Abstract
Characterization of glutamatergic input to dorsal raphe (DR) serotonin (5-HT) neurons is crucial for understanding how the glutamate and 5-HT systems interact in psychiatric disorders. Markers of glutamatergic terminals, vGlut1, 2, and 3, reflect inputs from specific forebrain and midbrain regions. Punctate staining of vGlut2 was homogenous throughout the mouse DR whereas vGlut1 and vGlut3 puncta were less dense in lateral wing (lwDR) compared to the ventromedial (vmDR) subregion. The distribution of glutamate terminals was consistent with the lower mEPSC frequency found in the lwDR; however, it was not predictive of glutamatergic synaptic input with local activity intact, as sEPSC frequency was higher in the lwDR. We examined the morphology of recorded cells to determine if variations in dendrite structure contributed to differences in synaptic input. Although lwDR neuronshad longer, more complex dendrites than vmDR neurons, glutamatergic input was not correlated with dendrite length in the lwDR, suggesting that dendrite length did not contribute to subregional differences in sEPSC frequency. Overall, glutamatergic input in the DR was the result of selective innervation of subpopulations of 5-HT neurons and was rooted in the topography of DR neurons and the activity of glutamate neurons located within the midbrain slice. Increased glutamatergic input to lwDR cells likely synergizes with previously reported increased intrinsic excitability of lwDR cells to increase 5-HT output in lwDR target regions. Because the vmDR and lwDR are involved in unique circuits, subregional differences in glutamate modulation likely result in diverse effects on 5-HT output in stress-related psychopathology.
Keywords: excitatory postsynaptic current, immunohistochemistry, lateral wing, ventromedial, whole-cell recording
Introduction
Dysregulation of glutamate neurotransmission and its effect on the serotonin (5-HT) system likely underlie a wide range of psychiatric disorders. Glutamate has an important influence over dorsal raphe (DR) 5-HT activity (Pan and Williams, 1989; Adell et al., 2002) and modulates 5-HT output to target brain regions (Tao and Auerbach, 1996; Tao et al., 1997; Tao and Auerbach, 2000; Celada et al., 2001; Tao and Auerbach, 2003). While the 5-HT-cell body dense ventromedial subregion of the DR (vmDR) is a common target of studies of 5-HT neurons, there are seldom explicit descriptions of the lateral wing subregion (lwDR). Emerging evidence suggests that, compared to 5-HT cells of the vmDR, lwDR 5-HT cells are distinct in their afferent input, efferent projections, and neurochemical content (reviewed in Abrams et al., 2004; Johnson et al., 2004; Michelson et al., 2007; Lowry et al., 2008). In addition, the intrinsic excitability of lwDR 5-HT neurons may make them more likely to be activated by a stressor (Crawford et al., 2010). Though our understanding of the influence of glutamate on the 5-HT systems is incomplete, we hypothesized that that there is differential glutamatergic input to various subfields of the raphe.
The three isoforms of the vesicular glutamate transporter protein (vGlut) have been used as presynaptic markers of glutamate input originating from distinct brain regions: vGlut1 is associated with input from the medial prefrontal cortex and other cortical regions (reviewed in Fremeau et al., 2004; Takamori, 2006) while vGlut2 marks projections from subcortical regions, including midbrain regions adjacent to the raphe (Hioki et al., 2010) and the lateral habenula (Barroso-Chinea et al., 2007). The third marker, vGlut3, is expressed in the soma and axon terminals of neurons that typically release other types of neurotransmitters, including 5-HT neurons (Fremeau et al., 2002; Gras et al., 2002; Fremeau et al., 2004a; Herzog et al., 2004; Gras et al., 2002; Hioki et al., 2004; Shutoh et al., 2008; Commons, 2009; Jackson et al., 2009; Hioki et al., 2010; Mintz and Scott, 2006; Schafer et al., 2002; Amilhon et al., 2010). It is unknown whether the distribution of vGlut-labeled terminals across raphe subregions serves as a useful proxy for relative glutamatergic synaptic input to 5-HT neurons.
Input to vmDR 5-HT neurons mediated by AMPA/kainate glutamate receptors has been demonstrated in the slice preparation in vitro (Liu et al., 2002; Haj-Dahmane and Shen, 2005; Lemos et al., 2006; Kirby et al., 2007; Haj-Dahmane and Shen, 2009). However, to our knowledge, glutamatergic synaptic input to lwDR neurons has never been characterized. Thus, we revisited vGlut immunohistochemistry with specific attention to the vmDR and lwDR subregions. We then investigated whether the distribution of glutamate terminals in the vmDR and lwDR would predict the levels of glutamatergic synaptic input to 5-HT cells using whole cell electrophysiology. Finally we characterized morphological and topographical factors of recorded neurons to determine whether they contribute to subregional variations in glutamatergic modulation.
Materials and Methods
Animals
Adult male 5-HT-YFP mice or their wild-type littermates were used at 2–4 months of age. These mice contain 5-HT neurons that express yellow fluorescent protein (YFP) under the control of the 5-HT specific Pet-1 promoter (Scott et al., 2005). The membrane characteristics of YFP-labeled 5-HT neurons did not differ from wild type 5-HT neurons (Crawford et al., 2010). The mice were on a background containing predominantly C57Bl/6. Mice were housed in a standard animal facility with lights on 06:00 to 18:00. Animal protocols were approved by the Institutional Animal Care and Use Committee and were conducted in accordance to the NIH Guide for the Care and Use of Laboratory Animals.
Immunohistochemistry
Adult male wild type mice were euthanized by pentobarbital via intraperitoneal injection, followed by transcardial perfusion of 20 ml 0.9% saline then 50–60 ml of 4% paraformaldehyde in phosphate buffer (PB), pH 7.4. Brains were postfixed in the same solution for 2 hours. Brains were then sunk for several days in 30% sucrose in phosphate buffer containing 0.1% (w/v) azide, frozen in dry ice and cut into 30 μm-thick floating sections on a cryostat. Sections were washed in phosphate buffered saline (PBS) and blocked in a PBS blocking solution containing 0.03% (v/v) Triton-X, 0.04% (w/v) bovine serum albumin (BSA), and 0.1% (w/v) azide. Tissue was then incubated in primary antibody diluted in the blocking solution overnight at room temperature. See Table 1 for primary antibodies used in this study. Tissue was then washed in PBS containing 0.03% (v/v) Triton-X, 0.04% (w/v) bovine serum albumin (BSA), then incubated for 2 hours in fluorescent secondary antibody diluted in the same solution. Secondary antibodies used included goat anti-guinea pig Alexa Fluor 647 (1:200, Invitrogen) and donkey anti-mouse Alexa Fluor 488 (1:200, Invitrogen, Carlsbad, CA). Finally, sections were washed in PBS, mounted in 0.05M PB, air dried in the dark, and coverslipped with Fluormount-G (Southern Biotech, Birmingham, AL) or ProLong Antifade (Invitrogen) coverslipping medium. Dual immunohistochemistry was performed sequentially where vGlut staining was followed by TPH staining. Control experiments that omitted primary antibody yielded no staining (data not shown). Images were captured using a Leica DMR fluorescent microscope (Leica Microsystems, Bannockburn, IL, 20X objective, NA 0.5) and OpenLab 3.0.9 software (Improvision, Lexington, MA). Confocal images of 30 μm slices were obtained on an Olympus Fluoview FV1000 confocal microscope(Olympus, Center Valley, PA) using Fluoview confocal software (FV10-ASW v1.7, Olympus). Images were pseudocolored and optimized by adjusting levels using Adobe Photoshop 6.0 (Adobe Systems Incorporated; San Jose, CA).
Table 1.
Antibodies used in this study
| Primary Antibody | Source | Host Species | Immunogen | Dilution | Specificity Reference | |
|---|---|---|---|---|---|---|
| TPH | Monoclonal Anti-Tryptophan Hydroxylase | Sigma, # T0678 | Mouse | Recombinant rabbit tryptophan hydroxylase. | 1:500 | Rosin D et al. J Comp Neurol 2006 Nov; 499(1):64–89 |
| vGlut1 | Anti-Vesicular Glutamate Transporter 1 (VGLUT1) | Millipore, # AB5905 | Guinea Pig | Synthetic peptide from rat VGLUT1 protein with no overlap to VGLUT2. | 1:1000 | Hilgen G et al., J Comp Neurol. 2011 Feb 15;519(3):433–50. |
| vGlut2 | Anti-Vesicular Glutamate Transporter 2 (VGLUT2) | Millipore, # AB5907 | Guinea Pig | Synthetic peptide from rat VGLUT2 protein. | 1:1000 | Yasaka T et al., Pain. 2010 Nov;151(2):475–88. |
| vGlut3 | Anti-Vesicular Glutamate Transporter 3 (VGLUT3) | Millipore, # AB5421 | Guinea Pig | Synthetic peptide from rat VGLUT3 protein. | 1:1000 | Johnson J et al., J Comp Neurol. 2004 Sep 27;477(4):386–98. |
Immunohistochemical identification of each neuronrecorded in patch clamp configuration was completed as previously described (Beck et al., 2004; Lemos et al., 2006; Kirby et al., 2008). In brief, a standard immunohistochemistry protocol was used on 200 μm-thick slices using mouse anti-TPH (1:200, Sigma) along with secondary donkey anti-mouse Alexa Fluor 488 (1:200, Invitrogen) and streptavidin-conjugated Pacific Blue (1:100, Invitrogen). TPH labeling of biocytin filled cells was imaged on a Leica DMR fluorescent microscope (Leica Microsystems) using OpenLab 3.0.9 software (Improvision) and confirmed on a Leica DMIRE2 confocal microscope (Leica Microsystems, Bannockburn, IL) using Leica confocal software (version 2.5, Leica Microsystems). The dendritic morphology of filled neurons was analyzed as described below.
