level of 3 animals exposed to the HFD was greatly increased compared with those exposed to the control diet. Difference in expression between these two groups is significantly increased. C) Npas2 expression level of 4 animals was greatly decreased (<0.5-fold) compared with those fed the control diet. Change in expression was significantly decreased in these animals. D) Fetal hepatic Per1 is up-regulated 4.23-fold in HFD-exposed animals (P<0.001). Expression in reversed diet animals trends toward that of animals fed the control diet but is still significantly changed (1.26-fold) compared with that for the control diet (P<0.01). E) Expression of Rev-erb-α is up-regulated 2.7-fold with HFD exposure (P=0.011) and returns to control diet levels with diet reversion.
To more formally investigate this possibility, our fetal cohorts were stratified into two groups, based on whether they displayed an increase in Npas2 expression (n=3) or a decrease in Npas2 expression (n=4). With stratification, the change between control diet- and HFD-exposed offspring is significant. In HFD-exposed animals with an up-regulation of Npas2, there is a 7.3-fold increase in expression (P<0.001) compared with that for the control group (Fig. 2B). A significant change is similarly observed in the fetal liver among offspring with down-regulated Npas2 expression under conditions of maternal HFD exposure (0.64-fold, P=0.04; Fig. 2C).
Npas2 is a transcriptional regulator of E-box-containing circadian genes. Expression levels of Bmal1, the dimerization partner of Npas2, as well as those of the downstream effectors Cry1, Cry2, Per1, Per2, Rev-erb-α, and ROR-α were characterized. In the fetal liver, expression of Per1 was significantly up-regulated 4.23-fold (P<0.01) in HFD-exposed animals (Fig. 2D). In animals exposed to the reversed diet, the expression levels trended back to control levels (1.26-fold) but were still significantly different from those of control diet animals (P<0.01) Fetal hepatic Rev-erb-α was also up-regulated in HFD-exposed animals (2.72-fold, P=0.011; Fig. 2E) and returned to control levels in reversed diet animals.
Changes in Npas2 expression are not accompanied by changes in promoter methylation
In an effort to determine the epigenetic modifications that accompany the changes in Npas2 expression in HFD-exposed animals (and would thereby potentially be regulating transcription), detailed methylation status of each of 100 CpG dinucleotides within the entirety of the 2.2-kb proximal promoter was analyzed with bisulfite modification and sequencing (Fig. 3A). Of note, contained within this functional promoter are two ROREs, which have been demonstrated to be critical for integrating the phase-antiphase maintenance of Npas2 expression (52). Because we and others have shown in other models that maternal exposure resulting in altered fetal CpG methylation in critical xenobiotic response elements (XREs) is significantly correlated with increased fetal gene transcription (53, 54), we reasoned that the differences in Npas2 gene expression between control and HFD-exposed fetal offspring could potentially be due to differential methylation surrounding the RORE. For our analysis, the functional promoter region was partitioned into 3 contiguous regions by virtue of proximity to the RORE: region I, retaining both ROREs (−1699 to −170); region II, containing the transcriptional start site (−170 to +110); and region III, incorporating the first noncoding exons (+110 to +575). Each of these regions embedded near equivalent numbers of CpG dinucleotides, and none contained other transcriptional binding elements.
The methylation status, as determined by bisulfite modification and sequencing for each individual CpG dinucleotide across the entirety and regional partitions of the 2.2-kb promoter, is depicted in Fig. 3B and summarized in Table 2. No significant variance in methylation at any given CpG dinucleotide between control and HFD-exposed animals was observed. Although a statistically significant difference between control and HFD-exposed animals in region III (P=0.006) was found, a difference in methylation of <3% is unlikely to represent a physiologically relevant variation, given the large changes reported in Npas2 gene expression.
Table 2.
Summary of Npas2 promoter methylation
| Npas2 promoter region | RORE in region | Diet | Clones sequenced (n) | CpG/total CG + TG | Methylated CpG (%) |
|---|---|---|---|---|---|
| I | Yes | Control | 32 | 13/1074 | 1.21 |
| High fat | 44 | 18/1574 | 1.14* | ||
| II | No | Control | 12 | 2/566 | 0.35 |
| High fat | 24 | 4/755 | 0.53# | ||
| III | No | Control | 12 | 17/520 | 3.27 |
| High fat | 19 | 4/635 | 0.63@ | ||
| Total | Control | 56 | 32/2160 | 1.48 | |
| High fat | 87 | 26/2964 | 0.88$ |
P = 0.911;
P = 1.0;
P = 0.013;
P = 0.081.
