Abstract
A large body of work supports the proposal that transplantation of olfactory ensheathing cells (OECs) into nerve or spinal cord injuries can promote axonal regeneration and remyelination. Yet, some investigators have questioned whether the transplanted OECs associate with axons and form peripheral myelin, or if they recruit endogenous Schwann cells that form myelin. Olfactory bulbs from transgenic mice expressing the enhanced green fluorescent protein (eGFP) under the control of the 2-3-cyclic nucleotide 3-phosphodiesterase (CNPase) promoter were studied. CNPase is expressed in myelin-forming cells throughout their lineage. We examined CNPase expression in both in situ in the olfactory bulb and in vitro to determine if OECs express CNPase commensurate with their myelination potential. eGFP was observed in the outer nerve layer of the olfactory bulb. Dissociated OECs maintained in culture had both intense eGFP expression and CNPase immunostaining. Transplantation of OECs into transected peripheral nerve longitudinally associated with the regenerated axons. These data indicate that OECs in the outer nerve layer of the olfactory bulb of CNPase transgenic mice express CNPase. Thus, while OECs do not normally form myelin on olfactory nerve axons, their expression of CNPase is commensurate with their potential to form myelin when transplanted into injured peripheral nerve.
1. Introduction
The only example of successful regeneration from peripheral neurons into the central nervous system (CNS) is within the olfactory system, where axons regenerate throughout life from the nasal mucosa into the olfactory bulbs of the brain. A specialized glia cell, the olfactory ensheathing cell (OEC), spans the CNS-peripheral nervous system (PNS) junction and is thought to bridge the gap to allow peripheral axons to penetrate the brain. Indeed, transplantation of cultured OECs leads to enhanced regeneration and remyelination of injured peripheral nerve [1, 2].
A large body of work supports the proposal that transplantation of OECs into various spinal cord injury and demyelination models can promote axonal regeneration, remyelination, and functional recovery [2–12]. Yet, some investigators have questioned whether the transplanted OECs form peripheral myelin, or if they recruit endogenous SCs that form myelin [13, 14]. These events are not mutually exclusive in that transplanted OECs could both facilitate SC invasion into the spinal cord and as well as myelinate axons. It is important to note that Franklin et al. [11] demonstrated myelination in the spinal cord by an OEC cell line, strongly suggesting that OECs can indeed remyelinate axons [9].
Although OECs do not form myelin on fine caliber olfactory nerve fibers during normal development, numerous studies have shown that OECs can remyelinate both CNS [15–18] and PNS [1, 2] axons in a variety of lesion models. This discrepancy between the normal developmental fate OECs in vivo and their differentiation when transplanted into demyelinated regions has raised the question of whether the myelination observed in OEC transplanted lesions is due to contamination of OEC preparations with Schwann cells, oligodendrocyte precursor cells (OPCs), or even neural stem cells [13, 19, 20].
The enzyme 2′,3′-cyclic nucleotide 3′-phosphodiesterase or CNPase is expressed in both oligodendrocytes and SCs and is considered a marker for myelin-forming cells, although it is also found in other cells, including lymphocytes and photoreceptors as well as some neurons in long-term culture [21]. CNPase is both membrane bound and linked to microtubules and is the third most abundant myelin protein in the CNS, representing 4% of CNS myelin proteins. The role of this enzyme is not yet clear, although over expression mutations suggest that CNPase plays a role in myelin compaction [22, 23]. CNPase is the earliest myelination-specific protein expressed by oligodendrocytes and is expressed in both myelinating and nonmyelinating oligodendrocytes and SCs. CNPase is therefore considered to be marker for the potential of cells to produce myelin, rather than an indication of actual myelination and evidence of CNPase expression by OECs would therefore provide strong support for the idea that OECs are capable of forming myelin.
Studies using immunostaining with antiCNPase antibodies yielded ambiguous and conflicting results for CNPase expression by OECs from the olfactory bulb and olfactory neuroepithelium. CNPase staining was observed on some, but not all presumptive OECs in explant cultures from the olfactory bulb [24], but not on presumptive OECs in dissociated cultures from the nasal epithelium cultured on astrocyte feeder layers [25]. Immunostaining of developing olfactory bulb focused on CNPase staining of oligodendrocytes and did not report CNPase staining of OECs [26]. It is not clear therefore whether CNPase is expressed by OECs only in specific environments, or whether levels of CNPase may be too low to reliably detect with standard antibody staining protocols.
The recent development of a transgenic mouse in which an enhanced green fluorescent protein (eGFP) is linked to expression of CNPase [21] has provided an opportunity to evaluate CNPase expression by OECs in a variety of environments. Since the use of a reporter gene eliminates problems with both false positive and false negative antibody staining, GFP transgenic mice would allow detection of CNPase expression without the need to optimize staining protocols to specific tissue or culture conditions. In this study we have examined CNPase-linked eGFP expression by OECs in the olfactory bulb and in dissociated cell culture. The results indicate that OECs express CNPase in the outer nerve layer of the olfactory bulb as well as in culture, thus indicating that OECs express an important enzyme required for myelination. This provides further evidence of the intrinsic capability of OECs to myelinate axons upon transplantation.
