Abstract
Medicinal benefits of Allium vegetables, including garlic, have been documented throughout the written history. Health benefits of garlic and other Allium vegetables (e.g., onions), such as lipid lowering and anticancer effects, are credited to metabolic byproducts, including diallyl trisulfide (DATS). Evidence for anticancer effects of garlic derives from both population-based case-control studies, and clinical and laboratory investigations using purified garlic constituents such as DATS. Studies have shown that DATS can offer protection against chemically-induced neoplasia as well as oncogene-driven spontaneous cancer development in experimental rodents. Mechanisms underlying cancer chemopreventive effects of DATS are not completely understood, but known pharmacological responses to this natural product include alteration in carcinogen-metabolizing enzymes, cell cycle arrest, induction of apoptotic cell death, suppression of oncogenic signal transduction pathways, and inhibition of neoangiogenesis. This article reviews mechanisms and targets of cancer chemoprevention by DATS.
Keywords: Garlic, Diallyl Trisulfide, Apoptosis, Chemoprevention
Introduction
Historical perspective on medicinal uses of garlic has been elegantly reviewed by Rivlin1 and Rahman2. Scientific literature is also packed with convincing experimental evidence demonstrating efficacy of Allium vegetables and/or their metabolic byproducts against a number of chronic diseases, including cardiovascular problems, diabetes, infections, and cancer3-6. Health benefits of garlic and other Allium vegetables are attributed to sulfur-containing compounds, which are generated upon processing (cutting or chewing) of these edible plants7. Evidence for anticancer effects of Allium vegetables derives from both population-based case-control studies8-12 and laboratory findings13-15. For example, You et al8 studied the association of Allium vegetable intake with the risk of gastric cancer in a population-based case-control study involving 564 patients and more than 1100 normal healthy subjects. This study concluded that the subjects with Allium vegetable intake were at a significantly lower risk of developing stomach cancer compared with low intake8. Similar epidemiological associations have been noted for esophageal cancer9, prostate cancer10, pancreatic cancer11, and endometrial cancer12 to name a few. These epidemiological observations have undoubtedly sparked interest among biologists to identify bioactive anticancer constituents from Allium vegetables. Major findings pertaining to cancer chemopreventive pharmacology of one such naturally-occurring compound, diallyl trisulfide (DATS), are summarized in this article.
Biochemistry of DATS Production
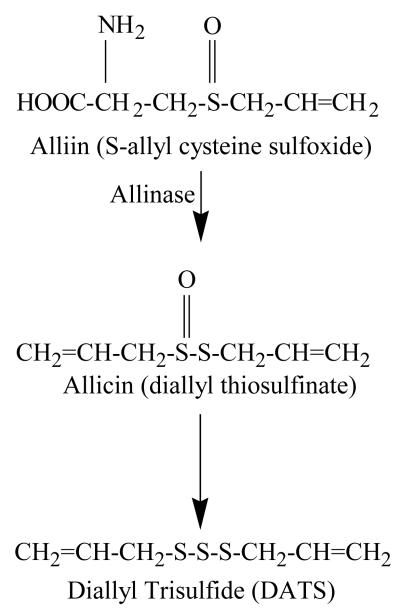
Biochemical synthesis of DATS (CH2=CH-CH2-S-S-S-CH2-CH=CH2) begins with γ-glutamyl-S-alk(en)yl-L-cysteine, which is hydrolyzed and oxidized to produce alliin (Figure 1)7. Alliin is the odorless precursor of DATS (also referred as allitridi in some publications). Processing of garlic (cutting or chewing) generates a vacuolar enzyme (allinase), which acts upon alliin to give rise to allicin and other alkyl alkane-thiosulfinates7. Allicin and related thiosulfinates are decomposed to yield various sulfur compounds including DATS. It has been estimated that one gram of fresh garlic may contain 900-1100 μg of DATS15.
Figure 1.
Biochemical synthesis and chemical structure of diallyl trisulfide.
In Vivo Evidence for Anticancer/Chemopreventive Activity of DATS in Experimental Rodents
Belman and colleagues16 were the first to show inhibition of chemically-induced skin carcinogenesis in mice by garlic oil. Published results documenting efficacy of DATS against cancer in experimental rodents are summarized in Table 1. Treatment of female A/J mice p.o. with 20 μmol DATS 96- and 48-hour prior to administration of 2 mg oral benzo[a]pyrene (BP), an environmental carcinogen abundant in cigarette smoke and barbecued food, resulted in 85% decrease in forestomach tumor multiplicity17. On the other hand, the number of pulmonary adenoma resulting from BP administration was not significantly reduced by DATS administration17. Oral administration of 25 μmol DATS, twice 48 hour apart, significantly inhibited forestomach cancer multiplicity induced by BP18. Gavage of 6 μmol DATS thrice weekly to male athymic mice subcutaneously implanted with PC-3 human prostate cancer cells caused retardation of xenograft growth without causing weight loss19. For example, twenty days after PC-3 cell injection the average tumor volume in vehicle-treated control mice (565 ± 112 mm3) was about 3-fold higher compared with DATS-treated mice19. The DATS treatment in this study was started on the day of tumor cell implantation19. Shankar et al20 also reported growth inhibitory effect of oral DATS administration (40 mg/kg, 5 times/week) against PC-3 cells orthotopically implanted in male BALB/c nude mice. Interestingly, co-treatment of PC-3 xenograft bearing mice with DATS (40 mg/kg, 5 times/week) and TRAIL (15 mg/kg administered intravenously on day 2, 8, 15, and 22) was more effective in inhibiting prostate tumor growth than either agent alone20. Intravenous administration of polybutylcyanoacrylate nanoparticle of DATS (1.5 mg/kg every alternate day for 14 days) significantly retarded the growth of orthotopically implanted HepG2 cells in nude mice21. Interestingly, DATS alone was not effective in this hepatocellular carcinoma xenograft model21. Intraperitoneal administration of 50 mg DATS/kg body weight to BALB/c nude mice with CT-26 murine colon cancer allograft significantly inhibited tumor growth22. Our group used a transgenic mouse model (Transgenic Adenocarcinoma of Mouse Prostate mice; commonly abbreviated as TRAMP mice) to determine efficacy of DATS for prevention of prostate cancer23. Incidence of poorly-differentiated carcinoma in the dorsolateral prostate of mice treated with 2 mg DATS/mouse (thrice/week) was lower by 41% (P= 0.035) in comparison with control mice. Moreover, the area occupied by the poorly-differentiated prostate cancer in mice administered with 2 mg DATS was lower by about 76% (P= 0.0189) compared with control mice23. Topical application of DATS at 5 or 25 μmol dose significantly inhibited incidence (22% inhibition by 25 μmol DATS) and multiplicity (25.6% and 71.1% inhibition by 5 and 25 μmol DATS, respectively) of skin papilloma in a two-stage chemically-induced skin model in female ICR mice24. Interestingly, in a medium-term bioassay in rats involving single i.p. injection of 200 mg diethylnitrosamine followed by two-third partial hepatectomy at week 3, gavage of 150 mg DATS/kg body weight two weeks after carcinogen challenge caused a significant increase, not reduction, in number and area of GST-P positive foci in the liver25. This single study suggests that DATS may have tumor promoting effects in some models of chemically-induced cancer25.
Table 1.