Npas2 histone code: reprogramming of Npas2 is accompanied by changes in H3K14ac levels at the RORE
We hypothesized that the change in Npas2 gene expression may be correlated with a change in histone modifications in proximity to the functional promoter. Because of our previous findings demonstrating an increase in fetal hepatic H3K14ac after maternal HFD exposure (9), we focused on changes in promoter occupancy of this modification. Because H3K14ac is traditionally associated with active chromatin (55–58), characterization of the changes in Npas2 promoter occupancy of a known repressive modification, trimethylation of lysine 9 of histone H3 (H3K9me3), was performed in parallel (59–61). Fetal hepatic H3K9me3 levels do not change with maternal diet exposure (9).
To measure promoter occupancy of modified histones (acetylation of methylation), ChIP with real-time PCR using oligos flanking the functional Npas2 promoter was performed. As a control for nonspecific ChIP enrichment, the first intron of Npas2 was used because it is a region that does not manifest differential occupancy of either modification. For each sample, a mock IP without a primary antibody was used to determine background (nonspecific) binding. Results are presented as percentage IP, which is the relative amount of DNA precipitated by the chromatin modification specific antibody compared with the total amount of input DNA.
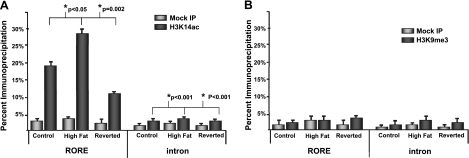
With use of an antibody against H3K14, there was significant enrichment for the RORE promoter region in animals exposed in utero to the maternal HFD (Fig. 4A). Compared with control diet-exposed animals (18.8%), animals exposed to the maternal HFD in utero showed a significant increase in enrichment of promoter occupancy with H3K14ac (27.6%, P<0.05). In the reversed diet offspring, enrichment drops to ∼11% (Fig. 4A). In the first intron of Npas2, a statistically significant increase in H3K14ac occupancy in HFD-exposed offspring was observed (2.3 vs. 3.7%, P<0.05), but the enrichment in the intron was 10-fold less than that in the RORE-containing promoter region (Fig. 4A). There were no significant changes in the mock IP samples, thereby validating the specificity of our ChIP analysis.
Figure 4.
Exposure to a maternal HFD in utero is associated with changes in occupancy of the Npas2 promoter RORE of H3K14ac but not H3K9me3. A) ChIP of extracts from fetal hepatic tissue with an antibody specific for H3K14 acetylation shows enrichment in the RORE region of the Npas2 promoter in animals exposed to a maternal HFD in utero. This enrichment does not persist in animals exposed to a reversed diet. B) ChIP with an antibody specific for trimethylation of lysine 9 of histone H3 does not show enrichment of the RORE of Npas2 due to maternal diet.
Given our observed enrichment of Npas2 RORE flanking promoter occupancy with H3K14ac ChIP (a marker of actively transcribed chromatin) (55–58), we next sought to analyze correlation of promoter occupancy with the general trend of increased expression of this gene (Fig. 2B). To achieve this, a Pearson correlation was determined between the expression of Npas2 in each HFD-exposed animal (as determined by ΔΔCT) and percentage IP. There is a significant correlation between gene expression levels and promoter occupancy as measured by H3K14ac ChIP (r=0.798, P<0.05).
In contrast, we observed that were no significant changes in promoter occupancy of H3K9me3 within the RORE of the Npas2 promoter in our model (Fig. 4B), and the enrichment of H3K9me3 was minimally increased relative to mock IP. When we again investigated correlation between H3K9me3 promoter occupancy and gene expression, we observed a nonsignificant correlation (0.449, P=0.186).
Disruption of circadian gene expression is consistent with a high-fat postweaning diet
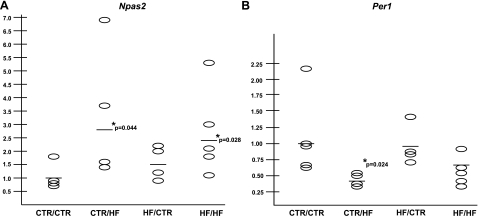
Four cohorts of juvenile (1-yr-old) animals, grouped both by diet exposure in utero (either control diet or HFD) and by postweaning diet (control diet or HFD), respectively (CTR/CTR, CTR/HF, HF/CTR, and HF/HF), were studied. Hepatic expression levels of Npas2 were examined in each of the 4 cohorts. The overall expression of Npas2 was significantly up-regulated only in the animals fed the HFD postweaning, regardless of in utero diet exposure (CTR/HF, 2.7-fold, P=0.044; HF/HF, 2.3-fold, P=0.028; Fig. 5A). In contrast, animals exposed to a maternal HFD in utero and subsequently weaned to a control diet (HF/CTR) do not differ significantly from the CTR/CTR group (1.5-fold, P=0.182; Fig. 5A). Hepatic expression of Per1 in the juvenile animals only differed from CTR/CTR in the CTR/HF cohort (0.4-fold, P=0.024). Expression in the HF/CTR (0.9-fold, P=0.753) and HF/HF (0.6-fold, P=0.093) groups was not significantly different from that in the CTR/CTR group (Fig. 5B). Expression levels of Bmal1, Cry1, Cry2, Per2, Rev-erb-α, and Ror-α were also determined for all four groups. No significant changes between groups was observed (data not shown).
