Abstract
Processing food extensively by thermal and nonthermal techniques is a unique and universal human practice. Food processing increases palatability and edibility and has been argued to increase energy gain. Although energy gain is a well-known effect from cooking starch-rich foods, the idea that cooking meat increases energy gain has never been tested. Moreover, the relative energetic advantages of cooking and nonthermal processing have not been assessed, whether for meat or starch-rich foods. Here, we describe a system for characterizing the energetic effects of cooking and nonthermal food processing. Using mice as a model, we show that cooking substantially increases the energy gained from meat, leading to elevations in body mass that are not attributable to differences in food intake or activity levels. The positive energetic effects of cooking were found to be superior to the effects of pounding in both meat and starch-rich tubers, a conclusion further supported by food preferences in fasted animals. Our results indicate significant contributions from cooking to both modern and ancestral human energy budgets. They also illuminate a weakness in current food labeling practices, which systematically overestimate the caloric potential of poorly processed foods.
Keywords: caloric value, nutrition label, weight, energy balance, human evolution
Energy availability is a routine constraint on metabolic processes, including growth, disease suppression, and reproduction, and therefore, it is a key variable for human nutrition and evolutionary fitness. Cooking has long been recognized to increase the energy available from starch (1–3), but how it changes the rate of energy gain from eating meat is an unsolved problem with practical and theoretical implications. Because meat is nearly always eaten cooked, the contribution of meat cooking to human nutritional energetics is potentially great: meat is the largest source of protein in all affluent countries except Japan (4), and dependence on meat is growing rapidly among developing nations (5), with annual global consumption of meat expected to reach 376 million tons by 2030 (6). The energetic consequences of cooking meat would also have been important in human evolution ever since fire was controlled, minimally 300,000–400,000 y ago, a period when meat is inferred to have been a large component of the diet and energy would have routinely been in short supply (7–9).
The standard Atwater system of energy assessment is based on measuring the total metabolizable nutrient content of an edible item (gross food energy content minus energy lost in urine, feces, secretions, and gases) and finds that cooking tends to have minimal impact on meat energy value (10) (Table S1). However, this conclusion is suspect, because the Atwater system ignores potentially important effects (1). Heat-induced protein denaturation, loss of structural integrity, and deactivation of microbes are expected to increase meat energy value (2, 11, 12), whereas dripping loss, Maillard reactions, formation of protein covalent bonds, and toughening of muscle fibers should reduce it (10, 13–15). The integrated effects of these mechanisms are unknown and can be understood only by studying consumer energy balance. In addition, to isolate the unique contributions of heat, the effects of cooking must be compared against the effects of nonthermal processing.
To address these problems, we investigated the effects of unprocessed, pounded, and/or cooked diets on body mass and food preference in a model omnivorous mammal, the mouse (Mus musculus), a species known to exploit both meat (16) and starch-rich foods (17) in natural contexts. We validated our protocol with diets consisting of tubers, a starch-rich item in which significant positive effects of cooking were predictable based on prior studies of starch digestibility (2) but underestimated by the Atwater convention (10) (Table S2). We then repeated the experiment using meat. Both tuber and meat experiments also allowed us to assess the relative energetic advantages of cooking and nonthermal processing, a previously unstudied problem.
Based on a counterbalanced within-subjects study design, adult male CD-1 mice were fed organic sweet potato (Ipomoea batatas) or organic lean beef (Bos taurus) in four experimental diets: raw and whole (RW), raw and pounded (RP), cooked and whole (CW), and cooked and pounded (CP) (Fig. S1). Each diet was administered ad libitum for 4 d, with a 6-d washout period of ad libitum chow between consecutive diets. Mice were housed individually in cages designed to minimize coprophagy and permit assessment of food intake and activity level (Fig. S2). We used change in body mass to index the energy gained or lost, accounting for intake and activity. In addition, to evaluate whether food preferences of naïve and/or experienced mice aligned with energetic value, we conducted preference tests before and after the cycle of feeding trials.
Results
Effects of Food Processing on Tuber Diets.
Cooking tends to increase weight gain in animals reared on starch-rich foods (1). Heat gelatinizes starches, transforming resistant semicrystalline structures into amorphous compounds that are readily hydrolyzed to sugars and dextrins (3), resulting in higher consumer-specific digestibility (2). We, therefore, expected to find a positive effect of cooking on the energetic value of our tuber diets, which consisted of ∼40% starch when raw on a dry-weight basis (Table S3). By contrast, the effect of nonthermal processing on tubers has never been investigated with respect to energy. Pounding reduces the structural integrity of tubers by damaging cell walls and releasing cellular water, thereby decreasing firmness (18, 19). Pounding also tends to fractionate raw tubers, reducing ingested particle size and probably, therefore, masticatory and gastric effort (20). These transformations should have a positive effect on net energy gain. However, pounding is unlikely to disrupt the starch granules themselves, which are typically 2–100 μm in diameter and thus, too small to be regularly damaged by mechanical treatment (21). We, therefore, hypothesized that pounding would improve net energy gain but that the advantages of pounding would be small compared with the advantages of cooking.