In Vivo Efficacy of diallyl trisulfide against cancer in experimental rodents.
| Species (sex) | Model | DATS dose/route | Outcome | Ref. |
|---|---|---|---|---|
| A/J mice (female) | BP1-induced | 20 μmol DATS2, oral | Forestomach cancer- 85% inhibition | 17 |
| Lung adenoma- no effect | 17 | |||
| A/J mice (female) | BP-induced | 25 μmol DATS, oral | Forestomach cancer- 86% inhibition | 18 |
| BALB/c nude mice (male) | PC-3 xenograft (subcutaneous) |
6 μmol DATS, oral, three times/wk |
Prostate tumor growth inhibition | 19 |
| BALB/c nude mice (male) | PC-3 xenograft (orthotopic) |
40 mg/kg DATS, oral, five times/wk |
Prostate tumor growth inhibition and potentiation of TRAIL efficacy |
20 |
| BALB/c nude mice (male) | HepG2 xenograft (orthotopic) |
1.5 mg/kg, i.v, DATS-PBCA-NP3 |
Hepatocellular cancer growth inhibition |
21 |
| BALB/c nude mice (female) | CT26 allograft | 50 mg/kg DATS, i.p., every fourth day |
Colon cancer growth inhibition | 22 |
| TRAMP4 mice (male) | Spontaneous | 1 or 2 mg DATS/mouse, oral, thrice/wk for 13 wk |
Inhibition of incidence and burden of poorly-differentiated prostate cancer |
23 |
| ICR mice (female) | two-stage skin papilloma model (DMBA5 + TPA6) |
5 or 25 μmol DATS, topical, twice/wk for 20 wk |
Inhibition of skin papilloma incidence (25 μmol DATS) and multiplicity (5 and 25 μmol DATS) |
24 |
| F344 Rats (male) | DEN7 + PH8 liver model |
150 mg/kg DATS, gavage five times/wk for 8 wk |
Increase in number and area of GST-P positive foci in the liver |
25 |
Abbreviations: BP, benzo[a]pyrene;
DATS, diallyl trisulfide;
DATS-PBCA-NP, polybutylcyanoacrylate nanoparticle of DATS;
TRAMP, Transgenic Adenocarcinoma of Mouse Prostate;
DMBA, 7,12-dimethylbenz(a)anthracene;
TPA, 12-O-tetradecanoylphorbol-13-acetate;
DEN, diethylnitrosamine;
PH, partial hepatectomy.
Pharmacokinetics of DATS
Pharmacokinetic parameters for DATS have been measured in Wistar rats after a single injection of 10 mg DATS administered via a jugular vein cannula26. Blood DATS concentration-time curves were analyzed using two-compartment analysis. The maximum blood concentration (Cmax) of DATS was 5516.9 μg/L (~31 μM)26 but peaked rapidly with a Tmax (time to reach Cmax) of about 1 min. Further studies are needed to determine the pharmacokinetic behavior and oral bioavailability of DATS in humans because this knowledge is integral for dose and schedule optimization in future clinical investigations.
Clinical Investigation of DATS
A double-blinded placebo-controlled interventional study was conducted in China using large doses of DATS in combination with selenium27. Inclusion criteria included at least one of the following: medical history of stomach disorder, family history of tumor, or smoking and/or alcohol consumption. A total of 2,526 and 2,507 individuals were randomly enrolled into the interventional and control arms, respectively, from villages of Shandong Province, China27. The interventional group of subjects was administered orally with 200 mg of synthetic DATS every day plus 100 μg of selenium every other day for one month of each year during November 1989 to December 1991. The control subjects received 2 placebo capsules. Large doses of DATS were well tolerated by all subjects without any harmful side effects. In the first five year follow-up between 1992 and 1997 after stopping the treatment, a decline in cancer morbidity rates was observed for the interventional group27. The relative risk, after adjustment for age, gender, and other confounders, for all tumors and gastric cancer were 0.67 (95% confidence level 0.43 - 1.03) and 0.48 (95% confidence level 0.21 - 1.06), respectively27. For male subjects, the relative risk for all tumors and gastric cancer were 0.51 (95% confidence level 0.30 - 0.85) and 0.36 (95% confidence level 0.14 - 0.92), respectively. However, a similar association was not evident in the female sub-group. This study not only established safety of large doses of DATS administration in humans but also demonstrated its cancer chemopreventive effect27.
Effect of DATS on Drug Metabolizing Enzymes
Suppression of cytochrome P450-dependent monooxygenases (collectively referred to as Phase 1 drug-metabolizing enzymes), which are responsible for metabolic activation of chemical carcinogens, and/or induction of Phase 2 carcinogen detoxifying enzymes (e.g., glutathione S-transferase, NADPH:quinone oxidoreductase) is considered major mechanism for cancer chemoprevention by naturally-occurring dietary bioactive compounds. Female A/J mice treated p.o. with 25 μmol DATS twice, 48-hour apart, and sacrificed 48 hours after the second administration exhibited a modest yet statistically significant decrease in activity of epoxide hydrolase in the forestomach28 compared with control mice. Interestingly, hepatic epoxide hydrolase activity was about 2.6-fold higher in DATS-treated mice compared with control mice28. DATS administration had no effect on Phase 1 ethoxyresorufin O-deethylase activity in either liver or lung28. Glutathione S-transferase (GST) activity towards ultimate carcinogenic metabolite of BP was significantly increased by DATS administration in liver and forestomach, but not in the lung28. However, quantitation of individual GST subunit protein levels from the lung of control and DATS-treated mice revealed induction of Pi class GST subunit by about 1.8-fold29. Interestingly, compared with control mice, DATS-treated mice exhibited a robust increase in protein levels of other classes of GST subunits in addition to Pi class GST subunit30. For example, the levels of hepatic Alpha class GST subunit α1 (mGSTA1 according to revised nomenclature), α3 (mGSTA3), and α4 (mGSTA4) were increased by about 1.7-, 8.0-, and 2.2-fold, respectively, upon DATS administration compared with control30. Even more robust induction of mGSTA1 and mGSTA2 by DATS administration was observed in the forestomach, a target organ for BP-induced cancer in A/J mice30. Noticeably, a dimer consisting of mGSTA1 and mGSTA2 subunit is exceptionally efficient in catalyzing glutathione conjugation and hence detoxification of the ultimate carcinogenic metabolite of BP compared with other classes of GSTs31. A 1.4- to 2.1-fold increase in protein levels of Mu class GST subunits was also evident in the liver of DATS-treated mice in comparison with control30. These studies provided evidence for DATS-mediated induction of carcinogen detoxifying GST subunits in the liver as well as in target organs in mice29,30. DATS treatment also resulted in a significant increase in NADPH:quinone oxidoreductase activity in the forestomach and lung of female A/J mice compared with control, and upregulation of its protein levels in the forestomach32. Five-day feeding of rats with 89 mg DATS/kg/day resulted in a robust increase in activity of NAD(P)H:quinone oxidoreductase in the liver (2.7-fold increase), kidney (5.5-fold increase), spleen (3.1-fold increase), lung (6.5-fold increase), forestomach (2.8-fold increase), and heart (2.5-fold increase) compared with those of control animals33. DATS-mediated increase in GST activity in rats was much less pronounced (e.g., only 1.4-fold increase in the liver) compared with the effect observed on NADPH:quinone oxidoreductase activity33. Together, these observations point towards species-related differences (mice versus rats) in DATS-mediated induction of Phase 2 enzymes29,30,32,33. NADPH:quinone oxidoreductase and heme oxygenase mRNA levels were increased by more than 4.5-fold upon 6-hour treatment with 100 μM DATS in HepG2 cells. There was also an increase in luciferase reporter activity mediated by antioxidant response element, and the protein levels of transcription factor Nrf234. DATS-mediated increase in luciferase activity was significantly attenuated by ectopic expression of a dominant negative Nrf2 and Keap1 and antioxidants34. In a study using rat primary hepatocytes, 24- and 48-hour exposure to 25 and/or 50 μM DATS caused an increase in activities of glutathione peroxidase and glutathione reductase35. Intracellular levels of reduced glutathione were also increased in rat hepatocytes after treatment with DATS35. On the other hand, rat hepatoma 4HIIE cells failed to show elevation of GST or NADPH:quionone oxidoreductase activity after 48-hour treatment with 50 or 100 μM DATS36. DATS was shown to inhibit (a) nuclear RNA synthesis in isolated rat liver nuclei, (b) 17β-estradiol binding to calf thymus DNA after liver microsome activation, and (c) RNA polymerase activity in the presence of a versatile epoxide-forming oxidant, dimethyldioxirane37. In a study involving HepG2 cells, DATS treatment inhibited BP-induced ethoxyresorufin O-deethylase activity, a marker enzyme for CYP1, by 70-95% at 100-1,000 μM concentrations38. Immunoblotting studies revealed DATS-mediated suppression of BP-inducible CYP1A2 protein38. Accordingly, levels of BP-7,8-diol were significantly reduced in DATS-treated microsomes38. These results suggest that the protective mechanism of DATS on BP-induced carcinogenesis is possibly also related to suppression of CYP1-mediated bioactivation of this carcinogen.
