Abstract
A burst of action potentials in hippocampal neurons is followed by a slow afterhyperpolarization (sAHP) that serves to limit subsequent firing. A reduction in the sAHP accompanies acquisition of several types of learning, whereas increases in the sAHP are correlated with cognitive impairment. The present study demonstrates in vitro that activity-dependent bidirectional plasticity of the sAHP does not require synaptic activation, and depends on the pattern of action potential firing. Whole-cell current-clamp recordings from CA1 pyramidal neurons in hippocampal slices from young rats (p14–24) were performed in blockers of synaptic transmission. The sAHP was evoked by action potential firing at gamma-related (50 Hz, gamma-AHP) or theta frequencies (5 Hz, theta-AHP), two firing frequencies implicated in attention and memory. Interestingly, when the gamma-AHP and theta-AHP were evoked in the same cell, a gradual potentiation of the gamma-AHP (191±32%) was observed that was blocked using Ca2+ channel blockers nimodipine (10 µM) or ω-conotoxin MVIIC (1 µM). In experiments that exclusively evoked the sAHP with 50 Hz firing, the gamma-AHP was similarly potentiated (198±44%). However, theta-burst firing pattern alone resulted in a decrease (65±1%) of the sAHP. In these experiments, application of the h-channel blocker ZD7288 (25 µM) selectively prevented enhancement of the gamma-AHP. These data demonstrate that induction requirements for bidirectional AHP plasticity depend on the pattern of action potential firing, and result from distinct mechanisms. The identification of novel mechanisms underlying AHP plasticity in vitro provides additional insight into the dynamic processes that may regulate neuronal excitability during learning in vivo.
Keywords: intrinsic excitability, intrinsic plasticity, afterhyperpolarization, theta-burst, gamma-related firing, h-channels, L-type Ca2+ channels, N-type Ca2+ channels, P/Q-type Ca2+ channels
INTRODUCTION
Action potentials (APs) in hippocampal neurons are followed by a post-burst afterhyperpolarization (AHP) that is dependent upon several outward Ca2+-dependent K+ currents, most notably the IAHP and sIAHP (Storm, 1990; Sah, 1996), but see Gu and colleagues (2005). The AHP serves to limit firing in response to sustained excitation (Madison & Nicoll, 1984; Lancaster & Nicoll, 1987; Schwindt et al., 1988), and is highly sensitive to changes in intracellular Ca2+ (Storm, 1987; Lancaster & Zucker, 1994; Velumian and Carlen, 1999). Modulation of the AHP by alterations in intracellular Ca2+ can be achieved through several pathways, including Ca2+ entry through L-type (Rascol et al., 1991; Moyer et al., 1992; Gamelli et al., 2009), N-type (Shah & Haylett, 2000), and P/Q-type (Pineda et al., 1999) voltage-dependent Ca2+ channels, and through calcium-induced calcium release (Shah & Haylett, 2000). Elucidating the mechanisms underlying plasticity of the AHP is important because reductions in the slow AHP (sAHP) are posited as a general mechanism for increasing neuronal excitability, which is involved in learning (Disterhoft et al., 1986; Disterhoft et al., 1988; Coulter et al., 1989; Disterhoft et al., 1996; Moyer et al., 1996; Thompson et al., 1996; Saar et al., 1998; Moyer et al., 2000; Saar et al., 2001; Oh et al., 2003; Tombaugh et al., 2005; Disterhoft & Oh, 2006; Ohno et al., 2006; Zelcer et al., 2006; Disterhoft & Oh, 2007; Kaczorowski & Disterhoft, 2009). Preliminary studies suggest that the pattern of action potential firing can influence the magnitude of the AHP (Kaczorowski et al., 2003), but few studies have explored how differences in activity patterns may induce long-lasting changes in the sAHP.
Theta-burst firing has been shown previously to induce a long-lasting activity-dependent reduction of the sAHP/IsAHP (Kaczorowski et al., 2007) that mimics changes observed after learning (see Disterhoft and Oh, 2007 for review). Although theta-burst firing is often thought to be a more physiologically relevant activity pattern (Ranck, 1973; Larson & Lynch, 1986; Larson et al., 1986; Otto et al., 1991), evidence suggests some CA1 pyramidal neurons exhibit gamma-related firing between 40 – 80 Hz in vivo (Barnes et al., 1990; Soltesz & Deschenes, 1993; Senior et al., 2008; Colgin et al., 2009) that may play a role in spatial and temporal encoding and memory (Barnes et al., 1990; Lisman & Idiart, 1995; Csicsvari et al., 2003; Lisman, 2005; Senior et al., 2008; Colgin et al., 2009). Although prior work suggests high-frequency somatic action potential firing in response to depolarizing constant current pulses enhances the sAHP (Borde et al., 1995), a systematic study of the effect of gamma-related firing (using brief current pulses at fixed frequency, 50 Hz) on plasticity of the sAHP in CA1 neurons has not been performed. Moreover, a direct comparison of these two activity patterns matched for the number of action potentials generated has not been performed to date. Therefore, the present report investigated how two different activity patterns (gamma-related firing and theta-burst firing), which have been observed in vivo during spatial navigation and memory tasks (Barnes et al., 1990; Otto et al., 1991; Senior et al., 2008; Colgin et al., 2009; Colgin & Moser, 2010), would interact to modulate the sAHP within the same cell, as well as in isolation, and to explore underlying mechanisms.
The primary candidate mechanisms underlying activity-dependent modulation of the sAHP are notably the IAHP and sIAHP (Borde et al., 1995; Borde et al., 1999; Borde et al., 2000; Le Ray et al., 2004; Kaczorowski et al., 2007), as well as alterations in voltage-gated and store-operated Ca2+ influx (Borde et al., 1995). In addition to IAHP and sIAHP, activity-induced increases in intracellular Ca2+ are likely to activate signaling pathways that can modify the AHP (Pedarzani et al., 1998), although other mechanisms are possible (Pedarzani and Storm, 1996). These modifications are thought to occur through Ca2+-triggered activation of kinases or adenylyl cyclases (Jonas & Kaczmarek, 1996).
The hyperpolarization-activated mixed cation channel (h-channel) is a strong candidate for Ca2+-dependent modulation, and the hyperpolarization-activated mixed cation current (Ih) has been shown to regulate neuronal excitation by altering the size and kinetics of the AHP (Storm, 1990; Maccaferri et al., 1993; Maccaferri et al., 1994; Poolos et al., 2002; Gu et al., 2005). Additionally, Ih is modulated by intracellular cAMP (DiFrancesco & Tortora, 1991), which has been shown to induce a shift in the activation curve of Ih to more depolarized potentials (DiFrancesco, 1993; Pape, 1996). Moreover, the magnitude and source of intracellular Ca2+ increases has been shown to affect Ih (Fan et al., 2005). The present work combines pharmacology and electrophysiology to demonstrate pattern-specific differences in the amount or source of Ca2+ and/or Ih modulation contribute to AHP plasticity independent of synaptic activation.
MATERIALS AND METHODS
Transverse Hippocampal Slices and Internal Solutions
All animal procedures were approved by the Northwestern University Animal Care and Use Committee. Male P14–28 Wistar rats were used. After deep halothane anesthesia, the rat was decapitated and the brain quickly removed and placed into ice-cold artificial cerebral spinal fluid (aCSF, in mM): 125 NaCl, 25 glucose, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2 (pH 7.5, bubbled with 95%O2/5%CO2). A blocking cut was made to obtain near-horizontal slices to maintain intact CA1 apical dendrites within the hippocampal slice. Slices (300 µm) of the medial hippocampus and adjacent cortex were made using a Leica vibratome (Wetzlar, Germany). The slices were first incubated at 34°C in bubbled aCSF for 30 minutes, and then held at room temperature in bubbled aCSF for 1–4 hours before use.
For whole-cell recordings, electrodes prepared from thin-walled capillary glass were filled with 115 mM potassium methylsulfate (KMeth)-based internal solution and had a resistance of 3–5 MΩ. Internal solution also contained 20 KCl, 10 Na-PCr, 10 HEPES, 2 MgATP, 0.3 NaGTP and 0.10% Biocytin. KOH was used to adjust the pH to 7.3. KMeth-based solution was used based on previous work showing this internal anion best recapitulates the AHP compared to perforated-patch configuration, and the appropriate controls were conducted (Kaczorowski et al, 2007). Unless otherwise stated, chemicals were obtained from Sigma (St. Louis, MO). Potassium methylsulfate was purchased from ICN Biomedical (Aurora, OH).