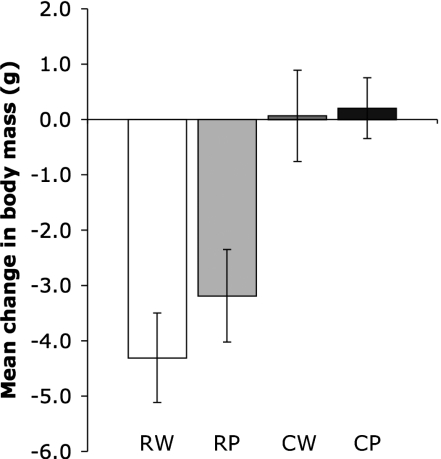
We found that net energy gain over 4 d was improved both by cooking and pounding [two-way repeated measures ANOVA (2 × 2 RM ANOVA); cooking: P < 0.001, pounding: P = 0.036]. The positive effects of cooking exceeded the effects of pounding, with mice maintaining weight on cooked diets but losing weight on raw diets, regardless of whether those diets were whole or pounded (Fig. 1). Cooking improved a pounded diet (paired t test; RP < CP; P < 0.001), but pounding did not improve a cooked diet (paired t test; CW < CP; P = 0.707). As expected under effective washout dynamics, changes in body mass during the washout periods after each diet produced the inverse result across diets (2 × 2 RM ANOVA; cooking: P < 0.001, pounding: P = 0.003) and for the RP–CP contrast (paired t test; RP > CP; P < 0.001) (Fig. S3).
Fig. 1.
Changes in body mass on tuber diets. Mean cumulative change in body mass [±95% confidence interval (CI)] over 4 d in mice (n = 17) fed standardized ad libitum diets of organic sweet potato (I. batatas) served raw and whole (RW), raw and pounded (RP), cooked and whole (CW), and cooked and pounded (CP). Diets were administered based on a counterbalanced within-subjects study design.
Differences in activity level did not drive energy gains, because we observed no differences in wheel running across diets (2 × 2 RM ANOVA; cooking: P = 0.098, pounding: P = 0.395). Food intake, by contrast, was higher for processed diets and therefore, contributed to differential energy gains. Food intake was analyzed both on a fresh-weight basis (grams of food fed minus grams of food remaining after 24 h) and a dry-weight basis (adjusted for water content by freeze-drying diets and refusals to constant mass). Under both measures, cumulative food intake over each trial [±95% confidence interval (CI)] was higher on processed diets compared with the RW diet: fresh-weight basis: RW, 83.0 ± 5.8 g; RP, 101.2 ± 7.2 g; CW, 99.4 ± 6.5 g; CP, 94.7 ± 6.3 g; dry-weight basis: RW, 19.5 ± 1.3 g; RP, 23.6 ± 1.8 g; CW, 30.4 ± 2.1 g; CP, 29.7 ± 2.3 g. This result was probably driven at least partially by the relative ease of masticating a processed diet (22), because mice consumed large quantities of all diets (mean daily fresh-weight intake across diets: 23.6 ± 0.9 g, representing 69.3% of mean body mass of 34.1 ± 0.4 g). Pounding significantly increased food intake on a fresh-weight basis (2 × 2 RM ANOVA; cooking: P = 0.181, pounding: P = 0.004, cooking × pounding: P < 0.001), and cooking and pounding were both significant positive influences on food intake on a dry-weight basis (2 × 2 RM ANOVA; cooking: P < 0.001, pounding: P = 0.024, cooking × pounding: P = 0.012). The difference between fresh- and dry-weight results with respect to cooking is consistent with the relatively low water contents of cooked diets on a fresh-weight basis (Table S3). Under both measures of intake, the interaction between cooking and pounding may have been driven by slower consumption of the CP treatment, which was typically licked rather than bitten.
Our intake results suggest that, beyond the known effects of cooking on starch digestibility (2), two additional mechanisms contributed to higher energy gains on processed tuber diets. First, mastication was likely facilitated by the physical effects of pounding and the physical and chemical effects of cooking on the mechanical properties of tuber diets. Second, water lost during cooking led to increased gross energy density in cooked diets (Table S3). Jointly, these factors would have reduced digestive costs per calorie consumed, because diet-induced thermogenesis increases with masticatory effort (23) and the proportion of meal energy lost to diet-induced thermogenesis scales closely with meal mass (24). These mechanisms, as well as potential differences in the physiological maxima of mice and humans with respect to mastication and digestion, require additional assessment.
