Abstract
The BCL-2 family of proteins regulates apoptosis by controlling mitochondrial outer membrane permeabilization (MOMP). Within the family there are numerous protein-protein interactions that influence MOMP; however, defining the ultimate signal that commits a cell to apoptosis remains controversial. We chose to examine the function of the BH3-only protein, p53 upregulated modulator of apoptosis (PUMA), to define its contribution to MOMP and cooperation with the direct activator proteins. PUMA is a potent regulator of MOMP and our data suggest that this function is attributed to two distinct mechanisms which both rely on PUMA binding to the anti-apoptotic BCL-2 proteins: de-repression and sensitization. Here we will define these interactions and discuss our experiments that suggest PUMA cooperates with direct activator proteins to efficiently induce MOMP and apoptosis.
Keywords: apoptosis, BCL-2 family, cytochrome c release, MOMP, PUMA
Introduction
During cellular respiration, the mitochondrial network is responsible for the production of adenosine-5′-triphosphate (ATP) through a series of electron transport reactions that occur between the inner and outer mitochondrial membranes (IMM and OMM, respectively).1 For mitochondrial ATP generation to be most efficient, the integrity of the mitochondrial membranes must remain intact to promote the localization of mitochondrial proteins and maintenance of the energy-producing electrochemical gradient across the IMM. Accordingly, cellular survival is contingent upon preserving mitochondrial integrity to sustain organelle function and cellular ATP levels.
Following irreparable cellular stress, the mitochondrial pathway of apoptosis commits the targeted cell to apoptosis via compromising the integrity of the OMM by inducing mitochondrial outer membrane permeabilization (MOMP) which leads to the release of intermembrane space (IMS) proteins, such as cytochrome c.2 Once in the cytosol, cytochrome c cooperates with apoptotic protease activating factor 1 (APAF-1) and deoxyadenosine-5′-triphosphate (dATP) to promote dimerization and activation of caspase-9.3 This induces the cleavage and activation of the executioner caspases (e.g., caspases-3 and -7) that are responsible for precipitating the morphological phenotypes of apoptosis (e.g., DNA fragmentation, plasma membrane blebbing) and rapid phagocytosis of the dying cell.4
The BCL-2 family of proteins directly regulates cellular fate by integrating the cellular environment to either preserve or compromise the OMM.2 The anti-apoptotic BCL-2 proteins (e.g., BCL-2, BCL-xL, MCL-1) promote survival by actively sequestering the pro-apoptotic BH3-only members (e.g., BAD, BID, BIM, PUMA) within the BCL-2 family (Fig. 1). The BH3-only proteins are expressed and activated following cellular stress scenarios and are hypothesized to promote MOMP by interacting with the anti-apoptotic BCL-2 proteins (i.e., ‘de-repressor/sensitizer’ functions) or inducing the oligomerization and pore-forming function of the pro-apoptotic BCL-2 effector proteins, BAK and BAX (i.e., ‘direct activator’ function).5,6 The dual action of the BH3-only proteins bifurcates this BCL-2 family subgroup into de-repressor/sensitizer and direct activator members (Fig. 2). Defining these discrete functions and their relationship to MOMP and apoptosis were the focus of our recent paper, “Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins.7”
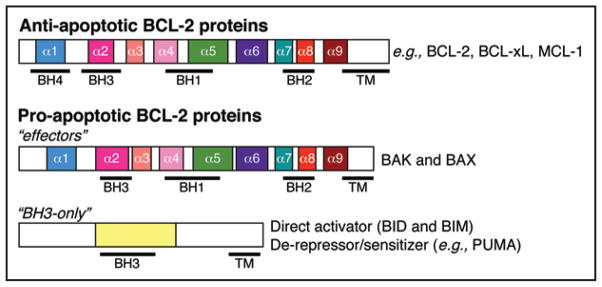
Figure 1.