DATS-Mediated Inhibition of Cancer Cell Proliferation and Cell Cycle Arrest
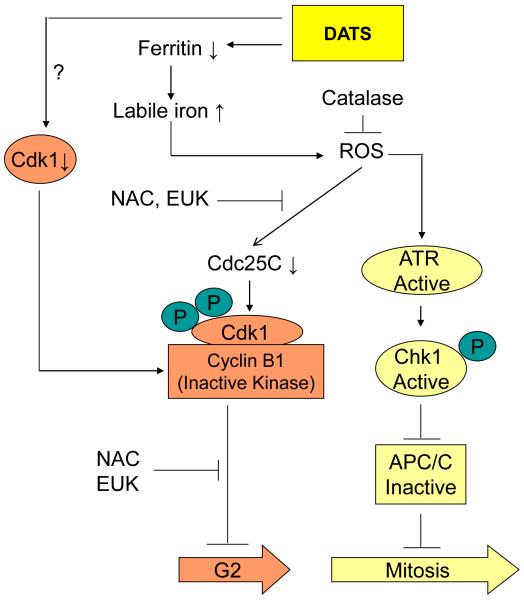
Milner and colleagues were the first to demonstrate anti-proliferative effect of DATS against cancer cells39. DATS-mediated suppression of cancer cell proliferation is associated with cell cycle arrest40-53, which has been reported in human liver cancer cells40, gastric cancer cells41, colon cancer cells42, prostate cancer cells43-48, lung cancer cells50,51, bladder cancer cells52, and skin cancer cells53. Most of these studies have revealed G2/M phase or mitotic arrest upon DATS treatment, but the mechanisms underlying blockade of cell cycle progression are better characterized in prostate cancer cells43-48. DATS-mediated G2/M phase cell cycle arrest in prostate cancer cells was associated with reactive oxygen species (ROS)-dependent hyperphosphorylation and destruction of the cell division cycle 25C phosphatase43. Notably, DATS-mediated G2/M phase cell cycle arrest occurred selectively in cancerous cells because a normal prostate epithelial cell line (PrEC) was resistant to cell cycle arrest by DATS43. Follow-up studies revealed that the ROS generation by DATS treatment in prostate cancer cells was caused by an increase in the level of labile iron due to c-Jun N-terminal kinase (JNK)-mediated degradation of the iron storage protein ferritin45. Further investigation indicated that DATS-treated prostate cancer cells were also arrested in prometaphase partly due to checkpoint kinase 1-dependent inactivation of the anaphase promoting complex/cyclosome44,46,47. A mechanistic model for DATS-induced cell cycle arrest in prostate cancer cells is schematically depicted in Figure 2. DATS-mediated cell cycle arrest in J5 human liver cancer cells was accompanied by accumulation of cyclin B1 and down-regulation of cyclin-dependent kinase (Cdk)740. Even though mitotic markers were not examined in this study, accumulation of cyclin B1 is suggestive of mitotic arrest by DATS in J5 cells40. In H358 human lung cancer cells, DATS treatment resulted in induction of cyclin B1, and down-regulation of total and Tyr15 phosphorylated Cdk1 (inactive kinase) and cell division cycle 25C phosphatase50.
Figure 2.
Molecular mechanisms of DATS-induced cell cycle arrest in human prostate cancer cells43-48. The DATS treatment causes degradation of ferritin to cause an increase in levels of labile iron leading to ROS generation. The DATS-induced ROS generation results in down-regulation of Cdc25C, which is attenuated in the presence of antioxidants such as N-acetylcysteine and superoxide dismutase and catalase mimetic EUK134. The DATS treatment down-regulates Cdk1 expression in prostate cancer cells, but the mechanism of this effect is not yet clear. Mitotic arrest resulting from DATS exposure, characterized by accumulation of cyclin B1 and securin, is caused by checkpoint kinase 1-dependent inactivation of anaphase-promoting complex/cyclosome.
Using HCT-15 and DLD-1 human colon cancer cells as a model42, it was shown that DATS treatment disrupted microtubule network formation. DATS treatment also inhibited tubulin polymerization in an in vitro cell-free system42. Peptide mass-mapping by liquid chromatography-tandem mass spectrometry of DATS-treated tubulin revealed modification of cysteine residues Cys-12β and Cys354β42. DATS-mediated disruption of microtubule network formation has also been observed in HT-29 human colon cancer cell line49. Because DATS-mediated mitotic arrest was irreversible46 and a fraction of cells arrested in mitosis were driven to apoptosis47, it is reasonable to conclude that cell cycle arrest is an important event in suppression of cancer cell proliferation by DATS.
Molecular Mechanisms of DATS-Induced Apoptosis
Numerous publications have concluded that apoptosis induction is an important mechanism for anticancer activity of DATS. The first report on DATS-induced apoptosis was published by Milner and colleagues who observed DNA fragmentation in DATS-treated A549 human lung cancer cells39. Elucidation of the mechanism(s) underlying DATS-induced apoptosis has been the topic of intense research in the last decade. Table 2 summarizes studies delineating mechanism of DTS-induced apoptosis with some functional experiments to test validity of the observed molecular changes. Most studies implicate involvement of Bcl-2 family proteins in regulation of DATS-mediated apoptosis. For example, work from our group has revealed that DATS-induced apoptosis in PC-3 and DU145 human prostate cancer cells, which are androgen-independent and lack functional wild-type p53, is associated with a decrease in Bcl-2 protein level as well as its hyperphosphorylation leading to reduced Bcl-2:Bax interaction and activation of caspase-9 and caspase-354. DATS-mediated hyperphosphorylation of Bcl-2 in PC-3 and DU145 cells is mediated by ROS-dependent activation of JNK, and to a smaller extent by activation of extracellular signal-regulated kinase 1/254. DATS treatment decreased Bcl-2 and Bcl-xL protein levels and caused induction of proapoptotic multidomain protein Bak in LNCaP human prostate cancer cell line55, which is androgen-responsive and expresses wild-type p53. While ectopic expression of Bcl-2 or Bcl-xL did not confer any protection of cell death resulting from DATS exposure in the LNCaP cells55, partial protection against cell death was discernible in the PC-3 cell line54. These observations point towards cell line-specific differences in DATS-induced apoptotic mechanisms. RNA interference of Bax and Bak proteins also conferred partial but significant protection against DATS-induced apoptosis in LNCaP cells55. Similar to PC-3 and DU145 cells54, DATS-induced apoptosis in LNCaP cells was accompanied by ROS generation. In LNCaP cells, both ROS generation and apoptosis resulting from DATS treatment were significantly attenuated in the presence of N-acetylcysteine55. Collectively, these studies indicated that DATS-induced apoptosis was not influenced by the androgen-receptor or the p53 status54,55. In the context of androgen-receptor signaling, DATS treatment has been shown to cause transcriptional repression and inhibition of nuclear translocation of androgen-receptor in LNCaP cells, its androgen-independent variant LNCaP-C4-2, and in a cell line derived from spontaneously developing prostate tumor of a TRAMP mouse (TRAMP-C1 cells)56. Moreover, DATS-mediated prevention of prostate cancer development in TRAMP mice was associated with a significant decrease in expression of androgen-receptor in the poorly-differentiated cancer56. Thus, suppression of androgen receptor signaling by DATS probably contributes to its anticancer effect in prostate cancer.
Table 2.