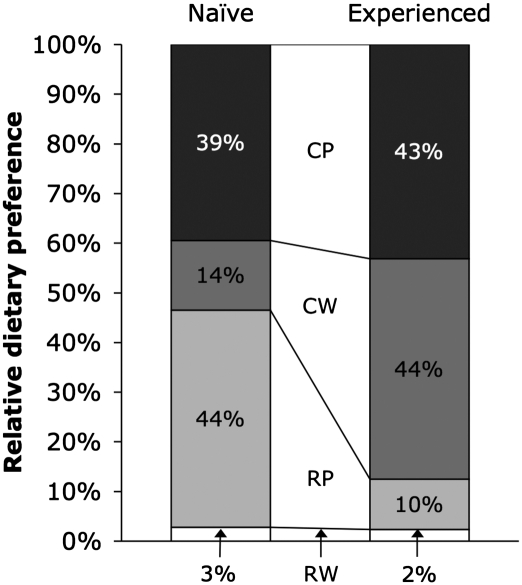
If processed diets provided superior energy returns compared with unprocessed diets and cooked diets were, in turn, superior compared with pounded diets, consumers are expected to recognize the advantages. We indeed found strong preferences for processed diets as well as a strong shift in preference from pounded diets to cooked diets over the course of the study (Fig. 2). Food preferences were assessed on fasted mice in the naïve (before exposure to any tuber diet) and experienced (after exposure to all tuber diets) conditions using two metrics: first bite (diet consumed first given concurrent presentation of all diets) and total intake (grams consumed in 3 h corrected for desiccation). Whereas naïve mice selected pounded diets in 14 of 17 cases (χ2; P = 0.008) and cooked diets in 9 of 17 cases (χ2; P = 0.808) on the basis of first bite, after experience, the same mice selected cooked diets in 17 of 17 cases (χ2; P < 0.001) and pounded diets in just 8 of 17 cases (χ2; P = 0.808). Measured by total intake, naïve mice consumed pounded diets preferentially (2 × 2 RM ANOVA; cooking: P = 0.388, pounding: P < 0.001), with pounded diets representing 84.1% of total grams ingested compared with 54.0% for cooked diets. By contrast, experienced mice consumed cooked diets preferentially (2 × 2 RM ANOVA; cooking: P < 0.001, pounding: P = 0.147), with cooked diets representing 75.0% of grams ingested compared with 59.4% for pounded diets. Because preference trials were conducted on fasted mice, it is reasonable to assume that mice selected foods at least partly on the basis of their energetic advantages.
Fig. 2.
Food preferences on tuber diets. Relative preferences among mice (n = 17) in the naïve (before exposure to any tuber diet) and experienced (after exposure to all tuber diets) conditions for organic sweet potato (I. batatas) served raw and whole (RW), raw and pounded (RP), cooked and whole (CW), and cooked and pounded (CP). Values shown reflect composite data from the two metrics of preference used in this study: first bite (diet consumed first given concurrent presentation of all diets) and total intake (grams consumed in 3 h corrected for desiccation). The composite value for a given diet is calculated as the average of the percentage of first bites and the percentage of total intake attributable to that diet. Naïve mice strongly preferred pounded tuber treatments (composite value χ2; cooking: P = 0.489, pounding: P < 0.001), whereas experienced mice strongly preferred cooked tuber treatments (composite value χ2; cooking: P < 0.001, pounding: P = 0.519).
Effects of Food Processing on Meat Diets.
To assess the energetic impact of food processing, it was necessary to maintain mice on a 100% meat diet for a measurable period. Mice of this species (M. musculus) readily consume meat, and in some ecological contexts, they have been observed to inflict intensive predation pressure on vertebrate populations (16). Nevertheless, pure meat diets are not expected to be beneficial for omnivorous species. In humans, lean meat diets that derive a majority proportion of their calories from protein lead to rabbit starvation, a condition of negative energy balance resulting from the high costs of protein digestion and the limited capacity of the liver for urea synthesis (25, 26). We, therefore, expected mice to lose body mass on all experimental meat diets, with relative loss of body mass indicating the relative values of the underlying diets.
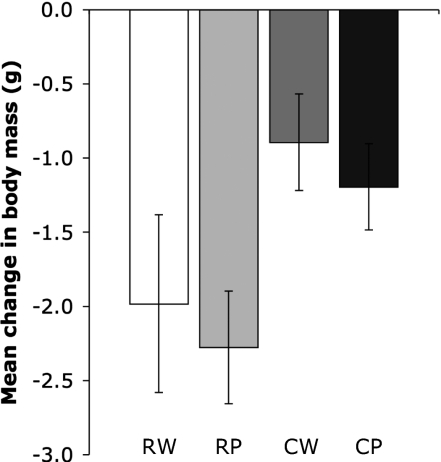
Although mice lost weight on all diets, we observed that cooking but not pounding had a positive effect on energy gain (2 × 2 RM ANOVA; cooking: P < 0.001, pounding: P = 0.138) (Fig. 3). Cooking improved a pounded diet (paired t test; RP < CP; P < 0.001), but the reverse was not true (paired t test; CW < CP; P = 0.141). As expected, washout periods resulted in inverse changes on body mass across diets (2 × 2 RM ANOVA; cooking: P = 0.004, pounding: P = 0.281) and for the RP–CP contrast (paired t test; RP > CP; P = 0.002) (Fig. S3).
Fig. 3.
Changes in body mass on meat diets. Mean cumulative change in body mass (±95% CI) over 4 d in mice (n = 16) fed standardized ad libitum diets of organic beef (B. taurus) eye round served raw and whole (RW), raw and pounded (RP), cooked and whole (CW), and cooked and pounded (CP). Diets were administered based on a counterbalanced within-subjects study design.
