Abstract
Background:
The central sulcus may be located through magnetic resonance imaging (MRI) by identifying the ipsilateral inverted Omega shape. In a brain with a lesion in this area, its identification becomes a hard task irrespective of the technique applied. The aim of this study is to show the usefulness of the contralateral Omega sign for the location of tumors in and around the central sulcus. We do not intend to replace modern techniques, but to show an easy, cheap and relatively effective way to recognize the relationship between the central sulcus and the lesion.
Methods:
From July 2005 through December 2010, 43 patients with lesions in and around the central sulcus were operated using the contralateral Omega sign concept. Additionally, 5 formalin-fixed brains (10 hemispheres) were studied to clarify the anatomy of the central sulcus where the Omega shape is found.
Results:
The central sulcus has three genua. The middle genu is characterized by an inverted Omega-shaped area in axial sections known as the Omega sign. On anatomical specimens, Omega was 11.2 ± 3.35 mm in height, on average, and 18.7 ± 2.49 mm in width, at the base. The average distance from the medial limit of the Omega to the medial edge of the hemisphere was 24.5 ± 5.35 mm. Identification of the Omega sign allowed for the topographic localization of the contralateral central sulcus in all our surgical cases but one.
Conclusion:
The contralateral Omega sign can be easily and reliably used to clarify the topographic location of the pathology. Hence, it gives a quick preoperative idea of the relationships between the lesion and the pre- and post-central gyri.
Keywords: Anatomy, central sulcus, magnetic resonance imaging, Omega sign
INTRODUCTION
During neurosurgical planning, the potential risks for postoperative deficits resulting from damage to the eloquent cortex must be addressed.[25] Treatment of lesions adjacent to the sensorimotor cortex requires localization of the central sulcus.[11] The central sulcus may be located through magnetic resonance imaging (MRI) by identifying the ipsilateral inverted Omega shape which corresponds to its medial genu[26] [Figure 1]. The usefulness of this structural landmark is limited where the lesion distorts the normal cerebral anatomy.[9]
Figure 1.
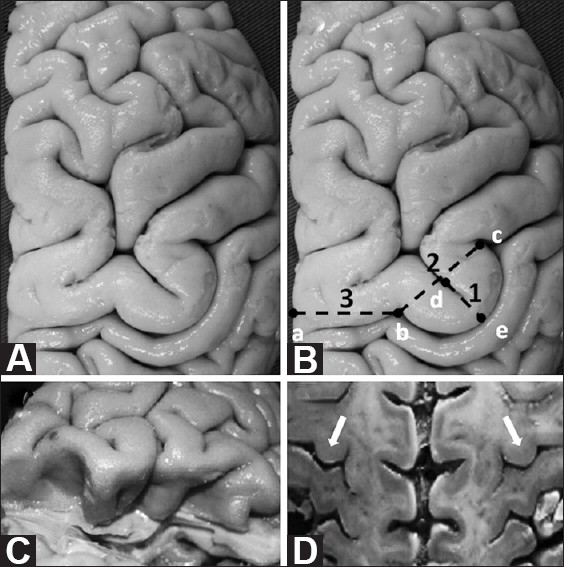
(A and B) Superior view of the Omega sign in a right brain. (B) Measurements taken in this study are highlighted in this diagram, based on the specimen shown in (A): 1(d-e) Omega height; 2(b-c) Omega width; 3(a-b) distance between the medial edge of the hemisphere and the medial limit of the Omega. (C) Post-central gyrus excised to expose pre-central gyrus. Note that the Omega shape of the central sulcus remains deep within the sulcus. (D) An axial section through both hemispheres resembles an MRI axial section. Note that both Omegas are at the same anterior posterior level, an anatomical fact that enables the use of the contralateral Omega sign for preoperative planning when the ipsilateral anatomy is distorted owing to a disease process
The aim of this study is to show the usefulness of the contralateral Omega sign, using MRI, for the topographic location of the central sulcus and the sensorimotor cortex in the pathological hemisphere. We do not intend to replace modern techniques, but to show an easy, cheap and relatively effective way to recognize the relationship between the central sulcus and the lesion.
