Abstract
Background:
High-grade primary and metastatic central nervous system (CNS) tumors are common, deadly, and refractory to conventional therapy and continue to be therapeutically challenging. A key nodal transcriptional factor, the signal transducer and activator of transcription 3 (STAT3), drives the fundamental components of tumor malignancy and metastases in the CNS by enhancing proliferation, angiogenesis, invasion, metastasis, and immunosuppression. The introduction of STAT3 inhibitors in clinical trials for this patient population is imminent.
Methods:
STAT3 inhibitors have been extensively tested in a variety of preclinical murine models.
Results:
The STAT3 inhibitor, WP1066, has displayed marked efficacy with minimal toxicity against malignancy in murine models, including established intracerebral tumors. The mechanism of this in vivo efficacy of the STAT3 blockade agents is a combination of direct tumor cytotoxicity and immune cytotoxic clearance.
Conclusions:
Given their direct antitumor cytotoxic effects, STAT3 inhibitors may exert therapeutic activity in the monotherapy setting but may also have compelling use as immunotherapeutic modulators or as a salvage therapy.
Keywords: Central nervous system, cytotoxic agent, glioma, immunotherapy, metastasis, signal transducer and activator of transcription 3
INTRODUCTION
The median survival time for patients for patients with the more malignant types of gliomas such as glioblastoma multiforme (GBM) is still a dismal 15 months despite multimodality therapy, including temozolomide (TMZ).[58] The prognosis is even worse for patients with brain metastasis from cancers such as melanoma, whose median survival time remains at 5 months.[50] Metastatic tumors are among the most common brain tumors, and in the United States there are an estimated 150,000 cases per year. Disease metastatic to the central nervous system (CNS) accounts for 20% of cancer deaths annually, a rate that can be traced to an increase in the median survival time of patients with cancer because of modern therapies, increased availability of advanced imaging techniques for early detection, and vigilant surveillance protocols for monitoring recurrence. Additionally, most systemic treatments (i.e., chemotherapeutic agents, which may penetrate the brain poorly) can transiently weaken the blood-brain barrier (BBB) and allow systemic disease to be seeded in the CNS, leaving the brain a safe haven for tumor growth.[43] Most of these patients with brain metastases are excluded from clinical trials owing to their poor prognosis. We are seeing an increased incidence of brain metastasis secondary to the increasing longevity of these patients.[4] There is clearly an unmet clinical need to develop therapeutic strategies that exert efficacy against CNS malignancies. Only now are pharmaceutical companies coming to the realization that there is a great untapped market for these agents, but few developmental chemists and translational researchers have considered CNS activity when developing drugs.
The signal transducer and activator of transcription 3 (STAT3) pathway is a potent regulator of tumorigenesis, tumor-mediated immune suppression, and metastasis to the brain. A variety of growth factors and cytokines activate STAT3 by phosphorylating the tyrosine residue in the STAT3 transactivation domain. Phosphorylated STAT3 (p-STAT3) then translocates into the nucleus and induces the expression of a wide variety of target genes involved in tumorigenesis. STAT3 is overexpressed almost ubiquitously in malignancies, including gliomas,[1] and propagates tumorigenesis by preventing apoptosis and enhancing proliferation, angiogenesis, invasiveness, and metastasis.[21,66] The STAT3 pathway becomes constitutively active in diverse tumor-infiltrating immune cells, markedly impairing their antitumor effector responses[67] and enhancing the functional activity of immunosuppressive regulatory T cells (Tregs).[29] Glioma cancer stem cells (gCSCs) are also dependent on the STAT3 pathway,[56] including for their immunosuppressive properties.[59,61] Targeting a molecular hub of both tumor-mediated immune suppression and tumorigenesis is a highly novel therapeutic strategy.