Activity levels and food intake could, in theory, drive differences in net energy gain separately from the underlying values of the meat diets, but they proved not to do so. Activity levels, as indexed by wheel running, were not different across diets (2 × 2 RM ANOVA; cooking: P = 0.143, pounding: P = 0.710). Cumulative food intake (±95% CI) did vary across diets, but cooked diets were generally associated with lower rather than higher intakes: fresh-weight basis: RW, 64.5 ± 3.2 g; RP, 69.1 ± 2.8 g; CW, 53.7 ± 2.7 g; CP, 55.1 ± 2.9 g; dry-weight basis: RW, 20.8 ± 0.8 g; RP, 19.3 ± 0.9 g; CW, 18.6 ± 0.9 g; CP, 18.7 ± 0.9 g. On a fresh-weight basis, food intake over the 4 d of feeding was significantly lower for cooked diets and higher for pounded diets (2 × 2 RM ANOVA; cooking: P < 0.001, pounding: P = 0.025). On a dry-weight basis, cooking lowered food intake and pounding had no effect (2 × 2 RM ANOVA; cooking: P = 0.010, pounding: P = 0.097). Taken together with observed changes in body mass, these data show that mice experienced higher absolute energy gain on cooked diets despite lesser food intake. Pounding, by contrast, had no positive effect.
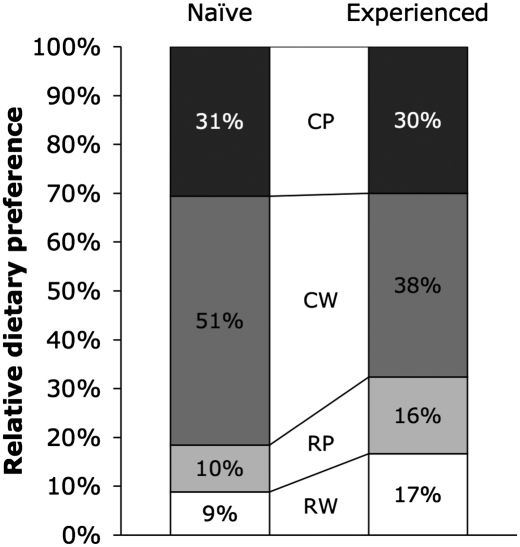
Preference data again conformed to the superior effects of cooking. In both the naïve and experienced conditions, mice preferred cooked diets (Fig. 4). On the basis of first bite, naïve mice selected cooked diets in 14 of 16 cases (χ2; P = 0.003) compared with pounded diets in 7 of 16 cases (χ2; P = 0.617), and experienced mice selected cooked diets in 12 of 16 cases (χ2; P = 0.046) compared with pounded diets in 6 of 16 cases (χ2; P = 0.317). On the basis of total intake, naïve mice consumed cooked diets preferentially (2 × 2 RM ANOVA; cooking: P = 0.012, pounding: P = 0.132), with cooked diets representing 75.6% of total grams ingested compared with 36.6% for pounded diets. Experienced mice also tended to select cooked diets (percentage of total grams ingested; cooked: 60.3%, pounded: 53.8%), although this preference did not reach statistical significance (2 × 2 RM ANOVA; cooking: P = 0.084, pounding: P = 0.448). The mechanisms governing preference for cooked meat in naïve mice are unknown. Visual, olfactory, and tactile cues are all possible.
Fig. 4.
Food preferences on meat diets. Relative preferences among mice (n = 16) in the naïve (before exposure to any meat diet) and experienced (after exposure to all meat diets) conditions for organic beef (B. taurus) eye round served raw and whole (RW), raw and pounded (RP), cooked and whole (CW), and cooked and pounded (CP). Values shown reflect composite data from the two metrics of preference used in this study: first bite (diet consumed first given concurrent presentation of all diets) and total intake (grams consumed in 3 h corrected for desiccation). The composite value for a given diet is calculated as the average of the percentage of first bites and the percentage of total intake attributable to that diet. Mice preferred cooked meat diets in both the naïve condition (composite value χ2; cooking: P < 0.001, pounding: P = 0.049) and experienced condition (composite value χ2; cooking: P < 0.001, pounding: P = 0.386).