2. Methods
2.1. Isolation and Characterization of OECs from CNP-EGFP Mouse
The CNP-EGFP mouse has been described previously [15, 27]. Freshly isolated OECs were obtained as reported previously [16, 28, 29]. Olfactory bulbs were removed from 4- to 8-week-old transgenic mice expressing GFP-CNPase and dissected free of meninges. The caudal one-third of the bulb was removed and discarded along with as much white matter as possible to isolate the outer nerve layer. Tissue was minced finely with a pair of scalpel blades (#10) on plastic culture dishes, and nonadherent tissue was washed from culture dishes and incubated for 25 min in collagenase A (0.75 mg/mL; Roche, Indianapolis, I, USA), collagenase D (0.75 mg/mL; Roche), and papain (12 U/mL; Worthington, Lakewood, NJ, USA) in calcium-free complete saline solution with trace cysteine for 25 min at 37°C on a rotary shaker in a CO2 incubator. The tissue suspension was then centrifuged for 7 min at 300 ×g, and, the supernatant was discarded. The pellet was resuspended in 2 mL of Dulbecco's modified medium (DMEM, Invitrogen, Carlsbad, Calif, USA) with 10% fetal calf serum (FCS) using gentle mechanical trituration; first with a 5 mL culture pipette and then with two fire-polished silicone-coated pasture pipettes with successively reduced diameters. The volume of media was immediately increased to 20 mL, and undissociated pieces of tissue were allowed to settle for 2 min before transferring the cell suspension to another culture tube and centrifuging as before. Cells were washed twice, resuspended, and preplated for 1 h in a culture flask at 37°C in a CO2 incubator. Nonadherent cells were gently washed off with DMEM, and the cells were centrifuged and resuspended three times in DMEM. Then cells were counted and concentrated to 3.0 × 104 cells/μL just prior to transplantation. P75NGFR- and S100-positive cells were counted in short-term cultures made from cell suspensions used for transplantation to assess purity of the cells. Over 95% of the cells were positive for p75NGFR and S100.
2.2. Immunostaining
To identify OECs, immunostaining for p75NGFR, a characteristic marker for OECs, was performed on cultured OECs. The cells were preincubated in normal goat blocking serum prior to incubation with the primary rabbit anti-p75NGFR monoclonal antibody (1 : 1000; Chemicon, Temecula, Calif, USA) followed by incubation with a fluorescein isothiocyanate- (FITC-) conjugated IgG (1 : 2000, Molecular Probes, Leiden, The Netherlands) secondary antibody for p75NGFR. Photographs were taken on a Spot RT Color CCD.
2.3. Immuno-EM
CNPase transgenic mice were deeply anesthetized (50 mg/kg sodium pentobarbital, i.p.) and perfused transcardially with PBS followed by 4% paraformaldehyde/0.02% glutaraldehyde in phosphate-buffered saline (PBS). Olfactory bulbs were excised, postfixed overnight in 4% paraformaldehyde, and embedded in 3% agar for vibratome sectioning. Free-floating sections (thickness 150 μm) were incubated in 2% normal goat serum for 30 min and then in rabbit anti-GFP antibody (1 : 2000; Chemicon) overnight at 4°C. The sections were incubated overnight with an anti-rabbit biotinylated secondary antibody (Sigma, St. Louis, Mo, USA) and then incubated for 1 h using a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, Calif, USA). The sections were postfixed with 1% osmium tetroxide for 4 h, dehydrated in graded ethanol, and embedded in Epox-812 (Ernest Fullam, Latham, NY, USA). Ultrathin sections were cut as described above but were not counterstained.
2.4. Induction of Nerve Crush Lesion and eGFP-OECs Transplantation Procedure
The Veterans Affairs Connecticut Healthcare System Institutional Animal Care and Use Committee approved all animal protocols. Experiments were performed in accordance with National Institutes of Health guidelines for the care and use of laboratory animals. Adult Sprague Dawley rats (200–225 g) were used for these experiments (n = 12). The rats were anesthetized with ketamine (75 mg/kg i.p.) and xylazine (10 mg/kg i.p.). The sciatic nerve was surgically exposed in anesthetized rats and injured by nerve crush lesion with fine microforceps for 40 seconds. This procedure completely transects all axons within the nerve and the animals showed signs of complete nerve transection [30]. The lesion site was standardized at the level of the piriformis tendon in the thigh. Cultured eGFP-expressing OECs from rat were detached from the culture flasks and resuspended in culture medium and adjusted to a concentration of 30,000 cells/μL. 2 μL of the cell suspension or vehicle alone (sham control) was injected 5.0 mm by using a Hamilton microsyringe caudally and distally into the crush lesion site. The animals survived for 5 weeks at which time they were intracardially perfused with 4% paraformaldehyde in phosphate buffer followed by removal of nerves for histological analysis.