MATERIALS AND METHODS
From July 2005 through December 2010, 43 patients harboring lesions in and around the central sulcus were operated. Lesions comprised 19 metastatic processes, 13 parasagittal meningiomas, 7 gliomas, 2 cavernous angiomas, 1 arteriovenous malformation and 1 tuberculous granuloma. The contralateral Omega sign and central sulcus were preoperatively identified on MRI in all patients but one.
Five formalin-fixed, cadaveric human brains (10 hemispheres) were studied. Measurements were taken with a caliper (Draper, Japan; accuracy: 0.02 mm).
RESULTS
Central sulcus and Omega sign
The central sulcus is one of the most important and consistent landmarks in the convexity of the brain. It separates motor from sensory areas, and frontal from parietal lobes. Starting medially, it descends anteriorly along the lateral surface of the hemisphere. It resembles an “S” in appearance with three distinct curves or genua: the superior and inferior genua, which are convex anteriorly, and the middle genu – the deepest curve – which is concave anteriorly. This remarkable shape of the middle genu of the central sulcus has been compared to an inverted Greek letter Omega [Figure 1A–D]. The Omega signs corresponding to both hemispheres are approximately at the same coronal level [Figure 1D].
Omega measurements
The characteristics of the middle genu of the central sulcus were analyzed on 10 formalin-fixed, cadaveric human hemispheres (5 brains). Omega was 11.2 ± 3.35 mm in height, on average, and 18.7 ± 2.49 mm in width, at the base. The average distance between the medial edge of the hemisphere and the medial limit of the Omega averaged 24.5 ± 5.35 mm [Table 1 and Figure 1B].
Table 1.
Measurements at Omega (N = 10)
Localization of the contralateral Omega
Between March 2006 and December 2010, MR images (axial sections) of 43 patients were carefully studied preoperatively to identify the central sulcus using the Omega sign contralateral to the disease process. Some illustrative cases are shown.
The contralateral Omega sign could be accurately identified in all patients but one, as a consequence of an arachnoid cyst distorting the anatomy on the contralateral brain; thus identification on sagittal sections of the posteriorly oriented hook shape of the ipsilateral pre-central gyrus[3] was alternatively used for surgical planning.
Illustrative cases
Patient 1
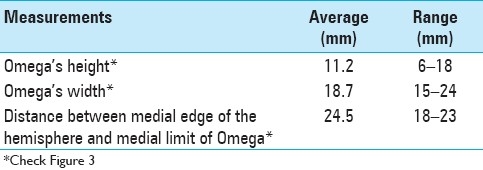
A 43-year-old female presented with seizures. MRI showed a lesion at the right superior frontal gyrus posteriorly, which was partially distorting normal right-brain anatomy. On the opposite side, a clear Omega sign was found and used to locate the central sulcus and the sensorimotor cortex in the pathological hemisphere. Following appropriate resection, the histopathologic diagnosis was a grade II astrocytoma. No motor deficits were seen postoperatively [Figure 2].
Figure 2.
(a and b) Pre-op axial MRI. Imaging reveals a lesion involving the right superior frontal gyrus posteriorly and partially distorting normal right-brain anatomy. On the opposite side, the Omega shape is highlighted in white. (c) Post-op axial MRI. Imaging demonstrates appropriate excision. Histologically, a grade II astrocytoma was found. (d) Follow-up. No motor deficits seen at neurological examination
Patient 2
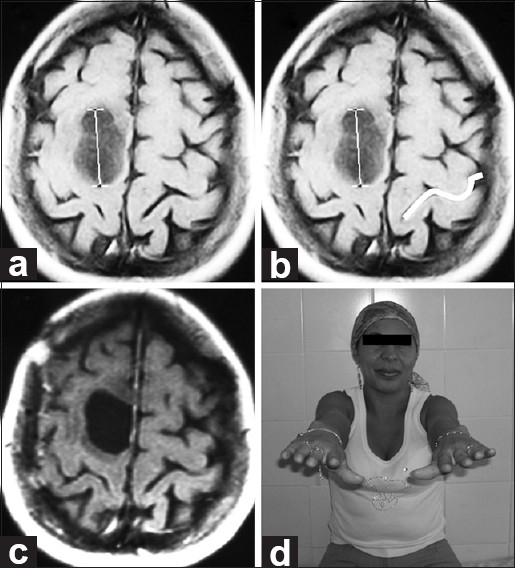
A 53-year-old female with a history of breast cancer presented with mild right crural paresis. MRI showed a lesion involving most of the left post-central gyrus and distorting normal left-brain anatomy. The Omega sign on the opposite side was used for preoperative planning. Total removal was accomplished with complete recovery, and no deficits were seen postoperatively [Figure 3].