We propose three candidate mechanisms for the positive effects of cooking observed in this study. First, consumer-specific digestibility may have been improved through heat-induced denaturation of protein. In this process, proteins unwind from their tightly bound structures when heated, adopting a random coil configuration that increases their susceptibility to proteolytic enzymes in the small intestine (27) before access by gut bacteria. Such susceptibility serves to increase the proportion of protein digested by the consumer compared to the proportion digested by gut bacteria; this result is especially important, because the products of microbial fermentation of protein seem to return little energy to the consumer (2). This mechanism is supported by evidence that heat-induced denaturation of egg protein contributed to increased ileal digestibility in humans (91–94% for cooked eggs compared with 51–65% for raw eggs) (28, 29). Second, diet-induced thermogenesis may have been reduced because of the compromised structural integrity of cooked meat. Although heat tends to toughen muscle fibers, it gelatinizes the collagen in the muscle matrix, easing separation of muscle fibers. This separation facilitates mastication and increases the surface area of meat exposed to gastric acids and enzymes (30, 31). Reduced structural integrity was the mechanism advanced to explain why pythons experienced 13% lower diet-induced thermogenesis after meals of cooked vs. raw meat (10). Third, cooking may have killed pathogens present in the meat samples, reducing the metabolic cost of immune defense (2). In humans, the ingestion of common meat-borne pathogens such as Escherichia coli, Salmonella, and Campylobacter can produce fever and other costly symptoms. Fever alone has been shown to increase resting energy expenditure by 7–13% for each 1 °C increase in body temperature (32, 33). The metabolic cost of immune defense arising from food-borne pathogens has not been investigated experimentally but is a promising area for additional study.
Discussion
Our integrated energy assessment protocol shows that cooking increases the energy gained from meat as well as tubers and that, in both foods, it does so to a greater extent than pounding. These results suggest three main conclusions for human nutrition in the past and present.
First, the adoption of cooking would have helped ancestral humans thrive. Meat and tubers have been exploited by humans for at least 2 million y, and the energetic resources of these foods are believed to have provided critical support for the evolution of costly increases in activity, birth rate, body size, and brain size (34). Meat would have been a preferred food, but its pursuit would require a large energetic investment with low rates of success (35). Tubers, by contrast, were less preferred but more consistently available, and this consistency would have made investments in the high-risk pursuit of meat possible (36). The proportions of animal and plant foods consumed by ancestral humans are unknown, but the parallel effects of cooking that we found suggest that the adoption of cooking would have led to energetic gains whether meat or tubers predominated. Moreover, because we found the effects of cooking to be incremental to the effects of pounding for both foods, the adoption of cooking was likely advantageous even if pounding methods were already in widespread use.
Second, energetic gains associated with cooking remain critical today (20). Meat and tubers continue to be staple foods worldwide (25, 37), and the energetic gains conferred by cooking contribute to explaining why these foods are typically cooked before eating. Although the energy contributed globally by cooking is not quantified, our results hint at large potentials. We note that modern raw foodists who eat meat and high‐quality plant products experience such high rates of chronic energy deficiency, despite processing their foods heavily by nonthermal means (38, 39), that a majority of females experience subfecundity (39). This finding suggests that, in humans, the caloric gains conferred by cooking may be not merely advantageous but also necessary for normal biological function.
Third, current food labeling practices take inadequate account of food processing. Our observation that mice fed cooked meat retained higher body mass despite lesser intake (fresh- or dry-weight basis) indicates that cooking increases the energy extracted per gram of meat fed. This finding contrasts with the predictions of conventional nutritional assays, which on the basis of the Atwater system, return roughly equivalent metabolizable energy densities for raw and cooked meat on a dry-weight basis (Table S1). The problem with the Atwater system is that it ignores changes in digestibility, costs of digestion, and costs of immune defense, all of which are likely influenced by food processing. The result is nutritional inaccuracy. For example, cooking is known to increase the proportion of starch digested at the terminal ileum before access by most gut bacteria (2). Starch that resists digestion in the small intestine delivers only a proportion of its metabolizable energy to the consumer, because the short-chain fatty acids produced during bacterial fermentation of starch generate less ATP than the glucose produced by starch hydrolysis in the small intestine. Additionally, the fatty acids are consumed as fuel for gut bacteria, and there are further losses from the production of combustible gases (40–42). Cooking, thus, increases the energetic value of starch to an extent greater than is represented by the Atwater system. Such differences between the calorie values determined by the Atwater system and the physiological outcomes observed in this study are relevant for the manipulation of diets to increased or decreased caloric loading. For instance, our results indicate that human dieters who count calories and eat similar mixed diets but cook them to different extents would experience different weight gain outcomes at comparable levels of physical activity. This prediction is consistent with recent long-term data indicating that preparation-specific factors affect the relationship between caloric consumption and weight gain in humans (43). Given the prevalence of both obesity and malnutrition today, additional attempts to quantify the energetic significance of cooking in humans will have important practical implications in the design of optimal diets.
Materials and Methods
Research Models.
Male CD-1 mice (M. musculus) were acquired at 12 wk of age (Model 022; Charles River). Mice were maintained and tested under controlled conditions (21 ± 1 °C, 30–50% humidity, 12 h light/dark cycle) at the Biological Research Infrastructure barrier facility at Harvard University. Adults were used to minimize change in body mass between trials, and residual growth was controlled by counterbalancing the order of treatments. Males were used to avoid a potentially confounding effect of ovarian function on energy expenditure (44). Mice participated in feeding trials for either tubers (n = 17) or meat (n = 16) but not both. All procedures were approved by the Institutional Animal Care and Use Committee at Harvard University (Protocol 29–06).
Experimental Cage Setup.