To target this pathway, several novel inhibitors of the STAT3 pathway have been designed and synthesized that show marked activity against intracerebral tumors in vivo both by directly interfering with tumorigenicity and by reversing tumor-mediated immune suppression. Our data demonstrate the highly promising potential application of one of these, WP1066, which we have been extensively developing. We hypothesize that by targeting multifactorial signaling mediated by the STAT3 pathway and reversing immunosuppression, we can improve survival in patients with gliomas and brain metastasis – a typically underserved patient population that continues to have a dismal survival prospect.
BACKGROUND
STAT3 is a key transcription factor that drives the fundamental components of malignancy and metastasis including those in gliomas. In many tumors, STAT3 is constitutively active including the unphosphorylated form which is also capable of inducing oncoproteins.[65] GBM tumors and glioma cell lines have been shown to have high levels of both in comparison to normal brain tissue.[46] We have shown that STAT3 is overexpressed in most gliomas and is a negative prognostic marker for survival in patients who have malignant glioma.[1] STAT3 has also been shown to be a master regulator for mesenchymal transformation in brain tumors.[6] The STAT3 pathway is also either likely to be or is actually overexpressed in other types of more benign CNS tumors, such as choroid plexus papilloma,[39] ependymoma,[7] and meningioma.[25] STAT3 has also been shown to be crucial to tumorigenesis and a mediator of poor prognosis in a wide variety of other malignancies, such as gastric and colon cancers,[14] renal cancer,[20] ovarian cancer,[38] squamous cell cancer,[37,55] hepatocellular carcinoma,[62] and anaplastic large cell lymphoma.[27] A study by Xie et al.[64] confirmed the importance of STAT3 in melanoma metastasis by showing that only highly metastatic melanoma cell lines, but not poorly metastatic ones, have elevated levels of activated STAT3 (p-STAT3). Furthermore, blockade of activated STAT3 prevents the invasiveness of melanoma cells, inhibits tumor growth, and prevents metastasis, suggesting that STAT3 activation is the crucial event in melanoma metastasis. Subsequent tissue microarray studies of brain metastasis in human melanoma patients have demonstrated higher levels of expression of activated STAT3 in melanoma brain metastasis specimens compared with those from parenchymal tumors.[63] Thus, this ubiquitous pathway is upregulated in almost all malignancies studied thus far, especially within CNS tumors.
STAT3 has also been implicated as a key regulator of immunosuppression in patients with cancer, and is therefore considered a potential target for immunotherapy.[67] The p-STAT3 pathway is induced in the immune cells within the tumor microenvironment,[32] which downregulates their antitumor immune responses. Specifically, p-STAT3 has been shown to suppress macrophage activation and to limit their inflammatory responses,[44] reduce natural killer (NK) cell and neutrophil cellular cytotoxicity, and reduce the expression of major histocompatibility complex (MHC) II, CD80, CD86, and interleukin (IL)-12 in dendritic cells, rendering them unable to stimulate T cells and to generate effective antitumor immunity.[32] STAT3 activation enhances the suppressive activities of human Tregs by upregulating FoxP3 expression.[68] Tumor-associated macrophages (MΦ)/microglia have been shown to become polarized via the STAT3 pathway toward immunosuppressive and tumor supportive phenotypes (M2) that then contribute to angiogenesis and tumor invasion.[35] Cumulatively, these data indicate that STAT3 is a key pathway that prevents the immune system from recognizing and eliminating malignancy from our bodies.
Finally, the p-STAT3 pathway is a fundamental hub for the control of gCSCs,[56] which are undifferentiated cells with the capacity for self-renewal and a high proliferative potential. The gCSCs confer the resistance to chemotherapy and radiation that is observed in cancer patients.[3,40] Many investigators believe that without targeting this subpopulation of cells therapeutically, tumors will continue to persist and recur. Implantation of gCSCs in mice recapitulates the key characteristics of human malignancies.[52] The gCSCs frequently have overexpression of p-STAT3, which results in proliferation and tumorigenic potential.[10] We have also shown that the gCSCs are highly immunosuppressive,[59] and when we block STAT3 in the gCSCs with WP1066, there is nearly complete reversal of tumor-mediated immune suppression.[59,61] The use of p-STAT3 inhibitors to overcome tumor-mediated immune suppression may result in the ability of the immune system to recognize and eradicate the tumor, which the tumor typically prevents it from doing. Cumulatively, these data indicate that targeting the STAT3 pathway is a multipronged approach that is truly unique.