Whole-Cell Electrophysiology
Electrophysiology recordings were conducted as previously described (Beck et al., 2004; Lemos et al., 2006; Crawford et al., 2010). In brief, 5-HT-YFP mice were sacrificed using decapitation. The midbrain was rapidly dissected and blocked while submerged in cold artificial cerebral spinal fluid (aCSF) solution where NaCl was replaced with sucrose (248mM), and cut with a Leica VT1000s vibratome (Leica Microsystems, Bannockburn, IL) to generate 200 μm thick brain slices. Slices were maintained in a holding chamber containing aCSF and bubbled with 95%O2/5%CO2 at 36°C for 1 hour and then at room temperature until used. Tryptophan (5 μm) was included in the holding chamber to maintain 5-HT synthesis prior to recording. This concentration of tryptophan is similar to that found in cerebrospinal fluid (Chaouloff, 1986).
Individual slices were then placed in a recording chamber and continuously perfused with 32–34°C aCSF solution bubbled with 95%O2/5%CO2 with a solution flow rate of 1.5–2.0ml/min. The composition of the aCSF was (in mM): 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2.0 MgSO4, 2.5 CaCl2, 10 dextrose, and 26 NaHCO3. Neurons were visualized using Nikon E600 (Optical Apparatus, Ardmore, PA) upright microscope fitted with a 40x water-immersion objective, DIC, and infrared filter. Ventromedial (vmDR) and lateral wing (lwDR) 5-HT neurons were targeted based on topographical expression of YFP and later confirmed using immunohistochemical detection of the synthetic enzyme marker TPH. To visualize YFP-positive 5-HT cells for recording, a fluorescent lamp and yellow-fluorescence filter were used. The image was generated on a computer monitor using a CCD camera and Nikon Elements software (Optical Apparatus). Whole-cell recording pipettes fabricated on a Sutter Instruments pipette puller (P-97, Sutter Instrument, Novato, CA) had a resistance of 6–10MΩ when filled with an intracellular solution of 130mM Kgluconate, 5mM NaCl, 10mM Na-phosphocreatinine, 1mM MgCl2, 0.02mM EGTA, 10mM HEPES, 2mM MgATP, 0.5mM Na2GTP, and 0.1% biocytin (pH 7.3). Voltage clamp recordings were conducted at a holding potential of −60mV such that spontaneous excitatory postsynaptic currents (sEPSCs) were downward and spontaneous inhibitory postsynaptic currents were upward; only downward events were analyzed. In experiments where the AMPA-kainate receptor blocker DNQX (20 μm) was added at the end of the recordings, all downward events were abolished (data not shown). Recordings were collected online with a Multiclamp 700B amplifier, Digidata 1320 A/D converter, and Clampex 9.0 software (Molecular Devices, Union City, CA). Membrane properties of the cell were monitored as was the access resistance of the patch pipette during recordings that lasted a minimum of 20 minutes. In a separate experiment miniature EPSCs (mEPSCs) were measured in the presence of the action-potential (AP) blocker tetrodotoxin (TTX, 1 μm) after a wash-in period that lasted a minimum of 5 minutes. Recordings were discarded if access resistance increased over 5x the initial access resistance noted prior to patching onto the cell. Any cells that showed physiological signs of instability, baseline holding currents below - 30pA, or resting membrane potential above −50mV were excluded from data analysis. Reported values do not incorporate a junction potential of approximately +15mV, as calculated using Clampex software. Following the experiment, DR slices were fixed for 2–3 hours with 4% paraformaldehyde and processed for immunohistochemistry.
Electrophysiology Data Analysis
Current clamp recordings using visualized whole cell patch clamp techniques were analyzed using Clampfit 9.0 (Molecular Devices). Resting membrane potential (RMP) and membrane resistance were measured as previously described (Beck et al., 2004; Crawford et al., 2010). Membrane input resistance was determined from the slope of voltage-current plot generated using current pulses ranging from −100 to 0pA. The time constant tau was determined for each cell by fitting a single exponential function to the first 200ms of the membrane potential response to a −20pA hyperpolarizing input current step. MiniAnalysis (Synaptosoft; Decatur, GA) was used to analyze EPSC data as has been reported in the past (Lemos et al., 2006). The parameters for synaptic event analysis were optimized for each cell with the threshold set beyond the maximum values of the all-points noise histogram for a segment of the trace containing no detectable synaptic events. The threshold generally ranged from 5 to 8pA. The MiniAnalysis program provides a summary table for each cell containing values for mean and median frequency, amplitude, rise time (10–90%), decay time (50%) and event half-width. For each cell, 200 randomly chosen events were manually filtered to exclude multiple peaks and combined to obtain an averaged EPSC for each cell. The decay tau reported was the tau obtained from the single exponential fit of the 10–90% decay of the averaged EPSC. The area-under-curve of the averaged EPSC was the charge per EPSC event; this value was multiplied by the frequency to obtain mean phasic current. For each parameter, group averages were obtained by compiling the mean and/or median values for each cell. Histograms and cumulative probability plots describing all events from all cells in each group were compared using the Kolmogorov-Smirnov (K-S) test. To determine differences in the populations of events recorded in the 2 subregions, OriginPro 8.1 software (Origin Lab Corporation, Northampton, MA) was used to generate Gaussian fits of event histograms using the Peak Analyzer function. The fit was generated by choosing 1–5 peaks in order to minimize the reduced Chi squared value.
Neuron Morphology Analysis
Biocytin-filled neurons filled during whole-cell patch clamp recording were processed for immunohistochemical detection as described above. Tissue slices were imaged on a Leica DMR fluorescent microscope to confirm the subregional location of the neuron and TPH content. Cells were then imaged on a Leica DMIRE2 confocal microscope (Leica Microsystems) using Leica confocal software (version 2.5, Leica Microsystems). For confocal image stacks, slices were imaged using a 20x objective lens and optical z-slices of 0.8 μm thickness were taken to capture the entire extent of the dendritic tree. The confocal stacks were then analyzed using Neurolucida (v8 and v9, MBF Bioscience Inc., Williston, VT). The cell soma and dendrite were traced using the autoneuron feature and then manually edited if necessary to ensure accurate tracing. Branch order was assigned using centrifugal branch order. Neurolucida Explorer was used to obtain measurements of traced neurons. The neuron soma characteristics included enclosed volume, surface area, mean length, total cross-sectional area, and mean cross-sectional area. The dendrite parameters included the number of nodes, i.e. branch points, the number of ends, the total dendrite length and the mean dendrite length. In addition data were obtained for number of branches, total length, and mean length according to branch order. A dendrogram was generated for each cell to obtain the length of the longest segment and the longest soma-to-tip dendritic length. Sholl analysis was performed using radial shells at 20 μm intervals. Polar histograms with 30 degree bins were obtained for each cell to ascertain directional preferences for dendrite orientation in the medial-lateral and dorsal-ventral directions. Because morphology analysis was conducted in 200 μm-thick brain slices, some of the axons and dendrites of analyzed cells could have been severed. While this problem applies to all neurons, it could potentially lead to underestimation of true dendritic length, especially of longer dendrites. Despite these caveats, observed differences between vmDR and lwDR 5-HT neuron morphology were an important, relevant proxy for 5-HT neurons in the intact brain. It should be noted that recordings targeted neurons deep within the 200 μm-thick slice and obviously severed dendrites were uncommon in confocal images of filled neurons.
The relationship between the electrophysiological parameters and morphological characteristics of recorded neurons was examined using Pearson’s correlation test unless otherwise noted. To examine the functional significance of topographical location of recorded neurons, an atlas of recorded neurons was used to assign a pair of coordinates to every cell with the origin of the x-y coordinate system positioned at the base of the aqueduct. The x, y coordinate of any cell positioned on the left half of the brain slice was transformed to allow comparison with the majority of cells on the right side of the brain slice. Because neither sEPSC parameters nor morphology measurements demonstrated a relationship with rostral-caudal position (data not shown), cell position was collapsed across the rostral-caudal axis. The x, y coordinates were then analyzed along with electrophysiological and morphological data using statistical tests of correlation.
Statistics
Reported values are mean ± standard error of the mean (s.e.m.); P values were generated using Students t-test unless otherwise noted and P< 0.05 was deemed significant. Outlying values (3 standard deviations from the mean) were excluded from group means but are denoted in scatter plots. After exclusion of outliers, any data that were not normally distributed according to the D’Agostino and Pearson omnibus normality test were compared using non-parametric statistical tests, i.e., Mann-Whitney test or Spearman’s correlation test. Statistical analysis was performed using Prism 5 (GraphPad Software, La Jolla, CA) and OriginPro 8.1 (Origin Lab Corporation).