Figure 3.
(a and b) Pre-op axial MRI. Imaging reveals a lesion involving most of the left post-central gyrus and distorting normal left-brain anatomy. On the opposite side, the Omega shape is highlighted in white. (c) Post-op axial MRI. Imaging demonstrates appropriate excision. Histologically, a breast cancer metastasis was found. (d) Follow-up. No motor deficits seen at neurological examination
Patient 3
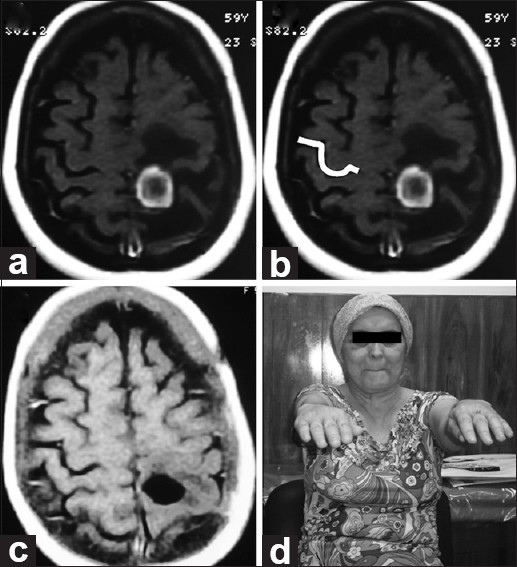
A 51-year-old female with no history of disease presented with seizures and mild right paresis, which was identified as a result of writing problems. On MRI, a ring-enhancing pre-central lesion with edema was found on the right superior frontal gyrus posteriorly that clearly distorted normal right-brain anatomy. Cancer screening tests were negative. A clearly demarcated Omega sign on the opposite side was used for preoperative planning. Complete excision was accomplished and no gross postoperative deficits were seen. Histopathology revealed a tuberculous granuloma [Figure 4].
Figure 4.
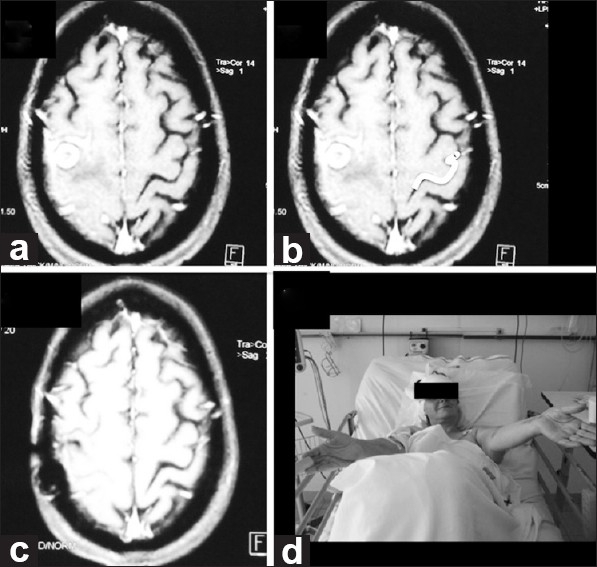
(a and b) Pre-op axial MRI. Imaging reveals a lesion in the pre central area distorting normal right-brain anatomy. On the opposite side, the Omega shape is highlighted in white. (c) Post-op axial MRI. Imaging demonstrates appropriate excision. Histologically, tuberculous granuloma was found. (d) Follow-up. No motor deficits seen at neurological examination
Patient 4
A 63-year-old male with a history of lung cancer presented with severe hemicranial headache. MRI showed a small lesion at the right superior frontal gyrus posteriorly, which was slightly distorting normal right-brain anatomy. A contralateral Omega sign helped to guide the identification of the central sulcus. Complete excision was performed, with no postoperative deficits [Figure 5].