RESULTS AND DISCUSSION
WP1066 is an analogue of caffeic acid that is a very potent and specific inhibitor of p-STAT3.[36,45] It has been shown to induce apoptosis in gliomas by downregulating the antiapopototic proteins Mcl-1, Bcl-X Land c-Myc, while activating Bax.[24] In other types of solid malignancy, WP1066 has been shown to inhibit vascular endothelial growth factor (VEGF) production by the tumor and associated in vivo angiogenesis,[19,33] indicating that inhibition of STAT3 results in a multiplicity of anti-tumor mechanisms. When WP1066 was administered to mice orally, toxicity was minimal. Detailed histological examination of the spleen, kidney, lung, heart, and bone marrow demonstrated no significant abnormalities in mice given the 40-mg/kg dose by oral gavage. Because WP1066 is a potent immune activator, nonspecific immune reactivity in the CNS remains a consideration; however, Luxol fast blue staining of the CNS failed to demonstrate any evidence of CNS autoimmunity. Thus, preliminary toxicology has demonstrated that WP1066 has an extremely desirable profile.[29]
Bioavailability studies after oral administration yielded mean peak plasma concentrations greater than 2 μM. Furthermore, kinetic data indicate that WP1066 has a marked ability to achieve excellent CNS penetration, exceeding 30 μM concentrations within the CNS of mice with an intact BBB, and the brain drug levels were long lasting (weeks). These doses markedly exceed the direct antitumor effects on cancer stem cells and tumor-mediated immune suppression.
Other studies have demonstrated statistically significant suppression of tumor growth in mice with head and neck carcinoma,[34] pancreatic cancer,[2,15] bladder cancer,[8] glioma,[24] B-cell non-Hodgkin's lymphoma and myeloma,[28] and chronic myelogenous leukemia,[48] when given WP1066. Treatment of established tumors in vivo with WP1066 has resulted in decreased tumor proliferation, tumor volume, and angiogenesis/vascular proliferation.[34] To determine whether treatment with WP1066 is efficacious against intracerebral tumors established in mice, the mice were treated with WP1066. Median survival durations and rates were markedly enhanced when WP1066 was administered, and 80% of WP1066-treated animals survived long term compared with 0% of control mice treated with the injection vehicle control (P = 0.0076).[29] Similar results have been seen in other animal model systems of intracranial gliomas.[31] Specifically, using the RCAS/Ntv-a system, mice were engineered to coexpress platelet-derived growth factor receptor (PDGF)-B + B-cell lymphoma (Bcl)-2 under the control of the glioneuronal-specific Nestin promoter. In mice expressing both PDGF-B + Bcl-2 that were treated with WP1066, there was a 55.5% increase in median survival time (P < 0.01), with an associated inhibition of intratumoral p-STAT3 and macrophages. These data demonstrate the compelling efficacy of WP1066 against established tumors in the CNS in multiple murine models.