The BCL-2 family of proteins is divided into three functional groups based on their composition of BCL-2 homology (BH) domains. The anti-apoptotic members (e.g., BCL-2, BCL-xL and MCL-1) contain four BCL-2 homology domains (designated BH1-4). The pro-apoptotic effectors (BAK and BAX) contain BH1-3 domains. The BH3-only proteins are structurally diverse and contain only one conserved region, the BH3 (leucine-x-x-x-x-aspartic acid, x = any amino acid). The BH3-only proteins are subdivided into direct activators (BID and BIM) and de-repressors/sensitizers (e.g., PUMA). The alpha helices of each protein are designated, and the regions contained within each BH domain are illustrated by bold lines under each protein. The hydrophobic carboxyl terminal transmembrane domain (TM) of each protein is based on in silico predictions and/or structural data and is not necessarily present in each member.
Figure 2.
Mechanisms of pro-apoptotic effector activation by the BH3-only proteins. (A) Central to MOMP is the activation and oligomerization of BAX or BAK. These proteins, once activated by a BH3-only protein, create oligomeric proteolipid pores in the OMM that permit the release of IMS proteins to the cytosol (e.g., cytochrome c). Through a transient interaction with BAX or BAK, the direct activator BH3-only proteins (C8-BID and BAX are shown in this example), induce MOMP and cytochrome c release. (B and C) A subset of BH3-only proteins, the de-repressors/sensitizers, cannot induce the activation of BAX or BAK alone. In this scenario (B), a direct activator BH3-only protein is sequestered by an anti-apoptotic BCL-2 protein. Following stress, a de-repressor/sensitizer BH3-only protein is induced, either by transcriptional upregulation or post-translational modification, and this protein then binds to an anti-apoptotic BCL-2 protein promoting the release of a sequestered, direct activator BH3-only protein. In this example, C8-BID is tonically sequestered by BCL-xL and the induction of PUMA allows for the release of C8-BID to engage MOMP (B). (C) Cells are sensitized to undergo MOMP when de-repressor/sensitizer BH3-only proteins are constitutively inhibiting anti-apoptotic BCL-2 proteins and any future induction of BID or BIM cannot be tolerated. In this example, BCL-xL is inhibited by PUMA and the induction of C8-BID engages MOMP; in the absence of PUMA expression, the MOMP signal would have been inhibited by BCL-xL.
To explore the de-repressor/sensitizer and direct activator functions of the BH3-only proteins, we studied the cooperation between p53 upregulated modulator of apoptosis (PUMA) and other BH3-only proteins (i.e., BID and BIM) in the context of anti-apoptotic (e.g., BCL-2, BCL-xL and/or MCL-1) and effector BCL-2 proteins (i.e., BAK and/or BAX). PUMA was chosen as the foremost BH3-only protein because of its essential role in multiple apoptotic pathways, and ability to bind and inhibit all anti-apoptotic BCL-2 proteins (Table 1).6,8–12 Due to this promiscuous binding, it was possible to examine the requirement and consequences for the de-repressor/sensitizer and direct activator BH3-only proteins in the setting of a fully inhibited anti-apoptotic BCL-2 protein repertoire.
Table 1.