Electrophysiological Recording
Slices were transferred to a recording chamber mounted on a Zeiss Axioskop (Oberkochen, Germany) where they were submerged in oxygenated aCSF at 33–34°C. Neurons were visualized with infrared differential interference contrast video microscopy. High-resistance seals (>1 GΩ) were obtained under visual control on the somata of CA1 pyramidal neurons; brief suction was applied to the patch in order to gain whole-cell access to the neuron. Once in whole-cell configuration, cells were evaluated on a number of criteria and accepted for use only if they had a resting membrane potential (Vm) < −58 mV, access resistance (Rs) < 40 MΩ, input resistance (RN) > 30 MΩ, and an action potential amplitude greater than 70 mV relative to action potential threshold (see data acquisition and analysis). All recordings were done in current-clamp mode using a Dagan current-clamp amplifier; cells were held at −66 mV by manually adjusting the holding current by less than ±100 pA. The electrode capacitance and series resistance (Rs) were monitored, compensated, and recorded frequently throughout the duration of the recording. In all experiments, GABAA receptors, ionotropic glutamate receptors, and muscarinic acetylcholine receptors were blocked by the addition of SR95531 (2 mM), kynurenic acid (2 mM) and atropine (1 µM) to the standard aCSF solution.
Stimulation and Recording Protocols
In the first set of experiments, AHPs were triggered by alternating between two distinct patterns of somatic current injection in the same cell (once every 3 minutes or once per minute for a total of 30 minutes, Figure 1A, B). Gamma-related firing consisted of a train of 50 action potentials at 50 Hz (gamma-AHP, black), and a theta-burst firing that consisted of 10 bursts of 5 action potentials at 100 Hz with an inter-burst frequency of 5 Hz (theta-AHP, grey). Under no circumstances were action potential failures or bursts (doublets) observed in response to brief current step used to evoke the AHP. Note that the stimulation described herein results from somatically generated action potentials, and occurs in the presence of synaptic blockers.
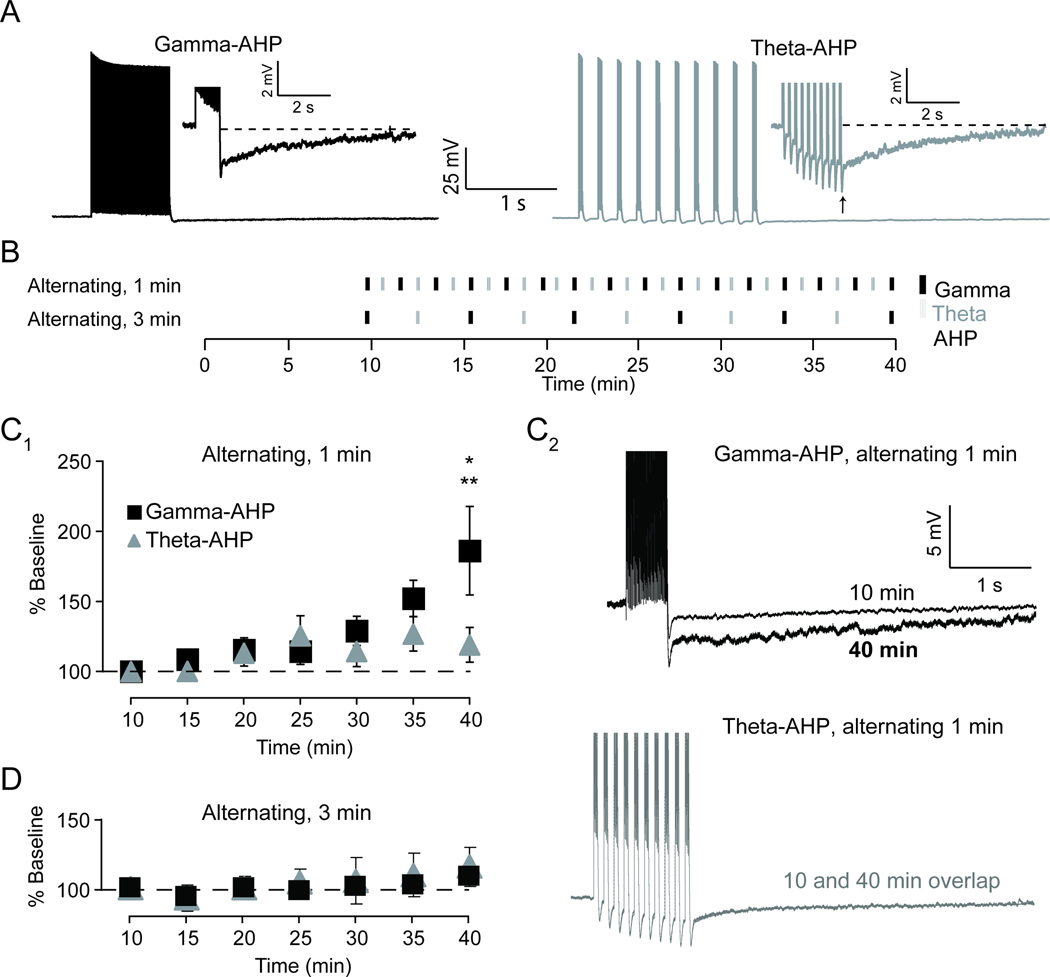
Figure 1. Alternating 50 Hz and theta-burst firing produced an increase in the afterhyperpolarization (AHP) triggered with 50 Hz firing (gamma-AHP) in CA1 pyramidal neurons from hippocampal slice of the rat.
A, Typical example of the post-burst AHP triggered using a train of action potentials at 50 Hz (black) compared to using theta-burst pattern firing (grey). Arrow denotes the peak of the AHP. Inset shows AHP on an expanded scale. AHP integral was measured as area under the dashed line. B, Schematic diagram illustrates the stimulation protocols used to examine plasticity of the AHP. Alternating protocol used somatic action potential firing at 50 Hz (black) and theta-burst firing (grey) in an alternating fashion. This served as both the induction protocol and the stimulus used to evoke AHPs (gamma-AHP and theta-AHP, respectively). C1, Plot showing alternating stimuli at 1 min intervals resulted in a gradual increase in the gamma-AHP relative to baseline (10 min). The gamma-AHP was significantly increased compared to baseline (* p< 0.05) and to theta-AHP measured at 40 minutes (** p< 0.05) in recordings from neurons exposed to alternating 50 Hz and theta-burst firing. C2, Top, representative trace of the gamma-AHP triggered at an early time point (10 min; thin black) superimposed on the gamma-AHP triggered at a late time point (40 min; thick black). Bottom, representative trace of the theta-AHP triggered at an early time point (10 min; dotted grey) superimposed on the gamma-AHP triggered at a late time point (40 min; grey). D, Plot showing alternating stimuli at 3 minute intervals had no effect on integral of the gamma-AHP or theta-AHP relative to baseline (10 min).
In the second set of experiments, cells were exposed to one of the two stimulation patterns once every minute for a total of 40 minutes (Figure 3A). All current injections used to trigger action potentials were brief (2 ms) somatic current injections (1 nA). In general, data collection began within 10 minutes after membrane rupture. In all experiments, initial measurements of the RN were performed directly following capacitance and series resistance compensations, approximately 2–3 minutes after gaining access to the neuron. RN was monitored at least once every 3 min for the duration of the recordings and determined by Ohm’s Law from the voltage response to an 800 ms, −50 pA current step.
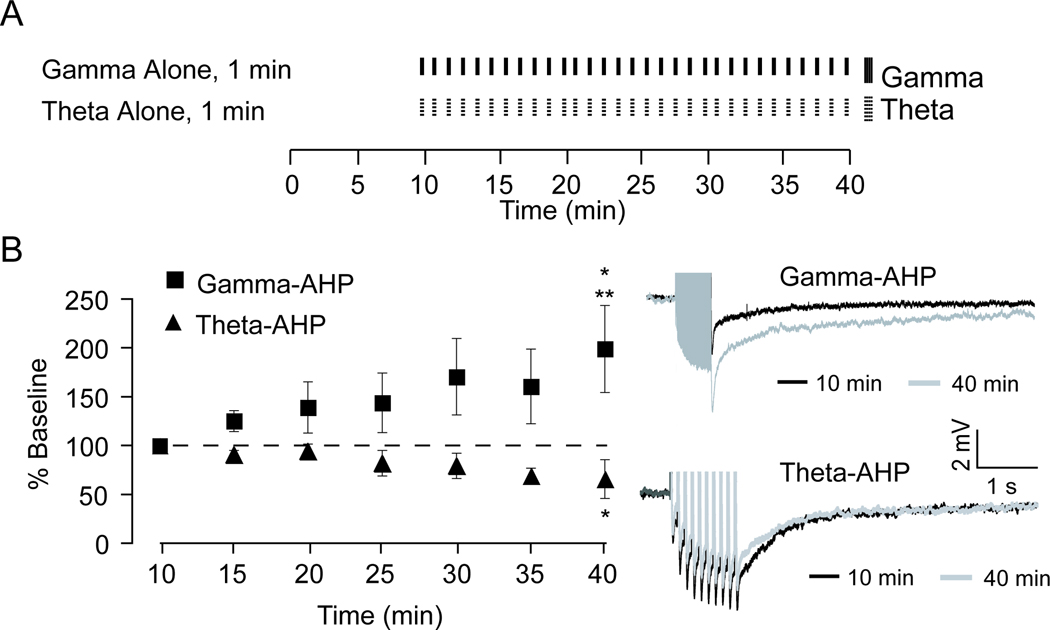
Figure 3. Bidirectional plasticity of the AHP depends on the firing pattern.