3. Results
GFP expression in the cortex of the CNPase-eGFP transgenic mouse is strong in oligodendrocytes of cortical white matter (Figure 1(a)). Expression is also observed in Schwann cells of peripheral nerve (Figure 1(b)). Sections through the olfactory bulb in the CNPase-eGFP transgenic mouse indicate intense CNPase expression in the outer nerve layer of the olfactory bulb, the site where OECs are localized and interior regions of the bulb which are rich in oligodendrocytes (Figure 1(c)). Additionally, CNPase was strongly expressed in the olfactory nerve as it enters the olfactory bulb (Figure 1(c); arrow). A higher power image of the olfactory nerve from Figure 1(c) shows GFP expression in the nuclei and cytoplasm of the OECs (Figure 1(e)). Immunohistochemistry for CNPase of the olfactory bulb shows staining in deep white matter as well as in the outer nerve layer (Figure 1(d)).
Figure 1.
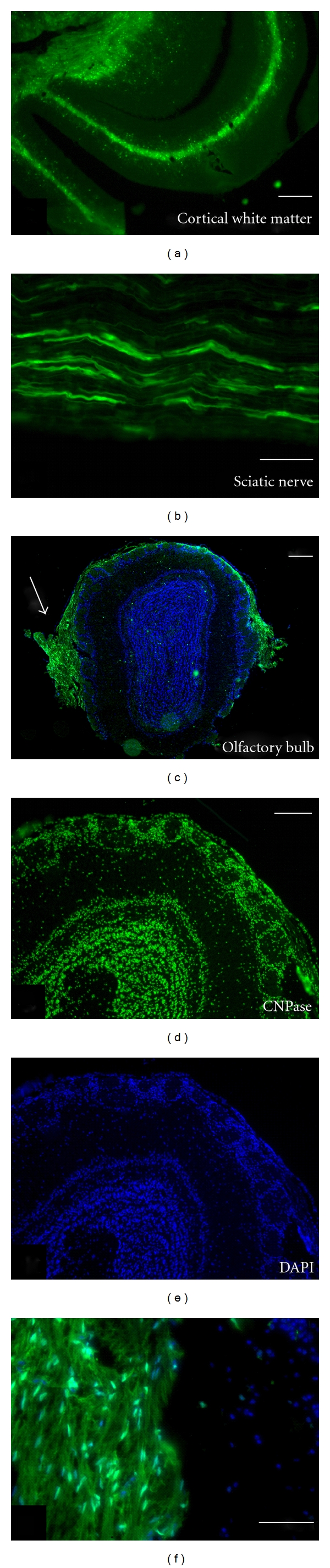
CNPase expression is characteristic of myelinating cells. (a) In the transgenic mouse where GFP is under the control of CNPase, GFP expression can be observed in cortical white matter (a) and sciatic nerve (b). The GFP is present in oligodendrocytes in white matter of the CNS and Schwann cells in the peripheral nerve. (c) Cross-section of the olfactory bulb from the CNPase mouse showing GFP expression in the olfactory nerve (arrow) and the outer nerve layer of the olfactory bulb (OB) where OECs are present as the only glia cell type. (f) Higher power image of the olfactory nerve from (c) showing GFP expression in the nuclei and cytoplasm of OECs in the outer nerve layer. (d) and (e) Immunohistochemistry for CNPase in a wild type mouse OB showing CNPase expression in deep white matter and outer margins of the bulb. Note that the round glomeruli are devoid of CNPase. Nuclei have been counterstained with DAPI (blue) in (c), (e), and (f). Scale bars: (a) = 8 μm, (b) = 12 μm, (c) = 500 μm, (d) = 150 μm and pertains to (d) and (e), (f) = 3 μm.
3.1. Colocalization of p75NGFR with CNPase in OECs in the Olfactory Bulb
The low affinity NGF receptor, p75NGFR, is expressed by OECs and Schwann cells. Cells in the olfactory nerve layer of eGFP-CNPase transgenic mice showed colocalization of GFP expression with the p75NGFR receptor (Figures 2(a)–2(c)). OECs can readily be identified in olfactory nerve as glial cells ensheathing large numbers of nonmyelinated olfactory nerve fibers and are distributed in the outer nerve layer of the olfactory bulb. For more detailed cellular localization of CNPase, immunoperoxidase staining of the olfactory bulb with a GFP antibody was performed and semithin plastic sections counterstained with toluidine, blue were obtained for more precise localization of eGFP in the OECs of the olfactory bulb and nerves. The eGFP (CNPase expressing cells) was localized in structurally well-defined OECs in the outer nerve layer of the olfactory bulb (Figure 2(d); higher magnification in Figure 2(e)). The cytoplasmic processes of the OECs wrapped bundles of nonmyelinated axons projecting within the outer nerve layer (Figure 2(e)). Thus, coexpression of p75 and CNPase within OECs in the olfactory bulb argues for the remyelination potential of OECs.
Figure 2.