Figure 5.
Disruption of hepatic circadian gene expression in juvenile animals is consistent with postweaning exposure to an HFD. A) Npas2 expression level is significantly increased in juvenile animals exposed to an HFD diet during the postweaning phase (CTR/HF, 2.7-fold, P=0.044; HF/HF, 2.3-fold, P=0.028). Animals exposed to the HFD in utero and weaned to a control diet do not show disrupted Npas2 expression (HF/CTR, 1.5-fold, P=0.182). B) Expression of Per1 differed from the CTR/CTR group only in the animals exposed in utero to a control diet and weaned to an HFD (CTR/HF, 0.4-fold, P=0.024).
DISCUSSION
It has been postulated that obesity begets obesity. We have previously demonstrated in a primate model of maternal HFD exposure (3) that the fetal histone code is reprogrammed (9) and that these alterations are associated with an altered fetal metabolome (62). The results of our study reported here further our understanding of the molecular and physiology mechanisms by which in utero HFD exposure modifies the fetal histone code at a systems biology level. Specifically, in this study we demonstrate that the primate peripheral circadian machinery is 1) established and transcriptionally functional during latter fetal development (d 130 of the 170- to 175-d gestation) and 2) dysregulated in both fetal and postnatal (up to 1 yr of life) liver due to HFD exposure. Extensive investigation of the epigenetic mechanisms regulating the transcription of the key molecular component in this system (Npas2; ref. 63; Fig. 1) allowed us to identify the critical role of HFD exposure in modifying acetylation of H3K14 to determine promoter occupancy and gene transcription, rather than altered CpG dinucleotide methylation in the promoter. Because alterations and disruptions of circadian rhythms are correlated with obesity and cardiovascular disease (reviewed in refs. 64, 65), the contribution of maternal diet to reprogramming of the fetal circadian rhythm is of interest in furthering our understanding of current childhood obesity trends.
Although our observations are novel in approach and significance, they are not without supportive observations from other mammalian systems. Glucose and lipid homeostasis are known to display circadian variation, and an HFD in a murine model amplifies the diurnal variation in glucose tolerance in a Clock-dependent fashion (19, 25). Similar to glucose homeostasis, peripheral Clock family members in liver and adipose tissue demonstrate robust and coordinated expression of the circadian oscillation system as well as Clock-controlled downstream effectors (23). Temporally restricted feeding or an alteration in diet content (calorically dense/high-fat) results in a coordinated phase shift in circadian expression of the major oscillator genes and their downstream targets in adipose tissues (20, 23). We provide evidence in this study that the peripheral circadian metabolic clock in the developing primate fetus can be altered in utero by virtue of maternal diet exposure, as evidenced by our observed changes in circadian gene expression patterns in fetal liver (Fig. 1B, C).
In conjunction with embryonic deletion experiments (63), our results demonstrating increasing low mRNA copy number with intact transcriptional oscillation are consistent with the notion that Npas2 is a critical orchestrator of the peripheral circadian system during development. We observe herein that subtle changes in copy number of Npas2 due to maternal HFD exposure (Fig. 1B, C) are associated with a large change in copy numbers of Per2 and Rev-erb-α only in HFD-exposed animals. Major components of energy homeostasis, including the sleep-wake cycle, thermogenesis, feeding, and glucose and lipid metabolism are subjected to circadian regulation that synchronizes energy intake and expenditure with changes in the perceived external environment (reviewed in refs. 66, 67) Recent and emerging evidence demonstrating that oscillation of Clock family genes can regulate circadian rhythmicity in vitro in other central (paraventricular hypothalamus and mediobasal hypothalamus) and peripheral tissues (liver, muscle, and pancreas) is consistent with the notion that fetal circadian and metabolic processes of nutrient homeostasis are linked at multiple levels (37, 68, 69). Therefore, studies focusing on regulation of the peripheral clock are of central importance in determining the molecular mechanisms of childhood obesity after maternal HFD exposure in utero.