Figure 5.
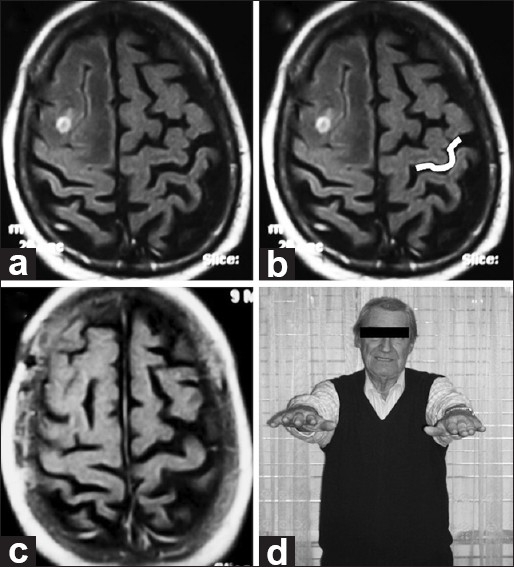
(a and b) Pre-op axial MRI. Imaging reveals a small lesion with perilesional edema involving the right middle frontal gyrus posteriorly and distorting normal right-brain anatomy. On the opposite side, the Omega shape is highlighted in white. (c) Post-op axial MRI. Imaging demonstrates appropriate excision. Histologically, a lung cancer metastasis was found. (d) Follow-up. No motor deficits seen at neurological examination
Patient 5
A 49-year-old female presented with an abrupt-onset headache. Computed tomography (CT) and MRI showed a lesion on the right superior frontal gyrus posteriorly which was slightly distorting normal right-brain anatomy. On the opposite side, an Omega sign was well delineated for preoperative planning. Complete excision was accomplished; pathologically, the lesion was diagnosed as a cavernous angioma [Figure 6].
Figure 6.
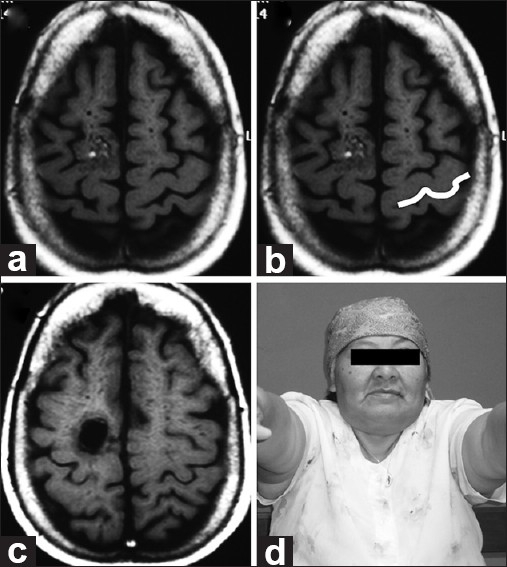
(a and b) Pre-op axial MRI. Imaging reveals a lesion involving the right superior frontal gyrus posteriorly and slightly distorting normal right-brain anatomy. On the opposite side, the Omega has been highlighted in white. (c) Post-op axial MRI demonstrating appropriate excision. Histologically, a cavernoma is found. (d) Follow-up. No motor deficits seen at neurological examination
Patient 6
A 52-year-old female presented with mild left hypoesthesia and paresis. MRI showed an extraaxial lesion compressing the left frontal and parietal lobes and distorting normal left-brain anatomy. The contralateral Omega sign was used for preoperative planning. Complete excision was accomplished, with no deficits postoperatively. Histology revealed a meningothelial meningioma [Figure 7].
Figure 7.