Molecular mechanisms of diallyl trisulfide (DATS)-induced apoptosis in cancer cells.
| Tumor TypeRef. | Cell Line | DATS Dose | Observed Changes | Functional Studies and Outcome |
|---|---|---|---|---|
| Prostate54 | PC-3, DU145 | 20-40 μM | ↑ROS,↑P-JNK,↑P-Bcl-2, ↓Bcl-2:Bax Interaction |
Protection against apoptosis by Bcl-2 and catalase overexpression |
| Prostate55 | LNCaP | 10-40 μM | ↓Bcl-2, ↓Bcl-xL, ↑Bax, ↑Bak, ↑ROS |
No effect on apoptosis by Bcl-2 or Bcl-xL overexpression, but protection by Bax/Bak siRNA |
| Prostate57 | PC-3, DU145 | 40-80 μM | ↓P-Akt, ↓GSK3-α/β, ↓P-BAD, ↓IGF-1R, ↓PI3K ↓14-3-3β:BAD interaction |
Apoptosis inhibition by over- expression of constitutively active Akt and caspase inhibitors |
| Prostate58 | LNCaP, DU145 | 20-40 μM | ↓P-JAK2→↓P-STAT3 ↓STAT3 dimer formation |
No effect on apoptosis by IL-6 mediated activation of STAT3 |
| Prostate59 | PC-3, LNCaP | 20-40 μM | ↓XIAP, ↑survivin, ↑cIAP1 |
Inhibition of apoptosis by ectopic expression of XIAP but modest effect of survivin or cIAP1 siRNA |
| Lung50 | H358, H460 | 10-40 μM | ↓Bcl-2, ↓Bcl-xL, ↑Bax ↑Bak, ↑BID |
Apoptosis inhibition by Bax and/or Bak siRNA, but no effect of BID knockdown |
| Lung51 | A549 | 12.5-100 μM | ↓Bcl-2, ↑P-JNK, ↓P-ERK ↑p53, ↑survivin, ↑ROS |
Inhibition of apoptosis by JNK inhibitor and antioxidants |
| Breast69 | MDA-MB-231 | 10-100 μM | ↑ROS, ↑P-ASK1, ↑JNK, ↑P-Bim |
Protection against cytotoxicity by JNK inhibitor |
It is intriguing to note that DATS treatment causes only a modest increase in protein levels of Bax and Bak in LNCaP cells and no increase in Bax level in the PC-3 cells yet knockdown of these proteins confers statistically significant protection against DATS-induced apoptosis54,55. Even though the mechanism(s) by which Bax and Bak regulate DATS-induced cell death are not fully elucidated, it is possible that DATS induces a conformational change and oligomerization of Bax/Bak resulting in their translocation to the mitochondria. This speculation is partially supported by the following correlative observations: (a) certain apoptotic stimuli cause Bax activation in an ROS-dependent manner and DATS treatment causes ROS generation51,55; (b) microtubule damaging agents induce Bax activation, and DATS treatment is shown to disrupt tubulin network42.
In PC-3 and DU145 cells, DATS treatment resulted in suppression of P-AktSer473/Thr308, P-GSK3α/βSer21/9, P-BADSer155, total IGF-R protein level, and total PI3K protein level57. Net outcome of these alterations was reduced interaction between 14-3-3β and BAD leading to mitochondrial translocation of BAD57. Furthermore, DATS-induced apoptosis in DU145 cells was significantly attenuated by ectopic expression of constitutively active Akt57. DATS-mediated activation of caspase-3 and apoptosis were also inhibited in the presence of a pan-caspase inhibitor (zVAD-fmk) and a caspase-9 specific inhibitor (zLEHD-fmk)57.
Using LNCaP and DU145 cells as models, we have investigated the role of signal transducer and activator of transcription 3 (STAT3)58, which is activated in prostate cancer, in regulation of DATS-induced apoptosis. DATS treatment resulted in suppression of constitutive (DU145) as well as IL-6-induced (LNCaP) phosphorylation of STAT3Tyr705, which correlated with inhibition of Janus-activated kinase 2 phosphorylation58. Constitutive and/or IL-6-induced nuclear translocation of P-STAT3 and STAT3 dimerization was also inhibited markedly on treatment with DATS in LNCaP and DU145 cell lines58. Inhibition of prostate cancer development in TRAMP mice by DATS correlated with a visible decrease in the levels of P-STAT358. Interestingly, IL-6-mediated activation of STAT3 largely failed to confer any protection against proapoptotic response to DATS in both cells. Likewise, DATS-mediated inhibition of cell migration was either not affected or minimally reversed by the IL-6 treatment or ectopic expression of constitutively active STAT358. These findings indicate that activation of STAT3 is largely dispensable for proapoptotic response to DATS, which should be viewed as a therapeutic advantage for this chemopreventive agent.
We have systematically studied the role of inhibitor of apoptosis family proteins in regulation of DATS-induced apoptosis using PC-3, LNCaP or DU145 cells59. Level of X-linked inhibitor of apoptosis (XIAP) protein was decreased upon 8-hour treatment with DATS59. In contrast, DATS-treated PC-3 and LNCaP cells exhibited marked induction of survivin and cIAP1 proteins59. Dorsolateral prostates from DATS-treated TRAMP mice exhibited statistically significant down-regulation of XIAP and induction of survivin protein compared with those of control mice59. Ectopic expression of XIAP conferred significant protection against DATS-induced apoptosis59. On the other hand, DATS-induced apoptosis was only marginally affected by RNA interference of survivin or cIAP159. These results indicate that DATS-induced apoptosis in prostate cancer cells is mediated by suppression of XIAP protein expression, and that XIAP represents a viable biomarker of DATS response in future clinical investigations.
Similar to the prostate cancer cells, DATS-induced apoptosis in H358 and H460 human lung cancer cell lines was associated with downregulation of Bcl-2 and Bcl-xL, and up-regulation of proapoptotic Bax, Bak, and BID protein expression50. BID protein was dispensable for DATS-induced apoptosis as evidenced by comparable sensitivity of SV40-immortalized mouse embryonic fibroblasts derived from wild-type and BID knockout mice. RNA interference of Bax and/or Bak significantly protected against DATS-induced apoptosis50. In human lung A549 adenocarcinoma cell line, DATS-induced apoptosis was accompanied by a marked and progressive increase in intracellular Ca2+level39. In another study, DATS treatment caused downregulation of Bcl-2 (but not Bcl-xL), increased activation of JNK (but not p38), caused induction of p53 and survivin (but not Bax or Fas), and ROS generation in A549 cells51. Similar to the prostate cancer cells54,55, DATS-induced apoptosis in A549 cells was significantly reduced in the presence of JNK inhibitor and N-acetylcysteine51.
DATS was shown to cause apoptosis in HCT-15 and DLD-1 human colon cancer cells but the mechanism was not studied42. In BGC823 human gastric cancer cell line, DATS-induced apoptosis was associated with suppression of Bcl-2 expression and caspase-3 activation, but no change in Bax expression60. Functional studies to determine the role of Bcl-2 were not carried out by these investigators60. Caspase-3 activation by DATS treatment was shown in MGC803 gastric cancer cell line61. DATS treatment caused up-regulation of protein of programmed cell death 5 in gastric cancer cells62. A few studies have used proteomics and gene expression techniques to identify targets of DATS in gastric cancer cells. In DATS-treated BGC823 cells, a total of 41 unique proteins were detected with significant changes in their expression levels, including GST-Pi, voltage-dependent anion channel-1, Annexin I, Galectin, and S100A1163. Noticeably, nearly 50% of the DATS-modulated proteins (19/41) are associated with apoptotic pathways. DATS-inducible differentially expressed genes were identified in BGC823 gastric cancer cells using a highly specific subtractive hybridization of cDNA representational difference analysis64. A total of 14 cDNA fragments (11 upregulated and 3 downregulated by DATS treatment) were isolated and confirmed by reverse Northern blot analysis64. A proteomic study of DATS-treated Saos-2 osteosarcoma cells revealed alterations in 27 unique proteins (18 downregulated and 9 upregulated), of which 13 are related to either cell cycle or apoptosis65. However, functional studies to confirm the role of altered proteins in DATS-mediated cell cycle arrest or apoptosis are lacking. Similar to the prostate cancer cells54, DATS was much more effective against Caco-2 and HT-29 human colon cancer cells compared with diallyl disulfide or diallyl sulfide66. Iitsuka et al67 studied the relationship between lipophilicity and inhibitory activity against HT-29 colon cancer cell growth of natural and synthetic trisulfides. Compounds with 3-carbon chains were found to be stronger in terms of growth inhibition67.