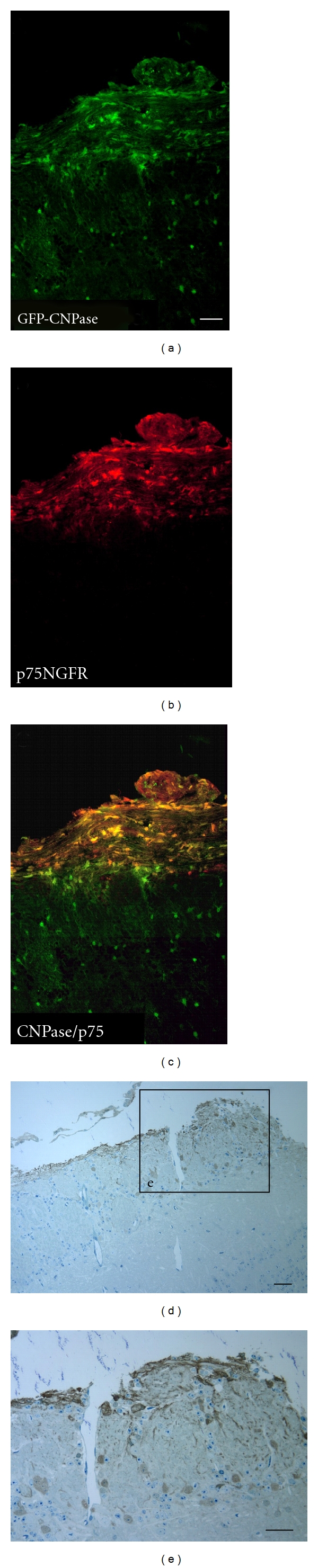
Colocalization of p75NGFR/CNPase and Immuno-EM. (a) GFP expression in the CNPase mouse in the olfactory nerve as it enters the OB and in scattered cells in the deep OB white matter. Immunostaining for p75 colocalizes with GFP in the olfactory nerve, but not in deep white matter (a–c). Immunoperoxidase staining for GFP in toluidine blue plastic sections (1 um) shows that the GFP was present in OECs in the outer nerve layer (d, e). Scale bars: in (a), pertains to (a–c) = 10 μm, (d) = 7, (e) = 10 μm.
3.2. Cultured OECs from the CNPase Transgenic Mouse Maintain Their GFP Expression
The CNPase expression observed in situ was maintained in culture when cells were prepared for cell transplantation (Figures 3(a)–3(d)). Confirmation of OEC identity was established by p75 (Figure 3(b)) immunostaining characteristic of OECs. OECs are the only cells expressing p75NGFR in the olfactory bulb. Dissociated OECs derived from the olfactory bulb and maintained in culture for 4 days had both intense p75NGFR immunostaining and eGFP-CNPase expression (Figure 3(d)).
Figure 3.
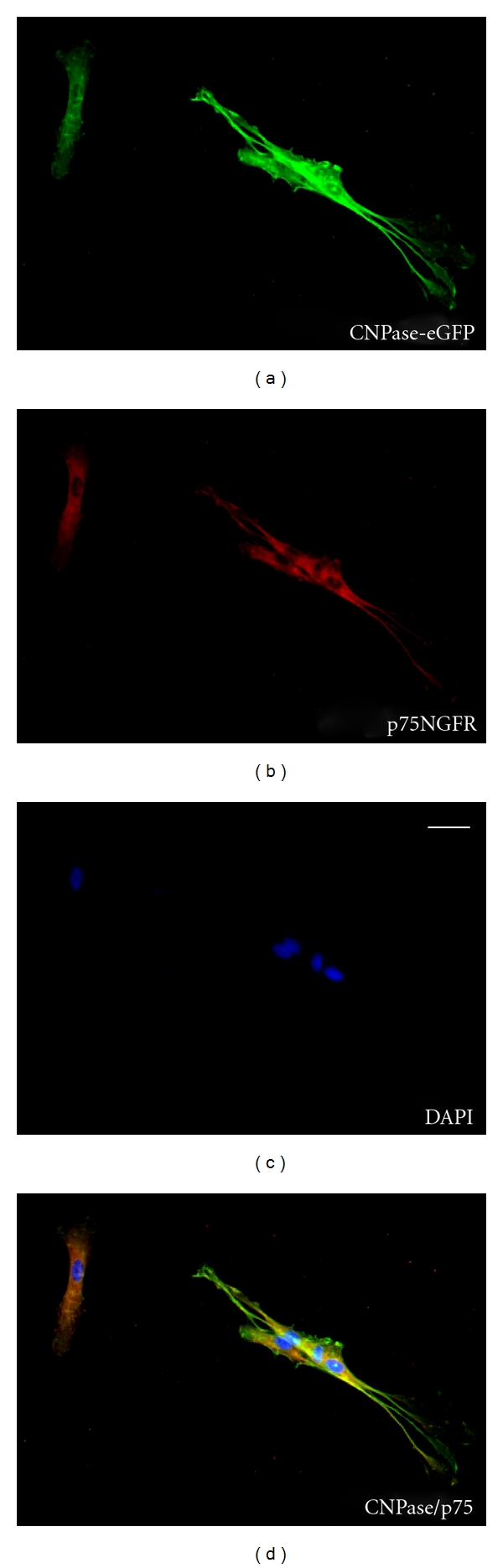
Cultured OECs from the CNPase transgenic mouse maintain their GFP expression. (a–d) Immunostaining of cultured olfactory bulb OECs derived from the eGFP-CNPase mouse indicates colocalization with p75. Nuclei stained blue with DAPI. Note the small cluster of spindle-shaped OECs and a more flattened OEC (upper left) both colocalize GFP (CNPase) and p75. Scale bar: (a) = 5 μm.