A, Schematic diagram illustrates the stimulation protocols used to examine plasticity of the AHP. Neurons received either somatic action potential firing at 50 Hz (black) or theta-burst firing (dotted). B, Plot showing that 50 Hz firing alone produced a significant enhancement of the integral of the gamma-AHP measured at 40 min, relative to baseline (* p < 0.05) and to theta-AHP measured at 40 minutes (** p< 0.05). Theta-burst firing alone produced a significant reduction, relative to baseline, of the integral of the theta-AHP (* p < 0.05). Top right, representative trace of the gamma-AHP triggered at 10 min (black) superimposed on the gamma-AHP triggered at 40 min (grey). Bottom right, representative trace of the theta-AHP triggered at 10 min (black) superimposed on the theta-AHP triggered at 10 min (grey).
Data acquisition and Analysis
Data were transferred to a computer using an ITC-16 digital-to-analog converter (InstruTech, Port Washington, NY). IgorPro (Wavemetrics, Lake Oswego, OR) software was used for acquisition and analysis. Statistical tests were performed using SigmaPlot software (Systat Software Inc., San Jose, CA). Significance was determined by repeated measures ANOVA and Fisher’s least significant difference t-tests where appropriate (unless otherwise noted). All results are reported as mean ± SEM.
The AHP was defined as the membrane potential beginning at the peak negative value (relative to the initial baseline) following the last spike (Figure 1A, upward arrow) and ending when the voltage had decayed to 95% of that peak value (see Kaczorowski et al., 2007). The integral of the AHP was calculated and shown as the area under the red dotted line (Figure 1A, inset). For ease of comparisons, the integral of the AHP was defined as the sAHP, given that the mAHP comprises less than 100 ms of the entire AHP lasting for tens of seconds (Storm, 1990; Stocker et al., 1999). Our prior work shows that > 90% of the integral of the AHP triggered by either gamma-related or theta-burst firing is blocked by the broad spectrum calcium channel blocker Cd2+ (200 µM) or noradrenaline (10 µM), indicating that the majority of the AHP integral is driven by the Ca2+-dependent sIAHP (Kaczorowski et al., 2007). The apamin-sensitive AHP contributes minimally to the integral of the AHP using the present stimulation protocols and recording solutions (Kaczorowski, 2006; Kaczorowski et al., 2007).
RESULTS
Whole-cell recordings were obtained from CA1 pyramidal neurons in rat hippocampal slices. All cells were regular spiking neurons that demonstrated a prominent AHP (a negative membrane potential relative to baseline) in response to action potential firing.
Differential effects of activity patterns on AHP plasticity
First, the AHP was assessed in response to two activity patterns, a gamma-related 50 Hz train (gamma-AHP) and theta-burst pattern (theta-AHP) of action potentials (n = 19). Analysis of results revealed that the integral of the gamma-AHP was less than the theta-AHP (gamma-AHP, −9.6 mV·s ± 1; theta-AHP, −11.8 mV·s ± 1; t18 = 3.93, p = 0.001, paired-t-test). AHPs measured at 10 min served as the baseline in subsequent comparisons aimed at examining the effects of repeated stimulation using gamma-related and theta-burst activity patterns on AHP plasticity.
Next, the AHP was assessed over time in response to two alternating activity patterns, a gamma-related 50 Hz train (gamma-AHP) and theta-burst pattern (theta-AHP) of action potentials (Figure 1B). When AHPs were evoked once per minute (Figure 1C), the integral of the gamma-AHP significantly increased to 186 ± 31% of baseline across 40 min (F6,132 = 3.72, p = 0.002). Post-hoc tests revealed a significant difference between the AHP integral measured at baseline, −9.6 ±1 mV·s, relative to 40 min, −15.8 ± 3.2 mV·s (t18 = 3.66, p = 0.002). Surprisingly, saturation of the gamma-AHP was not observed over the duration of the experiment. In contrast to the gamma-AHP, the integral of the theta-AHP was comparable to baseline across 40 min (119 ± 12%; baseline, −11.8 ±1 mV·s; 40 min, −12.0 ± 1.2 mV·s; F6,132 = 1.75, p = 0.13). Analysis of the results revealed a significant difference in the % change in AHP from baseline of the gamma-AHP compared to the theta-AHP at 40 minutes (t36 = 2.24, p = 0.032). Note that plasticity of the AHP was not observed in experiments when AHPs were triggered once every three minutes (n = 8, see Figure 1D), which were modeled after the low-activity protocol reported previously (Kaczorowski et al., 2007). Taken together, these data suggest repeated firing at gamma-frequency results in the activity-dependent enhancement of the sAHP and reflects a decrease in intrinsic excitability.
Blockade of L-type or N- and P/Q-type Ca2+ channels disrupt potentiation of the gamma-AHP
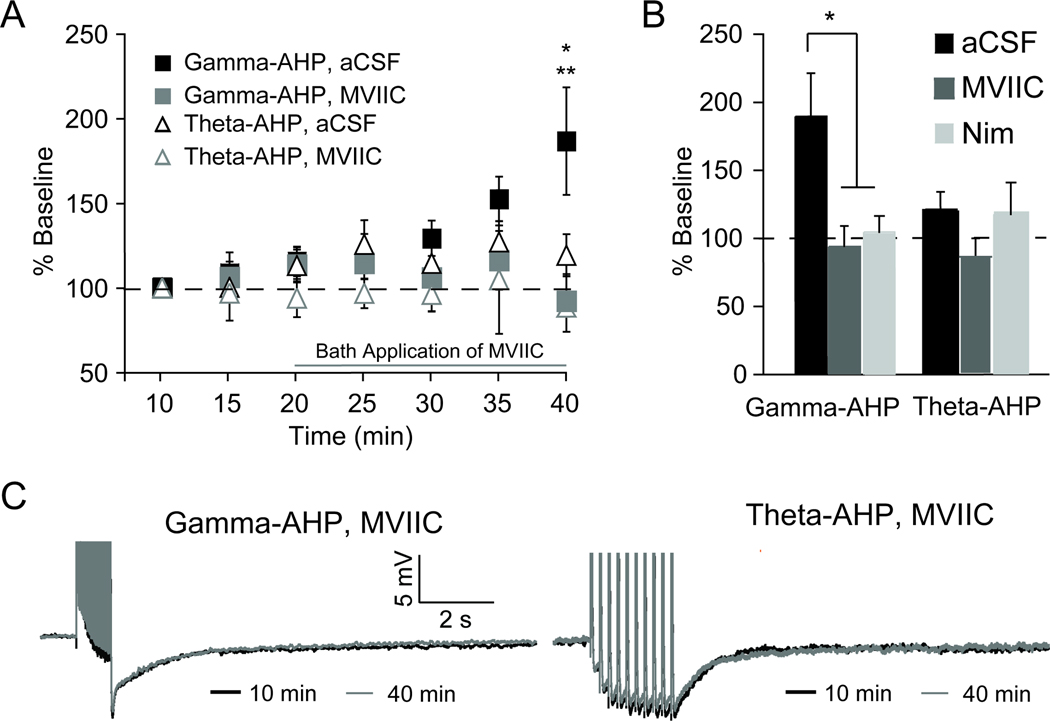
Previous work has shown that high-frequency firing in response to depolarizing constant current pulses increases the amplitude of the AHP, as well as IAHP and sIAHP, that depends on L-type Ca2+ channels or release from internal stores (Borde et al., 1999; Borde et al., 2000; Le Ray et al., 2004). Therefore, we hypothesized that the potentiation of the gamma-AHP observed herein may be sensitive to Ca2+ channel blockade as well. First, the immediate effect of N-P/Q-type Ca2+ channels on the AHP was assessed. Bath application of the N-P/Q-type Ca2+ channel blocker ω-conotoxin MVIIC (1 µM) had no general effect on the sAHP. Statistical analysis of the results using paired t-tests revealed no meaningful difference in the integral of the gamma-AHP or theta-AHP immediately (5–10 min) following bath application of ω-conotoxin MVIIC (gamma-AHP: aCSF −10.8 ± 2.0 mV·s vs. MVIIC −10.6 ± 1.5 mV·s, t7 = 0.14, p = 0.90; theta-AHP: aCSF −13.2 ± 2.5 mV·s vs. MVIIC −11 ± 1.5 mV·s, t7 = 1.88, p = 0.10). The integral of the gamma-AHP and theta-AHP in ω-conotoxin MVIIC were 114 ± 17% and 96 ± 8% of baseline (aCSF), respectively.