In T24 bladder cancer cells, correlative studies revealed suppression of P-AktSer473 and P-PDK1Ser241 phosphorylation, downregulation of Bcl-2. and Bax induction upon treatment with DATS52. DATS-induced apoptosis in A375 and BCC skin cancer cells was accompanied by ROS generation, collapse of mitochondrial membrane potential, and caspase-3 and caspase-9 cleavage53. DATS treatment caused apoptosis in MCF-768 and MDA-MB-231 human breast cancer cells69. Correlative studies showed induction of p53 and Bax mRNA and protein, and upregulation of Fas and downregulation of Akt and Bcl-2 mRNA in DATS-treated MCF-7 cells68. In MDA-MB-231 cells, DATS treatment resulted in ROS production, which was detected through glutaredoxin (GRX), a redox-sensing molecule, and subsequently GRX was dissociated from apoptosis signal-regulating kinase 1 (ASK1). Dissociation of GRX from ASK1 resulted in the activation of ASK1 and JNK-Bim pathway. A JNK inhibitor blocked DATS-induced Bim phosphorylation and protected cells from DATS-induced cytotoxicity69. Caspase-8 and p38 mitogen-activated protein kinase were implicated in DATS-induced apoptosis in CNE2 human nasopharyngeal cells70. In T98G and U87MG human glioblastoma cells, DATS treatment caused apoptosis in association with ROS generation and caspase-3 activation71.
Evidence for DATS-Mediated Apoptosis In Vivo
A few studies have looked at DATS-induced apoptosis in vivo. Our laboratory was the first to show that DATS administration (6 μmol DATS, which equates to ~1 mg DATS/mouse or roughly 50 mg DATS/kg body weight, three times/week for 20 days) to PC-3 tumor bearing mice resulted in increased number of terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)-positive apoptotic bodies19. DATS-induced apoptosis in vivo in PC-3 tumor xenografts correlated with statistically significant increase in protein levels of Bax and Bak in the tumor19. Unlike cellular data54,55, however, the levels of Bcl-2, Bcl-xL, or BID were not altered by DATS administration in PC-3 xenografts in vivo19. In an orthotopic PC-3 xenograft model, five weekly injection of 40 mg DATS/kg body weight for 4 weeks resulted in increased TUNEL-positive apoptotic cells20. DATS-mediated downregulation of Bcl-2 and Bcl-xL, upregulation of death receptor 4, death receptor 5, Bax, and Bak, and activation of caspase-8 were also reported in the orthotopic PC-3 xenografts20. Reasons behind discrepancy in DATS effects in subcutaneous19 versus orthotopic PC-3 xenografts20 in the context of Bcl-2 and Bcl-xL expression are not clear but likely relate to route and frequency of DATS administration. Administration of a hepatic-targeted polybutylcyanoacrylate nanoparticles of DATS (1.5 mg/kg, every other day for 14 days) to orthotopic HepG2 xenograft bearing BALB/c mice resulted in increased TUNEL-positive apoptotic bodies in the experimental tumors compared with control tumors21. Level of Bcl-2 protein was significantly lower in HepG2 tumors from DATS nanoparticle group compared with those of control mice, but there were no significant differences in the expression of Fas, Fas ligand, and Bax21.
To our surprise, prevention of prostate cancer development in TRAMP mice by DATS administration (1 or 2 mg DATS/day, thrice/week for 13 weeks) was not associated with increased apoptosis as judged by TUNEL-assay23. Several possibilities exist to explain discrepancies in the results between cultured human prostate cancer cells54,55 and TRAMP model23. One possibility relates to the frequency and dose of DATS administration. A more intensive dosing regimen, such as higher dose and/or daily administration of DATS, may be required to elicit apoptotic response in the dorsolateral prostate of TRAMP mice in vivo. Likewise, the possibility that earlier treatment with DATS (e.g., starting at 4 weeks of age) leads to increased apoptosis as well as even greater protection against prostate carcinogenesis in TRAMP mice cannot be ignored. Additional work is needed to systematically explore these possibilities.
Inhibition of Angiogenesis and Other Effects
DATS treatment inhibited capillary-like tube formation and migration of human umbilical vain endothelial cells72. Anti-angiogenic effect of DATS correlated with suppression of VEGF secretion, down-regulation of VEGF receptor-2 protein level and inactivation of Akt72. Even though DATS treatment inhibited migration of PC-3 cells, formation of new blood vessels was comparable in PC-3 tumor xenografts from control and DATS-treated mice as judged by immunohistochemical analysis of CD3119. Interestingly, DATS treatment resulted in suppression of neoangiogenesis (based on CD31 and Factor VIII staining) coupled with reduction in levels of VEGF in the orthotopic PC-3 xenografts20. However, similar to subcutaneous PC-3 tumor xenograft study19, DATS treatment did not inhibit angiogenesis (CD31 staining) in the TRAMP study23.
Among other noticeable effects, DATS has been shown to: (a) augment activation of T cells and enhance anti-tumor function of macrophages73, (b) reduce lipopolysaccaride-induced expression of inducible nitric oxide synthase, nitric oxide production, and activation of nuclear factor-κB transcription factor in RAW 264.7 cells74, (c) modify membrane rigidity in tumor cells and platelet membrane75, and (d) reverse cancer chemotherapy drug resistance in a osteosarcoma cell line by lowering level of P-glycoprotein76. To the contrary, DATS-mediated inhibition of P-glycoprotein function was not evident in another study77.
Because age is a known risk factor for some cancers (e.g., prostate cancer), we raised the question of whether DATS treatment affects lifespan78. We addressed this question using C. elegans as a model78. Treatment of worms with 5-10 μM DATS increased worm mean lifespan even when treatment was started during young adulthood. DATS administration increased the lifespan of daf-2 and daf-16 mutants, but not the eat-2 mutants78. Microarray experiments demonstrated that a number of genes regulated by oxidative stress and the skn-1 transcription factor were altered by DATS treatment78. Consistently, DATS treatment caused induction of the skn-1 target gene gst-4, and this induction was dependent on skn-178. We also found that the effect of DATS on worm lifespan depended on skn-1 activity in both in the intestine and neurons. Together these results indicate that DATS increases C. elegans lifespan by enhancing the function of the pro-longevity transcription factor skn-178. Further studies are needed to determine the anti-aging effect of DATS in other experimental systems prior to its promotion as a pro-longevity remedy in humans.
Concluding Remarks and Future Directions
Research over the years has revealed that DATS targets multiple pathways to inhibit cancer development, including potentiation of carcinogen detoxification, cell cycle arrest, induction of apoptosis, suppression of oncogenic signaling, and inhibition of angiogenesis. Because DATS exhibits other pharmacological effects, such as cardiovascular and anti-microbial effects, this compound can be classified as a promiscuous agent. This property is not unique to DATS because many other promising dietary cancer chemopreventive agents (e.g., cruciferous vegetable constituent sulforaphane) function similarly79,80. However, promiscuity may be an advantage for cancer chemopreventive agents because pathogenesis of cancer is complex involving abnormalities in multiple checkpoints and signaling pathways. Future research on DATS should focus on pharmacokinetics, bioavailability, and clinical investigations of DATS. Because DATS targets multiple signal transduction pathways, it is also plausible that this agent may prove useful in combination chemoprevention regimens involving mechanistically distinct agents.
Acknowledgement
The work cited in this article from the authors’ laboratory was supported by the United States Public Health Service Grant RO1 CA113363-07, awarded by the National Cancer Institute.