3.3. OECs Transplanted into Injured Peripheral Nerve Remyelinate Regenerating Axons
OECs prepared from olfactory bulb and transplanted into transected peripheral nerves remyelinate the regenerated axons [2]. The transplanted OECs, shown in green, aligned longitudinally with the regenerated axons (Figure 4(a)). The sciatic nerve crush model (axonotmesis) used completely transects all axons within the nerve. The images for Figure 4 were obtained several millimeters distal to the crush site indicating that regenerated axon were remyelinated by the transplanted OECs. Images of the sciatic nerve at 5 weeks post-OEC transplantation demonstrated an abundance of eGFP-OECs distributing along the injured nerve (Figure 4(a) with inset in (a)). eGFP can be seen in the nuclei and cytoplasm around regenerating peripheral nerve axons. The transplanted eGFP-OECs are longitudinally oriented and associated with neurofilament- (NF-) stained axons (inset Figure 4(a)). Longitudinal sections of a group of regenerated axons demonstrated GFP-OECs surrounding the axon (Figure 4(b)), and importantly, the remyelinated axons have nodes of Ranvier (Figure 4(b)) with appropriate sodium channel Nav1.6 expression (Figure 4(c)) flanked by the Caspr immunostained paranodes (Figure 4(d); overlay is shown in Figure 4(e)).
Figure 4.
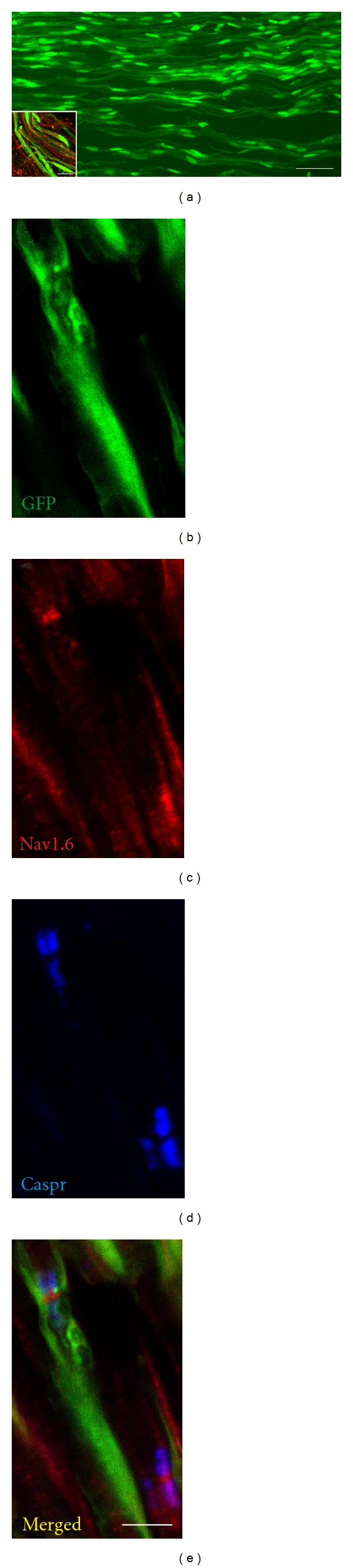
OECs transplanted into injured peripheral nerve remyelinate regenerating axons. (a) GFP can be seen in the nuclei and cytoplasm around regenerating peripheral nerve axons. The inset indicates that the GFP elements are longitudinally oriented with neurofilament- (NF-) stained regenerated axons. (b–e) Longitudinal section of a group of regenerated axons with GFP cells (b) surrounding the axons and sodium channel Nav1.6 (c) being flanked by the Caspr immunostained paranodes (d) indicating that the transplanted OECs can remyelinate regenerated axons which form appropriate sodium channels at the newly formed nodes of Ranvier (overlay in (e)). Scale bars: (a) = 50 μm (a), (b–e), and (a) inset in (a) = 5 μm.
4. Discussion
Here we demonstrate that OECs in the outer nerve layer of the olfactory bulb express CNPase, the universal marker for myelinating cells. CNPase expression in OECs is maintained in highly purified cultures and OECs transplanted into injured peripheral nerves remyelinate regenerated nerve fibers. A difficulty in comparing results regarding the remyelinating potential from OEC transplantation studies from various laboratories is that differences are present in the age of the animals used for cell harvesting, purification procedures, and lesion models into which the cells were transplanted. OECs used in the present study were prepared relatively acutely from the outer nerve layer of the adult olfactory bulb; a CNS area rich in OECs in vivo [18]. The degree of cell purity (>95%) in our cell suspension as assessed using p75NGFR/S100 immunostaining was about the same as in other studies where immunopanning techniques were used [14, 31] or where OECs were prepared from embryonic tissue [32]. Mitotic inhibitors and stimulators of cell proliferation and differentiation were used in those studies. In our cell preparation method from adult tissue, we did not use mitotic inhibitors nor did we stimulate proliferation and differentiation in vivo. Contamination by SCs, which are also p75/S100 positive, in our cultures would be problematic in the interpretation that adult OECs are able to form peripheral-like myelin. However, one would expect at best a very minor contamination of SCs possibly associated with blood vessel innervation [14] or meningeal cells [32]. Such minor contamination could not account for the vast majority (>95%) of our cells displaying a p75NGFR/S100+ phenotype in relatively acute cell suspensions.