Mice were housed individually in cages designed to allow the assessment of food intake and activity level as well as maintenance of diet integrity (Fig. S2). Standard cage bottoms were fitted with a wire mesh floor to minimize coprophagy and facilitate the daily collection of food refusals. High-ceiling filtered cage tops were fitted with an exercise wheel bearing a lightweight neodymium magnet and an external magnetic cycle counter (BC 506; Sigma) such that the sensor of the counter was positioned directly above the orbit of the magnet. This setup allowed the number of wheel rotations to be counted daily as an index of activity level. Treatment diets (including chow during washout periods) were administered to mice in Petri dishes with fitted tops perforated by holes, each 1.6 cm in diameter. The purposes of the fitted tops were to minimize contamination of food and prevent burrowing that would otherwise result in diets becoming inaccessible beneath the mesh floor. To prevent toppling, the feeding apparatus was secured to the mesh floor using a pair of sterile plastic-coated neodymium magnets. Cages were sterilized, and fresh liners, cotton nestlet, and water were provided daily.
Preparation of Tuber and Meat Diets.
Mice were fed standardized diets of organic sweet potato (I. batatas) or organic beef (B. taurus) eye round in four preparations: RW, RP, CW, and CP (Fig. S1). To avoid freezing, which is known to affect material and nutritive properties in both tubers (45) and meat (46), foods were sourced daily from a local specialty market (Savenor's, Cambridge, MA). Foods were processed under sterile conditions in batches of uniform size, and all pounded samples were prepared by one researcher (G.S.W.) to maximize consistency across trials. Diets were prepared within 2 h of use and were allowed to equilibrate to room temperature before feeding. For RW, tubers were cut into cylindrical samples (diameter = 4.0 cm, height ∼1.2 cm), symmetrical with respect to the long axis of the tuber and avoiding the cortex, and then weighed into ad libitum rations (40.0 ± 0.5 g). Meat was sliced into cubes (∼1.5 cm per side) and weighed into ad libitum rations (30.0 ± 0.5 g). For RP, RW samples were rationed and placed inside a sterile Ziploc bag, pounded manually using 50 strokes of a mallet, and then crushed using 50 passes of a mortar and pestle. For CW, RW samples were roasted in a convection oven at 232 °C. RW tuber rations were arranged in a grid on aluminum foil and roasted for 35 min, a time empirically determined by polarized light microscopy to gelatinize surface starch granules. RW meat rations were placed into Pyrex Petri dishes to allow for the retention of drippings and were roasted for 12 min, achieving an internal temperature of 65–70 °C, equivalent to medium-well done. For CP, CW tuber rations were pounded and crushed as described for RP. Meat samples were roasted after pounding to ensure retention of drippings. RP meat rations were placed into Pyrex Petri dishes and roasted as described for CW.
Feeding Protocol.
Mice were reared for 4 consecutive d on each of the RW, RP, CW, and CP treatment diets, with the order of treatments counterbalanced across individuals. Between consecutive treatment diets, mice were reared for 6 d on ad libitum chow (Prolab Isopro RMH 3000; PMI Nutrition). All diets were presented at the same time each day to ensure data collection in 24-h increments. During this daily intervention, mice were weighed in a glass beaker, and body weight was recorded (±0.05 g) during a period of inactivity. Food refusals from the previous 24 h were collected, weighed fresh for analysis of fresh-weight intake, and later, freeze-dried to constant mass for analysis of dry-weight intake. Wheel running data from the previous 24 h were recorded, and the counter was reset.
Preference Tests.
Food preference tests were administered to mice 3 d before the start of feeding trials, when mice had not yet been exposed to any food except chow, and again 3 d after the conclusion of feeding trials, when mice had been exposed to all diets. Twelve hours before the start of each preference test, food was removed from cages to encourage consumption of food on demand. Fasted mice were presented with 5.00 ± 0.05-g rations of all four diets concurrently, with the positioning of diets counterbalanced across cages. From the moment of food introduction, cages were observed, and the first diet ingested (first bite) was recorded by two researchers (R.N.C. and G.S.W.). In all cases, co-observations of first bite concurred. Mice were given access to diets for a total of 3 h. After the preference test, food refusals were collected and weighed. Consumption of each diet over the 3-h preference test (total intake) was determined after correcting refusals for desiccation. Desiccation was determined in each preference trial by measuring mass lost from rations in 20 control cages, which did not contain mice but were otherwise identical.
Statistical Analysis.
After confirming that our data were normally distributed, we assessed the empirical effects of cooking and pounding using 2 × 2 RM ANOVA, a test that accounts for within-subjects measurements and multiple comparisons of diet treatments. We considered two factors (cooking and pounding), each with two levels (unprocessed and processed), a data architecture that meets the RM ANOVA condition of sphericity by definition. Where we found significant effects of food processing on body mass, we used paired t tests to contrast RP–CP and CW–CP outcomes to assess whether the effects of cooking or pounding, respectively, were positive compared with a diet already processed by the other method. In all tests, ∝ = 0.05 (two-tailed).