Abbreviations
- DATS
Diallyl trisulfide
- TRAMP
Transgenic Adenocarcinoma of Mouse Prostate
- GST
Glutathione S-transferase
- BP
benzo[a]pyrene
- ROS
reactive oxygen species
- JNK
c-Jun N-terminal kinase
- Cdk
cyclin-dependent kinase
- STAT3
signal transducer and activator of transcription 3
- XIAP
X-linked inhibitor of apoptosis
- GRX
glutaredoxin
- ASK1
apoptosis signal-regulating kinase 1
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
References
- 1.Rivlin RS. Historical perspective on the use of garlic. J Nutr. 2001;131:951S. doi: 10.1093/jn/131.3.951S. [DOI] [PubMed] [Google Scholar]
- 2.Rahman K. Historical perspective on garlic and cardiovascular disease. J Nutr. 2001;131:977S. doi: 10.1093/jn/131.3.977S. [DOI] [PubMed] [Google Scholar]
- 3.Dausch JG, Nixon DW. Garlic: a review of its relationship to malignant disease. Preventive Med. 1990;19:346. doi: 10.1016/0091-7435(90)90034-h. [DOI] [PubMed] [Google Scholar]
- 4.Lau BHS, Tadi PP, Tosk JM. Allium sativum (garlic) and cancer prevention. Nutr Res. 1990;10:937. [Google Scholar]
- 5.Dorant E, van den Brandt PA, Goldbohm RA, Hermus RJJ, Sturmans F. Garlic and its significance for the prevention of cancer in humans: a critical review. Br J Cancer. 1993;67:424. doi: 10.1038/bjc.1993.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal KC. Therapeutic actions of garlic constituents. Med Res Rev. 1996;16:111. doi: 10.1002/(SICI)1098-1128(199601)16:1<111::AID-MED4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Block E. The chemistry of garlic and onions. Sci Am. 1985;252:114. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- 8.You WC, Blot WJ, Chang YS, Ershow A, Yang ZT, An Q, Henderson BE, Fraumeni JF, Wang TG. Allium vegetables and reduced risk of stomach cancer. J Natl Cancer Inst. 1989;81:162. doi: 10.1093/jnci/81.2.162. [DOI] [PubMed] [Google Scholar]
- 9.Gao CM, Takezaki T, Ding JH, Li MS, Tajima K. Protective effect of Allium vegetables against both esophageal and stomach cancer: a simultaneous case-referent study of a high-epidemic area in Jiangsu province, China. Jpn J Cancer Res. 1999;90:614. doi: 10.1111/j.1349-7006.1999.tb00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsing AW, Chokkalingam AP, Gao YT, Madigan MP, Deng J, Gridley G, Fraumeni JF. Allium vegetables and risk of prostate cancer: a population-based study. J Natl Cancer Inst. 2002;94:1648. doi: 10.1093/jnci/94.21.1648. [DOI] [PubMed] [Google Scholar]
- 11.Chan JM, Wang F, Holly EA. Vegetable and fruit intake and pancreatic cancer in a population-based case-control study in the San Francisco bay area. Cancer Epidemiol Biomarkers Prev. 2005;14:2093. doi: 10.1158/1055-9965.EPI-05-0226. [DOI] [PubMed] [Google Scholar]
- 12.Galeone C, Pelucchi C, Dal Maso L, Negri E, Montella M, Zucchetto A, Talamini R, La Vacchia C. Allium vegetables intake and endometrial cancer risk. Public Health Nutr. 2009;12:1576. doi: 10.1017/S1368980008003820. [DOI] [PubMed] [Google Scholar]
- 13.Herman-Antosiewicz A, Powolny AA, Singh SV. Molecular targets of cancer chemoprevention by garlic-derived organosulfides. Acta Pharmacol Sin. 2007;28:1355. doi: 10.1111/j.1745-7254.2007.00682.x. [DOI] [PubMed] [Google Scholar]
- 14.Powolny AA, Singh SV. Multitargeted prevention and therapy of cancer by Diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269:305. doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shukla Y, Kalra N. Cancer chemoprevention with garlic and its constituents. Cancer Lett. 2007;247:167. doi: 10.1016/j.canlet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Perchellet JP, Perchellet EM, Belman S. Inhibition of DMBA-induced mouse skin tumorigenesis by garlic oil and inhibition of two tumor-promotion stages by garlic and onion oils. Nutr Cancer. 1990;14:183. doi: 10.1080/01635589009514093. [DOI] [PubMed] [Google Scholar]
- 17.Sparnins VL, Barany G, Wattenberg LW. Effects of organosulfur compounds from garlic and onions on benzo[a]pyrene-induced neoplasia and glutathione S-transferase activity in the mouse. Carcinogenesis. 1988;9:131. doi: 10.1093/carcin/9.1.131. [DOI] [PubMed] [Google Scholar]
- 18.Hu X, Benson PJ, Srivastava SK, Xia H, Bleicher RJ, Zaren HA, Awasthi S, Awasthi YC, Singh SV. Induction of glutathione S-transferase π as a bioassay for the evaluation of potency of inhibitors of benzo(a)pyrene-induced cancer in a murine model. Int J Cancer. 1997;73:897. doi: 10.1002/(sici)1097-0215(19971210)73:6<897::aid-ijc23>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Xiao D, Lew KL, Kim YA, Zeng Y, Hahm ER, Dhir R, Singh SV. Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin Cancer Res. 2006;12:6836. doi: 10.1158/1078-0432.CCR-06-1273. [DOI] [PubMed] [Google Scholar]
- 20.Shankar S, Chen Q, Ganapathy S, Singh KP, Srivastava RK. Diallyl trisulfide increases the effectiveness of TRAIL and inhibits prostate cancer growth in an orthotopic model: molecular mechanisms. Mol Cancer Ther. 2008;7:2328. doi: 10.1158/1535-7163.MCT-08-0216. [DOI] [PubMed] [Google Scholar]
- 21.Zhang ZM, Yang XY, Deng SH, Xu W, Gao HQ. Anti-tumor effects of polybutylcyanoacrylate nanoparticles of diallyl trisulfide on orthotopic transplantation tumor model of hepatocellular carcinoma in BALB/c nude mice. Chin Med J. 2007;120:1336. [PubMed] [Google Scholar]
- 22.Wu PP, Huang WW, Chueh FS, Ko YC, Chiu TH, Lin JP, Kuo JH, Yang JS, Chung JG. Diallyl trisulfide (DATS) inhibits mouse colon tumor in mouse CT-26 cells allograft model in vivo. Phytomed. 2011 doi: 10.1016/j.phymed.2011.01.006. (in press) [DOI] [PubMed] [Google Scholar]
- 23.Singh SV, Powolny AA, Stan SD, Xiao D, Arlotti JA, Warin R, Hahm ER, Marynowski SW, Bommareddy A, Potter DM, Dhir R. Garlic constituent diallyl trisulfide prevents development of poorly differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP mice. Cancer Res. 2008;68:9503. doi: 10.1158/0008-5472.CAN-08-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrotriya S, Kundu JK, Na HK, Surh YJ. Diallyl trisulfide inhibits phorbol ester-induced tumor promotion, activation of AP-1, and expression of COX-2 in mouse skin by blocking JNK and Akt signaling. Cancer Res. 2010;70:1932. doi: 10.1158/0008-5472.CAN-09-3501. [DOI] [PubMed] [Google Scholar]
- 25.Takada N, Matsuda T, Otoshi T, Yano Y, Otani S, Hasegawa T, Nakae D, Konishi Y, Fukushima S. Enhancement by organosulfur compounds from garlic and onions of diethylnitrosamine-induced glutathione S-transferase positive foci in the rat liver. Cancer Res. 1994;54:2895. [PubMed] [Google Scholar]
- 26.Sun X, Guo T, He J, Zhao M, Yan M, Cui F, Deng Y. Determination of concentration of diallyl trisulfide in rat whole blood using gas chromatography with electron-capture detection and identification of its major metabolite with gas chromatography mass spectrometry. Yakugaku Zasshi. 2006;126:521. doi: 10.1248/yakushi.126.521. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Li HQ, Wang Y, Xu HX, Fan WT, Wang ML, Sun PH, Xie XY. An intervention study to prevent gastric cancer by micro-selenium and large dose of allitridum. Chin Med J. 2004;117:1155. [PubMed] [Google Scholar]