Drugs
All chemicals for making the sucrose aCSF and aCSF were purchased from Fisher Scientific (Pittsburgh, PA). Tetrodotoxin and all chemicals for the intracellular electrolyte solution were purchased from Sigma-Aldrich (St. Louis, MO). Bicuculline and DNQX were purchased from Tocris (Ellisville, MO).
Results
The distribution of vGlut proteins in the mouse DR varies between subregions
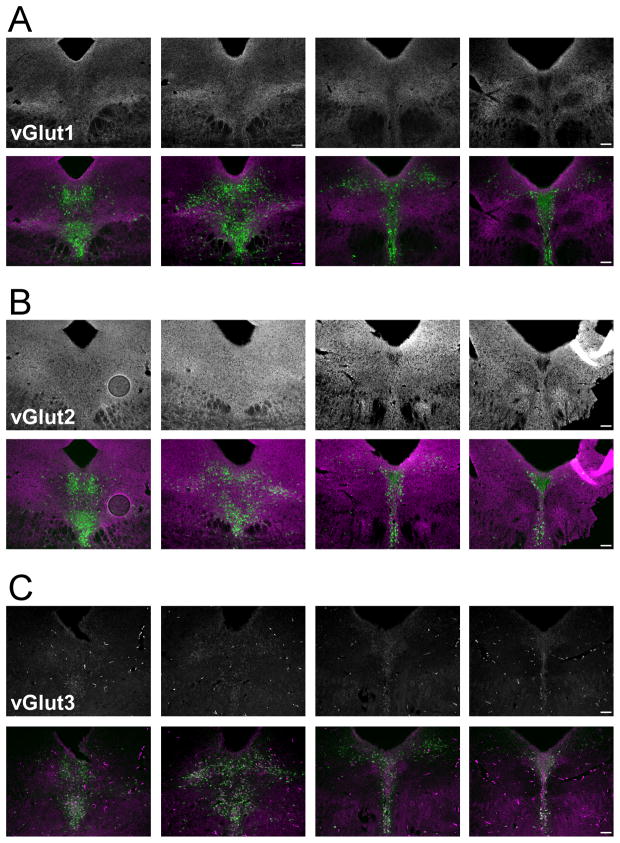
The distribution of vesicular glutamate transporter proteins 1, 2, 3 (vGlut1, 2, and 3) was examined in the DR of wild type mice. Dual immunohistochemistry was conducted to enable the use of the distribution of TPH neurons as an indicator of DR subregions and rostral-caudal level. Immunostaining for vGlut1 revealed punctate staining throughout every subregion of the DR, though an increased density of the punctate staining was present in bilateral regions of the ventral lwDR, just dorsal to the major longitudinal fasciculus (Fig. 1A). While this region of increased vGlut1 puncta partially overlapped with TPH immunopositive 5-HT neurons, the majority of 5-HT neurons were just dorsal to this region (Fig. 1A). Higher magnification confocal images demonstrated that despite the increased density in the ventral lwDR, the puncta surrounding the cluster of 5-HT cell bodies was actually lower in density than that seen surrounding cell bodies in the vmDR (Fig. 2A). Because reports of vGlut1 staining in the rat described homogenous puncta throughout the DR (Commons et al., 2005; Waselus and Van Bockstaele, 2007) this may represent a species difference.
Figure 1.
Immunostaining of vGlut proteins in the mouse DR. Fluorescent microscopy was used to generate the top panels showing the vGlut protein and the bottom panels shows the overlay of vGlut (magenta) and TPH, a marker of 5-HT neurons (green). A) Immunohistochemistry revealed the punctate pattern of vGlut1 staining through the rostral-caudal axis of the DR. Note the increased density of vGlut1 staining in the lwDR, ventral to the cluster of 5-HT neurons. B) The staining for vGlut2 puncta was dense and homogenous throughout the DR. C) Staining for vGlut3 was present in both puncta and in cell soma that were immunopositive for TPH. Images taken at 20x; see Figure 2 for confocal images at higher magnification. Scale bar 104.0 μm.
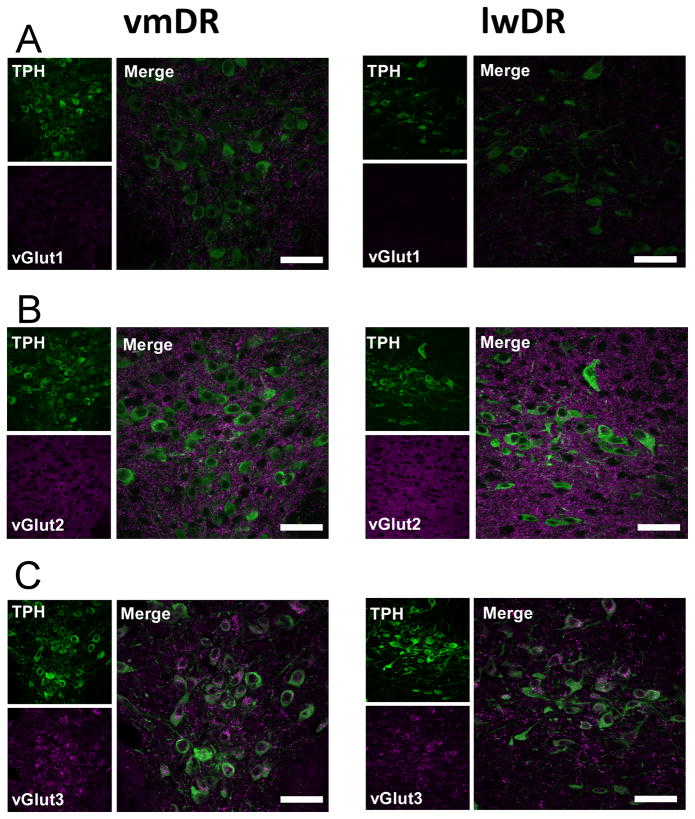
Figure 2.
Confocal images of vGlut proteins and TPH in the vmDR and lwDR subregions. High magnification (60x, voxel size = 0.621microns, 1 confocal plane) confocal images were taken to demonstrate the variations in punctate staining across the vmDR and lwDR subregions. In A, B, and C vGlut is shown in magenta, TPH in green. Panels to the left demonstrate staining in the vmDR while panels to the right show the lwDR. A) Confocal imaging confirms the lower density of vGlut1 punctate staining surrounding 5-HT neurons of the lwDR. B) Puncta expressing vGlut2 appeared to have the same density in the vmDR and the lwDR. C) Cell bodies and puncta expressing vGlut3 are shown. Note that several cell bodies also contained punctate staining. Comparison of the images of vGlut3 staining demonstrated that punctate staining of vGlut3 was slightly less dense in the lwDR than in the vmDR, though it is unclear if that was due to fewer terminals in the lwDR or due to fewer cell bodies containing punctate staining. Scale bar 42.4 μm.
Immunostaining of vGlut2 revealed homogeneous puncta that were much denser than vGlut1 puncta. Throughout all of the DR subregions, staining was present everywhere except the spaces occupied by cell bodies (Fig. 1B). Higher magnification confocal images revealed a clearer demonstration of the punctate nature of the staining (Fig. 2B). This was consistent with previous reports of vGlut2 staining in the rat (Commons et al., 2005; Waselus and Van Bockstaele, 2007).
Detection of vGlut3 revealed immunopositive cell bodies and punctate staining of presumed axon terminals throughout the neuropil (Fig. 1C). While the intensity of vGlut3 and TPH were not equal within every cell, the majority of the cell bodies containing vGlut3 also contained TPH in both the vmDR and lwDR. This was consistent with previous reports of vGlut3 in the mouse vmDR(Schafer et al., 2002; Amilhon et al., 2010)but differed from vGlut3 mRNA localization reported in the rat (Hioki et al., 2010). There were a few neurons that were singly stained for vGlut3 or TPH (data not shown). Puncta were less dense than vGlut1 or vGlut2 puncta and appeared less dense in the lwDR than in the vmDR (Fig. 2C). This was consistent with previous reports of punctate vGlut3 staining in the rat (Commons, 2009). Although colocalization of vGlut3 and 5-HT-labeled fibers in the supraependymal plexus just ventral to the aqueduct has been reported in the caudal DR of the rat, (Commons, 2009), this was not seen with our TPH immunostaining of the mouse.
Overall, the patterns of vGlut protein expression in the mouse resemble those seen in the rat. The degree of glutamatergic input to the lwDR may be different from that of the vmDR because vGlut3 terminals were lower in density in the lwDR and vGlut1 terminals were lower in the dorsal lwDR in regions surrounding 5-HT neurons. However, the distribution of vGlut-labeled puncta is limited in its ability to indicate the relative influence of glutamateon vmDR and lwDR 5-HT neurons. Therefore, electrophysiology was used to obtain another measurement of glutamatergic input to DR neurons.