In addition to the ability of WP1066 to enhance immune-mediated antitumor immune responses,[29] it also markedly inhibited Tregs in vivo.[30] We have also shown that WP1066 can upregulate costimulatory molecules (CD80, CD86) on ex vivo human microglia isolated from glioma patients, induce proinflammatory cytokine secretion essential for T effector responses, and induce impaired T cells to become activated and proliferate, indicating that STAT3 blockade is a potent approach for modulating immunosuppression.[22] Other investigators have shown that by ablating STAT3 in the hematopoietic cells in mice, there was marked enhancement of function within T cells, NK cells, and dendritic cells in tumor-bearing mice. This ablation of STAT3 in only the hematopoietic cells resulted in marked antitumor effects in vivo, indicating that STAT3 expression within the immune cells is what restrains the antitumor immune eradication.[32] This indicates that WP1066 could also be developed as an immunotherapeutic for malignancies. Finally, we have shown that WP1066 can target glioma cancer stem cells that are responsible for therapeutic resistance.[59,61]
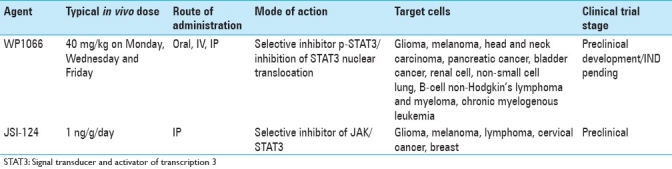
There are several other STAT3 inhibitory compounds that have therapeutic potential for introduction into clinical trials for patients with CNS malignancies [Table 1]. JSI-124 (cucurbitacin I)[54] has demonstrated prolonged survival in murine model systems with intracerebral tumors[13] and also has a favorable toxicity profile.[42] JSI-124 has in vitro activity against lymphoma[57] and cervical cancer[9] and in vivo growth inhibition of human breast carcinoma and syngeneic murine melanoma, but not in carcinomas that lack constitutive expression of p-STAT3.[5] Immunomodulatory properties of JSI-124 include the ability to overcome the dendritic cell differentiation block induced by tumors, resulting in the upregulation of MHC class II and costimulatory molecules and subsequent functional T-cell stimulation/activation.[41] In an in vivo sarcoma model in which JSI-124 had minimal direct tumor cytotoxicity, JSI-124 enhanced dendritic cell vaccination, resulting in marked tumor suppression that persisted for more than 4 weeks.[42] Furthermore, JSI-124 in vivo promoted a Th1 (cytotoxic effector) phenotype and enhanced the levels of glioma-infiltrating immune cells.[13] Immune-competent mice with intracerebral tumors treated with JSI-124 had prolonged survival, but this efficacy was not observed in an immune-incompetent background, indicating that the immune system played a role in the in vivo effect of JSI-124 in a manner similar to WP1066. Furthermore, when JSI-124 was combined with adoptive transfer of type I cytotoxic T lymphocytes, survival was further enhanced compared with treatment with either modality alone. The authors concluded that the inhibition of STAT3 could reverse the immunosuppressive immune microenvironment and promote the efficacy of adoptive transfer therapy.[13] Finally, although described as an inhibitor of janus kinase 2 (Jak2; which is upstream of STAT3), AZD1480 also possesses serious clinical potential for the treatment of patients with CNS malignancies. AZD1480 has demonstrated significant growth inhibition on a wide variety of human solid tumor xenografts[16] and myeloproliferative neoplasms[23,53] and is already in phase I clinical trials. Thus, there are several serious contenders that can block STAT3, which are likely to be introduced into clinical trials for patients with gliomas within the next few years.
Table 1.