Next, the effects of both the N-P/Q-type, as well as L-type Ca2+ channels, on plasticity of the AHP using application of either ω-conotoxin MVIIC (1 µM) or nimodipine (10 µM) were examined. The gamma-AHP and theta-AHP measured in aCSF (10 min) were compared to measures in ω-conotoxin MVIIC or nimodipine at 40 minutes (Figure 2). Analysis of the results revealed ω-conotoxin MVIIC was sufficient to occlude potentiation of the AHP; no significant difference in the gamma-AHP was observed at 40 min in ω-conotoxin MVIIC compared to baseline (gamma-AHP; baseline, −10.8 ± 2.5 mV·s; MVIIC, −8.7 ± 1.5 mV·s, t7 = 0.87, p = 0.41, paired t-test). Similar to previous work by Borde and colleagues (2000), blockade of Ca2+ via L-type Ca2+ channels using nimodipine was sufficient to block or mask potentiation of the AHP (gamma-AHP, 101 ± 11%, n = 5). These data suggest that potentiation of the gamma-AHP observed in normal aCSF after 40 min of repeated stimulation (186 ± 31% of baseline) was disrupted when either N-P/Q-type Ca2+ channels or L-type Ca2+ channels were blocked (92 ± 14% and 101 ± 11% of baseline, respectively). Given that no plasticity of the theta-AHP was observed in aCSF after 40 min of repeated stimulation (119 ± 12% of baseline), it was not surprising that neither ω-conotoxin MVIIC nor nimodipine differed from baseline (85 ± 15% and 114 ± 22% of baseline, respectively; Figure 2).
Figure 2. Potentiation of gamma-AHP is not observed using Ca2+ channel blockers.
A, Plot showing that enhancement of the gamma-AHP (control aCSF, filled black squares) is absent in the presence of the N- and P/Q-type Ca2+ channel blocker ω-conotoxin MVIIC (1 µM) (grey filled squares). ω-conotoxin MVIIC (1 µM) did not alter the theta-AHP (open grey triangles) relative to control experiments (open black triangles). B, Summary graph illustrates enhancement of gamma-AHP, was significantly reduced or blocked by either ω-conotoxin MVIIC (grey) or the L-type Ca2+ blocker nimodipine (Nim, 10 µM, light grey, *p < 0.05). Neither ω-conotoxin MVIIC nor nimodipine altered the theta-AHP compared to baseline. C, Left, representative trace of the gamma-AHP triggered under control conditions at 40 min (black) superimposed on the gamma-AHP triggered with ω-conotoxin MVIIC at 40 min (grey). Right, representative trace of the theta-AHP triggered under control conditions at 40 min (black) superimposed on the theta-AHP triggered with ω-conotoxin MVIIC at 40 min (grey).
Pattern-specific bidirectional AHP plasticity in vitro
In a second set of experiments, AHPs were triggered using only 50 Hz or only theta-burst firing. As when both stimulation patterns were applied in the same cell (Figure 2), the gamma-AHP was increased to 198 ± 44% of baseline when measured after 40 min of repeated 50 Hz gamma-related stimulation (Figure 3). The integral of the gamma-AHP at 40 min was significantly increased compared to the gamma-AHP measured at 10 min (gamma-AHP, baseline, −14.5 ± 2.6 mV·s vs. 40 min, −25 ± 2.6 mV·s, t5 = 2.98, p = 0.041, paired t-test). As predicted based on our prior work (Kaczorowski et al., 2007), a significant reduction in the amplitude of the theta-AHP to 65 ±19% baseline was observed after 40 min of theta-burst firing alone (theta-AHP, baseline, −18.5 ± 2.8 mV·s vs. 40 min, −10.2 ± 3.0 mV·s, t5 = 2.19, p = 0.049, one-tailed paired t-test) (Figure 3B). One important observation made herein was that in the gamma-only stimulated experiments, the input resistance (RN) gradually increased over the duration of recordings from 89 ± 7 MΩ to 151 ± 8 MΩ (t5 = 10.06, p = 0.001, paired t-test). RN was altered less in theta-only stimulated experiments, from 95 ± 7 MΩ to 122 ± 21 MΩ, and this change was not significant (t5 = 1.73, p = 0.16, paired t-test). A reduction in voltage sag, evidenced by an increase in the sag ratio (Δsteady-state voltage deflection/Δpeak voltage deflection), was greatest following 40 min gamma-related stimulation (50 Hz firing) relative to theta-burst stimulation (gamma-AHP, 107 ±1% of baseline; theta-AHP 104 ±1.3% of baseline). Based on the role of Ih in generation of depolarizing sag in CA1 hippocampal neurons (Maccaferri et al., 1993), these data hinted that alterations in Ih may contribute to plasticity of the sAHP that was directly tested by repeating these experiments in the presence of the h-channel blocker ZD7288.
H-current contributes to the size and shape of the AHP and plays a role in AHP plasticity
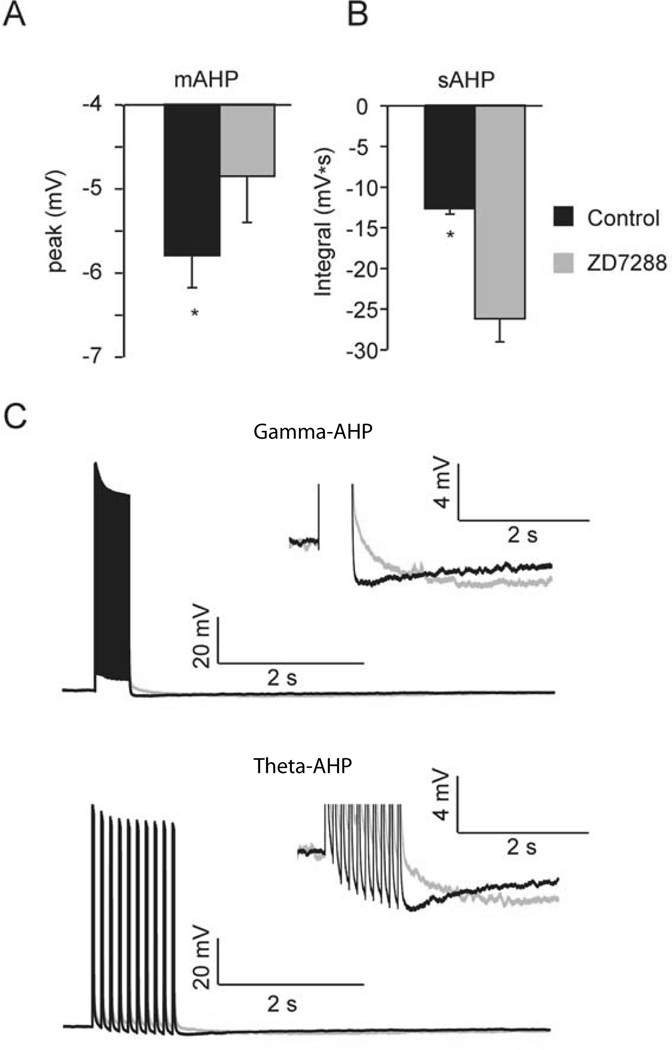
Prior to examining the effects of Ih on plasticity, the immediate effects of the h-channel blocker ZD7288 were first examined by comparing the gamma-AHP or theta-AHP (n = 5) in aCSF followed by bath application of ZD7288 (25 µM). Previous work suggests that blockade of Ih with ZD7288 or Cs+ reduces the medium component of the AHP (Storm, 1989; Williamson & Alger, 1990; Maccaferri et al., 1993; Gu et al., 2005), but enhances the sAHP (Gu et al., 2005). Therefore, consistent with previous reports, it was not surprising that ZD7288 (25 µM) reduced the peak of the AHP in 5 of 5 cells tested (Figure 4; gamma-AHP: −5.3 ± 0.06 mV in aCSF vs. −4.9 ± 0.1 mV: theta-AHP, −6.1 ± 1 mV in aCSF vs. −4.8 ± 1.7 mV). Moreover, the blockade of Ih using ZD7288 increased the integral of the gamma-AHP and theta-AHP to ~200% of baseline (gamma-AHP, aCSF, −13.4 ± 0.8 mV·s vs. ZD7288, −27.5 ± 4.0 mV·s: theta-AHP, aCSF, −12.2 ± 1.8 mV·s vs. ZD7288, −25.5 ± 7.8 mV·s), as shown previously (Gu et al., 2005).
Figure 4. Ih contributes to the size and shape of the AHP.