Lateral wing 5-HT neurons exhibit increased frequency of glutamate input that is dependent on the activity of local glutamate neurons
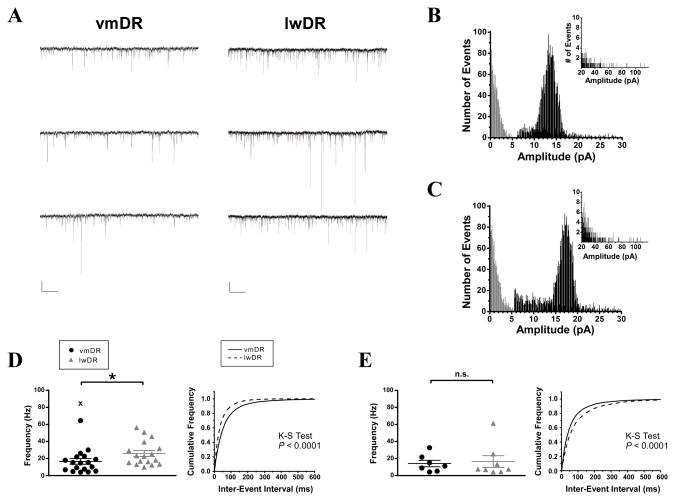
Glutamate input to 5-HT neurons mediated by AMPA-kainate receptors is present in the in vitro slice preparation (Liu et al., 2002; Haj-Dahmane and Shen, 2005; Lemos et al., 2006; Haj-Dahmane and Shen, 2009). However, as previous studies have primarily targeted the vmDR, it is unclear how local glutamatergic inputs may differ between the lwDR and vmDR subregions of the DR. Electrophysiology recordings were conducted in a total of 36 cells from 20 male 5-HT-YFP mice. Cells expressing YFP were targeted for recordings and later confirmed to be serotonergic using immunohistochemical detection of TPH. Outlying values occurring more than 3 standard deviations from the mean were identified. Because these cells may represent a distinct subpopulation, they were excluded from group means, but are presented in the scatter plots to demonstrate the heterogeneity of recordings in 5-HT neurons. In recordings of spontaneous excitatory postsynaptic current (sEPSC) activity in both subregions, the larger amplitudes were correlated with faster rise times (vmDR: R = −0.06, P=2.2E-16, lwDR: R = −0.04, P =7.9E-11, Spearman’s correlation test). This indicated that a small degree of electrotonic filtering was present in our voltage clamp recordings (Ling and Benardo, 1999).
Voltage clamp recordings of sEPSC were conducted in vmDR 5-HT neurons (n=19 neurons in 12 mice) and lwDR 5-HT neurons (n=17 neurons in 11 mice), revealing several striking differences (Table 2, PFigs. 3 and 4). The cumulative frequency histogram of all recorded events revealed an increased number of large-amplitude events in the lwDR compared to events in the vmDR (K-S test = 0.006). Figures 3B and C show the all events histogram for the cells in Figure 3A. Light gray area is noise. The insets provide a longer x-axis, demonstrating larger amplitude events.
Table 2.
Results of sEPSC recordings in DR 5-HT neurons
| Subregion | Frequency (Hz)* | Average Amplitude (pA) | Median Amplitude (pA) | Median Rise Time (ms) | Mean Phasic Current (pA) | Input Resistance (MΩ) (pA) |
|---|---|---|---|---|---|---|
| vmDR (n=19) | 16.7 ± 3.4 | 17.7 ± 1.4 | 13.5 ± 0.9 | 1.3 ± 0.03 | 1.4 ± 0.5 | 447.0 ± 45.1 |
| lwDR (n=17) | 25.9 ± 3.5 | 18.1 ± 0.7 | 14.2 ± 0.6 | 1.3 ± 0.03 | 1.1 ± 0.2 | 615.4 ± 69.5 |
Values are average ± s.e.m.
Mann-Whitney Test U = 86, P = 0.03
Figure 3.
Recordings of sEPSC events revealed increased frequency of glutamatergic synaptic input to lwDR 5-HT neurons, but not mEPSC activity. A) Raw data traces of sEPSC activity were obtained from representative vmDR (left trace) and lwDR (right trace) 5-HT neurons. Scale bar 512ms, 20pA. B) The all-events amplitude histogram shows the division between noise (in grey) and analyzed events (black) for the vmDR cell featured in A. The inset demonstrates large amplitude events using an enlarged scaling of the y-axis. C) The all-events amplitude histogram for the lwDR cell featured in A. In D-E, left panels demonstrate the average values obtained for each recorded cell, right panels demonstrate the cumulative probability plots of all recorded events. Outlying values denoted by the symbol “X” were not included in group means or cumulative probability plots but were indicated in scatter plots to demonstrate the heterogeneity of 5-HT neurons. D) The average frequency of sEPSC events was higher in lwDR neurons as shown in the left panel (U = 86, P= 0.03). The cumulative probability plots demonstrated a significant shift towards shorter inter-event intervals in the lwDR. E) There was no difference in average frequency of mEPSCs recorded in the lwDR (U = 25, P= 0.78). Cumulative probability plots of inter-event interval revealed that lwDR events were shifted towards longer inter-event intervals than vmDR events.
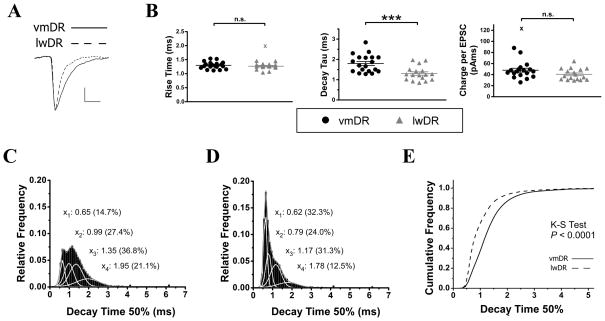
Figure 4.
sEPSC events recorded in lwDR 5-HT neurons demonstrate faster decay kinetics than events recorded in vmDR neurons. A) Enlarged averaged events show differences in decay kinetics between the two cells featured in Figure 3. Scale bar 3.0ms, 4.0pA. B) Summary scatter plots show group means of several sEPSC parameters. The rise time was comparable between vmDR (black) and lwDR (gray) neurons (t33=0.80, P=0.43). The decay tau obtained from an exponential fit of the averaged event (see Methods) was smaller in the lwDR, indicating faster decay kinetics (t34=3.73, P= 0.0007). The charge per sEPSC event was comparable between subregions (Mann Whitney U = 107, P = 0.13). C) The all-events histogram showing decay time-50% from all recorded events in vmDR neurons was analyzed using a Gaussian fit to obtain peak’s centers and percent area under the curve for each peak. D) The all-events histogram of decay time-50% of lwDR demonstrated a larger peak centered at 0.62ms and smaller peak centered at 1.78ms compared to the distribution of vmDR decay times. E) There was a significant leftward shift of lwDR decay times towards faster decay times. Outlying values aredenoted by the symbol “X”.
A monoexponential fit of the averaged postsynaptic event was generated for each cell, revealing a faster decay tau in lwDR neurons (1.3 ± 0.1ms) compared to vmDR neurons (1.8 ± 0.1ms, t34 = 3.73, P= 0.00007, Fig. 4B). This difference in kinetics was explored further by looking at the distribution of decay time-50% of all the events recorded in each subregion. The histogram revealed a population of fast-decaying events centered at a decay time-50% of 0.62ms that was larger in the lwDR (32.3% of all events, Fig. 4D) than in the vmDR (14.7% of all events, Fig. 4C). This resulted in a significant leftward shift of the lwDR cumulative frequency curve of decay time-50% (K-S test P< 0.0001, Fig. 4E). Surprisingly, despite presence of more narrow events, the glutamate-mediated phasic current to lwDR neurons remained comparable to that of vmDR neurons (1.3 ± 0.5pA, n=19 vmDR vs 1.1 ± 0.2pA, n=17 lwDR, Mann-Whitney U=126.0, P=0.27, Table 2). This was because the frequency of sEPSC events was significantly increased in lwDR neurons (25.9±3.5Hz, n=17) compared to vmDR neurons (16.7±3.4Hz, n=19, Mann-Whitney U=86.0, P=0.03, Fig. 3D).
To determine if differences in sEPSC frequency were due to the activity of local neurons within the slice, we asked whether the frequency of action potential (AP)-independent glutamatergic input differs between vmDR and lwDR neurons. Recordings of miniature EPSCs (mEPSCs) were conducted in the presence of the AP blocker TTX (1 μm). The average mEPSC frequency recorded in 5-HT neurons was comparable between the vmDR (14.2 ± 3.8Hz, n=7) and the lwDR (16.5 ± 6.9Hz, n=8, Mann-Whitney U=25.0, P=0.78, Fig. 3E). However, the cumulative probability plot of all recorded events revealed that the lwDR population of mEPSCs was shifted towards decreased frequency (i.e. larger inter-event intervals, K-S test, P< 0.0001, Fig. 3E). Thus, despite the higher spontaneous glutamatergic activity in the lwDR, basal, AP-independent activity was lower in the lwDR. This suggested that active glutamate neurons located within the midbrain slice contribute to the increased excitatory input to lwDR 5-HT cells.