Supplementary Material
Acknowledgments
We thank the National Science Foundation, Harvard Department of Human Evolutionary Biology, Harvard Museum of Comparative Zoology, and Stellenbosch Institute of Advanced Study for support. We benefited from assistance provided by Nancy Lou Conklin-Brittain, Jade Guedes, Stephen Secor, Jonathan Belanich, Bailey Nicholas, Christine Baugh, John Slyconish, Ernest Sergenti, and the staff of the Harvard Biological Research Infrastructure. Discussions with Andrew Cunningham, Katherine Zink, Victoria Wobber, Herman Pontzer, David Pilbeam, Charles Nunn, Zarin Machanda, Nathaniel Dominy, Peter Ellison, and Daniel Lieberman as well as comments from two anonymous reviewers improved this report.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 19101.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112128108/-/DCSupplemental.
References
- 1.Wrangham R. Catching Fire: How Cooking Made Us Human. New York: Basic Books; 2009. [Google Scholar]
- 2.Carmody RN, Wrangham RW. The energetic significance of cooking. J Hum Evol. 2009;57:379–391. doi: 10.1016/j.jhevol.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Tester RF, Qi X, Karkalas J. Hydrolysis of native starches with amylases. Anim Feed Sci Technol. 2006;130:39–54. [Google Scholar]
- 4.Smil V. Eating meat: Evolution, patterns, and consequences. Popul Dev Rev. 2002;28:599–639. [Google Scholar]
- 5.Delgado CL. Rising consumption of meat and milk in developing countries has created a new food revolution. J Nutr. 2003;133(Suppl 2):3907S–3910S. doi: 10.1093/jn/133.11.3907S. [DOI] [PubMed] [Google Scholar]
- 6.Walker P, Rhubart-Berg P, McKenzie S, Kelling K, Lawrence RS. Public health implications of meat production and consumption. Public Health Nutr. 2005;8:348–356. doi: 10.1079/phn2005727. [DOI] [PubMed] [Google Scholar]
- 7.Goren-Inbar N, et al. Evidence of hominin control of fire at Gesher Benot Ya'aqov, Israel. Science. 2004;304:725–727. doi: 10.1126/science.1095443. [DOI] [PubMed] [Google Scholar]
- 8.Roebroeks W, Villa P. On the earliest evidence for habitual use of fire in Europe. Proc Natl Acad Sci USA. 2011;108:5209–5214. doi: 10.1073/pnas.1018116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry AG, Brooks AS, Piperno DR. Microfossils in calculus demonstrate consumption of plants and cooked foods in Neanderthal diets (Shanidar III, Iraq; Spy I and II, Belgium) Proc Natl Acad Sci USA. 2011;108:486–491. doi: 10.1073/pnas.1016868108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Department of Agriculture, Agricultural Research Service USDA National Nutrient Database for Standard Reference (Release 24) 2011. Available at http://www.ars.usda.gov/ba/bhnrc/ndl. Accessed October 1, 2011.
- 11.Boback SM, et al. Cooking and grinding reduces the cost of meat digestion. Comp Biochem Physiol A Mol Integr Physiol. 2007;148:651–656. doi: 10.1016/j.cbpa.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Gaman PM, Sherrington KB. The Science of Food: An Introduction to Food Science, Nutrition and Microbiology. Oxford: Pergamon; 1996. [Google Scholar]
- 13.Bender A. Meat and Meat Products in Human Nutrition in Developing Countries. Rome: Food and Agriculture Organization of the United Nations; 1992. [Google Scholar]
- 14.Borenstein B, Lachance PA. In: Modern Nutrition in Health and Disease. 7th Ed. Shils ME, Young VR, editors. Philadelphia: Lea and Febiger; 1988. pp. 672–684. [Google Scholar]
- 15.Jenkins DJA. In: Modern Nutrition in Health and Disease. 7th Ed. Shils ME, Young VR, editors. Philadelphia: Lea and Febiger; 1988. pp. 1151–1166. [Google Scholar]
- 16.Wanless RM, Angel A, Cuthbert RJ, Hilton GM, Ryan PG. Can predation by invasive mice drive seabird extinctions? Biol Lett. 2007;3:241–244. doi: 10.1098/rsbl.2007.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown PR, Huth NI, Banks PB, Singleton GR. Relationship between abundance of rodents and damage to agricultural crops. Agric Ecosyst Environ. 2007;120:405–415. [Google Scholar]
- 18.Jarvis MC, Briggs SPH, Knox JP. Intercellular adhesion and cell separation in plants. Plant Cell Environ. 2003;26:977–989. [Google Scholar]
- 19.Waldron KW, Parker ML, Smith AC. Plant cell walls and food quality. Compr Rev Food Sci F. 2003;2:128–146. doi: 10.1111/j.1541-4337.2003.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 20.Wrangham RW, Conklin-Brittain NL. Cooking as a biological trait. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:35–46. doi: 10.1016/s1095-6433(03)00020-5. [DOI] [PubMed] [Google Scholar]