A thorough analysis of the chromatin modifications and promoter occupancy within the known promoter region of Npas2 provides a clear mechanism of differential expression due to HFD exposure. Multiple lines of evidence from lower eukaryotes and mammalian cell lines have shown that gene expression is regulated by a synergy between DNA methylation and post-translational histone modifications (70–72). Emerging evidence has shown that in addition to genomic base pair differences, gene expression can be silenced by nonallelic mechanisms including epigenetic influences such as covalent modifications of histones and DNA methylation (recently reviewed in ref. 73). We and other authors have previously observed that CYP1A1 is inducible in its placental expression among smokers and that well-characterized XREs in the proximal promoter are differentially methylated at CpG islands in lung tissue and placental tissue of smokers (53, 74). Given these published observations of others along with our prior observations, we hypothesized that nonallelic modulation of Npas2 expression in the developing fetal liver may occur under conditions of maternal HFD exposure. Because hypermethylation in key gene regulatory sequences at CpG islands is generally associated with gene silencing, we sought to comprehensively investigate the methylation status of the proximal promoter region of Npas2.
It remains unknown whether DNA methylation per se is required for the establishment or maintenance of the silent chromatin state or whether reactivation of the DNA methylation machinery occurs in response to maintained histone modifications with DNA replication events. In an effort to determine the role of DNA methylation in reprogramming of the fetal circadian gene expression rhythms, site-specific DNA methylation was analyzed in the promoter region of Npas2 in control and HFD-exposed animals. No biologically significant difference in methylation of any of the 100 CpG sites within the 2.2-kb region of the promoter studied was found, thereby providing the essential rationale for our studies on acetylation and promoter occupancy (Fig. 4).
Chromatin remodeling complexes temporally regulate the rhythmic gene expression exhibited by Clock genes in the circadian feedback loop. Transcriptional regulation is central to Clock family gene function. Given the known role of covalent modifications of histones to influence transcriptional induction, other investigators have examined the role of chromatin remodeling complexes in the temporal regulation of Clock genes. Two independent reports have recently emerged demonstrating a necessary and sufficient role for histone acetylation in regulating temporal circadian gene expression in mammalian tissues (75, 76). Given our previous report of increased H3K14ac in HFD-exposed fetal liver, a major finding of this work is that alteration of Npas2 expression is accompanied by an overall increase in promoter occupancy of H3K14ac and not the repressive H3K9me3 at the RORE.
Furthermore, we demonstrate with both the fetal and juvenile cohorts that HFD exposure specifically alters circadian transcription. In fetal animals exposed in utero to a maternal HFD, hepatic Npas2 expression is disrupted. However, in fetuses exposed to maternal obesity with a reversal to a control diet, expression levels are similar to those of control diet-exposed animals. In addition, juvenile animals fed a postnatal HFD, whether exposed in utero to a maternal control diet or HFD, have disrupted Npas2 expression. However, animals exposed in utero to a maternal HFD and consumed a control diet postnatally have Npas2 expression levels similar to those of controls.
The limitations of our NHP model system do not allow for analysis of circadian gene expression over a 24-h time period. Unlike rodent models of circadian rhythms, the macaques had singleton pregnancies. Therefore, we would not be able to generate the number of animals necessary to determine the rhythmicity of the gene expression in the fetal liver. Furthermore, studies of circadian rhythms in mice usually entail entraining them to a light-dark cycle followed by days in chronic darkness, which would not be permitted by our protocol. Follow-up of these studies in a rodent model of maternal HFD would allow for a more thorough analysis of the period length changes and true nature of the circadian disruption due to in utero maternal HFD exposure.
The results in the juvenile animals point to the strength of our observed association between consumption of an HFD and circadian disruption during critical windows of development. We find it of marked interest that Npas2 only differs in animals consuming a postnatal HFD at the time of biopsy (CTR/HF and HF/HF). In utero exposure to an HFD did not seem to persistently disrupt Npas2 in juvenile offspring, because consumption of a control diet at the time of biopsy (HF/CTR) was associated with reversal of Npas2 differential expression. In other words, altered fetal circadian gene expression during fetal life does not permanently nor persistently influence expression in juvenile life. The significance of these findings is 2-fold. First, they suggest that either in utero exposure or consumption of an HFD is capable of disruption of Npas2 expression. Second, this disruption is readily amenable to correction with cessation of the HFD postnatally and initiation of a control diet. The implications for programming are tremendous, because these results provide initial evidence that the instrumental peripheral hepatic circadian regulator is regulated by dietary exposure in the critical intervals of development (prenatally and postnatally) and can be readily reversed with dietary correction during these same critical intervals.
The results of this study show that fetal hepatic oscillatory expression of peripheral Clock family members is altered under conditions of a high-fat/calorically dense maternal diet. Emerging evidence suggests that the circadian genes are of pivotal significance in orchestrating the metabolic response at a cellular and molecular level. Our observations serve as a novel demonstration that this fetal system is under the potential influence of the maternal diet. By extension, we postulate that the maternal environment is capable of entraining the fetal metabolic response as a result of the unique vulnerability of Npas2 and downstream circadian regulated genes to HFD alterations in utero and the importance of the fetal circadian genes in scripting the fetal metabolic response.