Thus, despite the decreased density of glutamatergic puncta in the lwDR, lwDR 5-HT neurons receive more frequent glutamatergic synaptic events due to the activity of local glutamate neurons. However, we could not yet rule out the possibility that lwDR neurons had other properties that might help explain the differences in glutamatergic input. The striking difference between subregions and the variability within subregions suggested there might be features of the postsynaptic 5-HT cell that sculpt the incoming glutamatergic synaptic input.
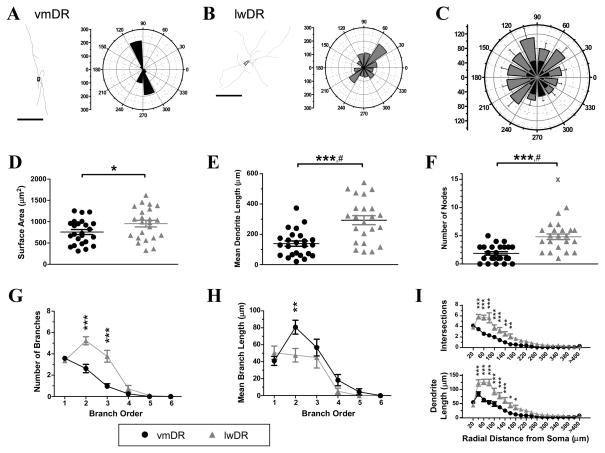
Lateral wing 5-HT neurons exhibit unique morphological characteristics including larger cell somas and increased dendritic complexity
We analyzed the morphology of 5-HT neurons that were filled with biocytin during whole-cell electrophysiology recordings. The cell bodies of lwDR 5-HT neurons were significantly larger than vmDR neurons in surface area (t45 =2.05, P = 0.046), mean length (Mann Whitney U=183, P=0.05), total area (t45= 2.02, P = 0.05) and a trend towards a larger enclosed volume and a larger cross-sectional area (Table 3, Fig. 5D). These results were consistent with previous descriptions of cell bodies in the DR where lwDR cell bodies had a larger average diameter than vmDR cell bodies (Steinbusch et al., 1981). Because the dendrites of 5-HT neurons of DR subregions have never been described, we also performed analysis of dendritic characteristics of vmDR and lwDR 5-HT cells. On average, lwDR 5-HT cells featured a larger number of dendrites with more nodes (Mann Whitney U = 75.5, P = <0.0001) and longer mean branch length (Mann Whitney U = 105, P = 0.0003) (Table 3, FFig. 5E–F). Looking more closely at dendritic branching structure, lwDR neurons have a larger number of 2nd and 3rd order branches(main effect of subregion 1.200=21.22, P<0.0001; main effect of branch order F5.200=101.2, P<0.0001; interaction between subregion and branch order F5.200=15.27, P<0.0001; Fig 5G). In addition, the mean branch length of 2nd order branches were longer in lwDR neurons (main effect of subregion F1.215=4.44, P=0.04; main effect of branch orderF5.215=34.63, P<0.0001; interaction between subregion and branch orderF5.215=2.37, P=0.04; Fig. 5H). Together this suggests that the increased dendrite length of lwDR neurons was specific for 2nd and 3rd order branches. Sholl analysis revealed that lwDR dendritic trees were significantly more complex than the dendritic trees of vmDR neurons (Fig. 5I), as was predicted by the higher number of nodes and ends measured in lwDR dendrites. Dendrites of lwDR featured more intersections than the dendrites of vmDR neurons (main effect of subregion F1,900=15.52, P=0.0003; main effect of radius F20,900=87.41, P<0.0001; interaction between subregion and radius F20,900=9.34, P<0.0001; Fig. 5I, upper panel), and had longer lengths of dendrites contained within several radial shells (main effect of subregion F1,840=18.35, P=0.0001; main effect of radius F20,840=101.6, P<0.0001; interaction between subregion and radius F20,840=10.89, P<0.0001; Fig. 5I, lower panel). Polar histograms were constructed for the dendritic tree of each cell and then averaged for each group to look for trends in orientation of dendrites within the coronal section. Directional analysis revealed that vmDR dendrites tended to be oriented in a dorso-ventral direction while lwDR dendrites demonstrated no preferred orientation (Fig. 5C). While most dendrites of vmDR neurons were contained wholly within the vmDR, the longest dendrites of vmDR 5-HT cells putatively pointed towards the MR and dmDR. While most dendrites of lwDR neurons were contained wholly within the lwDR, the longest dendrites of lwDR neurons tended to be oriented in the dorso-lateral, dorsal, medial, and ventromedial directions (Fig. 5C). These dendrites putatively pointed towards the ventrolateral periaqueductal gray, the aqueduct, the lateral edge of the dorsomedial DR, and the dorsolateral edge of the vmDR. A few lwDR dendrites ramified in the supraependymal plexus just ventral to the aqueduct.
Table 3.
Results of morphology analysis of 5-HT neurons
| Soma Analysis | Dendrite Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Subregion | Enclosed Volume | Mean Length (μm) | Total Area | Longest tip-to-tip length (μm) | Longest Segment (μm) | Number of trees | Number of Ends | Total Dendrite Length (μm) |
| vmDR (n=24) | 1821 ± 191 | 41.4 ± 1.6 | 2286 ± 239 | 198.0 ± 24.8 | 179.0 ± 24.3 | 3.6 ± 0.2 | 5.5 ± 0.4 | 461.7 ± 52.0 |
| lwDR (n=23) | 2473 ± 272 | 46.7 ± 2.5 | 3160 ± 365 | 287.7 ± 25.5 | 242.5 ± 23.8 | 3.4 ± 0.2 | 8.4 ± 0.6 | 970.8 ± 111.9 |
| Students t-test | t45=1.97, P=0.055 | t45=2.02, P=0.0496 | t45=0.67, P=0.64 | t44=3.93, P=0.0003 | ||||
| Mann-Whitney Test | U=183, P=0.050 | U=159, P=0.013 | U=175, P=0.033 | U=106, P=0.003 | ||||
Values are average ± s.e.m
Figure 5.
The 5-HT neurons of the lwDR possess somatic and dendritic morphology that is distinct from vmDR neurons. A representative tracing demonstrates the morphology of a vmDR neuron (A) and an lwDR neuron (B) as generated by Neurolucida. The corresponding polar histogram to the right of the trace demonstrates the dendritic length in each direction, using 30-degree bins. Scale bar 100 μm. C) The polar histograms were averaged across groups to generate an average polar histogram, showing orientation preferences for dendrites of vmDR neurons (black) and lwDR neurons (grey). The longest dendrites of vmDR neurons were oriented in the dorsal-ventral direction while the longest dendrites of lwDR neurons did not show a particular preference. D) The 5-HT neurons of the lwDR had large cell soma surface area (t45= 2.05, P = 0.046). E) Mean dendritic length was longer in lwDR neurons (Student’s t test with Welch’s correction t35 = 4.46, P< 0.0001). # denotes a significant difference in varianceF22,23 = 2.73, P = 0.02. F)Neurons of the lwDR also had higher numbers of nodes, i.e. branch points (Student’s t test with Welch’s correction t35 = 5.07, P< 0.0001). # denotes a significant difference in variance F21,23 = 2.451, P = 0.04. G) The increased number of branches seen in lwDR neurons was limited to 2nd and 3rd order dendrites. H) The increased mean branch length of lwDR neurons was limited to 2nd order branches. I) Dendritic tree complexity was quantified by Sholl analysis, revealing that lwDR neurons had more intersections and longer dendritic length in the region ranging from 40 to 160 μm from the cell soma. For G-I, * P< 0.05, ** P< 0.01, *** P< 0.001 Bonferroni post tests. See Results for additional results of ANOVA tests. Outlying values denoted by the symbol “X” were not included in group means.
The dendritic complexity of 5-HT neurons may contribute in part to the increased frequency of glutamatergic synaptic input
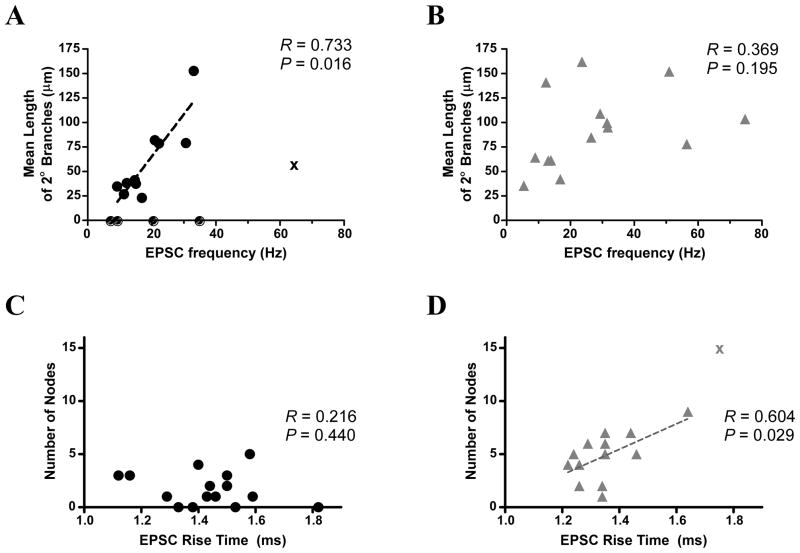
Differences in synaptic input to a neuron can sometimes be attributed to differences in the size of the dendritic tree (Hajos and Mody, 1997). Thus, the increased frequency of sEPSC observed in many lwDR neurons might be due to longer, more complex dendritic tree of lwDR neurons. To examine this relationship more closely we looked for correlations in morphological characteristics and sEPSC parameters obtained from the same neurons.