- 28.Srivastava SK, Hu X, Xia H, Zaren HA, Chatterjee ML, Agarwal R, Singh SV. Mechanism of differential efficacy of garlic organosulfides in preventing benzo(a)pyrene-induced cancer in mice. Cancer Lett. 1997;118:61. doi: 10.1016/s0304-3835(97)00237-1. [DOI] [PubMed] [Google Scholar]
- 29.Hu X, Singh SV. Glutathione S-transferases of female A/J mouse lung and their induction by anticarcinogenic organosulfides from garlic. Arch Biochem Biophys. 1997;340:279. doi: 10.1006/abbi.1997.9915. [DOI] [PubMed] [Google Scholar]
- 30.Hu X, Benson PJ, Srivastava SK, Mack LM, Xia H, Gupta V, Zaren HA, Singh SV. Glutathione S-transferases of female A/J mouse liver and forestomach and their differential induction by anti-carcinogenic organosulfides from garlic. Arch Biochem Biophys. 1996;336:199. doi: 10.1006/abbi.1996.0550. [DOI] [PubMed] [Google Scholar]
- 31.Hu X, Srivastava SK, Xia H, Awasthi YC, Singh SV. An alpha class mouse glutathione S-transferase with exceptional catalytic efficiency in the conjugation of glutathione with 7β,8α-dihydroxy-9α,10α-oxy-7,8,9,10-tetrahydrobenzo(a)pyrene. J Biol Chem. 1996;271:32684. doi: 10.1074/jbc.271.51.32684. [DOI] [PubMed] [Google Scholar]
- 32.Singh SV, Pan SS, Srivastava SK, Xia H, Hu X, Zaren HA, Orchard JL. Differential induction of NAD(P)H:quinone oxidoreductase by anti-carcinogenic organosulfides from garlic. Biochem Biophys Res Commun. 1998;244:917. doi: 10.1006/bbrc.1998.8352. [DOI] [PubMed] [Google Scholar]
- 33.Munday R, Munday CM. Relative activities of organosulfur compounds derived from onions and garlic in increasing activities of quinine reductase and glutathione transferase in rat tissues. Nutr Cancer. 2001;40:205. doi: 10.1207/S15327914NC402_18. [DOI] [PubMed] [Google Scholar]
- 34.Chen C, Pung D, Leong V, Hebbar V, Shen G, Nair S, Li W, Kong AN. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free Rad Biol Med. 2004;37:1578. doi: 10.1016/j.freeradbiomed.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 35.Wu CC, Lii CK, Tsai SJ, Sheen LY. Diallyl trisulfide modulates cell viability and the antioxidation and detoxification systems of rat primary hepatocytes. J Nutr. 2004;134:724. doi: 10.1093/jn/134.4.724. [DOI] [PubMed] [Google Scholar]
- 36.Chang HS, Ko M, Ishizuka M, Fujita S, Yabuki A, Hossain MA, Yamato O. Sodium 2-propenyl thiosulfate derived from garlic induces phase II detoxification enzymes in rat hepatoma H4IIE cells. Nutr Res. 2010;30:435. doi: 10.1016/j.nutres.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Yu FL, Bender W, Fang Q, Ludeke A, Welch B. Prevention of chemical carcinogen DNA binding and inhibition of nuclear RNA polymerase activity by organosulfur compounds as the possible mechanisms for their anticancer initiation and proliferation effects. Cancer Detect Prev. 2003;27:370. doi: 10.1016/s0361-090x(03)00135-1. [DOI] [PubMed] [Google Scholar]
- 38.Chun HS, Kim HJ, Choi EH. Modulation of cytochrome P4501-mediated bioactivation of benzo[a]pyrene by volatile allyl sulfides in human hepatoma cells. Biosci Biotechnol Biochem. 2001;65:2205. doi: 10.1271/bbb.65.2205. [DOI] [PubMed] [Google Scholar]
- 39.Sakamoto K, Lawson LD, Milner JA. Allyl sulfides from garlic suppress the in vitro proliferation of human A549 lung tumor cells. Nutr Cancer. 1997;29:152. doi: 10.1080/01635589709514617. [DOI] [PubMed] [Google Scholar]
- 40.Wu CC, Chung JG, Tsai SJ, Yang JH, Sheen LY. Differential effects of allyl sulfides from garlic essential oil on cell cycle regulation in human liver tumor cells. Food Chem Toxicol. 2004;42:1937. doi: 10.1016/j.fct.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Ha MW, Ma R, Shun LP, Gong YH, Yuan Y. Effects of allitridi on cell cycle arrest of human gastric cancer cells. World J Gastroenterol. 2005;11:5433. doi: 10.3748/wjg.v11.i35.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosono T, Fukao T, Ogihara J, Ito Y, Shiba H, Seki T, Ariga T. Diallyl trisulfide suppresses the proliferation and induces apoptosis of human colon cancer cells through oxidative modification of β-tubulin. J Biol Chem. 2005;280:41487. doi: 10.1074/jbc.M507127200. [DOI] [PubMed] [Google Scholar]
- 43.Xiao D, Herman-Antosiewicz A, Antosiewicz J, Xiao H, Brisson M, Lazo JS, Singh SV. Diallyl trisulfide-induced G2-M phase cell cycle arrest in human prostate cancer cells is caused by reactive oxygen species-dependent destruction and hyperphosphorylation of Cdc25C. Oncogene. 2005;24:6256. doi: 10.1038/sj.onc.1208759. [DOI] [PubMed] [Google Scholar]
- 44.Herman-Antosiewicz A, Singh SV. Checkpoint kinase 1 regulates diallyl trisulfide-induced mitotic arrest in human prostate cancer cells. J Biol Chem. 2005;280:28519. doi: 10.1074/jbc.M501443200. [DOI] [PubMed] [Google Scholar]
- 45.Antosiewicz J, Herman-Antosiewicz A, Marynowski SW, Singh SV. c-Jun NH2-terminal kinase signaling axis regulates diallyl trisulfide-induced generation of reactive oxygen species and cell cycle arrest in human prostate cancer cells. Cancer Res. 2006;66:5379. doi: 10.1158/0008-5472.CAN-06-0356. [DOI] [PubMed] [Google Scholar]
- 46.Herman-Antosiewicz A, Stan SD, Hahm ER, Xiao D, Singh SV. Activation of a novel ataxia-telangiectasia mutated and Rad3 related/checkpoint kinase 1-dependent prometaphase checkpoint in cancer cells by diallyl trisulfide, a promising cancer chemopreventive constituent of processed garlic. Mol Cancer Ther. 2007;6:1249. doi: 10.1158/1535-7163.MCT-06-0477. [DOI] [PubMed] [Google Scholar]
- 47.Xiao D, Zeng Y, Singh SV. Diallyl trisulfide-induced apoptosis in human cancer cells is linked to checkpoint kinase 1-mediated mitotic arrest. Mol Carcinogenesis. 2009;48:1018. doi: 10.1002/mc.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herman-Antosiewicz A, Kim YA, Kim SH, Xiao D, Singh SV. Diallyl trisulfide-induced G2/M phase cell cycle arrest in DU145 cells is associated with delayed nuclear translocation of cyclin-dependent kinase 1. Pharm Res. 2010;27:1072. doi: 10.1007/s11095-010-0060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosono T, Hosno-Fukao T, Inada K, Tanaka R, Yamada H, Iitsuka Y, Seki T, Hasegawa I, Ariga T. Alkenyl group is responsible for the disruption of microtubule network formation in human colon cancer cell line HT-29 cells. Carcinogenesis. 2008;29:1400. doi: 10.1093/carcin/bgn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao D, Zeng Y, Hahm ER, Kim YA, Ramalingam S, Singh SV. Diallyl trisulfide selectively causes Bax- and Bak- mediated apoptosis in human lung cancer cells. Env Mol Mut. 2009;50:201. doi: 10.1002/em.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu XJ, Hu Y, Lamy E, Mersch-Sundermann V. Apoptosis induction in human lung adenocarcinoma cells by oil-soluble allyl sulfides: triggers, pathways, and modulators. Env Mol Mut. 2009;50:266. doi: 10.1002/em.20467. [DOI] [PubMed] [Google Scholar]
