Abstract
Many different systems of bacterial interactions have been described. However, relatively few studies have explored how interactions between different microorganisms might influence bacterial development. To explore such interspecies interactions, we focused on Bacillus subtilis, which characteristically develops into matrix-producing cannibals before entering sporulation. We investigated whether organisms from the natural environment of B. subtilis—the soil—were able to alter the development of B. subtilis. To test this possibility, we developed a coculture microcolony screen in which we used fluorescent reporters to identify soil bacteria able to induce matrix production in B. subtilis. Most of the bacteria that influence matrix production in B. subtilis are members of the genus Bacillus, suggesting that such interactions may be predominantly with close relatives. The interactions we observed were mediated via two different mechanisms. One resulted in increased expression of matrix genes via the activation of a sensor histidine kinase, KinD. The second was kinase independent and conceivably functions by altering the relative subpopulations of B. subtilis cell types by preferentially killing noncannibals. These two mechanisms were grouped according to the inducing strain's relatedness to B. subtilis. Our results suggest that bacteria preferentially alter their development in response to secreted molecules from closely related bacteria and do so using mechanisms that depend on the phylogenetic relatedness of the interacting bacteria.
Cell–cell interactions are a feature common to all living systems. Bacteria are no exception, and numerous mechanisms that use secreted products as signaling molecules are known (1, 2). Among these, the so-called “quorum sensing” systems are perhaps the best studied (3, 4). In quorum sensing, all bacterial cells within a population produce secreted molecules. Only when population densities are high is there a response to these compounds, thus allowing the bacteria to coordinate their behavior. However, it is clear that there is much more to bacterial cell–cell interactions than simply counting numbers and coordinating behavior. Secreted molecules also play key roles in microbial development so that different cell fates can arise and coexist within a single-species population (5, 6). In addition, in settings where multiple species coexist, their interactions often are mediated through extracellular compounds. Development in one microbe can be influenced by small molecules secreted by other species (7, 8).
We have been interested in understanding the role of interspecies interactions in the well-studied developmental processes that the soil bacterium Bacillus subtilis undergoes during biofilm formation. Biofilms are aggregations of cells held together by an extracellular matrix (9, 10). The matrix has two main components, an exopolysaccharide synthesized by the products of the epsA-O operon, and amyloid fibers encoded by the products of the tapA operon (formerly yqxM) (9, 11, 12). Within biofilms, several different cell types coexist, including subpopulations of motile cells, matrix-producing cells, and dormant spores (5, 6).
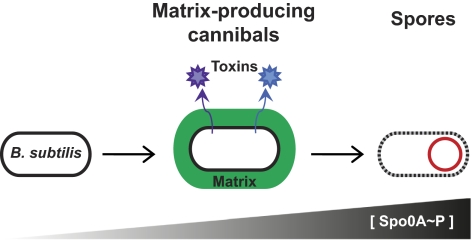
The regulation of the expression of the epsA-O and tapA operons has been studied extensively (10, 13–20). Both operons are controlled indirectly by the master regulator, phosphorylated Spo0A (Spo0A∼P) (11, 21, 22). This transcription factor modulates the expression of a large number of genes depending on its concentration (23, 24). Low levels of Spo0A∼P lead to the induction of transcription of the epsA-O and tapA operons, resulting in the production of extracellular matrix and thus biofilm formation (Fig. 1) (22). At high levels of Spo0A∼P, matrix genes are repressed, sporulation genes are induced, and these matrix-producing cells go on to become spores.
Fig. 1.
Diagram illustrating the development of B. subtilis into matrix-producing cannibals and then spores. At low Spo0A∼P levels B. subtilis activates genes required for the production of matrix and produces two cannibalism toxins. At high Spo0A∼P levels sporulation begins.
The matrix-producing cells have a second important physiological function: They are cannibals (Fig. 1) (25, 26). These cells produce two toxins, sporulation-delaying protein (SDP) and sporulation-killing factor (SKF) (25, 27). To survive, these cells also produce immunity proteins (28, 29). Both the toxin and immunity genes are under the control of Spo0A∼P. These toxins are able to kill B. subtilis cells not expressing immunity (those that have not yet phosphorylated Spo0A) as well as other microbes (30). This killing presumably provides the matrix-producing cannibal with nutrients and delays sporulation (25). The production of biofilm matrix thus is linked intrinsically to this mechanism of siblicide that prolongs the survival of this subpopulation of B. subtilis cells (25, 26).
The levels of Spo0A∼P are controlled by the action of membrane-bound histidine sensor kinases (KinA, KinB, KinC, KinD, and KinE) that directly and indirectly phosphorylate Spo0A in response to still poorly defined environmental cues (31). The activities of these kinases thus control differentiation in B. subtilis (31–33). KinC has a role in defining the matrix-producing cannibal subpopulation when it is activated by the self-produced molecule surfactin (34). Purified natural products functionally related to surfactin but produced by other bacteria also induce matrix synthesis in B. subtilis in a KinC-dependent manner (34). In addition, nisin, an antimicrobial produced by Lactococcus lactis, induces a hypercannibalism phenotype in B. subtilis (26). Thus, small molecules from numerous bacteria are capable of influencing differentiation in B. subtilis. This observation suggests that B. subtilis development may be influenced by the presence of other bacteria in natural settings.
We therefore hypothesized that other soil microbes, when grown next to B. subtilis, might trigger its differentiation into matrix-producing cannibals. To investigate these possible interspecies interactions, we developed a screen in which B. subtilis and soil microorganisms were cocultured. While the colonies grew, we monitored B. subtilis differentiation into matrix producers using a fluorescent reporter. In this way we identified a large number of soil organisms that induced differentiation in B. subtilis. The majority of these organisms were other members of the genus Bacillus. This result led us to explore how broadly distributed the ability to induce matrix production is among a range of phylogenetically diverse Bacillus species. We found that many Bacillus species induce matrix production in B. subtilis, and the mechanisms they use are grouped according to their phylogenetic relatedness to B. subtilis. We therefore suggest that the interspecies interactions that induce biofilm production in the environment correlate with phylogenetic relatedness.
Results
Screen to Identify Matrix-Inducing Soil Organisms.