Neurons were analyzed to assess correlations between measures of sEPSC frequency and length of 2nd order branches, as this is where the largest difference in dendritic morphology was seen. We found a significant correlation between the mean length of 2nd order branches of recorded neurons and sEPSC frequency in the vmDR subpopulation (R = 0.88, P =0.0009, Fig. 6A) but not in the lwDR subpopulation (R = 0.37, P = 0.20, Fig. 6B). This suggests that while the longer length of 2nd order dendritic branches may contribute to the increased sEPSC frequency measured in some vmDR neurons, it cannot explain the high sEPSC frequency seen in the lwDR.
Figure 6.
Correlation analysis reveals several relationships between sEPSC parameters and morphological characteristics. A) A significant correlation was found between the mean length of 2nd order branches and sEPSC frequency among vmDR neurons. B) There was no correlation seen in lwDR neurons, suggesting that the increased branch length of lwDR neurons cannot account for the increased sEPSC frequency seen in all lwDR neurons. C) There was no correlation between sEPSC rise time and the number of nodes in vmDR neurons. D) However, there was a significant correlation between slow rise times and a larger number of nodes in lwDR neurons, suggesting increased electrotonic filtering in lwDR neurons. Correlations in (A) and (D) analyzed by Spearmantest; correlations in (B) and (C) analyzed by Pearson test. See Methods for details.
Further analysis of sEPSC measurements and morphology revealed an additional relationship, present only in lwDR neurons, where slower rise times of lwDR sEPSC events were correlated to an increased number of nodes (R = 0.60, P = 0.03, Fig. 6D) along with the number of ends and the number and total length of 3rd order branches (data not shown). The correlation in the vmDR was not significant (R = −0.44, P = 0.12, Fig. 6C). This provides the first evidence for electrotonic filtering in DR 5-HT neurons and suggests that filtering may preferentially affect lwDR neurons with the most branch points and the longest dendrites.
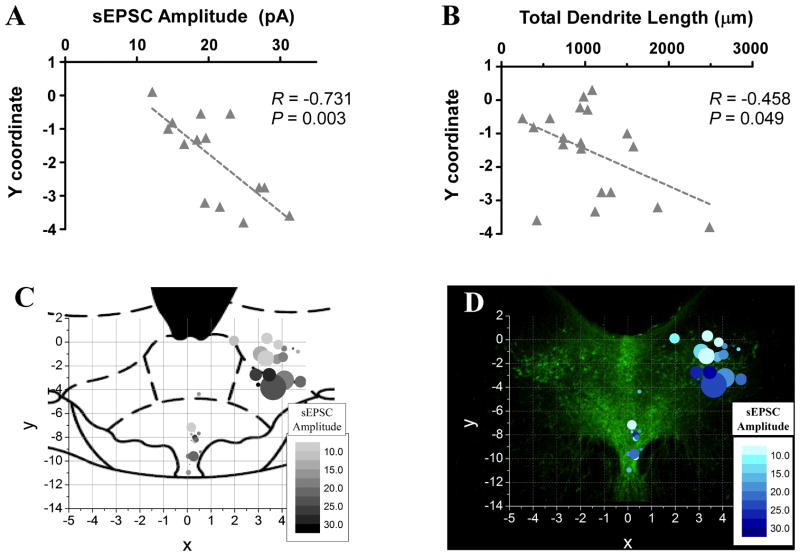
Topographical position within the lwDR subregion is correlated to particular features of sEPSCs and morphology
Because vGlut immunohistochemistry revealed specific topographical distributions of putative synaptic inputs, we examined the relationship between topographical position of DR neurons and the array of sEPSC measurements and morphology characteristics obtained from each cell. There was no correlation found between the x-coordinate, i.e. medial-lateral position, and any parameter. There was no correlation seen in vmDR neurons. In the lwDR a significant correlation was found between ventral position and several parameters including increased amplitude (R = −0.73, P= 0.003, Fig. 7A, D), increased charge per event (R = −0.53, P = 0.049), and longer total dendrite length (R = −0.458, P = 0.049, Fig. 7B, D). These data demonstrated that the ventral position of lwDR neurons was associated with increased sEPSC amplitude and longer dendrites. This suggested a functional consequence of the topographical organization of the lwDR that facilitates communication between a subset of lwDR neurons and synaptic terminals in the ventral lwDR and neighboring regions.
Figure 7.
Topographical position of lwDR 5-HT neurons is correlated to increased sEPSC amplitude and longer total dendrite length. A) After assigning x, y coordinates to the positions of recorded neurons, a negative correlation demonstrated the relationship between negative y-coordinate, i.e. ventral position within the lwDR, and increased sEPSC amplitude. B) A similar correlation was found between more ventral position within the lwDR and increased total dendrite length. C) This relationship is visualized in a bubble map overlaying an atlas image of the mid-rostral-caudal DR (adapted from Paxinos and Watson, 1997). Each point is positioned according to the x, y coordinate of the cell; the size of the point represents the total dendrite length of the cell and the color represents the average sEPSC amplitude recorded in that cell. Note that in the lwDR, larger points contain the darkest colors and are situated more ventral than the other cells. D) The same graph is mapped over an image of the mid-rostral-caudal DR where TPH staining more accurately demonstrates the distribution of 5-HT neurons.
Discussion
To understand how the glutamate and serotonin systems interact at the level of the DR, the relative distribution of glutamate terminals in two DR subregions was compared to measurements of glutamatergic synaptic input. Though vGlut2 puncta were homogenous through the DR, vGlut1 and vGlut3 puncta were less dense in the region surrounding lwDR 5-HT neurons. The relative density of vGlut puncta was predictive of AP-independent mEPSC events. However, it did not explain spontaneous glutamatergic input, as lwDR neurons had an increased sEPSC frequency that was dependent on the activity of local glutamate neurons within the slice. While lwDR neurons were found to have longer, more complex dendrites, correlations between sEPSC frequency and dendrite morphology were not significant in the lwDR, suggesting that increased dendrite length was not a major contributing factor to the increased excitatory synaptic input. Ventral position within the lwDR was associated with increased sEPSC amplitude and longer dendrite length, suggesting that topographical location, in addition to the activity of midbrain neurons shape glutamatergic modulation of lwDR neurons.
Methodological considerations for electrophysiology recordings of lwDR neurons
Voltage clamp recordings of sEPSC activity are optimal under conditions that enable clamping of the entire cell; however some degree of filtering due to incomplete space clamp is inherent in even the best of voltage clamp conditions (Spruston and Johnston, 2008). Our data revealed a negative correlation between event rise time and event amplitude, indicating a degree of electrotonic filtering of events. In the lwDR, but not the vmDR, cells with the longest dendrites had slower sEPSC event rise times. Thus, the greater filtering seen in the lwDR was most likely due to the cable properties of the longer, more complex lwDR dendrites, as branch points (i.e. nodes) can increase filtering (Rall et al., 1967; Ling and Benardo, 1999). Thus, the dendritic morphology of lwDR neurons contributed to their ability to filter input and thereby the actual amplitude of the events generated in the distal dendrites is probably greater in amplitude, due to the attenuation of the signals by filtering. As lwDR 5-HT neurons are selectively activated by a range of stressors (Commons 2008; Johnson et al., 2008), their unique morphology could thereby increase the resolution of input to the lwDR generated by the limbic stress response or in stress-related psychopathology.
In addition, the degree of filtering seen in lwDR neurons with the longest, most branched dendrites underscores the importance of proximity to synaptic inputs. The correlation found between ventral position within the lwDR, increased dendrite length, and increased sEPSC amplitude has implications for synaptic partners and the postsynaptic receptors that modulate the strength of that synaptic input. Ventrally located lwDR neurons may express more and different subtypes of AMPA receptors that enable cells to enhance the strength of glutamatergic input. Alternatively, the more ventral position situates the lwDR cell soma in closer proximity to major glutamatergic inputs, resulting in events that reach the soma with minimal filtering. Glutamate terminals that are situated closer to the ventral lwDR cell soma would be more effective at making the neuron fire. Interestingly, immunohistochemical detection of vGlut proteins in the ventral lwDR provides topographic evidence for potential glutamatergic input to the ventral lwDR.
The differences seen in the decay times also indicate potential differences in the kinetics of the glutamate receptors expressed by vmDR and lwDR neurons. Decay times histograms were much faster in terms of the peak distributions. The faster rise and decay times of the lwDR neurons may indicate subregional differences in AMPA receptor subunit composition or post-translational modification.