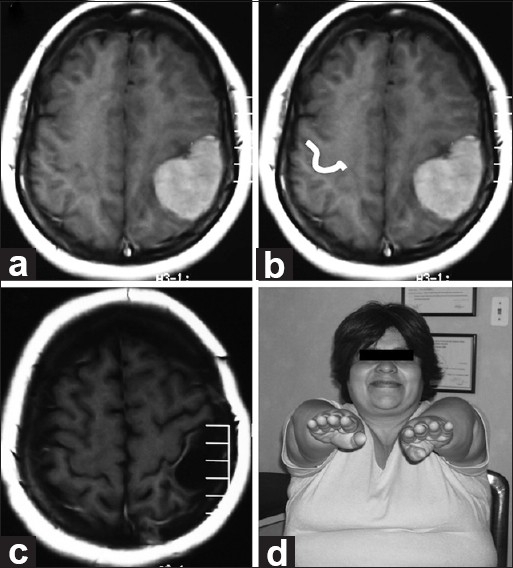
(a and b) Pre-op axial MRI. Imaging reveals an extra-axial lesion pushing the frontal and parietal left lobes and distorting normal left-brain anatomy. On the opposite side, the Omega shape has been highlighted in white. (c) Post-op axial MRI. Imaging demonstrates appropriate excision. Histologically, a meningothelial meningioma was found. (d) Follow-up. No motor deficits seen at neurological examination
DISCUSSION
Anatomical literature is controversial about the number and appearance of the curves that the central sulcus shows along its course. Classical anatomical books such as those of Rouviere[20] and Testut[23] describe the sulcus as having three curves: the superior and inferior genua, which are convex anteriorly, and the middle genu, which is convex posteriorly. On the other hand, Ono et al.[16] and Rhoton[19] describe only two genua: a superior curve, which is concave anteriorly, and an inferior curve, which is concave posteriorly. This discrepancy may be due to the smaller size of the superior genu. In our study, the central sulcus has been found to display three well-defined genua.
Classical general understanding has been that the motor cortical representation of the hand is located over the superior part of the pre-central gyrus.[17] In 1991, Salamon et al.[21] described a hook-like shape that corresponded to the hand projection area. In 1995, Naidich and Brightbill[14] described the cortical hand area as a knuckle. Also, in 1995, Puce et al.[18] described the motor hand area as a part of the pre-central gyrus that is located in front of the inverted Omega-shaped area of the central sulcus. Hence, the motor cortex hand area is located at the inverted Omega-shaped area on the pre-central gyrus, which becomes evident on an axial section through the central sulcus at the level of the interdigitation of the pre- and post-central gyri.[1]
MRI is a non-invasive type of study, which allows for a detailed analysis of cortical morphology.[26] Yousry et al.[26] could identify the central sulcus in 194 out of 198 hemispheres examined with MRI. In their study, failure in recognizing the central sulcus resulted from space-occupying lesions in three hemispheres and a porencephalic cavity in the remaining one. Yetkin et al.[25] reported that in 18 out of 23 studies, the anatomically identified central sulcus was compatible with functional MRI findings. In two patients with brain tumors, the central sulcus could not be identified because of the anatomical distortion caused by the disease, thereby confirming the observation that the anatomical identification of the central sulcus on a normal brain approaches 100%.
Three main techniques have reportedly been used to locate the central sulcus through MRI: (a) recognizing the ipsilateral Omega-shaped region corresponding to the motor hand area (Omega sign), (b) identifying the typical aspect of the crossroad formed by the superior frontal and pre-central sulci,[8] and (c) sequentially identifying the horizontal and ascending rami of the Sylvian fissure and the pre-central sulcus.[4,15] In our view, the Omega sign is a simple, easy-to-use and highly accurate technique in normal brains.[3,26] In a brain with a lesion, however, identification of the central sulcus becomes a hard task irrespective of the technique applied.