Signal transducer and activator of transcription 3 inhibitors with therapeutic potential for central nervous system malignancies
CURRENT AND FUTURE DEVELOPMENTS
The potential for using p-STAT3 inhibitors in the treatment of a variety of cancers, including CNS metastasis, is evident. After successful completion of phase I testing, an interesting application of these compounds will be in combination with other immunotherapeutic strategies. For example, p-STAT3 inhibitors could be used in combination with dendritic cell vaccinations. Alternatively, in the treatment of high-grade malignant gliomas, we have previously shown that a peptide vaccine (PEP-3-KLH/CDX-110) directed to the splice junction of type III variant of the epidermal growth factor receptor (EGFRvIII) is effective in the treatment of intracerebral tumors in both murine models[18] and human patients who have newly diagnosed GBM.[49,51] These trials were limited to GBM patients who underwent gross-total resections so that the immunosuppressive properties of the tumor were negated as much as possible. However, many GBM patients cannot have a surgical resection that places their disease in a microscopic residual infiltrative state, and therefore, agents that can counteract the immunosuppressive influence of a bulky tumor are essential. Within malignant glioma patients, there is a prominent Treg population in the systemic circulation[12] and marked infiltration within the tumors in the CNS.[17] These Tregs remain elevated even in patients receiving PEP-3-KLH.[49] Because the p-STAT3 agents inhibit Tregs, enhance cytotoxic responses, and reverse immune suppression, they have the potential to further enhance peptide-based vaccination strategies, such as with the PEP-3-KLH/CDX-110 vaccine, possibly including patients with bulky tumors who are unable to undergo surgical resection.
Emerging within the glioma literature is the concept that STAT3 may be upregulated during treatment failure. Specifically, treatment with imatinib (Gleevec®) resulted in the sustained activation of STAT3 in malignant cells.[11] Other investigators have suggested that anti-VEGF treatment can reduce the blood supply within gliomas, leading to a more hypoxic environment.[26] Moreover, we have recently demonstrated that hypoxia can induce the expression of p-STAT3.[60] Thus, it is possible that the failure of anti-VEGF strategies may be associated with upregulation of STAT3. If this is the case, then agents targeting the STAT3 pathway given in combination with anti-VEGF therapeutics may prevent or delay tumor recurrence and/or may be an appropriate salvage therapy.[47]
In summary, STAT3 blockade agents have multiple mechanisms of activity, including direct tumor cytotoxic effects and the ability to overcome the negative modulatory effects of the local tumor microenvironment, allowing for immunological recognition and clearance of cancer cells. The targets of STAT3 blockade agents are not only the STAT3 in tumor cells, but also the STAT3 in immune cells, which use the p-STAT3 pathway to prevent antitumor immune reactivity. The preclinical data thus far available are sufficiently compelling to justify the consideration of human clinical trials to test these agents. The small molecule inhibitors of p-STAT3 can be used to treat a wide variety of cancers but, in particular, may have a meaningful impact on patients with primary and metastatic tumors of the CNS, a markedly underserved patient population.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/163/89886
REFERENCES
- 1.Abou-Ghazal M, Yang DS, Qiao W, Reina-Ortiz C, Wei J, Kong LY, et al. The incidence, correlation with tumor-infiltrating inflammation, and prognosis of phosphorylated STAT3 expression in human gliomas. Clin Cancer Res. 2008;14:8228–35. doi: 10.1158/1078-0432.CCR-08-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao JJ, Fokt I, Szymanski S, Priebe W. Inhibition of constitutively active STAT3 by WP1066 suppresses proliferation and induces apoptosis in pancreatic cancer cells. Clin Cancer Res. 2005;11:9026S–7S. [Google Scholar]
- 3.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 4.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–72. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 5.Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–9. [PubMed] [Google Scholar]