We designed an interspecies-interaction microcolony screen to identify microbes able to induce matrix production in B. subtilis. To monitor B. subtilis matrix production, we used a reporter strain in which the promoter of the tapA operon (PtapA) drives the transcription of the YFP gene (yfp), PtapA-yfp (35). By coculturing this strain with random environmental microbes on agar plates, we could mimic a multispecies environment. Within this complex mixture of colonies, the fluorescent reporter allowed us to identify matrix-inducing interspecies interactions. Screening was performed in dilute, buffered LB medium (0.1× LB, pH 7) in which B. subtilis normally does not express the matrix genes (34).
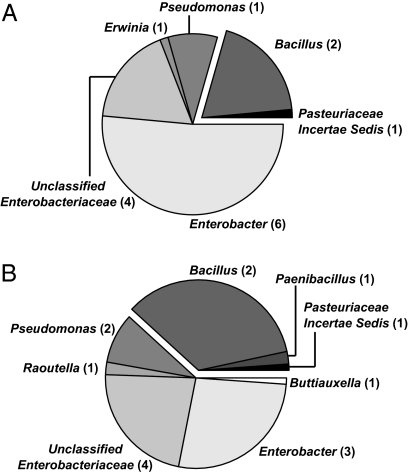
We first wanted to ensure that our chosen conditions permitted a diverse range of organisms to grow within the time scale of the screen. Therefore we plated a subset of the soil samples that would be used during screening in the absence of the B. subtilis reporter strain. Random microcolonies were isolated, and we sequenced their 16S rRNA genes. The environmental microbes that grew under these conditions were primarily Proteobacteria and Firmicutes, with representatives from a number of different genera (Fig. 2). The Firmicute representatives all fell within the order Bacillales. Rarefaction curves indicate that our sequence analysis accurately reflects the diversity of the bacteria that we cultivated (Fig. S1). Thus, although spore-forming Bacillus species are well represented in the cultivable soil isolates, our screen probed interactions between B. subtilis and a variety of phylogenetically diverse soil microbes.
Fig. 2.
The cultivable organisms growing under the microcolony coculture screen conditions are phylogenetically diverse. Soil organisms were selected systematically from random fields of view of soil A and soil B (grown as for coculture screen) and were isolated. (A) The 16S rRNA gene from 71 isolates from soil A and (B) 89 isolates from soil B were sequenced. The pie chart sectors are proportional to the number of representatives within each labeled group. The number of operational taxonomic units at the 99% level within that group is noted in parentheses. The darkly shaded exploded wedges represent the Firmicutes, whose representatives are all within the class Bacillales. All other sequences were from Proteobacteria.
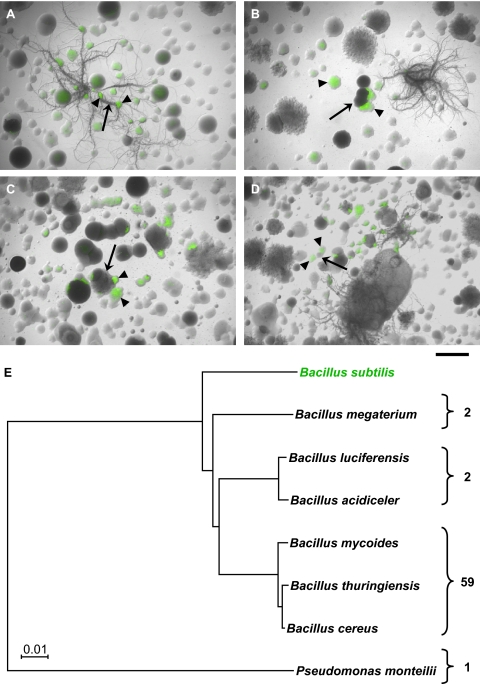
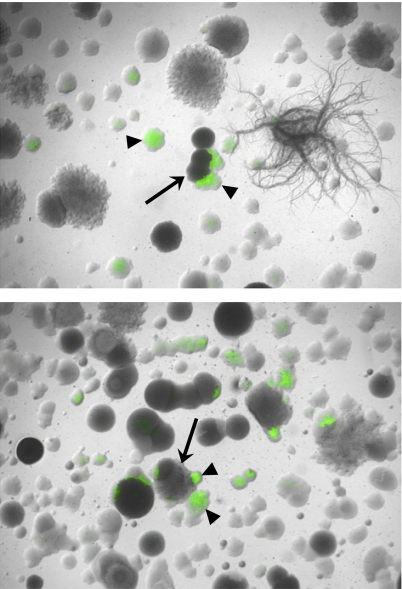
We conducted interspecies-interaction microcolony screens by mixing the PtapA-yfp B. subtilis reporter strain with soil samples from multiple sampling sites. These coculture plates were inspected visually using fluorescence microscopy to identify interspecies interactions resulting in matrix production in B. subtilis (i.e., activation of the fluorescent reporter, PtapA-yfp). Matrix-inducing soil microbes (nonfluorescent colonies adjacent to fluorescing B. subtilis reporter colonies) were identified frequently (Fig. 3 A–D). The hit rate in the screen was high (see Fig. 4 for quantification), and a subset of positive hits (fewer than 100 isolates) was selected for further analysis in a secondary screen. The secondary screen confirmed that the isolated soil microbes induced fluorescence in the reporter strain. We used both the B. subtilis matrix reporter strain (PtapA-yfp) and a cannibalism reporter strain (Pskf-yfp) in the secondary screen. As expected, in all cases in which we observed activation of PtapA-yfp, we also observed fluorescence from Pskf-yfp (26).
Fig. 3.
The microcolony coculture screens reveal interspecies interactions between B. subtilis and soil organisms. (A–D) Representative images of microcolony coculture screens of the B. subtilis PtapA-yfp reporter strain with four different soils. Bright-field images were overlaid with fluorescent images false-colored green. (Scale bar, 1 mm.) Fluorescent B. subtilis reporter colonies (arrowheads) are present in each field of view. Putative inducer colonies (arrows) are the nonfluorescent colonies in close proximity to the fluorescent reporter colonies. (E) Maximum-likelihood tree showing the identity of the organisms identified as PtapA-yfp inducers. The number of sequenced representatives within each group of organisms is given on the right.