A, Summary graph illustrates the peak of AHP is significantly reduced in ZD7288 (theta-AHP and train-AHP pooled for comparison, *p < 0.05). B, Summary graph illustrates the integral of AHP is significantly increased in ZD7288 (*p < 0.05). C, Top, representative trace of the train-AHP triggered in aCSF (black) superimposed on the train-AHP triggered in ZD7288 (grey). Inset shows train-AHP on an expanded scale. Bottom, representative trace of the theta-AHP triggered in aCSF (black) superimposed on the theta-AHP triggered in ZD7288 (grey). Inset shows theta-AHP on an expanded scale. Data from train-AHP and theta-AHP were similar, and pooled for comparison in shown in panel A.
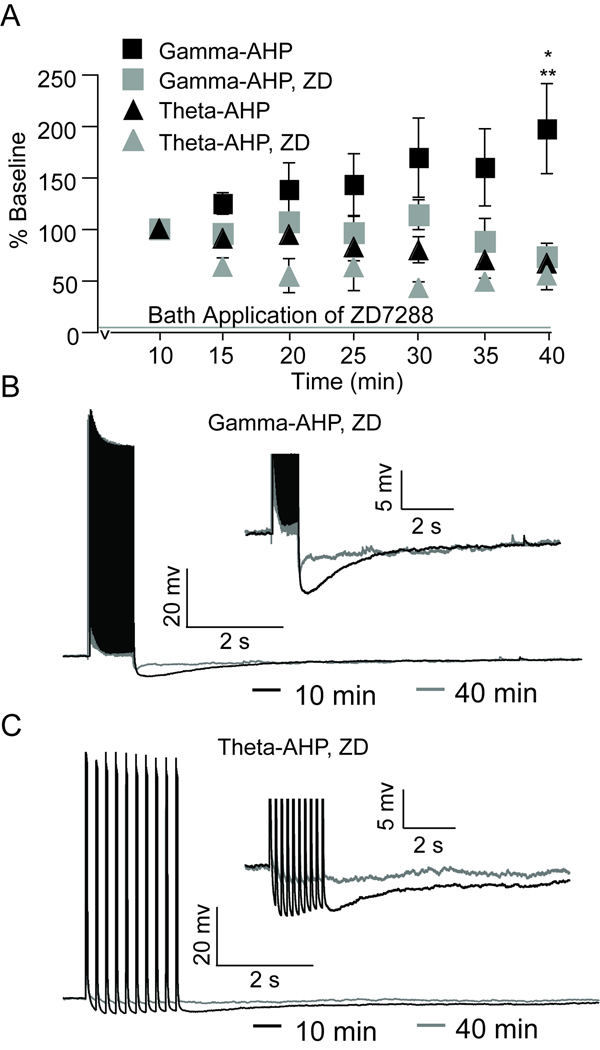
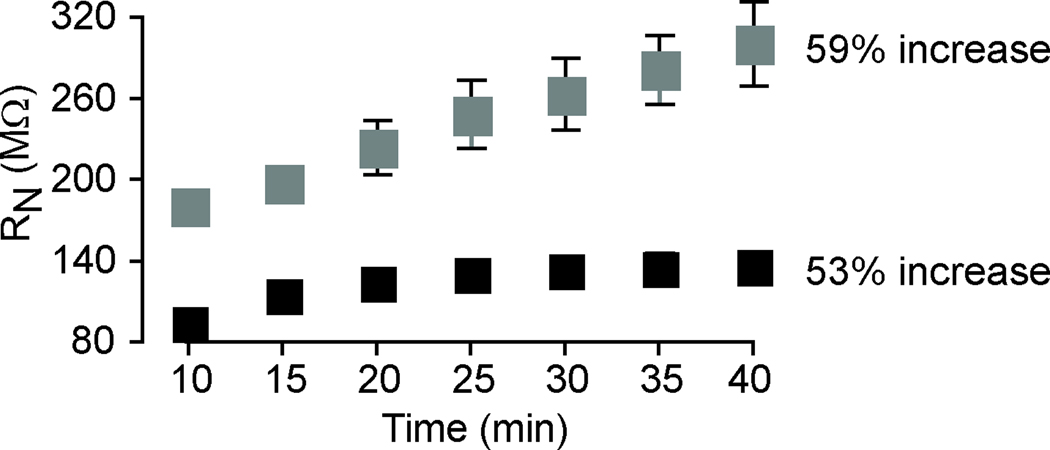
To test the hypothesis that plasticity of the AHP results from alterations in the availability and/or function of h-channels, the specific h-channel blocker ZD7288 was applied during repeated 50 Hz firing or theta-burst firing alone experiments (Figure 5). Blockade of h-channels prevented the enhancement of the gamma-AHP measured across 40 minutes of repeated 50 Hz gamma-related stimulation (72 ± 9% in ZD7288 compared to 198 ± 44% in aCSF, t9 = 3.08, p = 0.013), and revealed a decrease in the integral of the gamma-AHP that was qualitatively similar to that observed following 40 min of repeated theta-burst firing. Note that blockade of h-channels had no effect on the integral of the theta-AHP relative to aCSF measured after 40 minutes of repeated theta-burst firing (54 ±14% in ZD7288 compared to 65 ±19% in aCSF at 40 min, t8 = 0.42, p = 0.69). Therefore, it appears that when h-channels are not available for activation or modulation, the amplitude of the gamma-AHP in ZD7288 is comparable to the theta-AHP measured at 40 min in aCSF and ZD7288 (Figure 5). Although ZD7288 significantly increased RN compared to control conditions (Figure 6), statistical comparisons revealed no significant difference in the magnitude of RN in neurons exposed to gamma-related firing compared to theta-burst firing (t7 = 0.48, p = 0.64). Blockade of h-channels using ZD7288 did not prevent the gradual increase in RN, which increased from 179 ± 11 MΩ (10 minutes) to 300 ± 31 MΩ (40 minutes). This represents a 59% increase in RN using ZD7288 compared to a 53% increase in aCSF. Therefore, the effect of ZD7288 on AHP plasticity did not result from a non-specific stabilization of input resistance. Rather, h-channel blockers prevented enhancement of the AHP in spite of the general increase in RN that has been reported previously using KMeth-based internal solution (Kaczorowski et al., 2007). Further studies are required to determine the source of elevated RN described here and elsewhere (Kaczorowski et al., 2007).
Figure 5. Enhancement of gamma-AHP is blocked by h-channel blockers.
A, Plot showing that enhancement of the gamma-AHP (black squares), measured at 40 min, is blocked by the h-channel blocker ZD7288 (25 µM) (grey squares), whereas ZD7288 did not alter the theta-AHP (black triangles, control; grey triangles, ZD7288). B, Representative trace of the gamma-AHP triggered under ZD7288 at 10 min (black) superimposed on the gamma-AHP triggered under ZD7288 at 40 min (grey). Inset shows AHPs on an expanded scale. C, Representative trace of the theta-AHP triggered under ZD7288 at 10 min (black) superimposed on the theta-AHP triggered under ZD7288 at 40 min (grey). Inset shows AHPs on an expanded scale.
Figure 6. Input resistance (RN) increases in the presence of H-channel blocker ZD7288.
Time course and magnitude of enhancement of the RN measured in neurons exposed to gamma-related firing under control conditions (black) and in ZD7288 (grey). The magnitude of RN was significantly increased in ZD7288 at each time point (10 and 40 min) compared to the control condition (p < 0.05). However, the increase in RN over time was comparable in both conditions.
DISCUSSION
This study demonstrates that plasticity of the AHP is bidirectional, and the direction of plasticity depends on the pattern of action potential firing used to trigger the AHP. The sAHP is enhanced in response to high-frequency firing in the gamma frequency (50 Hz), or when alternated with theta-patterned stimulation. Remarkably, the results suggest that the availability of N-P/Q-type and L-type Ca2+ channels permit the activity-dependent enhancement of the gamma-AHP following repeated 50 Hz stimulation, but had no effect on the theta-AHP. Similarly, blockade of h-channels prevented plasticity of the gamma-AHP, but had no effect on plasticity of the AHP induced using theta-burst firing alone. Taken together, these data suggest activity-dependent alterations in N- and P/Q type Ca2+ channels and h-channels provide novel mechanisms to reduce neuronal excitability in response to gamma-related firing, as evidenced by an enhancement of the gamma-AHP. Future studies are necessary to determine whether or not activation of L-type and/or N-P/Q-type Ca2+ channels are coupled to alterations in Ih during the induction of gamma-AHP plasticity.
Contrary to plasticity induced by gamma-related activity, the induction and/or expression of AHP plasticity by theta-burst firing (theta-AHP) differed depending on whether theta-burst stimulation was used alone or in alternation with gamma-related firing. The theta-AHP was unchanged in experiments in which stimulation patterns were alternated, yet theta-burst patterned stimulation alone induced a reduction of the theta-AHP similar to previous work (Kaczorowski et al., 2007). Therefore, gamma-related firing appeared to prevent or mask the induction of theta-AHP plasticity. While a more detailed study is required to fully elucidate how different patterns of activity lead to changes in the sAHP, our results provide a framework to understand how these physiologically relevant firing patterns can alter the intrinsic excitability in principle neurons of the hippocampus – thus, providing additional computational power for information processing and storage.