- 6.Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–25. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cattaneo E, Magrassi L, De-Fraja C, Conti L, Di Gennaro I, Butti G, et al. Variations in the levels of the JAK/STAT and ShcA proteins in human brain tumors. Anticancer Res. 1998;18:2381–7. [PubMed] [Google Scholar]
- 8.Chakraborty A, Guha S, Helgason T, Szymanski S, Fokt I, Kazerooni R, et al., editors. Los Angeles, CA: American Association of Cancer Research Annual Meeting; 2007. A novel Jak2/STAT3 pathway inhibitor promotes apoptosis and blocks growth of bladder cancer cells. [Google Scholar]
- 9.Chen CL, Hsieh FC, Lieblein JC, Brown J, Chan C, Wallace JA, et al. Stat3 activation in human endometrial and cervical cancers. Br J Cancer. 2007;96:591–9. doi: 10.1038/sj.bjc.6603597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colman H, Sai K, Wang S, Popoff S, Aldape KD, Lang FF, et al. Effect of a small molecule inhibitor of the JAK2/STAT3 pathway on self-renewal of glioblastoma stem cells. J Clin Oncol. 2008;26:89s. [Google Scholar]
- 11.Dong Y, Jia L, Wang X, Tan X, Xu J, Deng Z, et al. Selective inhibition of PDGFR by imatinib elicits the sustained activation of ERK and downstream receptor signaling in malignant glioma cells. Int J Oncol. 2011;38:555–69. doi: 10.3892/ijo.2010.861. [DOI] [PubMed] [Google Scholar]
- 12.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 13.Fujita M, Zhu X, Sasaki K, Ueda R, Low KL, Pollack IF, et al. Inhibition of STAT3 promotes the efficacy of adoptive transfer therapy using type-1 CTLs by modulation of the immunological microenvironment in a murine intracranial glioma. J Immunol. 2008;180:2089–98. doi: 10.4049/jimmunol.180.4.2089. [DOI] [PubMed] [Google Scholar]
- 14.Gong W, Wang L, Yao J, Ajani JA, Wei D, Aldape K, et al. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res. 2005;11:1386–93. doi: 10.1158/1078-0432.CCR-04-0487. [DOI] [PubMed] [Google Scholar]
- 15.Guha S, Chakraborty A, Szymanski S, Fokt I, Abbruzzese J, Kazerooni R, et al. Los Angeles, CA: American Association of Cancer Research Annual Meeting; 2007. WP1066, a potent inhibitor of Jak2/STAT3 pathway inhibits pancreatic tumor growth both in vitro and in vivo. [Google Scholar]
- 16.Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–97. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Sun W, Qiao W, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14:5166–72. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 18.Heimberger AB, Crotty LE, Archer GE, Hess KR, Wikstrand CJ, Friedman AH, et al. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res. 2003;9:4247–54. [PubMed] [Google Scholar]
- 19.Horiguchi A, Asano T, Kuroda K, Sato A, Asakuma J, Ito K, et al. STAT3 inhibitor WP1066 as a novel therapeutic agent for renal cell carcinoma. Br J Cancer. 2010;102:1592–9. doi: 10.1038/sj.bjc.6605691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horiguchi A, Oya M, Shimada T, Uchida A, Marumo K, Murai M. Activation of signal transducer and activator of transcription 3 in renal cell carcinoma: A study of incidence and its association with pathological features and clinical outcome. J Urology. 2002;168:762–5. [PubMed] [Google Scholar]
- 21.Huang S. Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway: Clinical implications. Clin Cancer Res. 2007;13:1362–6. doi: 10.1158/1078-0432.CCR-06-2313. [DOI] [PubMed] [Google Scholar]
- 22.Hussain SF, Kong LY, Jordan J, Conrad C, Madden T, Fokt I, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67:9630–6. doi: 10.1158/0008-5472.CAN-07-1243. [DOI] [PubMed] [Google Scholar]
- 23.Ioannidis S, Lamb ML, Wang T, Almeida L, Block MH, Davies AM, et al. Discovery of 5-Chloro-N(2)-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]-N(4)-(5-methyl-1H-pyrazol-3-yl)pyrimidine-2,4-diamine (AZD1480) as a Novel Inhibitor of the Jak/Stat Pathway. J Med Chem. 2011;54:262–76. doi: 10.1021/jm1011319. [DOI] [PubMed] [Google Scholar]
- 24.Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I, et al. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene. 2007;26:2435–44. doi: 10.1038/sj.onc.1210031. [DOI] [PubMed] [Google Scholar]