Fig. 4.
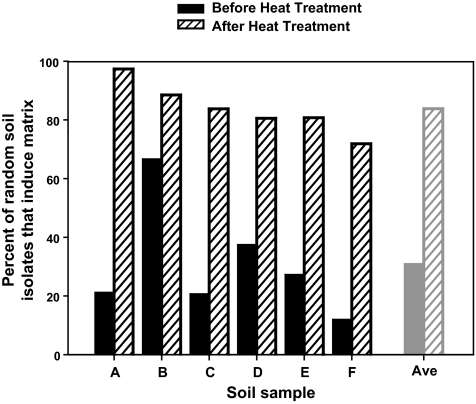
The ability of random soil isolates and endospores to induce the PtapA-yfp matrix reporter in B. subtilis is common. Random soil colonies from six soils used during coculture screening were tested for their ability to induce fluorescence from the B. subtilis PtapA-yfp reporter both before (dark bars) and after (hatched bars) treatment at 80 °C for 30 min. A total of 507 soil colonies were tested, with an average of 40 colonies examined for each bar.
Identification of Soil Isolates That Induce Matrix Gene Expression.
Next, we identified the soil isolates that induced fluorescence in the B. subtilis reporter strains. Sequencing of the 16S rRNA genes revealed that the bulk of the isolates were Bacillus species (Fig. 3E and Fig. S2). The majority of the sequenced strains were in the Bacillus cereus/thuringiensis/mycoides group, with a few representatives from other species, such as Bacillus megaterium and Bacillus luciferensis. B. subtilis was not identified among the 64 isolates sequenced. The only non-Bacillus isolate was identified as Pseudomonas monteilli. These results indicate that, by and large, in this assay B. subtilis responds to other members of the Bacillus genus.
A characteristic feature of the Bacillus genus is the ability of its constituents to form heat- and desiccation-resistant spores (36). Because of the preponderance of Bacillus species identified as matrix inducers, we wondered if the ability to induce matrix production in B. subtilis was a common feature of spore formers present in our soil samples. To test this possibility, we selected six soils used for screening and examined them either directly or after heat treatment (30 min at 80 °C) to select for Bacillus spores. These soil samples were plated at concentrations equivalent to those used during screening, and random colonies were selected from each sample. Organisms were isolated and assayed for their ability to induce fluorescence in the B. subtilis matrix reporter strain. The ability to induce matrix was pervasive among the random cultivable soil isolates, ranging from 12% to 67% (Fig. 4, dark bars), and an even higher percentage of spore formers from these soils (72–97%) was capable of inducing fluorescence in the reporter (Fig. 4, hatched bars).
We noted that the overall percentages of soil organisms able to induce the PtapA-yfp reporter (21% for soil A and 67% for soil B) (Fig. 4) were approximately proportional to the number of organisms within the Bacillales order from these soils (21% for soil A and 39% for soil B) (Fig. 2). Furthermore, more than 70% of Bacillus species present in the soil as spores can induce B. subtilis to produce matrix. Thus, the data are consistent with the conclusion that many of the environmental organisms capable of inducing matrix production in B. subtilis are likely other Bacillus species. In conjunction with our results from the specific isolates identified in the coculture screen, it is clear that close phylogenetic relatives of B. subtilis are particularly apt at stimulating biofilm formation in B. subtilis when grown in coculture.
To determine the phylogenetic breadth of Bacillus species that are capable of inducing matrix production in B. subtilis, we analyzed an assortment of 14 phylogenetically diverse, well-characterized Bacillus species, including many type strains. The majority of these other Bacillus species induced the B. subtilis matrix and cannibalism reporters under our conditions. We also examined the induction capability of the B. subtilis reporter itself, along with 13 other B. subtilis subsp. subtilis and B. subtilis subsp. spizizenii strains. Only two of these closely related strains induced the Pskf-yfp reporter (Fig. S3). Thus, in this assay, B. subtilis produces matrix in response to different Bacillus species more often than it does in response to B. subtilis strains. Because we selected our screening conditions to minimize self-induction of matrix production, this result was as anticipated. We next investigated the mechanism by which B. subtilis responds to the matrix-inducing molecule(s) from its phylogenetic relatives.
Role of Sensor Kinases in Matrix Induction.
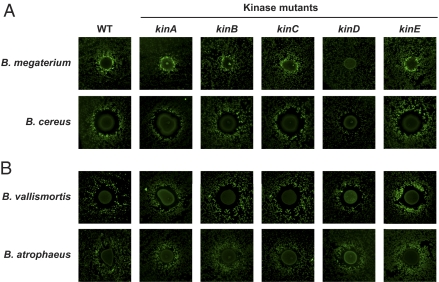
We wanted to determine which of the five sensor kinases known to phosphorylate Spo0A were involved in mediating the observed interspecies interactions. We thus compared the induction of Pskf-yfp in wild-type and kinase-mutant strains. If B. subtilis requires one of the sensor kinases to respond to the other Bacillus species and differentiate into matrix-producing cannibals, then no fluorescence would be expected in the corresponding sensor kinase-mutant reporter strain. The results obtained for four bacteria that induced Pskf-yfp are shown in Fig. 5. Interestingly, we observed no effects with mutants in kinA, kinB, kinC, or kinE, regardless of the inducing strain used. The matrix-induction produced by Bacillus megaterium and Bacillus cereus was abolished or reduced in the kinD mutant (Fig. 5A). Therefore, we concluded that, at least for these bacteria, the interspecies interactions proceeded along the anticipated Spo0A∼P pathway. However, the matrix-induction caused by the other two strains (Bacillus vallismortis and Bacillus atrophaeus) was not affected in any of the kinase mutants (Fig. 5B). This result led us to consider a possible role of cannibalism in this latter type of interaction.
Fig. 5.
kinD is necessary for some but not all interspecies interactions leading to induction of B. subtilis matrix production and cannibalism. Inducing organisms were tested for their ability to activate the Pskf-yfp reporter in wild-type and kinase deletion mutants of B. subtilis using the secondary screen format. (A) B. megaterium and B. cereus have reduced activation of the Pskf-yfp reporter in the absence of kinD. (B) B. vallismortis and B. atrophaeus do not require any of the sensor kinases for induction of the Pskf-yfp fluorescent reporter.