Using transgenic mice which express GFP only in cells which express CNPase, we were able to show that OECs from the outer nerve layer of the olfactory bulb express CNPase and that OEC preparations isolated from this tissue using our isolation methods also express CNPase. This expression is an important prerequisite to demonstrate the myelination potential of OECs.
Transplantation of OECs prepared from adult olfactory bulb into various traumatic spinal cord injury and nerve injury models have demonstrated improved functional recovery. Histologically, axonal regeneration, remyelination, and neuroprotection have been reported following OEC transplantation [33]. However, Li et al. (2007) report that while OECs remyelinated regenerated spinal cord axons that they did not remyelinated regenerated optic nerve axons [34], while Schwann cells did remyelinate some optic nerve axons. Reason for this difference is uncertain. We demonstrated that the migration properties of OECs and SCs are different in the X-irradiated spinal cord: OECs migrate extensively in both gray and white matter and SCs do not [35]. Indeed, a number of unique properties have been described for OECs to distinguish them from SCs [36]. While several groups point out unique properties following in vivo transplantation of OECs as compared to SCs such as the formation of cellular tunnels which provide a permissive environment for axonal regeneration and greater mobility in astrocytic regions [36] others suggest that the functional benefits of OEC transplantation may result from recruitment of endogenous SCs by the OECs [13, 19].
Currently, a number of clinical studies are underway exploring the potential clinical utility of OEC transplantation in spinal cord injury patients [37–41]. Better understanding of the ability of transplanted OECs to improve functional outcome and direct comparison to transplanted SCs in CNS injury will have an impact on the direction of future research directed toward clinical applications.
5. Conclusion
The results of this study indicate that OECs in the outer nerve layer of the olfactory bulb express CNPase, a universal marker for myelinating cells. CNPase expression in OECs is maintained in highly purified cultures and colocalizes in cells with p75 receptor expression. Moreover, in culture the OECs maintain strong eGFP-CNPase expression. OECs transplanted into injured peripheral nerves remyelinate regenerated nerve fibers which formed nodes of Ranvier with high density of sodium channels between the myelin segments formed by the transplanted OECs. These data demonstrate that OECs share the molecular machinery of CNPase expression with oligodendrocytes and Schwann cells indicating a third dominant myelinating cell type within the nervous system. These results encourage ongoing work with OECs as a therapeutic tool in peripheral nerve repair, in CNS trauma and demyelinating diseases.
Acknowledgments
This work was supported in part by the Medical and Research and Development Services of the Department of Veterans Affairs, the NIH, the National Multiple Sclerosis, Society and the German Research Foundation (FOR 1103: Ra 1901/1-1). The authors thank Heather Mallozzi and Margaret Borelli for excellent technical assistance.
Abbreviations
- CNPase:
2′-3′-cyclic nucleotide 3′-phosphodiesterase
- CNS:
Central nervous system
- DMEM:
Dulbecco's modified medium
- eGFP:
Enhanced green fluorescent protein
- FCS:
Fetal calf serum
- FITC:
Fluorescein isothiocyanate
- Nav1.6:
Sodium channel subtype 1.6
- NF:
Neurofilament
- OECs:
Olfactory ensheathing cells
- ONL:
Outer nerve layer
- OPC:
Oligodendrocyte precursor cells
- PBS:
Phosphate-buffered saline
- p75NGFR:
P75 nerve growth factor receptor
- PNS:
Peripheral nervous system.