Acknowledgments
The authors acknowledge the helpful comments of members of the Ignatia B. Van Den Veyver and Shannon M. Hawkins laboratories at Baylor College of Medicine.
This work was supported by the U.S. National Institutes of Health (NIH) Director New Innovator Award (DP2120OD001500-01 to K.A.T.), National Institute of Child Health and Human Development/National Institute of Diabetes and Digestive and Kidney Diseases (grants R01-DK080558-01 to R.H.L and K.A.T and DK79194 to K.L.G.), and the NIH Research Education and Career Horizon Institutional Research and Academic Career Development Award (grant K12-GM084897 to M.S.). The authors report no conflicts of interest or financial disclosures.
REFERENCES
- 1. Speiser P. W., Rudolf M. C., Anhalt H., Camacho-Hubner C., Chiarelli F., Eliakim A., Freemark M., Gruters A., Hershkovitz E., Iughetti L., Krude H., Latzer Y., Lustig R. H., Pescovitz O. H., Pinhas-Hamiel O., Rogol A. D., Shalitin S., Sultan C., Stein D., Vardi P., Werther G. A., Zadik Z., Zuckerman-Levin N., Hochberg Z. (2005) Childhood obesity. J. Clin. Endocrinol. Metab. 90, 1871–1887 [DOI] [PubMed] [Google Scholar]
- 2. Grove K. L., Grayson B. E., Glavas M. M., Xiao X. Q., Smith M. S. (2005) Development of metabolic systems. Physiol. Behav. 86, 646–660 [DOI] [PubMed] [Google Scholar]
- 3. McCurdy C. E., Bishop J. M., Williams S. M., Grayson B. E., Smith M. S., Friedman J. E., Grove K. L. (2009) Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J. Clin. Invest. 119, 323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Popkin B. M., Gordon-Larsen P. (2004) The nutrition transition: worldwide obesity dynamics and their determinants. Int. J. Obes. Relat. Metab. Disord. 28(Suppl. 3):S2–S9 [DOI] [PubMed] [Google Scholar]
- 5. Rich-Edwards J. W., Kleinman K., Michels K. B., Stampfer M. J., Manson J. E., Rexrode K. M., Hibert E. N., Willett W. C. (2005) Longitudinal study of birth weight and adult body mass index in predicting risk of coronary heart disease and stroke in women. BMJ 330, 1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slyper A. H. (2004) The pediatric obesity epidemic: causes and controversies. J. Clin. Endocrinol. Metab. 89, 2540–2547 [DOI] [PubMed] [Google Scholar]
- 7. Strauss R. S., Pollack H. A. (2001) Epidemic increase in childhood overweight, 1986–1998. JAMA 286, 2845–2848 [DOI] [PubMed] [Google Scholar]
- 8. MacLennan N. K., James S. J., Melnyk S., Piroozi A., Jernigan S., Hsu J. L., Janke S. M., Pham T. D., Lane R. H. (2004) Uteroplacental insufficiency alters DNA methylation, one-carbon metabolism, and histone acetylation in IUGR rats. Physiol. Genomics 18, 43–50 [DOI] [PubMed] [Google Scholar]
- 9. Aagaard-Tillery K. M., Grove K., Bishop J., Ke X., Fu Q., McKnight R., Lane R. H. (2008) Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J. Mol. Endocrinol. 41, 91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fu Q., McKnight R. A., Yu X., Wang L., Callaway C. W., Lane R. H. (2004) Uteroplacental insufficiency induces site-specific changes in histone H3 covalent modifications and affects DNA-histone H3 positioning in day 0 IUGR rat liver. Physiol. Genomics 20, 108–116 [DOI] [PubMed] [Google Scholar]
- 11. Hartil K., Vuguin P. M., Kruse M., Schmuel E., Fiallo A., Vargas C., Warner M. J., Durand J. L., Jelicks L. A., Charron M. J. (2009) Maternal substrate utilization programs the development of the metabolic syndrome in male mice exposed to high fat in utero. Pediatr. Res. 66, 368–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lane R. H., Flozak A. S., Ogata E. S., Bell G. I., Simmons R. A. (1996) Altered hepatic gene expression of enzymes involved in energy metabolism in the growth-retarded fetal rat. Pediatr. Res. 39, 390–394 [DOI] [PubMed] [Google Scholar]
- 13. Lane R. H., Tsirka A. E., Gruetzmacher E. M. (2000) Uteroplacental insufficiency alters cerebral mitochondrial gene expression and DNA in fetal and juvenile rats. Pediatr. Res. 47, 792–797 [DOI] [PubMed] [Google Scholar]
- 14. Bhargava S. K., Sachdev H. S., Fall C. H., Osmond C., Lakshmy R., Barker D. J., Biswas S. K., Ramji S., Prabhakaran D., Reddy K. S. (2004) Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N. Engl. J. Med. 350, 865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patton H. M., Sirlin C., Behling C., Middleton M., Schwimmer J. B., Lavine J. E. (2006) Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. J. Pediatr. Gastroenterol. Nutr. 43, 413–427 [DOI] [PubMed] [Google Scholar]
