Abstract
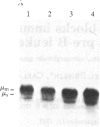
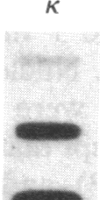
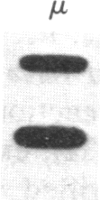
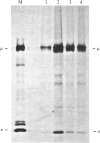
The murine pre-B leukemic cell line 70Z/3 contains both an unrearranged immunoglobulin kappa-light-chain gene and a functionally rearranged but silent kappa-light-chain gene. Mitogenic stimulation of growing 70Z/3 cells with bacterial lipopolysaccharide (LPS) activates kappa-light-gene transcription and results in a 10- to 20-fold increase in cytoplasmic kappa-light-chain mRNA. The induction of kappa gene expression by LPS was probed by using an inhibitor of protein synthesis. Concomitant treatment of 70Z/3 cells with LPS and cycloheximide failed to block kappa-light-chain mRNA accumulation, indicating that new protein synthesis is not required for the activation of kappa-gene expression. Treatment of 70Z/3 cells with cycloheximide alone resulted in kappa-mRNA induction equivalent to those produced by LPS alone. The kappa mRNA synthesized in the presence of cycloheximide was intact and able to direct the synthesis of kappa light chains. Nuclear transcription assays revealed that cycloheximide, like LPS, activated kappa-gene transcription. These findings indicate that the trans-acting factors necessary for kappa-light-chain-gene transcription are present in pre-B cells, but their activity is blocked by short-lived inhibitory proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calame K. L. Mechanisms that regulate immunoglobulin gene expression. Annu Rev Immunol. 1985;3:159–195. doi: 10.1146/annurev.iy.03.040185.001111. [DOI] [PubMed] [Google Scholar]
- Calame K., Rogers J., Early P., Davis M., Livant D., Wall R., Hood L. Mouse Cmu heavy chain immunoglobulin gene segment contains three intervening sequences separating domains. Nature. 1980 Apr 3;284(5755):452–455. doi: 10.1038/284452a0. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Darnell J. E., Jr Cycloheximide stimulates early adenovirus transcription if early gene expression is allowed before treatment. J Virol. 1983 Feb;45(2):683–692. doi: 10.1128/jvi.45.2.683-692.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans R. J., Denis K. A., Taylor A., Wall R. Expression of an immunoglobulin heavy chain gene transfected into lymphocytes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1292–1296. doi: 10.1073/pnas.81.5.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrat S., Kaempfer R. Control of biologically active interleukin 2 messenger RNA formation in induced human lymphocytes. Proc Natl Acad Sci U S A. 1984 May;81(9):2601–2605. doi: 10.1073/pnas.81.9.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggerding F., Raskas H. J. Effect of protein synthesis inhibitors on viral mRNA's synthesized early in adenovirus type 2 infection. J Virol. 1978 Jan;25(1):453–458. doi: 10.1128/jvi.25.1.453-458.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder P. K., Schmidt L. J., Ono T., Getz M. J. Specific stimulation of actin gene transcription by epidermal growth factor and cycloheximide. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7476–7480. doi: 10.1073/pnas.81.23.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff N., Wellauer P. K., Wall R. Intermolecular duplexes in heterogeneous nuclear RNA from HeLa cells. Cell. 1977 Apr;10(4):597–610. doi: 10.1016/0092-8674(77)90092-7. [DOI] [PubMed] [Google Scholar]
- Gaynor R. B., Hillman D., Berk A. J. Adenovirus early region 1A protein activates transcription of a nonviral gene introduced into mammalian cells by infection or transfection. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1193–1197. doi: 10.1073/pnas.81.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T., Kudo A., Watanabe T. Induction of immunoglobulin gene expression in mouse fibroblasts by cycloheximide treatment. J Exp Med. 1984 Dec 1;160(6):1937–1942. doi: 10.1084/jem.160.6.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Mains P. E., Sibley C. H. The requirement of light chain for the surface deposition of the heavy chain of immunoglobulin M. J Biol Chem. 1983 Apr 25;258(8):5027–5033. [PubMed] [Google Scholar]
- Maki R., Kearney J., Paige C., Tonegawa S. Immunoglobulin gene rearrangement in immature B cells. Science. 1980 Sep 19;209(4463):1366–1369. doi: 10.1126/science.6774416. [DOI] [PubMed] [Google Scholar]
- Makino R., Hayashi K., Sugimura T. C-myc transcript is induced in rat liver at a very early stage of regeneration or by cycloheximide treatment. Nature. 1984 Aug 23;310(5979):697–698. doi: 10.1038/310697a0. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Sharp P. A., Gefter M. L. RNA synthesis in isolated nuclei: identification and comparison of adenovirus 2 encoded transcripts synthesized in vitro and vivo. J Mol Biol. 1979 Nov 25;135(1):171–197. doi: 10.1016/0022-2836(79)90346-2. [DOI] [PubMed] [Google Scholar]