In order to overcome this problem, the following techniques are proposed: (a) functional MRI, based on the changes of local cerebral blood flow and oxygen consumption associated with neuronal activation (BOLD effect);[7,9,10,12] (b) magnetoencephalography (MEG), which topographically displays the activity of specific cerebral areas;[6,9,22] and (c) intraoperative cortical mapping.[2,5,13,24] Although useful, these techniques are not available to every neurosurgeon. Our findings support previous evidence that the contralateral Omega sign enables the reasonable location of the central sulcus and the development of an operative plan to preserve the eloquent cortex, even in anatomically distorted brains. The surgical experience in our series of 43 patients operated using this method is strongly suggestive of the usefulness of the contralateral Omega sign. There are two important points to remark. Localization of the contralateral Omega sign is easier in old patients with brain atrophy; in contrast, sometimes in parasagittal tumors, the edema can be present in both hemispheres, making its identification difficult.
CONCLUSION
The contralateral Omega sign found in MRI can be easily and reliably used to locate the central sulcus and the sensorimotor cortex on the side harboring the disease process. This technique may guide an understanding of the relationships of the lesion to the eloquent brain in and around the central sulcus in patients where ipsilateral anatomy is distorted. Furthermore, the Omega sign contributes to the development of a successful operative strategy.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/164/89892
Contributor Information
Alvaro Campero, Email: alvarocampero@yahoo.com.ar.
Pablo Ajler, Email: pablo.ajler@gmail.com.
Carolina Martins, Email: cmrecife@hotmail.com.
Juan Emmerich, Email: dremmerich@hotmail.com.
Luiz Felipe de Alencastro, Email: lfalencastro@hotmail.com.
Albert Rhoton, Jr, Email: rhoton@neurosurgery.ufl.edu.
REFERENCES
- 1.Alkadhi H, Kollias SS. Pli de passage fronto-pariétal moyen of Broca separates the moto homunculus. AJNR Am J Neuroradiol. 2004;25:809–12. [PMC free article] [PubMed] [Google Scholar]
- 2.Berger MS, Ojemann GA, Lettich E. Neurophysiological monitoring during astrocytoma surgery. Neurosurg Clin N Am. 1990;1:65–80. [PubMed] [Google Scholar]
- 3.Caulo M, Bastos A. Localization of the central area of the brain: How can it be done? 2000. [Last Accessed on 2011 Sep 5]. pp. 4–5. Available from: http://mni.mcgill.ca/neuroimage/pdf_files/oct2000.pdf .
- 4.Ebeling U, Steinmetz H, Huang YX, Kahn T. Topography and identification of the inferior precentral sulcus in MR imaging. AJR Am J Roentgenol. 1989;153:1051–6. doi: 10.2214/ajr.153.5.1051. [DOI] [PubMed] [Google Scholar]
- 5.Helmers SL. Evoked potentials for cortical mapping in children and adults. Tech Neurosurg. 2001;7:4–11. [Google Scholar]
- 6.Hund M, Rezai AR, Kronberg E, Capell J, Zonenshayn M, Ribary U, et al. Magnetoencephalographic mapping: Basic of a new functional risk profile in the selection of patients with cortical brain lesions. Neurosurgery. 1997;40:936–42. doi: 10.1097/00006123-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Inoue T, Shimizu H, Nakasato N, Kumabe T, Yoshimoto T. Accuracy and limitation of functional magnetic resonance imaging for identification of the central sulcus: Comparison with magnetoencephalography in patients with brain tumors. Neuroimage. 1999;10:738–48. doi: 10.1006/nimg.1999.0501. [DOI] [PubMed] [Google Scholar]
- 8.Kido DK, LeMay M, Levinson AW, Benson WE. Computed tomographic localization of the precentral gyrus. Radiology. 1980;135:373–7. doi: 10.1148/radiology.135.2.7367629. [DOI] [PubMed] [Google Scholar]