Role of Subpopulation Dynamics in Matrix Induction.
How did these Bacillus species stimulate matrix production if they did not lead to an increase in Spo0A phosphorylation via one of the known sensor kinases? One possibility is that the matrix-inducing strains might produce two or more compounds that activate two or more kinases, and the additive effect of these compounds might lead to the observed activation. Although this notion was possible, there was a more parsimonious explanation. We hypothesized that the observed increase in Pskf-yfp fluorescence could be attributed to an increase in the number of cells of the matrix-producing cannibal subpopulation rather than to changes in transcription mediated by the kinases. A cannibalistic toxin produced by the matrix-inducing strain to which the B. subtilis cells also were immune would have the effect of increasing the relative proportion of the matrix-producing cannibal subpopulation.
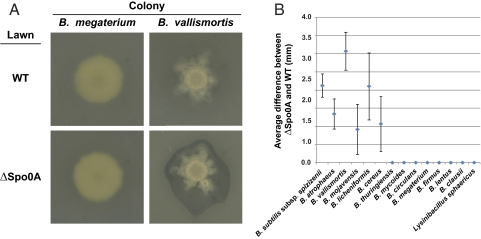
To investigate this possibility indirectly, we tested whether these matrix-inducing strains were able to kill a B. subtilis strain that could not produce cannibal toxins or resistance. Cells lacking the spo0A gene can never produce matrix-producing cannibals, because Spo0A∼P levels cannot accumulate to activate this development. We determined whether the matrix-inducing strains produced a larger killing halo on a lawn of a spo0A-mutant strain than on a wild-type B. subtilis lawn. We spotted the matrix-inducing strains on LB agar and let them grow until a mature colony was formed, allowing them time to produce any potential toxins. We then superimposed an agar slab embedded with either wild-type B. subtilis or spo0A mutant cells and examined the overlays after growth to determine the extent of the killing, if any. Some Bacillus species produced no halo (B. megaterium) or a halo of the same size with either B. subtilis strain, but others (such as B. vallismortis) produced a significantly larger killing zone on the spo0A-mutant cells than on wild-type cells (Fig. 6). Thus, B. subtilis possesses an Spo0A-dependent immunity to these strains, perhaps resulting from resistance to a cannibalism-like toxin. This immunity could result in the appearance of enhanced matrix production in B. subtilis because of the death of all subpopulations of cells not producing matrix and not immune to cannibalism.
Fig. 6.
B. subtilis possesses a Spo0A-dependent immunity to some Bacillus species. (A) B. megaterium and B. vallismortis colonies overlaid with embedded agar lawns of either wild-type (WT) or ΔSpo0A B. subtilis. B. megaterium does not kill either B. subtilis strain; B. vallismortis kills ΔSpo0A but not WT B. subtilis. (B) Graph showing quantification of the average difference in the halo size between the ΔSpo0A and WT overlays. At least three independent experiments were averaged, and each experiment was performed in duplicate. Error bars show SEM.
Verification That Induction Is via Secreted Molecules.
Although the chemical identity of these matrix-inducing compounds is not yet known, we verified that these developmental effects were the result of secreted compounds. We used approaches that allowed us to separate the production of compounds by the matrix inducers temporally and spatially from the matrix-induction assay of B. subtilis. This separation allowed us to test whether any secreted compounds induced the Pskf-yfp reporter even in the presence of additional nutrients. To do so we used three different approaches. We grew the matrix-inducing organisms and then removed the colony by coring out and UV-sterilizing the agar; we used cellophanes to separate the matrix inducers from the agar; and we examined the effects of conditioned medium (CM) on colony development as well as on fluorescence induction. The results from these assays indicate that different molecules are produced by the matrix-inducing organisms (Table S1).
Two Matrix-Inducing Mechanisms Cluster with the Relatedness of the Inducing Strain.
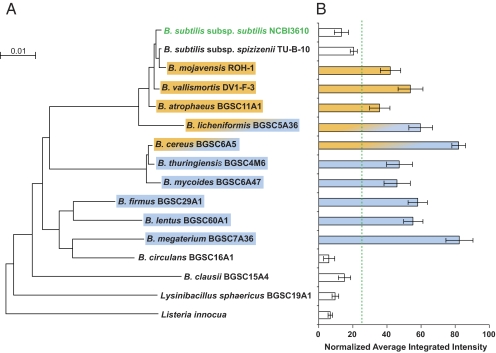
The microbes that induced B. subtilis matrix production via KinD and those that produced an Spo0A-specific zone of killing fell largely into two groups. We mapped the mechanism of matrix induction onto the phylogenetic tree of these strains (Fig. 7). The strains inducing B. subtilis matrix production via the Spo0A-dependent immunity mechanisms were the closest relatives of B. subtilis (including the two B. subtilis matrix-inducing strains). The strains inducing B. subtilis matrix production via the KinD-dependent mechanism were more distantly related to B. subtilis. Two strains, B. cereus and Bacillus licheniformis, induced matrix via both mechanisms.
Fig. 7.
The mechanisms used to induce matrix-producing cannibals in B. subtilis are segregated based on phylogeny. (A) Maximum-likelihood tree of the strains tested. The strains able to induce the PtapA-yfp and Pskf-yfp reporters in B. subtilis are highlighted by boxes. B. subtilis possesses an Spo0A∼P-dependent immunity to the organisms in orange; organisms in blue induce B. subtilis in a KinD-dependent manner. B. cereus and B. licheniformis demonstrated both activities. (B) Fluorescence induction for each strain was quantified from at least three independent experiments (error bars show SEM). The normalized average intensities of the inducers were significantly different from those of the noninducers using the Wilcoxon rank sum test (P = 0.0006). The boundary between these groups’ normalized average intensities is indicated by the green dashed line. An analysis of means test (α = 0.05) also indicates that the average intensities of the noninducing organisms are significantly below the mean threshold of the measured values (58).