References
- 1.Dombrowski MA, Sasaki M, Lankford KL, Kocsis JD, Radtke C. Myelination and nodal formation of regenerated peripheral nerve fibers following transplantation of acutely prepared olfactory ensheathing cells. Brain Research. 2006;1125(1):1–8. doi: 10.1016/j.brainres.2006.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radtke C, Aizer AA, Agulian SK, Lankford KL, Vogt PM, Kocsis JD. Transplantation of olfactory ensheathing cells enhances peripheral nerve regeneration after microsurgical nerve repair. Brain Research. 2009;1254(13):10–17. doi: 10.1016/j.brainres.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 3.Imaizumi T, Lankford KL, Kocsis JD. Transplantation of olfactory ensheathing cells or Schwann cells restores rapid and secure conduction across the transected spinal cord. Brain Research. 2000;854(1-2):70–78. doi: 10.1016/s0006-8993(99)02285-4. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Field PM, Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science. 1997;277(5334):2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Field PM, Raisman G. Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells. Journal of Neuroscience. 1998;18(24):10514–10524. doi: 10.1523/JNEUROSCI.18-24-10514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imaizumi T, Lankford KL, Burton WV, Fodor WL, Kocsis JD. Xenotransplantation of transgenic pig olfactory ensheathing cells promotes axonal regeneration in rat spinal cord. Nature Biotechnology. 2000;18(9):949–953. doi: 10.1038/79432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramón-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. Journal of Neuroscience. 1998;18(10):3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramón-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25(2):425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- 9.Keyvan-Fouladi N, Raisman G, Li Y. Functional repair of the corticospinal tract by delayed transplantation of olfactory ensheathing cells in adult rats. Journal of Neuroscience. 2003;23(28):9428–9434. doi: 10.1523/JNEUROSCI.23-28-09428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Féron F, Mackay-Sim A, Waite PME. Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain. 2002;125(1):14–21. doi: 10.1093/brain/awf014. [DOI] [PubMed] [Google Scholar]
- 11.Franklin RJM, Gilson JM, Franceschini IA, Barnett SC. Schwann cell-like myelination following transplantation of an olfactory bulb-ensheathing cell line into areas of demyelination in the adult CNS. Glia. 1996;17(3):217–224. doi: 10.1002/(SICI)1098-1136(199607)17:3<217::AID-GLIA4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 12.Plant GW, Christensen CL, Oudega M, Bunge MB. Delayed transplantation of olfactory ensheathing glia promotes sparing/regeneration of supraspinal axons in the contused adult rat spinal cord. Journal of Neurotrauma. 2003;20(1):1–16. doi: 10.1089/08977150360517146. [DOI] [PubMed] [Google Scholar]
- 13.Boyd JG, Lee J, Skihar V, Doucette R, Kawaja MD. LacZ-expressing olfactory ensheathing cells do not associate with myelinated axons after implantation into the compressed spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(7):2162–2166. doi: 10.1073/pnas.0303842101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takami T, Oudega M, Bates ML, Wood PM, Kleitman N, Bunge MB. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. Journal of Neuroscience. 2002;22(15):6670–6681. doi: 10.1523/JNEUROSCI.22-15-06670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imaizumi T, Lankford KL, Waxman SG, Greer CA, Kocsis JD. Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in the demyelinated dorsal columns of the rat spinal cord. Journal of Neuroscience. 1998;18(16):6176–6185. doi: 10.1523/JNEUROSCI.18-16-06176.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki M, Black JA, Lankford KL, Tokuno HA, Waxman SG, Kocsis JD. Molecular reconstruction of nodes of Ranvier after remyelination by transplanted olfactory ensheathing cells in the demyelinated spinal cord. Journal of Neuroscience. 2006;26(6):1803–1812. doi: 10.1523/JNEUROSCI.3611-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akiyama Y, Lankford KL, Radtke C, Greer CA, Kocsis JD. Remyelination of spinal cord axons by olfactory ensheathing cells and Schwann cells derived from a transgenic rat expressing alkaline phosphatase marker gene. Neuron Glia Biology. 2004;1(1):47–55. doi: 10.1017/S1740925X04000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato T, Honmou O, Uede T, Hashi K, Kocsis JD. Transplantation of human olfactory ensheathing cells elicits remyelination of demyelinated rat spinal cord. Glia. 2000;30(3):209–218. doi: 10.1002/(sici)1098-1136(200005)30:3<209::aid-glia1>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyd JG, Doucette R, Kawaja MD. Defining the role of olfactory ensheathing cells in facilitating axon remyelination following damage to the spinal cord. FASEB Journal. 2005;19(7):694–703. doi: 10.1096/fj.04-2833rev. [DOI] [PubMed] [Google Scholar]
- 20.Boyd JG, Jahed A, McDonald TG, et al. Proteomic evaluation reveals that olfactory ensheathing cells but not schwann cells express calponin. Glia. 2006;53(4):434–440. doi: 10.1002/glia.20299. [DOI] [PubMed] [Google Scholar]
- 21.Yuan X, Chittajallu R, Belachew S, Anderson S, McBain CJ, Gallo V. Expression of the green fluorescent protein in the oligodendrocyte lineage: a transgenic mouse for developmental and physiological studies. Journal of Neuroscience Research. 2002;70(4):529–545. doi: 10.1002/jnr.10368. [DOI] [PubMed] [Google Scholar]