- 16. Schwimmer J. B., Behling C., Newbury R., Deutsch R., Nievergelt C., Schork N. J., Lavine J. E. (2005) Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology 42, 641–649 [DOI] [PubMed] [Google Scholar]
- 17. Franks P. W., Hanson R. L., Knowler W. C., Sievers M. L., Bennett P. H., Looker H. C. (2010) Childhood obesity, other cardiovascular risk factors, and premature death. N. Engl. J. Med. 362, 485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. La Fleur S. E., Kalsbeek A., Wortel J., Fekkes M. L., Buijs R. M. (2001) A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes 50, 1237–1243 [DOI] [PubMed] [Google Scholar]
- 19. Rudic R. D., McNamara P., Curtis A. M., Boston R. C., Panda S., Hogenesch J. B., Fitzgerald G. A. (2004) BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2, e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Turek F. W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E., Laposky A., Losee-Olson S., Easton A., Jensen D. R., Eckel R. H., Takahashi J. S., Bass J. (2005) Obesity and metabolic syndrome in circadian clock mutant mice. Science 308, 1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ibuka N., Nihonmatsu I., Sekiguchi S. (1980) Sleep-wakefulness rhythms in mice after suprachiasmatic nucleus lesions. Waking Sleeping 4, 167–173 [PubMed] [Google Scholar]
- 22. Herzog E. D., Takahashi J. S., Block G. D. (1998) Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat. Neurosci. 1, 708–713 [DOI] [PubMed] [Google Scholar]
- 23. Zvonic S., Ptitsyn A. A., Conrad S. A., Scott L. K., Floyd Z. E., Kilroy G., Wu X., Goh B. C., Mynatt R. L., Gimble J. M. (2006) Characterization of peripheral circadian clocks in adipose tissues. Diabetes 55, 962–970 [DOI] [PubMed] [Google Scholar]
- 24. Barnea M., Madar Z., Froy O. (2009) High-fat diet delays and fasting advances the circadian expression of adiponectin signaling components in mouse liver. Endocrinology 150, 161–168 [DOI] [PubMed] [Google Scholar]
- 25. Horikawa K., Minami Y., Iijima M., Akiyama M., Shibata S. (2005) Rapid damping of food-entrained circadian rhythm of clock gene expression in clock-defective peripheral tissues under fasting conditions. Neuroscience 134, 335–343 [DOI] [PubMed] [Google Scholar]
- 26. Kohsaka A., Laposky A. D., Ramsey K. M., Estrada C., Joshu C., Kobayashi Y., Turek F. W., Bass J. (2007) High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 6, 414–421 [DOI] [PubMed] [Google Scholar]
- 27. Mendoza J., Pevet P., Challet E. (2008) High-fat feeding alters the clock synchronization to light. J. Physiol. 586, 5901–5910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stokkan K. A., Yamazaki S., Tei H., Sakaki Y., Menaker M. (2001) Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493 [DOI] [PubMed] [Google Scholar]
- 29. Reppert S. M., Weaver D. R. (2001) Molecular analysis of mammalian circadian rhythms. Annu. Rev. Physiol. 63, 647–676 [DOI] [PubMed] [Google Scholar]
- 30. Reppert S. M., Weaver D. R. (2002) Coordination of circadian timing in mammals. Nature 418, 935–941 [DOI] [PubMed] [Google Scholar]
- 31. Bae K., Lee C., Hardin P. E., Edery I. (2000) dCLOCK is present in limiting amounts and likely mediates daily interactions between the dCLOCK-CYC transcription factor and the PER-TIM complex. J. Neurosci. 20, 1746–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Debruyne J. P. (2008) Oscillating perceptions: the ups and downs of the CLOCK protein in the mouse circadian system. J. Genet. 87, 437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Siepka S. M., Yoo S. H., Park J., Lee C., Takahashi J. S. (2007) Genetics and neurobiology of circadian clocks in mammals. Cold Spring Harb. Symp. Quant. Biol. 72, 251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rutter J., Reick M., Wu L. C., McKnight S. L. (2001) Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 293, 510–514 [DOI] [PubMed] [Google Scholar]
- 35. Preitner N., Damiola F., Lopez-Molina L., Zakany J., Duboule D., Albrecht U., Schibler U. (2002) The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 [DOI] [PubMed] [Google Scholar]
- 36. Seron-Ferre M., Valenzuela G. J., Torres-Farfan C. (2007) Circadian clocks during embryonic and fetal development. Birth Defects Res. C Embryo Today 81, 204–214 [DOI] [PubMed] [Google Scholar]