- 9.Korvenoja A, Kirveskari E, Aronen HJ, Avikainen S, Brander A, Huttunen J, et al. Sensorimotor cortex localization: Comparison of magnetoencephalography, functional MR imaging, and intraoperative cortical mapping. Radiology. 2006;241:213–22. doi: 10.1148/radiol.2411050796. [DOI] [PubMed] [Google Scholar]
- 10.Krings T, Reul J, Spetzger U, Klusmann A, Roessler F, Gilsbach JM, et al. Functional magnetic resonance mapping of sensory motor cortex for image-guided neurosurgical intervention. Acta Neurochir (Wien) 1998;140:215–22. doi: 10.1007/s007010050088. [DOI] [PubMed] [Google Scholar]
- 11.Kumabe T, Nakasato N, Inoue T, Yoshimoto T. Primary thumb sensory cortex located at the lateral shoulder of the inverted omega-shape on the axial images of the central sulcus. Neurol Med Chir (Tokyo) 2000;40:393–403. doi: 10.2176/nmc.40.393. [DOI] [PubMed] [Google Scholar]
- 12.Majos A, Tybor K, Stefanczyk L, Góraj B. Cortical mapping by functional magnetic resonance imaging in patients with brain tumors. Eur Radiol. 2005;15:1148–58. doi: 10.1007/s00330-004-2565-0. [DOI] [PubMed] [Google Scholar]
- 13.Matz PG, Cobbs C, Berger MS. Intraoperative cortical mapping as a guide to the surgical resection of gliomas. J Neurooncol. 1999;42:233–45. doi: 10.1023/a:1006122122404. [DOI] [PubMed] [Google Scholar]
- 14.Naidich TP, Brightbill TC. The intraparietal sulcus: A landmark for localization of pathology on axial CT scans. Int J Neuroradiol. 1995;1:3–16. [Google Scholar]
- 15.Naidich TP, Valavanis AG, Kubik S. Anatomic relationships along the low-middle convexity: Part I - normal specimens and magnetic resonance imaging. Neurosurgery. 1995;36:517–32. doi: 10.1227/00006123-199503000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Ono M, Kubik S, Abernathey CD. Stuttgart: Georg Thieme Verlag; 1990. Atlas of the Cerebral Sulci. [Google Scholar]
- 17.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 18.Puce A, Constable RT, Luby ML, McCarthy G, Nobre AC, Spencer DD, et al. Functional magnetic resonance imaging of sensory and motor cortex: Comparison with electrophysiological localization. J Neurosurg. 1995;83:262–70. doi: 10.3171/jns.1995.83.2.0262. [DOI] [PubMed] [Google Scholar]
- 19.Rhoton AL., Jr The cerebrum. Neurosurgery. 2002;51(Suppl 1):S1–51. doi: 10.1097/00006123-200210001-00002. [DOI] [PubMed] [Google Scholar]
- 20.Rouviere H, Delmas A. 9th ed. Vol. 1994. Tomo 3 Barcelona: Masson; Anatomía Humana. Descriptiva, Topográfica y Funcional; p. 669. [Google Scholar]
- 21.Salamon G, Martini P, Ternier F, Vibert E, Murayama N, Khadr E. Topographical study of supratentorial brain tumors. J Neuroradiol. 1991;18:123–40. [PubMed] [Google Scholar]
- 22.Sobel DF, Gallen CC, Schwartz BJ, Waltz TA, Aung M, Copeland B, et al. Locating the central sulcus: Comparison of MR anatomic and magnetoencephalographic functional methods. AJNR Am J Neuroradiol. 1993;14:915–25. [PMC free article] [PubMed] [Google Scholar]
- 23.Testut L, Latarjet A. 9th ed. Tomo 2. Barcelona: Salvat Editores; 1958. Tratado de Anatomía Humana; p. 951. [Google Scholar]
- 24.Tróccoli G, Echevarría G, Torresi S, Villar D, D’Annuncio E, Campero A, et al. Resección de lesiones cerebrales en área rolándica con mapeo cortical intraoperatorio. Rev Argent Neuroc. 2006;20:1–6. [Google Scholar]
- 25.Yetkin FZ, Papke RA, Mark LP, Daniels DL, Mueller WM, Haughton VM. Location of the sensorimotor cortex: Functional and conventional MR compared. AJNR Am J Neuroradiol. 1995;16:2109–13. [PMC free article] [PubMed] [Google Scholar]
- 26.Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–57. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]