Discussion
We were interested in determining which soil microbes might influence B. subtilis development. To address this question, we devised a screen that allowed us to identify microbes that, when grown in the vicinity of B. subtilis, would induce B. subtilis to make an extracellular matrix. Although the growth conditions used permitted the growth of a phylogenetically diverse collection of microbes, secreted molecules leading to matrix production came primarily from members of the Bacillus genus. Thus, as B. subtilis evaluates its environment to control its development, the microbes that prove most influential are its close relatives, although primarily not those of the same species. Interestingly, matrix inducers more closely related to B. subtilis elicited this effect through a mechanism reminiscent of cannibalism, whereas more distantly related bacteria induced extracellular matrix formation via the activation of the sensor histidine kinase KinD (Fig. 7).
KinD was described recently as a “checkpoint” protein controlling the progression from matrix producers to spore formers (37). KinD appears to have a dual function, acting as a phosphatase until sufficient matrix is produced and then becoming a kinase that helps attain the high Spo0A∼P levels required for sporulation (37). It is possible that the Bacillus species identified in our screen produce matrices that somehow influence KinD activity.
Here we show that B. subtilis possesses an Spo0A-dependent immunity against the killing activities produced by Bacillus species that are closely related to it. As a result, when B. subtilis is grown near these close relatives, cells unable to induce the Spo0A regulon are killed. We propose that this killing could lead to an increase in the fraction of the population that is composed of matrix-producing cannibals. These close relatives probably do not produce the same toxins as B. subtilis, because no skf or sdp operons are found in their genomes. However, they may produce other toxins to which B. subtilis’ cannibalism immunity genes provide cross-immunity. The Spo0A-dependent immunity of B. subtilis to these close relatives may be related instead to more general, intrinsic mechanisms of cellular immunity, such as those under the control of the transcription factors sigma W or sigma X, which have a role in providing immunity to SDP and other antimicrobials in B. subtilis (38–40).
It is clear that the development of B. subtilis, and in particular the expression of matrix and cannibalism genes, can be influenced by extracellular bacterial molecules. The compound surfactin is produced by B. subtilis itself and acts as a quorum-sensing molecule to increase the subpopulation of matrix-producing cannibals via the action of the sensor kinase KinC (34). In addition, KinC was activated similarly by functionally related compounds produced by Actinobacteria such as valinomycin and amphotericin (34). Because of our screening conditions, it is not surprising that we did not find any Actinobacteria that induced matrix-producing cannibals in B. subtilis. However, our finding that B. subtilis possesses an Spo0A-dependent immunity to the killing action of other Bacillus species suggests that many antibiotics produced by soil bacteria could influence the development of B. subtilis.
Our understanding of the natural ecology of B. subtilis in the soil is incomplete (41, 42), and there are still serious challenges in knowing whether results obtained from bacteria in the laboratory are relevant to their counterparts in the soil (43). Studies examining how bacteria are distributed spatially in soil indicate that cells generally are clustered locally in small colonies (44, 45). Investigators looking specifically at Bacillus found them in groups of 1–40 cells depending on the substrate, averaging five cells per colony (43, 46). Although it seems feasible that cells at such proximities could interact, we also do not know the concentrations at which natural products are produced natively in soil (47). These gaps in our knowledge make it impossible to determine whether these molecules evolved for the purpose of altering bacterial development or whether that function is a nonadaptive by-product of another ecological role.
It is highly likely that the molecules mediating the observed interactions evolved for reasons unrelated the interactions themselves. Nevertheless, such molecules may be present in the microhabitat of B. subtilis and have the ability to influence its development. Thus, it is tempting to speculate about the consequences of such interspecies interactions in natural environments. These interactions could benefit the inducing organism by reducing their competition for resources: in B. subtilis, matrix-producing cannibals and swimming subpopulations are mutually exclusive (22, 48). Alternatively, increases in this subpopulation of B. subtilis cells could be advantageous to both interacting partners, allowing them to be enclosed jointly by a common matrix within a multispecies biofilm. Finally, matrix-producing cannibals could represent a bet-hedging strategy for B. subtilis with regards to survival in complex microbial communities (49). By maintaining a subpopulation of matrix-producing cannibal cells, B. subtilis safeguards those cells against antibiotics produced by other bacteria while also producing its own toxic arsenal. Our results show that B. subtilis increases its relative subpopulation of matrix-producing cannibal cells in response to antibiotics produced by closely related organisms. Thus, by allowing the death of a subpopulation of its own cells, B. subtilis may enhance its ability to survive when confronted with other organisms in coculture.
Methods
Strains and Culture Conditions.
Strains used and generated in this work are listed in Table S2.
The B. subtilis strain was NCIB3610 from our laboratory collection. New strains were generated by using SPP1 phage transduction (50). Lysate from ZK3757 (3610 amyE::Pskf-yfp, from our strain collection) was transduced into various kinase mutant strains, and spectinomycin-resistant colonies were selected. Recipient strains (from our strain collection) were HV1204 for ZK4814, CA051 for ZK4815, DL147 for ZK4816, DL153 for ZK4817, and HV1205 for ZK4818. All plates contained 20 mL, poured using a Wheaton Unispense liquid dispenser. Coculture and secondary screen plates were 0.1× LB broth (BD Difco), 100 mM 3-(N-morpholino)propanesulfonic acid (MOPS, pH 7) (Sigma), and 1.5% (wt/vol) agar. The halo overlay plates were 1× LB, 1.5% (wt/vol) agar. Liquid LB broth contained no agar.
Reporter Preparation.
B. subtilis reporter strains were cultured in LB broth to mid-log stage, diluted to an OD600 of 0.02, and regrown to mid-log stage at least twice to reduce background fluorescence levels. At mid-log stage after these dilutions, glycerol was added [15–20% (vol/vol) final solution], and aliquots were frozen at −80 °C. The number of colony-forming units per milliliter of aliquot was determined by plating serial dilutions on LB plates before use.
Soil Preparation.
Soil was collected from the Boston, MA, area. The top ∼0.5 cm of soil was discarded, and samples below that level were collected. Soil A and Soil B were collected at the same location 3 mo apart (in August and November, respectively). Sterile saline [0.85% (wt/vol) NaCl] was added at a ratio of 10 mL/g of soil, and this suspension was either vortexed for 1 min or blended in a Waring blender for three 1-min cycles, with 1-min rests on ice. Glycerol was added [15–20% (vol/vol) final solution], aliquots were frozen at −80 °C, and the number of colony-forming units per milliliter was determined as with the reporters.