- 37. Seron-Ferre M., Torres C., Parraguez V. H., Vergara M., Valladares L., Forcelledo M. L., Constandil L., Valenzuela G. J. (2002) Perinatal neuroendocrine regulation. Development of the circadian time-keeping system. Mol. Cell. Endocrinol. 186, 169–173 [DOI] [PubMed] [Google Scholar]
- 38. Walsh S. W., Ducsay C. A., Novy M. J. (1984) Circadian hormonal interactions among the mother, fetus, and amniotic fluid. Am. J. Obstet. Gynecol. 150, 745–753 [DOI] [PubMed] [Google Scholar]
- 39. Seron-Ferre M., Taylor N. F., Rotten D., Koritnik D. R., Jaffe R. B. (1983) Changes in fetal rhesus monkey plasma dehydroepiandrosterone sulfate: relationship to gestational age, adrenal weight and preterm delivery. J. Clin. Endocrinol. Metab. 57, 1173–1178 [DOI] [PubMed] [Google Scholar]
- 40. Socol M. L., Druzin M. L., Murata Y., Melmed S., Martin C. B., Jr., Hershman J. M. (1981) A cyclic variation in thyroid-stimulating hormone in the fetal rhesus monkey. Am. J. Obstet. Gynecol. 139, 712–714 [DOI] [PubMed] [Google Scholar]
- 41. El-Hennamy R., Mateju K., Bendova Z., Sosniyenko S., Sumova A. (2008) Maternal control of the fetal and neonatal rat suprachiasmatic nucleus. J. Biol. Rhythms 23, 435–444 [DOI] [PubMed] [Google Scholar]
- 42. Kovacikova Z., Sladek M., Bendova Z., Illnerova H., Sumova A. (2006) Expression of clock and clock-driven genes in the rat suprachiasmatic nucleus during late fetal and early postnatal development. J. Biol. Rhythms 21, 140–148 [DOI] [PubMed] [Google Scholar]
- 43. Kennaway D. J. (2002) Programming of the fetal suprachiasmatic nucleus and subsequent adult rhythmicity. Trends Endocrinol. Metab. 13, 398–402 [DOI] [PubMed] [Google Scholar]
- 44. Ohta H., Xu S., Moriya T., Iigo M., Watanabe T., Nakahata N., Chisaka H., Hanita T., Matsuda T., Ohura T., Kimura Y., Yaegashi N., Tsuchiya S., Tei H., Okamura K. (2008) Maternal feeding controls fetal biological clock. PLoS ONE 3, e2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Englund A., Kovanen L., Saarikoski S. T., Haukka J., Reunanen A., Aromaa A., Lonnqvist J., Partonen T. (2009) NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J. Circadian Rhythms 7, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 47. Chen R., Schirmer A., Lee Y., Lee H., Kumar V., Yoo S. H., Takahashi J. S., Lee C. (2009) Rhythmic PER abundance defines a critical nodal point for negative feedback within the circadian clock mechanism. Mol. Cell 36, 417–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sosniyenko S., Hut R. A., Daan S., Sumova A. (2009) Influence of photoperiod duration and light-dark transitions on entrainment of Per1 and Per2 gene and protein expression in subdivisions of the mouse suprachiasmatic nucleus. Eur. J. Neurosci. 30, 1802–1814 [DOI] [PubMed] [Google Scholar]
- 49. Porterfield V. M., Mintz E. M. (2009) Temporal patterns of light-induced immediate-early gene expression in the suprachiasmatic nucleus. Neurosci. Lett. 463, 70–73 [DOI] [PubMed] [Google Scholar]
- 50. Legates T. A., Dunn D., Weber E. T. (2009) Accelerated re-entrainment to advanced light cycles in BALB/cJ mice. Physiol. Behav. 98, 427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vollmers C., Gill S., DiTacchio L., Pulivarthy S. R., Le H. D., Panda S. (2009) Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl. Acad. Sci. U. S. A. 106, 21453–21458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamamoto T., Nakahata Y., Soma H., Akashi M., Mamine T., Takumi T. (2004) Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol. Biol. 5, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Suter M., Abramovici A., Showalter L., Hu M., Shope C. D., Varner M., Aagaard-Tillery K. (2010) In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism 59, 1481–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Suter M. A., Aagaard-Tillery K. M. (2009) Environmental influences on epigenetic profiles. Semin. Reprod. Med. 27, 380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Plata M. P., Kang H. J., Zhang S., Kuruganti S., Hsu S. J., Labrador M. (2009) Changes in chromatin structure correlate with transcriptional activity of nucleolar rDNA in polytene chromosomes. Chromosoma 118, 303–322 [DOI] [PubMed] [Google Scholar]