PUMA binds to the anti-apoptotic BCL-2 repertoire with nanomolar affinity
| Anti-apoptotic | PUMA KD (reported range) |
|---|---|
| A1 | 28.6 nM (5.7– 59 nM) |
| BCL-2 | 10.7 nM (3.3–18 nM) |
| BCL-xL | 6.1 nM (3–10 nM) |
| BCL-w | 15.1 nM (5.1–25 nM) |
| MCL-1 | 16.5 nM (5–31.9 nM) |
The dissociation constant (KD) between the PUMA BH3 domain peptide and recombinant anti-apoptotic proteins has been evaluated using several model systems. Listed here are the average KD values for the PUMA BH3 domain peptide and each anti-apoptotic protein, including published and unpublished (personal communication with Richard W. Kriwacki, Ph.D. and John C. Fisher at St. Jude Children’s Research Hospital, Memphis, TN, USA) ranges in parentheses.6,11,16
PUMA Functions within an Established BCL-2 Network
Here we define a BCL-2 network as a direct activator BH3-only protein (e.g., BID), an anti-apoptotic BCL-2 protein (e.g., BCL-xL) and a pro-apoptotic effector protein (e.g., BAX). We propose that the BCL-2 network directly controls at least two fundamental aspects of cellular fate. The first is to promote survival by inhibiting the direct activator proteins (e.g., BCL-xL inhibits BID), which keeps the effector molecules monomeric and the OMM intact. Secondly, when every member of the anti-apoptotic repertoire is associated with a direct activator protein due to pro-apoptotic stimulation (e.g., every BCL-xL molecule is associated with a molecule of BID), a subsequent direct activator molecule cannot be inhibited and is readily able to activate effector molecules (e.g., BID activates BAX) leading to MOMP. Experimentally, we were focused on observing the influence of PUMA within a BCL-2 network prior and subsequent to the presence of direct activator proteins.7
To begin investigations, we established a model system of continual cellular stress to probe the impact of PUMA on the function of an endogenous direct activator protein, BID. In response to tumor necrosis factor (TNF) treatment, BID is cleaved and activated by caspase-8 (referred to as caspase-8 BID, C8-BID), which is a potent inducer of MOMP and apoptosis. In our cellular stress model system, HeLa cells were routinely treated (6 hours treatment, 42 hours recovery) with sub-lethal doses of TNF to promote the accumulation of C8-BID. We speculated that multiple sub-lethal TNF treatments would allow for C8-BID generation, but it would be bound and inhibited by the anti-apoptotic proteins. This model system established a BCL-2 network that allowed us to test the consequences of subsequent PUMA addition.
We confirmed the TNF treated cells accumulated C8-BID, and then introduced exogenous PUMA via microinjection. The addition of PUMA caused the cells to undergo MOMP rapidly; it was not unusual for cells harboring C8-BID to release cytochrome c within an hour after PUMA addition suggesting that the anti-apoptotic repertoire can sequester considerably high levels of C8-BID. Indeed, mitochondria isolated from these cells were loaded with C8-BID on the anti-apoptotic repertoire (i.e., BCL-2 and MCL-1), which was released by PUMA addition. In vitro using isolated mitochondria, the anti-apoptotic repertoire-associated C8-BID also induced MOMP in the presence of PUMA. Throughout our studies, we also evaluated ABT-737, a small molecule inhibitor to a subset of the anti-apoptotic repertoire, which promoted C8-BID release, activity and MOMP, albeit to a lesser degree.13 In these scenarios, MOMP was always dependent upon the presence of C8-BID as cells and isolated mitochondria that did not harbor C8-BID were refractory to PUMA treatment.
These observations suggest: (i) in cells, anti-apoptotic BCL-2 proteins on the OMM can sequester substantial direct activator BH3-only protein function to inhibit MOMP, cytochrome c release and apoptosis; and (ii) inhibition of the anti-apoptotic BCL-2 repertoire by PUMA can release these direct activator proteins to rapidly induce MOMP, cytochrome c release and apoptosis.