Coculture and Secondary Screens.
Screen plates were grown at ambient room temperature (24–26 °C). For the coculture screen, reporter and soil aliquots were thawed and diluted independently in saline or 0.1× LB broth, and 25,000 cfu of each were spread simultaneously on plates using 3-mm glass beads. After 24–26 h of growth, plates were examined visually using a Zeiss Stemi SV6 stereoscope attached to a fluorescence illumination system (X-cite 120, Lumen Dynamics). Putative matrix-inducing soil colonies were selected and isolated. For the secondary screen, the test organisms were resuspended in 0.1× LB broth to an OD600 of 0.5, and 3 μL was spotted onto a dried plate freshly inoculated with a B. subtilis reporter microcolony lawn. After 24–26 h of growth, the plates were examined for fluorescence using a Typhoon 9600 fluorescence imager [GE Healthcare; 488-nm excitation, 526-nm emission, 500-V (photomultiplier tube), 100 μm resolution, 3 mm scan height].
Fluorescence Quantification.
Typhoon data files (.gel) were loaded into Metamorph 7.1 (Molecular Devices), and brightness and contrast were adjusted linearly. Thresholding eliminated the pixel intensity of the agar. The average maximum intensity plus the signal-to-noise ratio (from at least 12 independent regions containing no microcolonies) was used as the lower bound in an inclusive threshold. Concentric regions of interest were defined around each colony spot. From the innermost, the regions of interest enclosed (1) the colony spot itself, (2) the microcolonies immediately surrounding the colony spot (potential induction area), (3) a spacer region, and (4) distant microcolonies (background intensity values). The average integrated intensity per area was determined by the intensity of region 2 minus the intensity of region 4. The resulting values were normalized by the background values to account for interplate variability. Values from each replicate assay were scaled relative to the maximum measured intensity for that assay to reduce noise and allow comparisons between biological replicates. Values for at least three independent experiments were averaged.
Halo Overlay Assay.
Inducer strains were resuspended to OD600 = 0.5 in LB broth, spotted onto 20-mL LB plates, and grown for 5 d at 30 °C. Wild-type and ΔSpo0A B. subtilis strains were grown in LB medium at 37 °C to OD600 ∼1.0 and then were diluted 1:100 in LB agar [1.5% (wt/vol)] that had been melted and cooled to 50 °C. Ten-milliliter agar slabs embedded with B. subtilis were allowed to set and then were flipped onto the inducing strain colonies. After growth for 24 h at 30 °C, killing halos were measured as the distance between the outer edge of the colony and the B. subtilis halo, if any, and the difference between ΔSpo0A and wild-type halos was determined. Values for at least three independent experiments were averaged, with each experiment performed in duplicate.
Tests for Secreted Molecules.
Agar was 0.1× LB, 100 MOPS unless noted. For cored plates, 3 μL of the inducers at OD600 = 0.5 were spotted onto 15-mL plates and grown for 2 d at 30 °C. Agar plugs containing the colonies were removed using sterilized corers, and the plates were UV-irradiated for 1 h. For cellophane separations, sterile cellophane (500PUT; Innovia Films, Inc.) was placed on 15-mL agar plates. Five milliliters of agar and then 3 μL of the inducer strain were added, and colonies were grown for 2 d at 30 °C before cellophanes and the top agar was removed. Then 5 mL of agar was added to the plates, and B. subtilis microcolony lawns were spread as for secondary screens. For CM, matrix inducers were grown in 0.1× LB medium or 1× LB medium for 4 d at 30 °C. Cultures were centrifuged, and the supernatant was filtered to sterilize it. Concentrated CM was tested for induction activity in a secondary screen. Matrix preparations were performed on cell pellets from the liquid cultures as described (11). For morphological tests, 20 μL of CM from LB cultures was spotted three successive times onto Minimal Salts glycerol glutamate (MSgg) plates (22) before 2 μL of B. subtilis was added. Colonies were grown for 3 d at 30 °C and were examined for a wrinkly phenotype indicative of hypercannibalism (26).
Sequence Analysis and Phylogenetic Trees.
16S rRNA genes were amplified in 50-μL PCR reactions using the 27F and 1492R primers (51), Qiagen Taq polymerase, and a DYAD DNA Engine Peltier Thermal Cycler (MJ Research). Amplicons were purified using a Qiagen PCR purification kit and sequenced using the 27F primer at the Dana-Farber/Harvard Cancer Center DNA Resource Core. Gene sequences were inspected manually for base-caller errors and were trimmed by removing any ambiguous trailing or leading bases using Sequencher (GeneCodes, Inc.). For soil clone library analysis, all sequences were longer than 450 bp. Sequences were checked for chimeras using Bellerophon, version 3 (52), and putative chimeras were removed from downstream analyses. The sequences were grouped into operational taxonomic units (OTUs) based on 99% sequence identity, and one representative of each OTU was compared with sequences in the National Center for Biotechnology Information database using BLAST. Rarefaction analysis was carried out using a Rarefaction Calculator (http://www2.biology.ualberta.ca/jbrzusto/rarefact.php). The Chao-1 nonparametric species richness estimator was used to estimate the total number of similar clusters of sequenced isolates (53, 54). 16S rRNA sequences from the soil analysis were deposited in GenBank (accession nos. JF501232–JF501388). For inducing isolates, all sequences were longer than 700 bp except for one, which was 576 bp. For phylogenetic trees, sequences were inserted into SILVA database dendrogram version 98 in ARB using parsimony (55, 56). Maximum-likelihood trees were constructed in ARB with novel and reference sequences selected from the ARB database using randomized axelerated maximum likelihood for high performance computing (RAxML-VI-HPC) v. 2.2 under the GTRCAT model of evolution (57). For constructing the tree, a Listeria sp. was used as an outgroup and subsequently was pruned. Bootstrap values greater than 50% (of 1,000 replicates) are shown. 16S rRNA gene sequences of inducing isolates were deposited in GenBank (accession nos. JF496856–JF496919).