- 56. Nakayama J., Rice J. C., Strahl B. D., Allis C. D., Grewal S. I. (2001) Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292, 110–113 [DOI] [PubMed] [Google Scholar]
- 57. Martinato F., Cesaroni M., Amati B., Guccione E. (2008) Analysis of Myc-induced histone modifications on target chromatin. PLoS ONE 3, e3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. LeRoy G., Rickards B., Flint S. J. (2008) The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol. Cell 30, 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hiragami-Hamada K., Xie S. Q., Saveliev A., Uribe-Lewis S., Pombo A., Festenstein R. (2009) The molecular basis for stability of heterochromatin-mediated silencing in mammals. Epigenetics Chromatin 2, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Loyola A., Tagami H., Bonaldi T., Roche D., Quivy J. P., Imhof A., Nakatani Y., Dent S. Y., Almouzni G. (2009) The HP1α-CAF1-SetDB1-containing complex provides H3K9me1 for Suv39-mediated K9me3 in pericentric heterochromatin. EMBO Rep. 10, 769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vaquero A., Scher M., Erdjument-Bromage H., Tempst P., Serrano L., Reinberg D. (2007) SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature 450, 440–444 [DOI] [PubMed] [Google Scholar]
- 62. Cox J., Williams S., Grove K., Lane R. H., Aagaard-Tillery K. M. (2009) A maternal high-fat diet is accompanied by alterations in the fetal primate metabolome. Am. J. Obstet. Gynecol. 201, 281 e281–e289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dudley C. A., Erbel-Sieler C., Estill S. J., Reick M., Franken P., Pitts S., McKnight S. L. (2003) Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science 301, 379–383 [DOI] [PubMed] [Google Scholar]
- 64. Gangwisch J. E. (2009) Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes. Rev. 10(Suppl. 2), 37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ruger M., Scheer F. A. (2009) Effects of circadian disruption on the cardiometabolic system. Rev. Endocr. Metab. Disord. 10, 245–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Duez H., Staels B. (2009) Rev-erb-α: an integrator of circadian rhythms and metabolism. J. Appl. Physiol. 107, 1972–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Duez H., Staels B. (2008) Rev-erb α gives a time cue to metabolism. FEBS Lett. 582, 19–25 [DOI] [PubMed] [Google Scholar]
- 68. Lemos D. R., Downs J. L., Urbanski H. F. (2006) Twenty-four-hour rhythmic gene expression in the rhesus macaque adrenal gland. Mol. Endocrinol. 20, 1164–1176 [DOI] [PubMed] [Google Scholar]
- 69. Panda S., Antoch M. P., Miller B. H., Su A. I., Schook A. B., Straume M., Schultz P. G., Kay S. A., Takahashi J. S., Hogenesch J. B. (2002) Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109, 307–320 [DOI] [PubMed] [Google Scholar]
- 70. Fuks F., Burgers W. A., Brehm A., Hughes-Davies L., Kouzarides T. (2000) DNA methyltransferase Dnmt1 associates with histone deacetylase activity. Nat. Genet. 24, 88–91 [DOI] [PubMed] [Google Scholar]
- 71. Robertson K. D., Ait-Si-Ali S., Yokochi T., Wade P. A., Jones P. L., Wolffe A. P. (2000) DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nat. Genet. 25, 338– 342 [DOI] [PubMed] [Google Scholar]
- 72. Datta J., Ghoshal K., Sharma S. M., Tajima S., Jacob S. T. (2003) Biochemical fractionation reveals association of DNA methyltransferase (Dnmt) 3b with Dnmt1 and that of Dnmt 3a with a histone H3 methyltransferase and Hdac1. J. Cell. Biochem. 88, 855–864 [DOI] [PubMed] [Google Scholar]
- 73. Cheng X., Blumenthal R. M. (2010) Coordinated chromatin control: structural and functional linkage of DNA and histone methylation. Biochemistry 49, 2999–3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Anttila S., Hakkola J., Tuominen P., Elovaara E., Husgafvel-Pursiainen K., Karjalainen A., Hirvonen A., Nurminen T. (2003) Methylation of cytochrome P4501A1 promoter in the lung is associated with tobacco smoking. Cancer Res. 63, 8623–8628 [PubMed] [Google Scholar]
- 75. Etchegaray J. P., Lee C., Wade P. A., Reppert S. M. (2003) Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature 421, 177–182 [DOI] [PubMed] [Google Scholar]
- 76. Curtis A. M., Seo S. B., Westgate E. J., Rudic R. D., Smyth E. M., Chakravarti D., FitzGerald G. A., McNamara P. (2004) Histone acetyltransferase-dependent chromatin remodeling and the vascular clock. J. Biol. Chem. 279, 7091–7097 [DOI] [PubMed] [Google Scholar]