Defining the De-Repressor and Sensitizer Functions of PUMA
Seminal papers in the BCL-2 literature demonstrated that BH3 domain peptides (16–25 amino acid peptides containing the canonical BH3 motif ‘leucine-x-x-x-x-aspartic acid’, x = any amino acid) from distinct BH3-only proteins cooperate to promote MOMP and apoptosis.5,6 For example, BCL-xL inhibited BID-dependent MOMP, which was reversible by the addition of the BAD BH3 domain peptide.5,6 The cooperative nature of distinct BH3 domain peptides suggests that BH3-only proteins are functionally distinguishable based on their ability to either directly promote MOMP (i.e., activate BAK and/or BAX) or indirectly by exclusively binding to the anti-apoptotic repertoire. The binding of non-activating BH3-only proteins to the anti-apoptotic repertoire presents two mechanisms of action for this BH3-only protein subclass: (i) if this subclass of the BH3-only proteins is present before the direct activators proteins, then the anti-apoptotic repertoire cannot inhibit subsequent direct activator proteins and MOMP will proceed—this is referred to as ‘sensitization’ as very little direct activator BH3-only protein function is required to induce MOMP; and (ii) direct activator proteins can readily be sequestered and inhibited by the anti-apoptotic repertoire until displaced by a collateral non-activating BH3-only protein—this is referred to as ‘de-repression’ as the collateral BH3-only protein coordinately liberates the direct activator protein to promote MOMP while consequently inhibiting the anti-apoptotic repertoire. We chose to evaluate and define the sensitization and de-repression functions of PUMA by using biochemical and cellular model systems.
To determine if PUMA could sensitize mitochondria to direct activator proteins, freshly isolated liver mitochondria were pre-treated with PUMA protein allowing it to bind the anti-apoptotic repertoire. All unbound PUMA was washed away and then mitochondria were treated with picomolar concentrations of C8-BID. In the absence of PUMA pre-treatment, these doses of C8-BID were inhibited by the anti-apoptotic repertoire thus preserving OMM integrity. However, PUMA pre-treatment promoted MOMP for all doses of C8-BID tested (5–25 pM), which yielded a sensitization of 100–200 fold (normally 1 nM C8-BID was required for complete MOMP). These results were extended to the PUMA BH3 domain peptide and other biochemically defined direct activators (i.e., BIM and cytosolic p53) with similar sensitization effects.5,6,14
De-repression of direct activator function is demonstrated to be of clinical significance for anti-tumor strategies, such as in the treatment of chronic lymphocytic leukemia (CLL).15,16 Due to high affinity binding between PUMA and the anti-apoptotic repertoire, we proposed that PUMA would be a potent de-repressor BH3-only protein. To examine this hypothesis, HeLa cells were microinjected with recombinant proteins to establish an intracellular BCL-2 network and analyzed for MOMP. Introduction of C8-BID promoted the rapid release of cytochrome c which was inhibited by co-treatment with BCL-xL; and the inhibitory activity of BCL-xL was reversed by the addition of PUMA. We also tested freshly isolated liver mitochondria to observe PUMA-mediated de-repression. Mitochondria were pre-treated with low concentrations of direct activators (i.e., C8-BID, BIM or cytosolic p53) that do not induce MOMP due to association and inhibition by the anti-apoptotic repertoire. The ‘primed’ mitochondria were then treated with PUMA, which promoted potent de-repression of direct activator function from the anti-apoptotic repertoire and cytochrome c release. Finally, using a biochemically defined large unilamellar vesicle (LUV) system, we were also able to demonstrate PUMA-mediated de-repression of C8-BID from BCL-xL and MCL-1 to promote BAX activation and LUV permeabilization.17
Our data suggest PUMA is a potent sensitizer and de-repressor BH3-only protein, as PUMA-dependent MOMP proceeded with doses of direct activators that would normally be inhibited by the anti-apoptotic repertoire. Importantly, the sole addition of PUMA to the described model systems did not induce BAK and/or BAX activation or MOMP in the absence of direct activator function. We contend that synergy between the distinct direct activator and de-repressor/sensitizer functions of the BH3-only proteins efficiently engage BAK and/or BAX activation, MOMP and apoptosis.