AHP plasticity: new findings and comparison to previous reports
Previous reports have demonstrated that repeated suprathreshold somatic firing leads to an enhancement of the AHP (AHP-E) and potentiation of the sIAHP (Borde et al., 1995; Borde et al., 1999; Borde et al., 2000). However, recent work suggests the opposite, that suprathreshold somatic firing reduces the sAHP and the sIAHP (Kaczorowski et al., 2007). One interesting difference between these prior studies is the firing-frequency of the stimulation that was used; Borde and colleagues used high-frequency variable firing and Kaczorowski and colleagues (2007) used theta-burst firing to evoke the AHP. However, because Borde and colleagues (1995) allowed firing frequency and total action potential number to vary, it was premature to attribute differences in the direction of AHP plasticity to differences in firing frequency. Therefore, the present results extend earlier work to clarify the differential role of activity patterns in the regulation of the AHP (gamma-related versus theta-burst firing) using reproducible stimuli, matched in total action potentials, and evoked at fixed intervals. This approach allowed, for the first time, the direct comparison of different activity patterns on AHP plasticity.
Moreover, the present report demonstrates that plasticity of the sAHP is activity dependent. No change in sAHPs were observed in experiments where activity patterns were delivered less frequently (every 3 min compared to 1 min), thus resulting in 1/3 number of action potentials triggered. Because the present experiments were performed under blockers of fast excitatory and inhibitory transmission, for the first time, it is shown herein that the induction requirements for bidirectional sAHP plasticity do not depend on synaptic activation. Lastly, these data reveal the select involvement of N-P/Q-type Ca2+ channels (in addition to L-type Ca2+ channels reported by Borde et al. 2005) for plasticity of the gamma-AHP. These data suggest that activity-dependent potentiation of the AHP results from specific activity patterns (notably gamma-related firing), in part, from increases in Ca2+ influx via voltage-gated calcium channels and/or activation of their downstream targets, for example the sIAHP.
Modulation of Ih alters neuronal excitability in hippocampal neurons
This study also shows that blockade of Ih using ZD7288 masked or prevented the potentiation of the gamma-AHP observed under control conditions, and suggests that the availability of h-channels are important for plasticity of the gamma-AHP. Although prior work has shown that long-term exposure to ZD7288 can have nonspecific effects on neuron excitability (Chevaleyre & Castillo, 2002), it is unlikely that such nonspecific effects could explain the present results given that 1) blockers of fast excitatory and inhibitory synaptic transmission were present, 2) the time required to observe significant and nonspecific effects of ZD7288 (50 µM, twice the concentration used in this study) reported previously (Chevaleyre and Castillo, 2002) was longer than most of the recordings in this study (40 min), and 3) ZD7288 had a select effect on plasticity of the gamma-AHP.
The Ih–dependent AHP plasticity induced by repeated 50 Hz gamma-related firing described in the present report differs from recent work by Fan and colleagues (2005). They report that either synaptic theta-burst firing or somatic theta-burst firing in CA1 hippocampal neurons can drive a decrease in RN and reduce hippocampal neuronal excitability resulting from an NMDA receptor and CaMKII-dependent increase in Ih. Such an increase in Ih available at the resting membrane potential would have the opposite effect of ZD7288 on the AHP shown here and in previous work (Gu et al., 2005; Maccaferri et al., 1993; Storm, 1989; Williamson & Alger, 1990). Specifically, an increase in Ih is posited to increase the mAHP and reduce the sAHP. Moreover, the present results using somatic theta-burst firing show a trend towards an increase in RN that may indicate a reduction in Ih, and an increase in hippocampal neuronal excitability (evidenced by a reduction in the theta-AHP). These differences may be due to our use of synaptic blockers, including kynurenate, which would block activation of postsynaptic NMDA receptors posited to underlie plasticity described by Fan and colleagues (2005).
The present results also differ in that here I show blockade of Ih with ZD7288 did not significantly alter the induction of intrinsic plasticity (e.g. increased neuronal excitability evidenced by a reduction in the sAHP) following repeated theta-burst firing compared to control experiments. Differences in the number of action potentials and the frequency of the theta-burst stimulation, which is used as the induction protocol, may be responsible for the differences in the mechanisms underlying theta-burst induced plasticity, or lack thereof. Fan and colleagues (2005) used one epoch of theta-burst firing (150 spikes over 2 minutes), whereas the present report triggered the AHP using theta-burst firing once a minute for 40 minutes (2000 spikes). Therefore, it is possible that the brief theta-burst firing stimulation used by Fan and colleagues (2005) was not sufficient to induce alterations in neuronal excitability reported here.
Bidirectional modulation of the AHP may result from differences in the source or magnitude of Ca2+ entry or downstream targets
The present work demonstrates that the pattern of activity in the form of postsynaptic firing, in the absence of synaptic input, is a critical determinant of the plasticity of the AHP. Pattern-specific differences in the induction and/or expression of AHP plasticity may arise from differential involvement of voltage-gated channels located in the dendrites. Because somatic action potentials in a theta-burst pattern are expected to backpropagate more effectively into the dendritic tree than gamma-related firing at 50 Hz (Spruston et al., 1995), pattern specific differences in AHP plasticity may reflect differential activation and/or expression of voltage-gated channels in the dendrite. The unique activation of the distal dendrites by theta-burst firing could modify voltage-gated channels that are primarily expressed in the dendrites – and thus, more likely to be activated by theta-burst firing compared to gamma-related firing. An example of this was shown by Nolan and colleagues (2004) where modifications in h-channel expression substantially impacted synaptic plasticity in the distal portions of the dendrite, but not at synapses located in the proximal dendrite (Nolan et al., 2004). Alternatively, the spatial and temporal characteristics of intracellular Ca2+ rise and buffering may differ between gamma-related and theta-burst firing. Pattern specific differences in Ca2+ rise and buffering would be expected to alter the sAHP magnitude in response to subsequent stimulation (Powell et al., 2008; Goldberg et al., 2009), as well as differentially affect Ca2+-dependent plasticity similar to that described with regards to synaptic plasticity (Larson et al., 1986; Huang & Kandel, 1994).
It is possible that when alternating stimulation is used that there are two opposing effects: an amplification of the sAHP caused by the 50 Hz train stimulation and a reduction in the sAHP induced by theta-burst firing. This could explain why the theta-AHP is reduced in isolation but not in experiments that alternate activity patterns. However, this speculation is inconsistent with the observation that the rate and amplitude of gamma-AHP potentiation is comparable in experiments where 50 Hz firing was delivered alone or interleaved with theta-burst firing (Figures 1 and 3). Lastly, it is possible that different activity patterns lead to changes in calcium buffering that influence the sAHP (Powell et al., 2008). While a more detailed study is required to fully elucidate how different patterns of activity lead to changes in the sAHP, our results provide a framework to understand these mechanisms.
Bidirectional modulation of the AHP provides a synapse-independent mechanism that shapes excitability of hippocampus in response to neural activity and experience
Activity-dependent bidirectional modulation of the sAHP occurred in response to somatically generated action potentials. Because neuronal output in response to somatic current injection is determined by activation of intrinsic voltage-gated or Ca2+-activated ion channels, changes in the sAHP reported herein most likely reflect alterations in intrinsic postsynaptic excitability. Although GABAB receptors were not blocked in the present report, GABAB-receptor activation is unlikely to occur in response to postsynaptic firing (in the absence of synaptic stimulation). Furthermore, the present experiments were conducted in the presence of fast excitatory and inhibitory synaptic blockers that essentially isolate the postsynaptic neuron from activating downstream neurons that could potentially provide a source of GABA for activation of these receptors. Taken together, these data confirm that synaptic activation is not a requirement for induction of the sAHP plasticity reported here. Although a more detailed analysis of the signal transduction pathways involved in pattern-specific sAHP plasticity are important, the present report provides novel insight into the differential role of HVA Ca2+ channels and Ih in bidirectional pattern-specific sAHP plasticity.
Examinations of learning-related AHP alterations in CA1 pyramidal neurons have been observed following learning paradigms that depend upon the hippocampus, including trace eyeblink conditioning in rabbit and rat (de Jonge et al., 1990; Moyer et al., 1996; Moyer et al., 2000; Kuo et al., 2004), and spatial water maze training and contextual fear conditioning in rat and mouse (Oh et al., 2003; Tombaugh et al., 2005; Ohno et al., 2006; Kaczorowski & Disterhoft, 2009; McKay et al., 2009). Reductions in the AHP are posited to underlie, or at least facilitate, cellular mechanisms of hippocampus-dependent learning. In other studies, activation of metabotropic glutamate receptors have been shown to induce long-lasting suppression of the IAHP and sIAHP (Faber & Sah, 2002; Melyan et al., 2002; Sourdet et al., 2003; Ruiz et al., 2005). Here we show somatic-theta burst pattern firing, that mimics activity patterns observed during learning tasks in vivo, is sufficient to induce a reduction in the sAHP.