- 25.Johnson MD, O’Connell M, Vito F, Bakos RS. Increased STAT-3 and synchronous activation of Raf-1-MEK-1-MAPK, and phosphatidylinositol 3-Kinase-Akt-mTOR pathways in atypical and anaplastic meningiomas. J Neurooncol. 2009;92:129–36. doi: 10.1007/s11060-008-9746-7. [DOI] [PubMed] [Google Scholar]
- 26.Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SA, Fack F, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A. 2011;108:3749–54. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoury JD, Medeiros LJ, Rassidakis G, Yared MA, Tsioli P, Leventaki V, et al. Differential expression and clinical significance of tyrosine-phosphorylated STAT3 in ALK+ and ALK- anaplastic large cell lymphoma. Clin Cancer Res. 2003;9:3692–9. [PubMed] [Google Scholar]
- 28.Kong LY, Abou-Ghazal MK, Wei J, Chakraborty A, Sun W, Qiao W, et al. A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin Cancer Res. 2008;14:5759–68. doi: 10.1158/1078-0432.CCR-08-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong LY, Kapuria V, Bartholomeusz G, Talpaz M, Priebe W, Donato NJ. Antitumor Activity and Mechanism of Action of a Novel Stat3 Inhibitor, WP1066, Against Human B-Cell Non-Hodgkin's Lymphoma and Multiple Myeloma. Blood (ASH Annual Meeting Abstracts) 2005;106:1489. [Google Scholar]
- 30.Kong LY, Wei J, Sharma AK, Barr J, Abou-Ghazal MK, Fokt I, et al. A novel phosphorylated STAT3 inhibitor enhances T cell cytotoxicity against melanoma through inhibition of regulatory T cells. Cancer Immunol Immunother. 2009;58:1023–32. doi: 10.1007/s00262-008-0618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong LY, Wu AS, Doucette T, Wei J, Priebe W, Fuller GN, et al. Intratumoral mediated immunosuppression is prognostic in genetically engineered murine models of glioma and correlates to immunotherapeutic responses. Clin Cancer Res. 2010;16:5722–33. doi: 10.1158/1078-0432.CCR-10-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 33.Kupferman ME, Jayakumar A, Zhou G, Xie T, Dakak-Yazici Y, Zhao M, et al. Therapeutic suppression of constitutive and inducible JAK\STAT activation in head and neck squamous cell carcinoma. J Exp Ther Oncol. 2009;8:117–27. [PubMed] [Google Scholar]
- 34.Kupferman ME, Zhou G, Zhao M, Jasser S, Dakak-Yazici Y, Priebe W, et al. Washington, DC: 97th American Association of Cancer Research Annual Meeting; 2006. A novel inhibitor of STAT3 signaling in head and neck squamous cell carcinoma. [Google Scholar]
- 35.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–46. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 36.Madden T, Kazerooni R, Myer J, Culotta K, Donato N, Johansen M, et al. Washington, DC: Annual Meeting of the American Association for Cancer Research; 2006. The preclinical pharmacology of WP1066, a potent small molecule inhibitor of the JAK2/STAT3 pathway. [Google Scholar]
- 37.Masuda M, Suzui M, Yasumatu R. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–5. [PubMed] [Google Scholar]
- 38.McLoughlin RM, Jenkins BJ, Grail D, Williams A, Fielding C, Parker CR, et al. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9589–94. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merino B, Diez-Fernandez C, Ruiz-Gayo M, Somoza B. Choroid plexus epithelial cells co-express the long and short fo rm of the leptin receptor. Neurosci Lett. 2006;393:269–72. doi: 10.1016/j.neulet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Murat A, Migliavacca E, Gorlia T, Lambiv WL, Shay T, Hamou MF, et al. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. 2008;26:3015–24. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 41.Nefedova Y, Cheng P, Gilkes D, Blaskovich M, Beg AA, Sebti SM, et al. Activation of dendritic cells via inhibition of Jak2/STAT3 signaling. J Immunol. 2005;175:4338–46. doi: 10.4049/jimmunol.175.7.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nefedova Y, Nagaraj S, Rosenbauer A, Muro-Cacho C, Sebti SM, Gabrilovich DI. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 2005;65:9525–35. doi: 10.1158/0008-5472.CAN-05-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer. 1996;78:1781–8. [PubMed] [Google Scholar]