- 21.Englyst KN, Englyst HN. Carbohydrate bioavailability. Br J Nutr. 2005;94:1–11. doi: 10.1079/bjn20051457. [DOI] [PubMed] [Google Scholar]
- 22.Lucas P. Dental Functional Morphology: How Teeth Work. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- 23.Oka K, et al. Food texture differences affect energy metabolism in rats. J Dent Res. 2003;82:491–494. doi: 10.1177/154405910308200617. [DOI] [PubMed] [Google Scholar]
- 24.Secor SM. Specific dynamic action: A review of the postprandial metabolic response. J Comp Physiol B. 2009;179:1–56. doi: 10.1007/s00360-008-0283-7. [DOI] [PubMed] [Google Scholar]
- 25.Cordain L, et al. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am J Clin Nutr. 2000;71:682–692. doi: 10.1093/ajcn/71.3.682. [DOI] [PubMed] [Google Scholar]
- 26.Speth JD, Spielmann KA. Energy source, protein metabolism, and hunter-gatherer subsistence strategies. J Anthropol Archaeol. 1983;2:1–31. [Google Scholar]
- 27.Davies KJA, Lin SW, Pacifici RE. Protein damage and degradation by oxygen radicals. IV. Degradation of denatured protein. J Biol Chem. 1987;262:9914–9920. [PubMed] [Google Scholar]
- 28.Evenepoel P, et al. Amount and fate of egg protein escaping assimilation in the small intestine of humans. Am J Physiol Gastrointest Liver Physiol. 1999;277:935–943. doi: 10.1152/ajpgi.1999.277.5.G935. [DOI] [PubMed] [Google Scholar]
- 29.Evenepoel P, et al. Digestibility of cooked and raw egg protein in humans as assessed by stable isotope techniques. J Nutr. 1998;128:1716–1722. doi: 10.1093/jn/128.10.1716. [DOI] [PubMed] [Google Scholar]
- 30.Barham P. The Science of Cooking. Berlin: Springer; 2001. [Google Scholar]
- 31.McGee H. On Food and Cooking: The Science and Lore of the Kitchen. New York: Scribner; 2004. [Google Scholar]
- 32.DuBois EF. The Mechanisms of Heat Loss and Temperature Regulation. Palo Alto, CA: Stanford University Press; 1937. [Google Scholar]
- 33.Stettler N, Schutz Y, Whitehead R, Jéquier E. Effect of malaria and fever on energy metabolism in Gambian children. Pediatr Res. 1992;31:102–106. doi: 10.1203/00006450-199202000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Ungar PS. Evolution of the Human Diet: The Known, the Unknown, and the Unknowable. New York: Oxford University Press; 2007. [Google Scholar]
- 35.Hawkes K. Showing off: Tests of an hypothesis about men's foraging goals. Ethol Sociobiol. 1991;12:29–54. [Google Scholar]
- 36.Wrangham RW, Jones JH, Laden G, Pilbeam D, Conklin-Brittain NL. The raw and the stolen. Cooking and the ecology of human origins. Curr Anthropol. 1999;40:567–594. [PubMed] [Google Scholar]
- 37.Kearney J. Food consumption trends and drivers. Philos Trans R Soc Lond B Biol Sci. 2010;365:2793–2807. doi: 10.1098/rstb.2010.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fontana L, Shew JL, Holloszy JO, Villareal DT. Low bone mass in subjects on a long-term raw vegetarian diet. Arch Intern Med. 2005;165:684–689. doi: 10.1001/archinte.165.6.684. [DOI] [PubMed] [Google Scholar]
- 39.Koebnick C, Strassner C, Hoffmann I, Leitzmann C. Consequences of a long-term raw food diet on body weight and menstruation: Results of a questionnaire survey. Ann Nutr Metab. 1999;43:69–79. doi: 10.1159/000012770. [DOI] [PubMed] [Google Scholar]
- 40.Livesey G. The impact of complex carbohydrates on energy balance. Eur J Clin Nutr. 1995;49(Suppl 3):S89–S96. [PubMed] [Google Scholar]
- 41.Livesey G. Thermogenesis associated with fermentable carbohydrate in humans, validity of indirect calorimetry, and implications of dietary thermogenesis for energy requirements, food energy and body weight. Int J Obes Relat Metab Disord. 2002;26:1553–1569. doi: 10.1038/sj.ijo.0802168. [DOI] [PubMed] [Google Scholar]
- 42.Wiseman J. Variations in starch digestibility in non-ruminants. Anim Feed Sci Technol. 2006;130:66–77. [Google Scholar]
- 43.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curtis V, Henry CJK, Birch E, Ghusain-Choueiri A. Intraindividual variation in the basal metabolic rate of women: Effect of the menstrual cycle. Am J Hum Biol. 1996;8:631–639. doi: 10.1002/(SICI)1520-6300(1996)8:5<631::AID-AJHB8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 45.Mondy NI, Chandra S. Quality of potato tubers as affected by freezing. Am J Potato Res. 1979;56:119–132. [Google Scholar]
- 46.Ballin NZ, Lametsch R. Analytical methods for authentication of fresh vs. thawed meat: A review. Meat Sci. 2008;80:151–158. doi: 10.1016/j.meatsci.2007.12.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.