- Maroteaux L., Chen L., Mitrani-Rosenbaum S., Howley P. M., Revel M. Cycloheximide induces expression of the human interferon beta 1 gene in mouse cells transformed by bovine papillomavirus-interferon beta 1 recombinants. J Virol. 1983 Jul;47(1):89–95. doi: 10.1128/jvi.47.1.89-95.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather E. L., Nelson K. J., Haimovich J., Perry R. P. Mode of regulation of immunoglobulin mu- and delta-chain expression varies during B-lymphocyte maturation. Cell. 1984 Feb;36(2):329–338. doi: 10.1016/0092-8674(84)90226-5. [DOI] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. L., Oi V. T. Transfer and expression of immunoglobulin genes. Annu Rev Immunol. 1984;2:239–256. doi: 10.1146/annurev.iy.02.040184.001323. [DOI] [PubMed] [Google Scholar]
- Nelson K. J., Mather E. L., Perry R. P. Lipopolysaccharide-induced transcription of the kappa immunoglobulin locus occurs on both alleles and is independent of methylation status. Nucleic Acids Res. 1984 Feb 24;12(4):1911–1923. doi: 10.1093/nar/12.4.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Ralph P. Murine B cell leukemia line with inducible surface immunoglobulin expression. J Immunol. 1978 Aug;121(2):641–647. [PubMed] [Google Scholar]
- Parslow T. G., Granner D. K. Chromatin changes accompany immunoglobulin kappa gene activation: a potential control region within the gene. Nature. 1982 Sep 30;299(5882):449–451. doi: 10.1038/299449a0. [DOI] [PubMed] [Google Scholar]
- Parslow T. G., Granner D. K. Structure of a nuclease-sensitive region inside the immunoglobin kappa gene: evidence for a role in gene regulation. Nucleic Acids Res. 1983 Jul 25;11(14):4775–4792. doi: 10.1093/nar/11.14.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Immunoglobulin messenger RNAs in murine cell lines that have characteristics of immature B lymphocytes. Cell. 1979 Dec;18(4):1333–1339. doi: 10.1016/0092-8674(79)90243-5. [DOI] [PubMed] [Google Scholar]
- Picard D., Schaffner W. A lymphocyte-specific enhancer in the mouse immunoglobulin kappa gene. Nature. 1984 Jan 5;307(5946):80–82. doi: 10.1038/307080a0. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Rice D., Baltimore D. Regulated expression of an immunoglobulin kappa gene introduced into a mouse lymphoid cell line. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7862–7865. doi: 10.1073/pnas.79.24.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J. E., Gilliam A. C., Shen A., Tucker P. W., Blattner F. R. Unusual sequences in the murine immunoglobulin mu-delta heavy-chain region. Nature. 1983 Dec 1;306(5942):483–487. doi: 10.1038/306483a0. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Dieckmann B., Vannice J. L., Trahey M., McCormick F. Inhibition of protein synthesis stimulates the transcription of human beta-interferon genes in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3964–3968. doi: 10.1073/pnas.81.13.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins D. M., Paek I., Seeburg P. H., Axel R. Regulated expression of human growth hormone genes in mouse cells. Cell. 1982 Jun;29(2):623–631. doi: 10.1016/0092-8674(82)90178-7. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N., Kishimoto T., Kikutani H., Watanabe T., Yoshida N., Shimizu A., Yamawaki-Kataoka Y., Honjo T., Yamamura Y. Induction and regulation of immunoglobulin expression in a murine pre-B cell line, 70Z/3. I. Cell cycle-associated induction of sIgM expression and kappa-chain synthesis in 70Z/3 cells by LPS stimulation. J Immunol. 1980 Dec;125(6):2654–2659. [PubMed] [Google Scholar]
- Sidman C. B lymphocyte differentiation and the control of IgM mu chain expression. Cell. 1981 Feb;23(2):379–389. doi: 10.1016/0092-8674(81)90133-1. [DOI] [PubMed] [Google Scholar]
- Storb U., Denis K. A., Brinster R. L., Witte O. N. Pre-B cells in kappa-transgenic mice. Nature. 1985 Jul 25;316(6026):356–358. doi: 10.1038/316356a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Treisman R., Green M. R., Maniatis T. cis and trans activation of globin gene transcription in transient assays. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7428–7432. doi: 10.1073/pnas.80.24.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usa T., Morimoto Y., Fukuda T., Anami K., Setoguchi M., Maruyama Y. [Pharmacological studies on Y-8894. (III). Its effect on the abnormal electrocorticogram induced by destruction of the internal capsule]. Nihon Yakurigaku Zasshi. 1986 Oct;88(4):289–297. doi: 10.1254/fpj.88.289. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Kuehl M. Biosynthesis and regulation of immunoglobulins. Annu Rev Immunol. 1983;1:393–422. doi: 10.1146/annurev.iy.01.040183.002141. [DOI] [PubMed] [Google Scholar]
- Weintraub H. A dominant role for DNA secondary structure in forming hypersensitive structures in chromatin. Cell. 1983 Apr;32(4):1191–1203. doi: 10.1016/0092-8674(83)90302-1. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Whitlock C., Denis K., Robertson D., Witte O. In vitro analysis of murine B-cell development. Annu Rev Immunol. 1985;3:213–235. doi: 10.1146/annurev.iy.03.040185.001241. [DOI] [PubMed] [Google Scholar]