Supplementary Material
Acknowledgments
We thank Hera Vlamakis for helpful comments on the manuscript and the students from the spring, 2008 Harvard College Life Sciences 100 course (partially funded by a Howard Hughes Medical Institute undergraduate education grant) for identifying inducers in the coculture screen. E.A.S. was supported by a Helen Hay Whitney Postdoctoral Fellowship and by Harvard New England Regional Center of Excellence Grant AI057159. This work was supported by National Institutes of Health Grants GM58213 (to R.K.), GM82137 (to R.K.), and GM18568 (to R.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 19107.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. JF501232–JF501388 and JF496856–JF496919).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103630108/-/DCSupplemental.
References
- 1.Camilli A, Bassler BL. Bacterial small-molecule signaling pathways. Science. 2006;311:1113–1116. doi: 10.1126/science.1121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan RP, Dow JM. Diffusible signals and interspecies communication in bacteria. Microbiology. 2008;154:1845–1858. doi: 10.1099/mic.0.2008/017871-0. [DOI] [PubMed] [Google Scholar]
- 3.Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waters CM, Bassler BL. Quorum sensing: Cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 5.Lopez D, Vlamakis H, Kolter R. Generation of multiple cell types in Bacillus subtilis. FEMS Microbiol Rev. 2009;33:152–163. doi: 10.1111/j.1574-6976.2008.00148.x. [DOI] [PubMed] [Google Scholar]
- 6.López D, Kolter R. Extracellular signals that define distinct and coexisting cell fates in Bacillus subtilis. FEMS Microbiol Rev. 2010;34:134–149. doi: 10.1111/j.1574-6976.2009.00199.x. [DOI] [PubMed] [Google Scholar]
- 7.Straight PD, Kolter R. Interspecies chemical communication in bacterial development. Annu Rev Microbiol. 2009;63:99–118. doi: 10.1146/annurev.micro.091208.073248. [DOI] [PubMed] [Google Scholar]
- 8.Shank EA, Kolter R. New developments in microbial interspecies signaling. Curr Opin Microbiol. 2009;12:205–214. doi: 10.1016/j.mib.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branda SS, González-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci USA. 2001;98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamon MA, Lazazzera BA. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol Microbiol. 2001;42:1199–1209. doi: 10.1046/j.1365-2958.2001.02709.x. [DOI] [PubMed] [Google Scholar]
- 11.Branda SS, Chu F, Kearns DB, Losick R, Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol. 2006;59:1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- 12.Romero D, Aguilar C, Losick R, Kolter R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc Natl Acad Sci USA. 2010;107:2230–2234. doi: 10.1073/pnas.0910560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamon MA, Stanley NR, Britton RA, Grossman AD, Lazazzera BA. Identification of AbrB-regulated genes involved in biofilm formation by Bacillus subtilis. Mol Microbiol. 2004;52:847–860. doi: 10.1111/j.1365-2958.2004.04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kearns DB, Losick R. Cell population heterogeneity during growth of Bacillus subtilis. Genes Dev. 2005;19:3083–3094. doi: 10.1101/gad.1373905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu F, Kearns DB, Branda SS, Kolter R, Losick R. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol Microbiol. 2006;59:1216–1228. doi: 10.1111/j.1365-2958.2005.05019.x. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi K. Bacillus subtilis pellicle formation proceeds through genetically defined morphological changes. J Bacteriol. 2007;189:4920–4931. doi: 10.1128/JB.00157-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chai Y, Chu F, Kolter R, Losick R. Bistability and biofilm formation in Bacillus subtilis. Mol Microbiol. 2008;67:254–263. doi: 10.1111/j.1365-2958.2007.06040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu F, et al. A novel regulatory protein governing biofilm formation in Bacillus subtilis. Mol Microbiol. 2008;68:1117–1127. doi: 10.1111/j.1365-2958.2008.06201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chai Y, Kolter R, Losick R. Paralogous antirepressors acting on the master regulator for biofilm formation in Bacillus subtilis. Mol Microbiol. 2009;74:876–887. doi: 10.1111/j.1365-2958.2009.06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chai Y, Kolter R, Losick R. Reversal of an epigenetic switch governing cell chaining in Bacillus subtilis by protein instability. Mol Microbiol. 2010;78:218–229. doi: 10.1111/j.1365-2958.2010.07335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stöver AG, Driks A. Regulation of synthesis of the Bacillus subtilis transition-phase, spore-associated antibacterial protein TasA. J Bacteriol. 1999;181:5476–5481. doi: 10.1128/jb.181.17.5476-5481.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kearns DB, Chu F, Branda SS, Kolter R, Losick R. A master regulator for biofilm formation by Bacillus subtilis. Mol Microbiol. 2005;55:739–749. doi: 10.1111/j.1365-2958.2004.04440.x. [DOI] [PubMed] [Google Scholar]
- 23.Molle V, et al. The Spo0A regulon of Bacillus subtilis. Mol Microbiol. 2003;50:1683–1701. doi: 10.1046/j.1365-2958.2003.03818.x. [DOI] [PubMed] [Google Scholar]
- 24.Fujita M, González-Pastor JE, Losick R. High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J Bacteriol. 2005;187:1357–1368. doi: 10.1128/JB.187.4.1357-1368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González-Pastor JE, Hobbs EC, Losick R. Cannibalism by sporulating bacteria. Science. 2003;301:510–513. doi: 10.1126/science.1086462. [DOI] [PubMed] [Google Scholar]
- 26.López D, Vlamakis H, Losick R, Kolter R. Cannibalism enhances biofilm development in Bacillus subtilis. Mol Microbiol. 2009;74:609–618. doi: 10.1111/j.1365-2958.2009.06882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu WT, et al. Imaging mass spectrometry of intraspecies metabolic exchange revealed the cannibalistic factors of Bacillus subtilis. Proc Natl Acad Sci USA. 2010;107:16286–16290. doi: 10.1073/pnas.1008368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellermeier CD, Hobbs EC, Gonzalez-Pastor JE, Losick R. A three-protein signaling pathway governing immunity to a bacterial cannibalism toxin. Cell. 2006;124:549–559. doi: 10.1016/j.cell.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 29.González-Pastor JE. Cannibalism: A social behavior in sporulating Bacillus subtilis. FEMS Microbiol Rev. 2011;35:415–424. doi: 10.1111/j.1574-6976.2010.00253.x. [DOI] [PubMed] [Google Scholar]