Curiously, repetition of gamma-related firing resulted in an enhancement of the sAHP (i.e. decreased neuron excitability) that was qualitatively similar to that observed in hippocampal neurons from poor-learner mice expressing familial Alzheimer’s Disease (FAD) mutations (Kaczorowski et al., 2009). Remarkably, mice with FAD mutations are more susceptible to unprovoked seizures than their wild-type counterparts largely due hyperexcitable neurons in brain areas expressing beta-amyloid protofibrils (Minkeviciene et al., 2009) and beta-amyloid plaques (Busche et al., 2008). It is possible that gamma-related enhancement of the sAHP provides a mechanism to reduce disease-related neuron hyperexcitability that is capable of inducing seizures; with the caveat being this occurs at the expense of cognitive performance. Alternatively, gamma-related firing may restore basal levels of excitability following a learning event to prevent hyperexcitability following multiple learning episodes.
Overall, plasticity of intrinsic neuronal excitability is thought to increase the storage capacity of neurons and likely plays a critical role in learning and memory (Saar & Barkai, 2003; Zhang & Linden, 2003; Disterhoft et al., 2004; Oh et al., 2010). Therefore, the identification of novel mechanisms underlying AHP plasticity in vitro herein provides additional insight into the dynamic processes that likely influence learning and memory. The plasticity described here differs significantly from the induction of synaptic plasticity; synaptic stimulation at either gamma- or theta-frequencies induces rapid and long lasting potentiation of synaptic strength at Shaffer-collateral-CA1 synapses (Chen et al., 1999 and Larson et al., 1986, respectively) whereas sAHP induced by postsynaptic firing develops gradually and the direction of plasticity differs based on the activity pattern used. Postsynaptic firing at 50 Hz, gamma-related frequency, results in a gradual decrease in neuronal excitability that is opposite to the enhancement in neuronal excitability observed following repeated postsynaptic theta-burst firing. Both synaptic plasticity and intrinsic plasticity have been postulated as cellular mechanisms critically involved in learning and memory. Since it has yet to be elucidated how learning-related changes in neuron excitability and synaptic strength develop and interact during the acquisition of various hippocampal-dependent learning and memory tasks that can require minutes (e.g. contextual fear conditioning) to several days (e.g. spatial watermaze and trace eyeblink conditioning) of training, the present findings provide new information on the mechanisms underlying AHP changes and provide a model to further how such changes relate to learning processes.
Acknowledgments
The authors wish to thank Dr. Gianmaria Maccaferri and Dr. Shannon Moore for their advice and critical reading of the manuscript. Caroline Cook provided advice on the illustrations. This work was supported by the National Institutes of Mental Health grant F31 MH-067445 awarded to C.C.K. and the Department of Physiology at the Medical College of Wisconsin, Milwaukee, WI.
Footnotes
Disclosure Statement: There are no real or perceived conflicts of interest.
References
- Barnes CA, McNaughton BL, Mizumori SJ, Leonard BW, Lin LH. Comparison of spatial and temporal characteristics of neuronal activity in sequential stages of hippocampal processing. Prog Brain Res. 1990;83:287–300. doi: 10.1016/s0079-6123(08)61257-1. [DOI] [PubMed] [Google Scholar]
- Borde M, Bonansco C, Buno W. The activity-dependent potentiation of the slow Ca2+-activated K+ current regulates synaptic efficacy in rat CA1 pyramidal neurons. Pflugers Arch. 1999;437:261–266. doi: 10.1007/s004240050778. [DOI] [PubMed] [Google Scholar]
- Borde M, Bonansco C, de Sevilla F, Le Ray D, Buno W. Voltage-clamp analysis of the potentiation of the slow Ca2+-activated K+ current in hippocampal pyramidal neurons. Hippocampus. 2000;10:198–206. doi: 10.1002/(SICI)1098-1063(2000)10:2<198::AID-HIPO9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Borde M, Cazalets JR, Buno W. Activity-dependent response depression in rat hippocampal CA1 pyramidal neurons in vitro. J Neurophysiol. 1995;74:1714–1729. doi: 10.1152/jn.1995.74.4.1714. [DOI] [PubMed] [Google Scholar]
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- Chen HX, Otmakhov N, Lisman J. Requirments for LTP induction by pairing in hippocampal CA1 pyramidal cells. J Neurophysiol. 1999;82:526–532. doi: 10.1152/jn.1999.82.2.526. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Assessing the role of Ih channels in synaptic transmission and mossy fiber LTP. Proc Natl Acad Sci U S A. 2002;99:9538–9543. doi: 10.1073/pnas.142213199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Moser EI. Gamma oscillations in the hippocampus. Physiology (Bethesda) 2010;25:319–329. doi: 10.1152/physiol.00021.2010. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Lo Turco JJ, Kubota M, Disterhoft JF, Moore JW, Alkon DL. Classical conditioning reduces amplitude and duration of calcium-dependent afterhyperpolarization in rabbit hippocampal pyramidal cells. J Neurophysiol. 1989;61:971–981. doi: 10.1152/jn.1989.61.5.971. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- de Jonge MC, Black J, Deyo RA, Disterhoft JF. Learning-induced afterhyperpolarization reductions in hippocampus are specific for cell type and potassium conductance. Exp Brain Res. 1990;80:456–462. doi: 10.1007/BF00227987. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Coulter DA, Alkon DL. Conditioning-specific membrane changes of rabbit hippocampal neurons measured in vitro. Proc Natl Acad Sci U S A. 1986;83:2733–2737. doi: 10.1073/pnas.83.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft JF, Golden DT, Read HL, Coulter DA, Alkon DL. AHP reductions in rabbit hippocampal neurons during conditioning correlate with acquisition of the learned response. Brain Res. 1988;462:118–125. doi: 10.1016/0006-8993(88)90593-8. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM. Pharmacological and molecular enhancement of learning in aging and Alzheimer's disease. J Physiol Paris. 2006;99:180–192. doi: 10.1016/j.jphysparis.2005.12.079. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM. Alterations in intrinsic neuronal excitability during normal aging. Aging Cell. 2007;6:327–336. doi: 10.1111/j.1474-9726.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Thompson LT, Moyer JR, Jr, Mogul DJ. Calcium-dependent afterhyperpolarization and learning in young and aging hippocampus. Life Sci. 1996;59:413–420. doi: 10.1016/0024-3205(96)00320-7. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Wu WW, Ohno M. Biophysical alterations of hippocampal pyramidal neurons in learning, ageing and Alzheimer's disease. Ageing Res Rev. 2004;3:383–406. doi: 10.1016/j.arr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci. 2002;22:1618–1628. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, Johnston D. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in I(h) Nat Neurosci. 2005;8:1542–1551. doi: 10.1038/nn1568. [DOI] [PubMed] [Google Scholar]
- Gamelli AE, McKinney BC, White JA, Murphy GG. Deletion of the L-type calcium channel Ca(V)1.3 but not Ca(V)1.2 results in a diminished sAHP in mouse CA1 pyramidal neurons. Hippocampus. 2009 doi: 10.1002/hipo.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Teagarden MA, Foehring RC, Wilson CJ. Nonequilibrium calcium dynamics regulate the autonomous firing pattern of rat striatal cholinergic interneurons. J Neurosci. 2009;29:8396–8407. doi: 10.1523/JNEUROSCI.5582-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol. 2005;566:689–715. doi: 10.1113/jphysiol.2005.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- Jonas EA, Kaczmarek LK. Regulation of potassium channels by protein kinases. Curr Opin Neurobiol. 1996;6:318–323. doi: 10.1016/s0959-4388(96)80114-0. [DOI] [PubMed] [Google Scholar]
- Kaczorowski CC. Intrinsic plasticity in CA1 pyramidal neurons: Role of calcium(2+)-and sodium(+)-activated potassium currents in aging and Alzheimer's disease. Dissertation. 2006:1–291. [Google Scholar]
- Kaczorowski CC, Disterhoft J, Spruston N. Stability and plasticity of intrinsic membrane properties in hippocampal CA1 pyramidal neurons: effects of internal anions. J Physiol. 2007;578:799–818. doi: 10.1113/jphysiol.2006.124586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski CC, Disterhoft JF. Memory deficits are associated with impaired ability to modulate neuronal excitability in middle-aged mice. Learn Mem. 2009;16:362–366. doi: 10.1101/lm.1365609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski CC, Disterhoft JF, Spruston N. Differential Activity-dependent Plasticity of the Slow AHP Evoked by Tetanic Vs. Theta-burst Stimulation in CA1 Pyramidal Neurons: A Role for the Hyperpolarization-activated Cation Conductance. Society for Neuroscience Abstracts. 2003;369.13 [Google Scholar]