PUMA Reveals Covert Direct Activator BH3-Only Protein Function
Due to the potent de-repressor and sensitizer functions of PUMA, we speculated that basal and/or inducible expression of PUMA begets cells that are highly sensitive to future direct activator function and apoptosis. Indeed, initial studies described that exogenous PUMA expression was sufficient to induce apoptosis; however, the requirement for direct activator function in these contexts was not addressed.8–10 To identify if synergy between PUMA and endogenous direct activator proteins was responsible for PUMA-induced apoptosis, we transiently transfected wild type, bid−/−, bim−/− and bid−/−bim−/− mouse embryonic fibroblasts (MEFs) with PUMA cDNA. Exogenous expression of PUMA promoted apoptosis that was primarily dependent upon the presence of BIM; however, hyper-physiological levels of exogenous PUMA promoted apoptosis that was BIM-independent. The requirement for BIM in PUMA-mediated apoptosis suggested that either basal levels of BIM were sufficient to cooperate with PUMA, or the transient transfection of MEFs induced endogenous BIM activation that synergized with exogenous PUMA to promote apoptosis.
The MEF studies were extended to liver mitochondria derived from untreated wild type, bid−/−, bim−/− and bid−/−bim−/− animals. Interestingly, PUMA protein and BH3 domain peptide could induce modest, but appreciable levels of MOMP and cytochrome c release that was dependent upon the presence of BID and/or BIM. These results suggested that mitochondria from healthy primary tissues actively sequester direct activator proteins, which possibly result from previous cellular stress, and that additional direct activator expression is not necessarily required to observe PUMA function. The sequestered direct activator molecules may be a mechanism to record accumulated stress, which upon a certain threshold provide a rapid means to induce BAK and/or BAX activation. These scenarios highlight the importance and fidelity of the anti-apoptotic proteins at the OMM to inhibit unwarranted MOMP. Likewise, when PUMA is present, its de-repression activity rapidly liberates the sequestered direct activators from the anti-apoptotic repertoire to efficiently promote BAK and/or BAX activation.
Perspectives on PUMA-Mediated Regulation of Cellular Fate
The focus of our paper was to test the current hypotheses regarding BH3-only protein function, i.e., understanding the contribution of de-repression and sensitization to MOMP and apoptosis. We established several model systems to explore BH3-only protein function and utilized PUMA (protein and BH3 domain peptide) because of its ability to inhibit the entire anti-apoptotic repertoire. In the context of our model systems, PUMA displayed a distinct contribution to MOMP compared to direct activator BH3-only proteins. We propose that synergy between the distinct direct activator (e.g., BID or BIM) and de-repressor/sensitizer (e.g., PUMA) functions of the BH3-only proteins efficiently engage BAK and/or BAX activation, MOMP and apoptosis.
Cooperation between PUMA and BID/BIM is not undisputed as cytokine deprivation-induced apoptosis has recently been described to be dependent on puma and not bid and/or bim in HoxB8-immortalized myeloid progenitor cells.18 These data insinuate either: (i) PUMA may have additional pro-apoptotic functions that are distinct from mediating MOMP; or (ii) BID and BIM are not the only direct activator proteins (several non BCL-2 family proteins are already described to have direct activator function, e.g., cytosolic p53).14 The evidence for the latter stems from comparing the sensitivity of puma−/− and bak−/−bax−/− HoxB8-immortalized myeloid progenitor cells to cytokine deprivation-induced apoptosis. Specifically, bak−/−bax−/− cells displayed complete resistance whereas the majority of puma−/− cells still underwent apoptosis.18 It is possible for cytokine deprivation-induced apoptosis to be engaged most efficiently PUMA is required, but sustained pro-apoptotic signaling may promote the accumulation of direct activator function and eventual MOMP despite PUMA-mediated de-repression and sensitization function. We have observed similar dosage effects of direct activator proteins in the absence of PUMA expression.19
However, in the literature there are several notions that PUMA is not only capable of de-repression and sensitization, but also the direct activation of BAX.20,21 From a BAX peptide scan, it was determined that the carboxyl terminus region of BAX alpha helix 1 could directly interact with the PUMA BH3 domain. Recent structural investigations between BAX and the BIM BH3 peptide revealed a similar region of interaction; furthermore, cytosolic p53 has also been suggested to bind to a similar site on BAK.14,22,23 Although biochemical studies using LUVs combined with recombinant BAX and PUMA (protein or BH3 domain peptide) have not yielded similar results, these studies focused exclusively on BAX-mediated permeabilization and could not reveal PUMA-BAX interactions that did not induce BAX activation.6,7
While the fundamental components of PUMA-regulated MOMP are generally defined (anti-apoptotic, effectors and BH3-only proteins are involved), our continual challenge is to discover the mechanism(s) of action for individual members within the BCL-2 network that dictate cellular fate. When PUMA is expressed, what protein-protein interaction is the key event that leads to BAK and/or BAX activation? Our data suggest that PUMA regulates MOMP by interactions within the anti-apoptotic BCL-2 repertoire leading to de-repression and/or sensitization, but the initiating signal to induce MOMP and apoptosis is dependent upon direct activator function.