- 30.Nandy SK, Bapat PM, Venkatesh KV. Sporulating bacteria prefers predation to cannibalism in mixed cultures. FEBS Lett. 2007;581:151–156. doi: 10.1016/j.febslet.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Jiang M, Shao W, Perego M, Hoch JA. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol Microbiol. 2000;38:535–542. doi: 10.1046/j.1365-2958.2000.02148.x. [DOI] [PubMed] [Google Scholar]
- 32.Ireton K, Rudner DZ, Siranosian KJ, Grossman AD. Integration of multiple developmental signals in Bacillus subtilis through the Spo0A transcription factor. Genes Dev. 1993;7:283–294. doi: 10.1101/gad.7.2.283. [DOI] [PubMed] [Google Scholar]
- 33.de Hoon MJ, Eichenberger P, Vitkup D. Hierarchical evolution of the bacterial sporulation network. Curr Biol. 2010;20:R735–R745. doi: 10.1016/j.cub.2010.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.López D, Fischbach MA, Chu F, Losick R, Kolter R. Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc Natl Acad Sci USA. 2009;106:280–285. doi: 10.1073/pnas.0810940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vlamakis H, Aguilar C, Losick R, Kolter R. Control of cell fate by the formation of an architecturally complex bacterial community. Genes Dev. 2008;22:945–953. doi: 10.1101/gad.1645008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonenshein AL, Hoch JA, Losick R. Bacillus subtilis and Other Gram-Positive Bacteria: Biochemistry, Physiology, and Molecular Genetics. Washington: American Society for Microbiology; 1993. [Google Scholar]
- 37.Aguilar C, Vlamakis H, Guzman A, Losick R, Kolter R. KinD is a checkpoint protein linking spore formation to extracellular-matrix production in Bacillus subtilis biofilms. MBio. 2010 doi: 10.1128/mBio.00035-10. 10.1128/mBio.00035-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao M, Helmann JD. The Bacillus subtilis extracytoplasmic-function sigmaX factor regulates modification of the cell envelope and resistance to cationic antimicrobial peptides. J Bacteriol. 2004;186:1136–1146. doi: 10.1128/JB.186.4.1136-1146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butcher BG, Helmann JD. Identification of Bacillus subtilis sigma-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by Bacilli. Mol Microbiol. 2006;60:765–782. doi: 10.1111/j.1365-2958.2006.05131.x. [DOI] [PubMed] [Google Scholar]
- 40.Murray EJ, Strauch MA, Stanley-Wall NR. SigmaX is involved in controlling Bacillus subtilis biofilm architecture through the AbrB homologue Abh. J Bacteriol. 2009;191:6822–6832. doi: 10.1128/JB.00618-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholson WL. Roles of Bacillus endospores in the environment. Cell Mol Life Sci. 2002;59:410–416. doi: 10.1007/s00018-002-8433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Earl AM, Losick R, Kolter R. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 2008;16:269–275. doi: 10.1016/j.tim.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill IR, Gray TR. Application of the fluorescent-antibody technique to an ecological study of bacteria in soil. J Bacteriol. 1967;93:1888–1896. doi: 10.1128/jb.93.6.1888-1896.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grundmann GL. Spatial scales of soil bacterial diversity—the size of a clone. FEMS Microbiol Ecol. 2004;48:119–127. doi: 10.1016/j.femsec.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 45.O'Donnell AG, Young IM, Rushton SP, Shirley MD, Crawford JW. Visualization, modelling and prediction in soil microbiology. Nat Rev Microbiol. 2007;5:689–699. doi: 10.1038/nrmicro1714. [DOI] [PubMed] [Google Scholar]
- 46.Siala A, Hill IR, Gray TRG. Populations of spore-forming bacteria in an acid forest soil, with special reference to Bacillus-subtilis. J Gen Microbiol. 1974;81:183–190. [Google Scholar]
- 47.Davies J. Are antibiotics naturally antibiotics? J Ind Microbiol Biotechnol. 2006;33:496–499. doi: 10.1007/s10295-006-0112-5. [DOI] [PubMed] [Google Scholar]
- 48.Chai Y, Norman T, Kolter R, Losick R. An epigenetic switch governing daughter cell separation in Bacillus subtilis. Genes Dev. 2010;24:754–765. doi: 10.1101/gad.1915010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veening JW, Smits WK, Kuipers OP. Bistability, epigenetics, and bet-hedging in bacteria. Annu Rev Microbiol. 2008;62:193–210. doi: 10.1146/annurev.micro.62.081307.163002. [DOI] [PubMed] [Google Scholar]
- 50.Yasbin RE, Ganesan AT, Young FE. Bacteriophage interference in Bacillus subtilis 168. J Virol. 1974;13:916–921. doi: 10.1128/jvi.13.4.916-921.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic Acid Techniques in Bacterial Systematics. New York: John Wiley and Sons; 1991. [Google Scholar]
- 52.Huber T, Faulkner G, Hugenholtz P. Bellerophon: A program to detect chimeric sequences in multiple sequence alignments. Bioinformatics. 2004;20:2317–2319. doi: 10.1093/bioinformatics/bth226. [DOI] [PubMed] [Google Scholar]
- 53.Chao A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics. 1987;43:783–791. [PubMed] [Google Scholar]
- 54.Hughes JB, Hellmann JJ, Ricketts TH, Bohannan BJ. Counting the uncountable: Statistical approaches to estimating microbial diversity. Appl Environ Microbiol. 2001;67:4399–4406. doi: 10.1128/AEM.67.10.4399-4406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar Y, Westram R, Kipfer P, Meier H, Ludwig W. Evaluation of sequence alignments and oligonucleotide probes with respect to three-dimensional structure of ribosomal RNA using ARB software package. BMC Bioinformatic. 2006 doi: 10.1186/1471-2105-7-240. 10.1186/1471-2105-7-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pruesse E, et al. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 58.Wludyka PS, Nelson PR. An analysis-of-means-type test for variances from normal populations. Technometrics. 1997;39:274–285. [Google Scholar]