- 44.O’Farrell AM, Liu Y, Moore KW, Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: Evidence for Stat3-dependent and independent pathways. EMBO J. 1998;17:1006–18. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Priebe W, Fokt I, Szymanski S, Madden T, Bao JI, Lesyng B, et al. Design, synthesis and structure-activity relationships of novel Jak2/STAT3 signaling inhibitors. 97th Annual Meeting of the American Association for Cancer Research. 2006;47:LB–298. [Google Scholar]
- 46.Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–13. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- 47.Rapisarda A, Hollingshead M, Uranchimeg B, Bonomi CA, Borgel SD, Carter JP, et al. Increased antitumor activity of bevacizumab in combination with hypoxia inducible factor-1 inhibition. Mol Cancer Ther. 2009;8:1867–77. doi: 10.1158/1535-7163.MCT-09-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samanta A, Kantarjian H, Priebe W, Arlinghaus R, editors. Los Angeles, CA: 98th American Association of Cancer Research Annual Meeting; 2007. Cross talk between Jak2 and Lyn in Bcr-Abl signaling pathway in cells from Imatinib-sensitive and resistant chronic myelogenous leukemia (CML) [Google Scholar]
- 49.Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13:324–33. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sampson JH, Carter JH, Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 51.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–9. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–22. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 53.Scuto A, Krejci P, Popplewell L, Wu J, Wang Y, Kujawski M, et al. The novel JAK inhibitor AZD1480 blocks STAT3 and FGFR3 signaling, resulting in suppression of human myeloma cell growth and survival. Leukemia. 2011;25:538–50. doi: 10.1038/leu.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sebti SM, Jove R. US: 2004. Materials and methods for treatment of cancer and identification of anti-cancer compounds. inventors; None, assignee. [Google Scholar]
- 55.Shah NG, Trivedi TI, Tankshali RA. STAT3 expression in oral squamous cell carcinoma: association with clinicopathological parameters and survival. Int J Biol Markers. 2006;21:175–83. doi: 10.1177/172460080602100307. [DOI] [PubMed] [Google Scholar]
- 56.Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells. 2009;27:2383–92. doi: 10.1002/stem.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi X, Franko B, Frantz C, Amin HM, Lai R. JSI-124 (cucurbitacin I) inhibits Janus kinase-3/signal transducer and activator of transcription-3 signalling, downregulates nucleophosmin-anaplastic lymphoma kinase (ALK), and induces apoptosis in ALK-positive anaplastic large cell lymphoma cells. Br J Haematol. 2006;135:26–32. doi: 10.1111/j.1365-2141.2006.06259.x. [DOI] [PubMed] [Google Scholar]
- 58.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 59.Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, et al. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther. 2010;9:67–78. doi: 10.1158/1535-7163.MCT-09-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei J, Wu A, Kong LY, Wang Y, Fuller G, Fokt I, et al. Hypoxia potentiates glioma-mediated immunosuppression. PLoS One. 2011;6:e16195. doi: 10.1371/journal.pone.0016195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12:1113–25. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xi S, Gooding WE, Grandis JR. In vivo antitumor efficacy of STAT3 blockade using a transcription factor decoy approach: Implications for cancer therapy. Oncogene. 2005;24:970–9. doi: 10.1038/sj.onc.1208316. [DOI] [PubMed] [Google Scholar]
- 63.Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, et al. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–96. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 64.Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23:3550–60. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 65.Yang J, Chatterjee-Kishore M, Staugaitis SM, Nguyen H, Schlessinger K, Levy DE, et al. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005;65:939–47. [PubMed] [Google Scholar]
- 66.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 67.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 68.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–9. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]