Acknowledgments
This work was supported by NIH AI52735 and CA69381 (to D.R.G.), an NCI Cancer Center Core Grant P30CA21765 (at SJCRH) and the American Lebanese Syrian Associated Charities.
Abbreviations
- BAD
BCL-2 antagonist of cell death
- BAX
BCL-2 associated x protein
- BAK
BCL-2 antagonist killer 1
- BCL-2
B-cell CLL/lymphoma 2
- BCL-xL
BCL-2-related protein long isoform
- BID
BH3-interacting domain death agonist
- BIM
BCL-2 interacting mediator of cell death
- C8-BID
caspase-8 cleaved BID
- CLL
chronic lymphocytic leukemia
- IMM
inner mitochondrial membrane
- LUVs
large unilamellar vesicles
- MCL-1
myeloid cell leukemia 1
- MOMP
mitochondrial outer membrane permeabilization
- MEFs
mouse embryonic fibroblasts
- OMM
outer mitochondrial membrane
- PUMA
p53 upregulated modulator of apoptosis
- TNF
tumor necrosis factor
References
- 1.Vonck J, Schafer E. Supramolecular organization of protein complexes in the mitochondrial inner membrane. Biochim Biophys Acta. 2009;1793:117–24. doi: 10.1016/j.bbamcr.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–64. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ow YP, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol. 2008;9:532–42. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- 4.Pop C, Salvesen GS. Human caspases: Activation, specificity and regulation. J Biol Chem. 2009 doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–92. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 6.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–35. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Chipuk JE, Fisher JC, Dillon CP, Kriwacki RW, Kuwana T, Green DR. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proc Natl Acad Sci USA. 2008;105:20327–32. doi: 10.1073/pnas.0808036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–94. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 9.Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–8. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 10.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA. 2003;100:1931–6. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 12.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–9. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 13.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 14.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–4. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 15.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–21. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–65. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, et al. Bid, Bax and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–42. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 18.Jabbour AM, Heraud JE, Daunt CP, Kaufmann T, Sandow J, O’Reilly LA, et al. Puma indirectly activates Bax to cause apoptosis in the absence of Bid or Bim. Cell Death Differ. 2009;16:555–63. doi: 10.1038/cdd.2008.179. [DOI] [PubMed] [Google Scholar]
- 19.Chipuk JE, Bouchier-Hayes L, Kuwana T, Newmeyer DD, Green DR. PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science. 2005;309:1732–5. doi: 10.1126/science.1114297. [DOI] [PubMed] [Google Scholar]
- 20.Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, et al. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16:807–18. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 21.Gallenne T, Gautier F, Oliver L, Hervouet E, Noel B, Hickman JA, et al. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol. 2009;185:279–90. doi: 10.1083/jcb.200809153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–81. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietsch EC, Perchiniak E, Canutescu AA, Wang G, Dunbrack RL, Murphy ME. Oligomerization of BAK by p53 utilizes conserved residues of the p53 DNA binding domain. J Biol Chem. 2008;283:21294–304. doi: 10.1074/jbc.M710539200. [DOI] [PMC free article] [PubMed] [Google Scholar]