Functional significance of vGlut protein in the mouse DR
Collectively, our findings help to clarify the functional significance of the topography of vGlut labeling in the DR. The marker vGlut1 is associated with the axon terminals of cortex projections (Fremeau et al., 2004a; Takamori, 2006) and may mark terminals possessing increased potential for synaptic plasticity (Fremeau et al., 2004b; Fremeau et al., 2004a). The density of punctate labeling of vGlut1 terminals was highest in the lwDR, just ventral to the cluster of lwDR 5-HT neurons. Projections from the medial prefrontal cortex synapse primarily on GABA DR neurons (Jankowski and Sesack, 2004) that are preferentially located in the lateral regions of the DR (Stamp and Semba, 1995; Day et al., 2004). Thus, the increased density of vGlut1 puncta may represent projections from medial prefrontal cortex to GABA neurons of the DR. Given the role of medial prefrontal cortex input to the DR in mood disorders (Maier et al., 2006), the potential for increased synaptic plasticity at vGlut1 synapses in the DR is worthy of further investigation.
The dense and homogenous punctate staining of vGlut2 demonstrated the terminals of axons that could potentially originate from the lateral habenula (Barroso-Chinea et al., 2007), a prominent source of glutamate afferents to the DR that link the DR to the limbic forebrain (Wang and Aghajanian, 1977; Kalen et al., 1985; Araki et al., 1988; Peyron et al., 1998; Lee et al., 2003). Because cells that express vGlut2 mRNA are also found in midbrain regions outside of the DR such as the periaqueductal grey (Hioki et al., 2010), projections from vGlut2-expressing glutamate neurons could be intact within the brain slice preparation. Therefore, as a possible source of spontaneous excitatory input to the raphe, vGlut2-labeled terminals may originate from local, active glutamatergic inputs that selectively target lwDR 5-HT neurons.
Detection of vGlut3 revealed staining of cell bodies along with puncta that were slightly less dense in the lwDR. It is likely that vGlut3 plays a role in glutamate release from the terminals of 5-HT cells, as has been demonstrated in auditory cortex (Ruel et al., 2008; Seal et al., 2008). If so, then the vGlut3 stained glutamate neurons may also provide the spontaneous excitatory input to the raphe neurons. A recent study of the vGlut3 knockout mouse (Amilhon et al., 2010) suggests that vGlut3 had no effect on vesicular filling in the raphe, but may have increased vesicular filling in subsets of 5-HT terminals. Also, selective stimulation of eGFP-labeled 5-HT terminals induced an increase in the frequency of glutamatergic input to hippocampal interneurons (Varga et al., 2009). Further studies are needed to fully characterize the synaptic contributions of vGlut3-positive 5-HT neurons to glutamatergic neurotransmission in the DR and DR projection regions.
Functional consequences of the differential glutamatergic regulation of lwDR neurons
Based on the density of vGlut puncta alone, the expectation would be that lwDR 5-HT cells receive less glutamatergic input than vmDR 5-HT cells. While lower mEPSC frequency in the lwDR was consistent with this expectation, the higher sEPSC frequency suggested that the input to the lwDR is dependent on the activity of glutamate neurons that are 1) contained within the midbrain slice and 2) selectively target lwDR 5-HT neurons more so than vmDR 5-HT neurons. Though the literature is still not clear as to the role of vGlut in synaptic physiology (Takamori 2006), what can be concluded from our data is that midbrain glutamate neurons have a larger impact on lwDR than on vmDR 5-HT activity. The advent of optogenetics may help to clarify the role of individual vGlut proteins on vesicular filling, release probability, and the consequent activity of post-synaptic neurons in the future. Such techniques should be especially useful in regions such as the raphe nuclei where there is no clear fiber bundle or pathway that can be used to uniformly stimulate the diffusely distributed glutamatergic input.
As has been demonstrated in other brain regions, the morphology of post-synaptic cells can influence synaptic inputs. Compared to vmDR neurons, lwDR 5-HT neurons had a larger cell soma, more complex dendritic branching, and increased dendritic length, particularly in the 2nd and 3rd order dendrites. There was a correlation between the mean length of 2nd order branches and sEPSC frequency in the vmDR; however this correlation was not present in the lwDR. This suggests that these dendrites in lwDR neurons do not account for the increased glutamate input that was present in recordings.
The increased glutamate input to lwDR neurons has several functional ramifications. Our previous studies showed that compared to vmDR neurons, lwDR 5-HT neurons demonstrate an increased gain that is independent of synaptic input and attributable to intrinsic membrane properties (Crawford et al., 2010). The increased gain is an indication that the cell is more excitable to incoming synaptic input, i.e., a given degree of depolarization generates a higher frequency of action potentials. The increased glutamatergic input to lwDR neurons reported here likely synergizes with the increased gain, enabling an increased responsivity to glutamatergic input. It is likely no coincidence that the highest degree of glutamatergic input is going to those 5-HT cells whose morphology and intrinsic properties make them especially suited to integrate input. The extended dendritic tree of lwDR 5-HT neurons provides a likely milieu for integration of a variety of inputs in the intact brain, e.g., glutamate input as characterized above, inhibitory input from DR GABA neurons, noradrenergic input (Kim et al., 2004), CRF input (Roche et al., 2003), and other modulators of 5-HT activity. Stress-activated afferents to the lwDR would increase output in target regions that are crucial for the behavioral and physiological responses to those stressors. There is a presumed degree of overlap in projection regions of the lwDR and vmDR, but studies that explicitly include the lwDR have revealed important distinctions. While vmDR 5-HT neurons project to regions such as the medial prefrontal cortex, ventral hippocampus, and amygdala, lwDR projections tend to be limited to subcortical regions and include projections to midbrain and hindbrain regions responsible for panic and escape behaviors as well as vasoconstriction responses mediated by the sympathetic system (Abrams et al., 2004; Johnson et al., 2004; Michelsen et al., 2007; Lowry et al., 2008). In addition lwDR neurons have been implicated in regulation of vmDR neurons (Peyron et al., 1998). Together with the intrinsic membrane properties of lwDR 5-HT neurons, local glutamate neurons likely function to increase 5-HT output, particularly in lwDR target regions. Thus, glutamate may contribute to the role of lwDR neurons in descending pathways that regulate panic behaviors and sympathetic system in response to stress. Dysregulation of glutamatergic modulation of subsets of 5-HT neurons likely results in specific, diverse effects on 5-HT output to limbic brain regions and brainstem centers responsible for the behavioral and physiological components of a range of psychiatric disorders.
Acknowledgments
We thank Larry Tecott and Evan Deneris for providing the founders for the colony of mice used in these experiments. This research was supported by the following NIH grants MH075047 (SGB) and MH082611 (LKC).
Abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- AP
action potential
- DR
dorsal raphe
- lwDR
lateral wings of the DR
- mEPSC
miniature excitatory postsynaptic current
- sEPSC
spontaneous excitatory postsynaptic current
- vmDR
ventromedial DR
- vGlut
vesicular glutamate transporter
References
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Adell A, Celada P, Abellan MT, Artigas F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res Brain Res Rev. 2002;39:154–180. doi: 10.1016/s0165-0173(02)00182-0. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, Miot S, Gras C, Gardier AM, Gallego J, Hamon M, Lanfumey L, Gasnier B, Giros B, El Mestikawy S. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J Neurosci. 2010;30:2198–2210. doi: 10.1523/JNEUROSCI.5196-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki M, McGeer PL, Kimura H. The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain Res. 1988;441:319–330. doi: 10.1016/0006-8993(88)91410-2. [DOI] [PubMed] [Google Scholar]
- Barroso-Chinea P, Castle M, Aymerich MS, Perez-Manso M, Erro E, Tunon T, Lanciego JL. Expression of the mRNAs encoding for the vesicular glutamate transporters 1 and 2 in the rat thalamus. J Comp Neurol. 2007;501:703–715. doi: 10.1002/cne.21265. [DOI] [PubMed] [Google Scholar]
- Beck SG, Pan YZ, Akanwa AC, Kirby LG. Median and dorsal raphe neurons are not electrophysiologically identical. J Neurophysiol. 2004;91:994–1005. doi: 10.1152/jn.00744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouloff F, Laude D, Guezennec Y, Ilghozi JL. Motor activity increases tryptophan, 5-hydroxyindoleacetic acid, and homovanillic acid in ventricular cerebrospinal fluid of the conscious rat. J Neurochem. 1986;46:1313–6. doi: 10.1111/j.1471-4159.1986.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Commons KG. Evidence for topographically organized endogenous 5-HT-1A receptor-dependent feedback inhibition of the ascending serotonin system. Eur J Neurosci. 2008;27:2611–8. doi: 10.1111/j.1460-9568.2008.06235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG. Locally collateralizing glutamate neurons in the dorsal raphe nucleus responsive to substance P contain vesicular glutamate transporter 3 (VGLUT3) J Chem Neuroanat. 2009;38:273–281. doi: 10.1016/j.jchemneu.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Beck SG, Bey VW. Two populations of glutamatergic axons in the rat dorsal raphe nucleus defined by the vesicular glutamate transporters 1 and 2. Eur J Neurosci. 2005;21:1577–1586. doi: 10.1111/j.1460-9568.2005.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese BM, Phan KL. The role of glutamate in anxiety and related disorders. CNS Spectr. 2005;10:820–830. doi: 10.1017/s1092852900010427. [DOI] [PubMed] [Google Scholar]
- Crawford LK, Craige CP, Beck SG. Increased intrinsic excitability of lateral wing serotonin neurons of the dorsal raphe: a mechanism for selective activation in stress circuits. J Neurophysiol. 2010;103:2652–2663. doi: 10.1152/jn.01132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004a;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004b;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. The wake-promoting peptide orexin-B inhibits glutamatergic transmission to dorsal raphe nucleus serotonin neurons through retrograde endocannabinoid signaling. J Neurosci. 2005;25:896–905. doi: 10.1523/JNEUROSCI.3258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. Endocannabinoids suppress excitatory synaptic transmission to dorsal raphe serotonin neurons through the activation of presynaptic CB1 receptors. J Pharmacol Exp Ther. 2009;331:186–196. doi: 10.1124/jpet.109.153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Mody I. Synaptic communication among hippocampal interneurons: properties of spontaneous IPSCs in morphologically identified cells. J Neurosci. 1997;17:8427–8442. doi: 10.1523/JNEUROSCI.17-21-08427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Gilchrist J, Gras C, Muzerelle A, Ravassard P, Giros B, Gaspar P, El Mestikawy S. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience. 2004;123:983–1002. doi: 10.1016/j.neuroscience.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Hioki H, Fujiyama F, Nakamura K, Wu SX, Matsuda W, Kaneko T. Chemically specific circuit composed of vesicular glutamate transporter 3- and preprotachykinin B-producing interneurons in the rat neocortex. Cereb Cortex. 2004;14:1266–1275. doi: 10.1093/cercor/bhh088. [DOI] [PubMed] [Google Scholar]
- Hioki H, Nakamura H, Ma YF, Konno M, Hayakawa T, Nakamura KC, Fujiyama F, Kaneko T. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J Comp Neurol. 2010;518:668–686. doi: 10.1002/cne.22237. [DOI] [PubMed] [Google Scholar]
- Jackson J, Bland BH, Antle MC. Nonserotonergic projection neurons in the midbrain raphe nuclei contain the vesicular glutamate transporter VGLUT3. Synapse. 2009;63:31–41. doi: 10.1002/syn.20581. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J Comp Neurol. 2004;468:518–529. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Jaslove SW. The integrative properties of spiny distal dendrites. Neuroscience. 1992;47:495–519. doi: 10.1016/0306-4522(92)90161-t. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Lightman SL, Lowry CA. A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann N Y Acad Sci. 2004;1018:58–64. doi: 10.1196/annals.1296.006. [DOI] [PubMed] [Google Scholar]
- Johnson P, Lowry C, Truitt W, Shekhar A Disruption of GABAergic tone in the dorsomedial hypothalamus attenuates responses in a subset of serotonergic neurons in the dorsal raphe nucleus following lactate-induced panic. J Psychopharmacol. 2008;22:642–52. doi: 10.1177/0269881107082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalen P, Karlson M, Wiklund L. Possible excitatory amino acid afferents to nucleus raphe dorsalis of the rat investigated with retrograde wheat germ agglutinin and D-[3H]aspartate tracing. Brain Res. 1985;360:285–297. doi: 10.1016/0006-8993(85)91244-2. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pan YZ, Freeman-Daniels E, Rani S, Nunan JD, Akanwa A, Beck SG. Cellular effects of swim stress in the dorsal raphe nucleus. Psychoneuroendocrinology. 2007;32:712–723. doi: 10.1016/j.psyneuen.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci. 2008;28:12927–12937. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, Segev I. The role of single neurons in information processing. Nat Neurosci. 2000;3(Suppl):1171–1177. doi: 10.1038/81444. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 2003;963:57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Pan YZ, Ma X, Lamy C, Akanwa AC, Beck SG. Selective 5-HT receptor inhibition of glutamatergic and GABAergic synaptic activity in the rat dorsal and median raphe. Eur J Neurosci. 2006;24:3415–3430. doi: 10.1111/j.1460-9568.2006.05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling DS, Benardo LS. Restrictions on inhibitory circuits contribute to limited recruitment of fast inhibition in rat neocortical pyramidal cells. J Neurophysiol. 1999;82:1793–1807. doi: 10.1152/jn.1999.82.4.1793. [DOI] [PubMed] [Google Scholar]
- Liu R, Ding Y, Aghajanian GK. Neurokinins activate local glutamatergic inputs to serotonergic neurons of the dorsal raphe nucleus. Neuropsychopharmacology. 2002;27:329–340. doi: 10.1016/S0893-133X(02)00305-6. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Evans AK, Gasser PJ, Hale MW, Staub DR, Shekhar A. Topographic organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus. In: Monti JM, Pandi-Perumai SR, Jacobs BL, Nutt DJ, editors. Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Basel, Switzerland: Birkhauser Verlag AG; 2008. [Google Scholar]
- Maier SF, Amat J, Baratta MV, Paul E, Watkins LR. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin Neurosci. 2006;8:397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KA, Schmitz C, Steinbusch HW. The dorsal raphe nucleus--from silver stainings to a role in depression. Brain Res Rev. 2007;55:329–342. doi: 10.1016/j.brainresrev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Scott TJ. Colocalization of serotonin and vesicular glutamate transporter 3-like immunoreactivity in the midbrain raphe of Syrian hamsters (Mesocricetus auratus) Neurosci Lett. 2006;394:97–100. doi: 10.1016/j.neulet.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Williams JT. GABA- and glutamate-mediated synaptic potentials in rat dorsal raphe neurons in vitro. J Neurophysiol. 1989;61:719–726. doi: 10.1152/jn.1989.61.4.719. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3. San Diego: Academic Press; 1997. [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Puig MV, Artigas F, Celada P. Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: involvement of serotonin and GABA. Cereb Cortex. 2005;15:1–14. doi: 10.1093/cercor/bhh104. [DOI] [PubMed] [Google Scholar]
- Rall W, Burke RE, Smith TG, Nelson PG, Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol. 1967;30:1169–1193. doi: 10.1152/jn.1967.30.5.1169. [DOI] [PubMed] [Google Scholar]
- Ruel J, Emery S, Nouvian R, Bersot T, Amilhon B, Van Rybroek JM, Rebillard G, Lenoir M, Eybalin M, Delprat B, Sivakumaran TA, Giros B, El Mestikawy S, Moser T, Smith RJ, Lesperance MM, Puel JL. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. Am J Hum Genet. 2008;83:278–292. doi: 10.1016/j.ajhg.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277:50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci U S A. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, Lustig LR, Edwards RH. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–275. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutoh F, Ina A, Yoshida S, Konno J, Hisano S. Two distinct subtypes of serotonergic fibers classified by co-expression with vesicular glutamate transporter 3 in rat forebrain. Neurosci Lett. 2008;432:132–136. doi: 10.1016/j.neulet.2007.12.050. [DOI] [PubMed] [Google Scholar]
- Spruston N, Johnston D. Out of control in the dendrites. Nat Neurosci. 2008;11:733–734. doi: 10.1038/nn0708-733. [DOI] [PubMed] [Google Scholar]
- Stamp JA, Semba K. Extent of colocalization of serotonin and GABA in the neurons of the rat raphe nuclei. Brain Res. 1995;677:39–49. doi: 10.1016/0006-8993(95)00119-b. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW, Nieuwenhuys R, Verhofstad AA, Van der Kooy D. The nucleus raphe dorsalis of the rat and its projection upon the caudatoputamen. A combined cytoarchitectonic, immunohistochemical and retrograde transport study. J Physiol (Paris) 1981;77:157–174. [PubMed] [Google Scholar]
- Takamori S. VGLUTs: ‘exciting’ times for glutamatergic research? Neurosci Res. 2006;55:343–351. doi: 10.1016/j.neures.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Differential effect of NMDA on extracellular serotonin in rat midbrain raphe and forebrain sites. J Neurochem. 1996;66:1067–1075. doi: 10.1046/j.1471-4159.1996.66031067.x. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Regulation of serotonin release by GABA and excitatory amino acids. J Psychopharmacol. 2000;14:100–113. doi: 10.1177/026988110001400201. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Influence of inhibitory and excitatory inputs on serotonin efflux differs in the dorsal and median raphe nuclei. Brain Res. 2003;961:109–120. doi: 10.1016/s0006-8993(02)03851-9. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Influence of AMPA/kainate receptors on extracellular 5-hydroxytryptamine in rat midbrain raphe and forebrain. Br J Pharmacol. 1997;121:1707–1715. doi: 10.1038/sj.bjp.0701292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, Hangya B, Holderith N, Magee JC, Freund TF. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–53. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK. Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science. 1977;197:89–91. doi: 10.1126/science.194312. [DOI] [PubMed] [Google Scholar]
- Waselus M, Van Bockstaele EJ. Co-localization of corticotropin-releasing factor and vesicular glutamate transporters within axon terminals of the rat dorsal raphe nucleus. Brain Res. 2007;1174:53–65. doi: 10.1016/j.brainres.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]