- 52.Wang YB, Qin J, Zheng XY, Bai Y, Yang K, Xie LP. Diallyl trisulfide induces Bcl-2 and caspase-3 dependent apoptosis via downregulation of Akt phosphorylation in human T24 bladder cancer cells. Phytomed. 2010;17:363. doi: 10.1016/j.phymed.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 53.Wang HC, Yang JH, Hsieh SC, Sheen LY. Allyl sulfides inhibit cell growth of skin cancer cells through induction of DNA damage mediated G2/M arrest and apoptosis. J Agric Food Chem. 2010;58:7096. doi: 10.1021/jf100613x. [DOI] [PubMed] [Google Scholar]
- 54.Xiao D, Choi S, Johnson DE, Vogel V, Johnson CS, Trump DL, Lee YJ, Singh SV. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 55.Kim YA, Xiao D, Xiao H, Powolny AA, Lew KL, Reilly M, Zeng Y, Wang Z, Singh SV. Mitochondria-mediated apoptosis by diallyl trisulfide in human prostate cancer cells is associated with generation of reactive oxygen species and regulated by Bax/Bak. Mol Cancer Ther. 2007;6:1599. doi: 10.1158/1535-7163.MCT-06-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stan SD, Singh SV. Transcriptional repression and inhibition of nuclear translocation of androgen receptor by diallyl trisulfide in human prostate cancer cells. Clin Cancer Res. 2009;15:4895. doi: 10.1158/1078-0432.CCR-09-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao D, Singh SV. Diallyl trisulfide, a constituent of processed garlic, inactivates Akt to trigger mitochondrial translocation of BAD and caspase-mediated apoptosis in human prostate cancer cells. Carcinogenesis. 2006;27:533. doi: 10.1093/carcin/bgi228. [DOI] [PubMed] [Google Scholar]
- 58.Chandra-Kuntal K, Singh SV. Diallyl trisulfide inhibits activation of signal transducer and activator of transcription 3 in prostate cancer cells in culture and in vivo. Cancer Prev Res. 2010;3:1473. doi: 10.1158/1940-6207.CAPR-10-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SH, Bommareddy A, Singh SV. Garlic constituent Diallyl trisulfide suppresses X-linked inhibitor of apoptosis protein in prostate cancer cells in culture and in vivo. Cancer Prev Res. 2011 doi: 10.1158/1940-6207.CAPR-10-0323. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lan H, Lu YY. Allitridi induces apoptosis by affecting Bcl-2 expression and caspase-3 activity in human gastric cancer cells. Acta Pharmacol Sin. 2004;25:219. [PubMed] [Google Scholar]
- 61.Xiao XL, Peng J, Su Q, Xiang SL, Tang GH, Huang YS, Zhou XT. Diallyl trisulfide induces apoptosis of human gastric cancer cell line MGC803 through caspase-3 pathway. Chin J Cancer. 2006;25:1247. [PubMed] [Google Scholar]
- 62.Yang YH, Zhao M, Li WM, Chen YY, Kang B, Lu YY. Expression of programmed cell death 5 gene involves in regulation of apoptosis in gastric tumor cells. Apoptosis. 2006;11:993. doi: 10.1007/s10495-006-6714-6. [DOI] [PubMed] [Google Scholar]
- 63.Li N, Guo R, Li W, Shao J, Li S, Zhao K, Chen X, Xu N, Liu S, Lu Y. A proteomic investigation into a human gastric cancer cell line BGC823 treated with Diallyl trisulfide. Carcinogenesis. 2006;27:1222. doi: 10.1093/carcin/bgi306. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Lu YY. Isolation of Diallyl trisulfide inducible differentially expressed genes in human gastric cancer cells by modified cDNA representational difference analysis. DNA Cell Biol. 2002;21:771. doi: 10.1089/104454902320908423. [DOI] [PubMed] [Google Scholar]
- 65.Iitsuka Y, Tanaka Y, Hosono-Fukao T, Hosono T, Seki T, Ariga T. Relationship between lipophilicity and inhibitory activity against cancer cell growth of nine kinds of alk(en)yl trisulfides with different side chains. Oncol Res. 2010;18:575. doi: 10.3727/096504010x12767359113965. [DOI] [PubMed] [Google Scholar]
- 66.Jakubikova J, Sedlak J. Garlic-derived organosulfides induce cytotoxicity, apoptosis, cell cycle arrest, and oxidative stress in human colon carcinoma cell lines. Neoplasma. 2006;53:3. [PubMed] [Google Scholar]
- 67.Zhang YK, Zhang XH, Li JM, Sun DS, Yang Q, Diao DM. A proteomic study on a human osteosarcoma cell line Saos-2 treated with Diallyl trisulfide. Anti-Cancer Drugs. 2009;20:702. doi: 10.1097/CAD.0b013e32832e89c7. [DOI] [PubMed] [Google Scholar]
- 68.Malki A, El-Saadani M, Sultan AS. Garlic constituent diallyl trisulfide induced apoptosis in MCF-7 human breast cancer cells. Cancer Biol Ther. 2009;8:2174. doi: 10.4161/cbt.8.22.9882. [DOI] [PubMed] [Google Scholar]
- 69.Lee BC, Park BH, Kim SY, Lee YJ. Role of Bim in Diallyl trisulfide-induced cytotoxicity in human cancer cells. J Cell Biochem. 2011;112:118. doi: 10.1002/jcb.22896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ji C, Ren F, Xu M. Caspase-8 and p38MAPK in DATS-induced apoptosis of human CNE2 cells. Braz J Med Biol Res. 2010;43:821. doi: 10.1590/s0100-879x2010007500084. [DOI] [PubMed] [Google Scholar]
- 71.Das A, Banik NL, Ray SK. Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer. 2007;110:1083. doi: 10.1002/cncr.22888. [DOI] [PubMed] [Google Scholar]
- 72.Xiao D, Li MF, Herman-Antosiewicz A, Antosiewicz J, Xiao H, Lew KL, Zeng Y, Marynowski SW, Singh SV. Diallyl trisulfide inhibits angiogenic features of human umbilical vein endothelial cells by causing Akt inactivation and down-regulation of VEGF and VEGF-R2. Nutr Cancer. 2006;55:94. doi: 10.1207/s15327914nc5501_12. [DOI] [PubMed] [Google Scholar]
- 73.Feng ZH, Zhang GM, Hao TL, Zhou B, Zhang H, Jiang ZY. Effect of Diallyl trisulfide on the activation of T cell and macrophage-mediated cytotoxicity. J Tonji Med Univ. 1994;14:142. doi: 10.1007/BF02886794. [DOI] [PubMed] [Google Scholar]
- 74.Liu KL, Chen HW, Wang RY, Lei YP, Sheen LY, Lii CK. DATS reduces LPS-induced iNOS expression, NO production, oxidative stress, and NF-κB activation in RAW 264.7 macrophages. J Agric Food Chem. 2006;54:3472. doi: 10.1021/jf060043k. [DOI] [PubMed] [Google Scholar]
- 75.Tsuchiya H, Nagayama M. Garlic allyl derivatives interact with membrane lipids to modify the membrane fluidity. J Biomed Sci. 2008;15:653. doi: 10.1007/s11373-008-9257-8. [DOI] [PubMed] [Google Scholar]
- 76.Li J, Liu W, Zhao K, Zhang Y, Li X, Yang Q, Li Z, Li J. Diallyl trisulfide reverses drug resistance and lowers the ratio of CD133+ cells in conjunction with methotrexate in a human osteosarcoma drug-resistant cell subline. Mol Med Reports. 2009;2:245. doi: 10.3892/mmr_00000091. [DOI] [PubMed] [Google Scholar]
- 77.Nabekura T, Kamiyama S, Kitagawa S. Effects of dietary chemopreventive phytochemicals on P-glycoprotein function. Biochem Biophys Res Commun. 2005;327:866. doi: 10.1016/j.bbrc.2004.12.081. [DOI] [PubMed] [Google Scholar]
- 78.Powolny AA, Singh SV, Melov S, Hubbard A, Fisher AL. The garlic constituent Diallyl trisulfide increases the lifespan of C. elegans via skn-1 activation. Exp Gerontol. 2011 doi: 10.1016/j.exger.2011.01.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stan SD, Kar S, Stoner GD, Singh SV. Bioactive food components and cancer risk reduction. J Cell Biochem. 2008;104:339. doi: 10.1002/jcb.21623. [DOI] [PubMed] [Google Scholar]
- 80.Stan SD, Singh SV, Brand RE. Chemoprevention strategies for pancreatic cancer. Nature Rev Gastroenterol Hepatol. 2010;7:347. doi: 10.1038/nrgastro.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]