- 22.Gravel M, Peterson J, Yong VW, Kottis V, Trapp B, Braun PE. Overexpression of 2’,3’-cyclic nucleotide 3’-phosphodiesterase in transgenic mice alters oligodendrocyte development and produces aberrant myelination. Molecular and Cellular Neurosciences. 1996;7(6):453–466. doi: 10.1006/mcne.1996.0033. [DOI] [PubMed] [Google Scholar]
- 23.Yin X, Peterson J, Gravel M, Braun PE, Trapp BD. CNP overexpression induces aberrant oligodendrocyte membranes and inhibits MBP accumulation and myelin compaction. Journal of Neuroscience Research. 1997;50(2):238–247. doi: 10.1002/(SICI)1097-4547(19971015)50:2<238::AID-JNR12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Santos-Silva A, Cavalcante LA. Expression of the non-compact myelin protein 2’,3’-cyclic nucleotide 3’-phosphodiesterase (CNPase) in olfactory bulb ensheathing glia from explant cultures. Neuroscience Research. 2001;40(2):189–193. doi: 10.1016/s0168-0102(01)00204-8. [DOI] [PubMed] [Google Scholar]
- 25.Pixley SK. Characterization of olfactory receptor neurons and other cell types in dissociated rat olfactory cell cultures. International Journal of Developmental Neuroscience. 1996;14(7-8):823–839. doi: 10.1016/s0736-5748(96)00057-3. [DOI] [PubMed] [Google Scholar]
- 26.Gomes SS, Carvalho SL, Santiago MF, Lopez LB, Barradas PC, Cavalcante LA. Expression of 2’,3’-cyclic nucleotide 3’-phosphodiesterase (CNPase) in the developing olfactory bulb and subventricular zone rostral extension. Journal of Neuroscience Research. 2003;73(4):471–480. doi: 10.1002/jnr.10678. [DOI] [PubMed] [Google Scholar]
- 27.Yuan X, Chittajallu R, Belachew S, Anderson S, McBain CJ, Gallo V. Expression of the green fluorescent protein in the oligodendrocyte lineage: a transgenic mouse for developmental and physiological studies. Journal of Neuroscience Research. 2002;70(4):529–545. doi: 10.1002/jnr.10368. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki M, Lankford KL, Zemedkun M, Kocsis JD. Identified olfactory ensheathing cells transplanted into the transected dorsal funiculus bridge the lesion and form myelin. Journal of Neuroscience. 2004;24(39):8485–8493. doi: 10.1523/JNEUROSCI.1998-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki M, Hains BC, Lankford KL, Waxman SG, Kocsis JD. Protection of corticospinal tract neurons after dorsal spinal cord transection and engraftment of olfactory ensheathing cells. Glia. 2006;53(4):352–359. doi: 10.1002/glia.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hildebrand C, Kocsis JD, Berglund S, Waxman SG. Myelin sheath remodelling in regenerated rat sciatic nerve. Brain Research. 1985;358(1-2):163–170. doi: 10.1016/0006-8993(85)90960-6. [DOI] [PubMed] [Google Scholar]
- 31.Devon R, Doucette R. Olfactory ensheathing cells myelinate dorsal root ganglion neurites. Brain Research. 1992;589(1):175–179. doi: 10.1016/0006-8993(92)91182-e. [DOI] [PubMed] [Google Scholar]
- 32.Lakatos A, Smith PM, Barnett SC, Franklin RJM. Meningeal cells enhance limited CNS remyelination by transplanted olfactory ensheathing cells. Brain. 2003;126(3):598–609. doi: 10.1093/brain/awg055. [DOI] [PubMed] [Google Scholar]
- 33.Radtke C, Sasaki M, Lankford KL, Vogt PM, Kocsis JD. Potential of olfactory ensheathing cells for cell-based therapy in spinal cord injury. Journal of Rehabilitation Research and Development. 2008;45(1):141–152. doi: 10.1682/jrrd.2007.03.0049. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Li D, Raisman G. Transplanted Schwann cells, not olfactory ensheathing cells, myelinate optic nerve fibres. Glia. 2007;55(3):312–316. doi: 10.1002/glia.20458. [DOI] [PubMed] [Google Scholar]
- 35.Lankford KL, Sasaki M, Radtke C, Kocsis JD. Olfactory ensheathing cells exhibit unique migratory, phagocytic, and myelinating properties in the X-irradiated spinal cord not shared by Schwann cells. Glia. 2008;56(15):1664–1678. doi: 10.1002/glia.20718. [DOI] [PubMed] [Google Scholar]
- 36.Kocsis JD, Lankford KL, Sasaki M, Radtke C. Unique in vivo properties of olfactory ensheathing cells that may contribute to neural repair and protection following spinal cord injury. Neuroscience Letters. 2009;456(3):137–142. doi: 10.1016/j.neulet.2008.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H, Chen L, Wang H, et al. Safety of fetal olfactory ensheathing cell transplantation in patients with chronic spinal cord injury. A 38-month follow-up with MRI. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(4):439–443. [PubMed] [Google Scholar]
- 38.Féron F, Perry C, Cochrane J, et al. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128(12):2951–2960. doi: 10.1093/brain/awh657. [DOI] [PubMed] [Google Scholar]
- 39.Dobkin BH, Curt A, Guest J. Cellular transplants in China: observational study from the largest human experiment in chronic spinal cord injury. Neurorehabilitation and Neural Repair. 2006;20(1):5–13. doi: 10.1177/1545968305284675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lima C, Pratas-Vital J, Escada P, Hasse-Ferreira A, Capucho C, Peduzzi JD. Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study. Journal of Spinal Cord Medicine. 2006;29(3):191–204. doi: 10.1080/10790268.2006.11753874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senior K. Olfactory ensheathing cells to be used in spinal-cord repair trial. Lancet Neurol. 2002;1(5):p. 269. doi: 10.1016/s1474-4422(02)00145-x. [DOI] [PubMed] [Google Scholar]