- Kaczorowski CC, Sametsky E, Shah S, Vassar R, Disterhoft JF. Mechanisms underlying basal and learning-related intrinsic excitability in a mouse model of Alzheimer's disease. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo AG, Lee G, Disterhoft JF. Alteration of sIAHP underlies reduction of AHP in rat CA1 pyramidal neurons after trace eyebink conditioning. Society for Neuroscience Abstracts. 2004;741.13 [Google Scholar]
- Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Zucker RS. Photolytic manipulation of Ca2+ and the time course of slow, Ca(2+)-activated K+ current in rat hippocampal neurones. J Physiol. 1994;475:229–239. doi: 10.1113/jphysiol.1994.sp020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986;232:985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Le Ray D, Fernandez De Sevilla D, Belen Porto A, Fuenzalida M, Buno W. Heterosynaptic metaplastic regulation of synaptic efficacy in CA1 pyramidal neurons of rat hippocampus. Hippocampus. 2004;14:1011–1025. doi: 10.1002/hipo.20021. [DOI] [PubMed] [Google Scholar]
- Lisman J. The theta/gamma discrete phase code occurring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus. 2005;15:913–922. doi: 10.1002/hipo.20121. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Janigro D, Lazzari A, DiFrancesco D. Cesium prevents maintenance of long-term depression in rat hippocampal CA1 neurons. Neuroreport. 1994;5:1813–1816. doi: 10.1097/00001756-199409080-00032. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Mangoni M, Lazzari A, DiFrancesco D. Properties of the hyperpolarization-activated current in rat hippocampal CA1 pyramidal cells. J Neurophysiol. 1993;69:2129–2136. doi: 10.1152/jn.1993.69.6.2129. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, Matthews EA, Oliveira FA, Disterhoft JF. Intrinsic neuronal excitability is reversibly altered by a single experience in fear conditioning. J Neurophysiol. 2009;102:2763–2770. doi: 10.1152/jn.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melyan Z, Wheal HV, Lancaster B. Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron. 2002;34:107–114. doi: 10.1016/s0896-6273(02)00624-4. [DOI] [PubMed] [Google Scholar]
- Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fulop L, Penke B, Zilberter Y, Harkany T, Pitkanen A, Tanila H. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29:3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr, Power JM, Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci. 2000;20:5476–5482. doi: 10.1523/JNEUROSCI.20-14-05476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr, Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. J Neurophysiol. 1992;68:2100–2109. doi: 10.1152/jn.1992.68.6.2100. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci. 1996;16:5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Oh MM, Kuo AG, Wu WW, Sametsky EA, Disterhoft JF. Watermaze learning enhances excitability of CA1 pyramidal neurons. J Neurophysiol. 2003;90:2171–2179. doi: 10.1152/jn.01177.2002. [DOI] [PubMed] [Google Scholar]
- Oh MM, Oliveira FA, Disterhoft JF. Learning and aging related changes in intrinsic neuronal excitability. Front Aging Neurosci. 2010;2:2. doi: 10.3389/neuro.24.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Sametsky EA, Silva AJ, Disterhoft JF. Differential effects of alphaCaMKII mutation on hippocampal learning and changes in intrinsic neuronal excitability. Eur J Neurosci. 2006;23:2235–2240. doi: 10.1111/j.1460-9568.2006.04746.x. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H, Wiener SI, Wible CG. Learning-related patterns of CA1 spike trains parallel stimulation parameters optimal for inducing hippocampal long-term potentiation. Hippocampus. 1991;1:181–192. doi: 10.1002/hipo.450010206. [DOI] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Krause M, Haug T, Storm JF, Stuhmer W. Modulation of the Ca2+-activated K+ current sIAHP by a phosphatase-kinase balance under basal conditions in rat CA1 pyramidal neurons. J Neurophysiol. 1998;79:3252–3256. doi: 10.1152/jn.1998.79.6.3252. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF. Evidence that Ca/calmodulin-dependent protein kinase mediates the modulation of the Ca2+-dependent K+ current, IAHP, by acetylcholine, but not by glutamate, in hippocampal neurons. Pflugers Arch. 1996;431:723–728. [PubMed] [Google Scholar]
- Pineda JC, Galarraga E, Foehring RC. Different Ca2+ source for slow AHP in completely adapting and repetitive firing pyramidal neurons. Neuroreport. 1999;10:1951–1956. doi: 10.1097/00001756-199906230-00029. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Migliore M, Johnston D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci. 2002;5:767–774. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- Powell AD, Toescu EC, Collinge J, Jefferys JG. Alterations in Ca2+-buffering in prion-null mice: association with reduced afterhyperpolarizations in CA1 hippocampal neurons. J Neurosci. 2008;28:3877–3886. doi: 10.1523/JNEUROSCI.0675-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- Rascol O, Potier B, Lamour Y, Dutar P. Effects of calcium channel agonist and antagonists on calcium-dependent events in CA1 hippocampal neurons. Fundam Clin Pharmacol. 1991;5:299–317. doi: 10.1111/j.1472-8206.1991.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Sachidhanandam S, Utvik JK, Coussen F, Mulle C. Distinct subunits in heteromeric kainate receptors mediate ionotropic and metabotropic function at hippocampal mossy fiber synapses. J Neurosci. 2005;25:11710–11718. doi: 10.1523/JNEUROSCI.4041-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar D, Barkai E. Long-term modifications in intrinsic neuronal properties and rule learning in rats. Eur J Neurosci. 2003;17:2727–2734. doi: 10.1046/j.1460-9568.2003.02699.x. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. Eur J Neurosci. 1998;10:1518–1523. doi: 10.1046/j.1460-9568.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Long-lasting cholinergic modulation underlies rule learning in rats. J Neurosci. 2001;21:1385–1392. doi: 10.1523/JNEUROSCI.21-04-01385.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P. Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Spain WJ, Foehring RC, Chubb MC, Crill WE. Slow conductances in neurons from cat sensorimotor cortex in vitro and their role in slow excitability changes. J Neurophysiol. 1988;59:450–467. doi: 10.1152/jn.1988.59.2.450. [DOI] [PubMed] [Google Scholar]
- Senior TJ, Huxter JR, Allen K, O'Neill J, Csicsvari J. Gamma oscillatory firing reveals distinct populations of pyramidal cells in the CA1 region of the hippocampus. J Neurosci. 2008;28:2274–2286. doi: 10.1523/JNEUROSCI.4669-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Haylett DG. Ca(2+) channels involved in the generation of the slow afterhyperpolarization in cultured rat hippocampal pyramidal neurons. J Neurophysiol. 2000;83:2554–2561. doi: 10.1152/jn.2000.83.5.2554. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Deschenes M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. J Neurophysiol. 1993;70:97–116. doi: 10.1152/jn.1993.70.1.97. [DOI] [PubMed] [Google Scholar]
- Sourdet V, Russier M, Daoudal G, Ankri N, Debanne D. Long-term enhancement of neuronal excitability and temporal fidelity mediated by metabotropic glutamate receptor subtype 5. J Neurosci. 2003;23:10238–10248. doi: 10.1523/JNEUROSCI.23-32-10238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N, Schiller Y, Stuart G, Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science. 1995;268:297–300. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Intracellular injection of a Ca2+ chelator inhibits spike repolarization in hippocampal neurons. Brain Res. 1987;435:387–392. doi: 10.1016/0006-8993(87)91631-3. [DOI] [PubMed] [Google Scholar]
- Storm JF. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol. 1989;409:171–190. doi: 10.1113/jphysiol.1989.sp017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Moyer JR, Jr, Disterhoft JF. Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation. J Neurophysiol. 1996;76:1836–1849. doi: 10.1152/jn.1996.76.3.1836. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci. 2005;25:2609–2616. doi: 10.1523/JNEUROSCI.5023-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velumian AA, Carlen PL. Differential control of three after-hyperpolarizations in rat hippocampal neurones by intracellular calcium buffering. J Physiol. 1999;517:201–216. doi: 10.1111/j.1469-7793.1999.0201z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A, Alger BE. Characterization of an early afterhyperpolarization after a brief train of action potentials in rat hippocampal neurons in vitro. J Neurophysiol. 1990;63:72–81. doi: 10.1152/jn.1990.63.1.72. [DOI] [PubMed] [Google Scholar]
- Zelcer I, Cohen H, Richter-Levin G, Lebiosn T, Grossberger T, Barkai E. A cellular correlate of learning-induced metaplasticity in the hippocampus. Cereb Cortex. 2006;16:460–468. doi: 10.1093/cercor/bhi125. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]