Abstract
Persistent relapse to addictive drugs constitutes the most challenging problem in addiction therapy, and is linked to impaired prefrontal cortex regulation of motivated behaviors involving the nucleus accumbens. Using a rat model of heroin addiction, we show that relapse requires long-term potentiation (LTP)-like increases in synaptic strength in the prefrontal cortex projection to the nucleus accumbens. The increased synaptic strength was paralleled by dendritic spine enlargement in accumbens spiny neurons and required up-regulated surface expression of NMDA2b-containing receptors (NR2B). Accordingly, blocking NR2B before reinstating heroin-seeking prevented the induction of LTP-like changes in spine remodeling and synaptic strength, and inhibited heroin relapse. These data show that LTP-like neuroplasticity in prefrontal-accumbens synapses is initiated by NR2B stimulation and strongly contributes to heroin relapse. Moreover, the data reveal NR2B-containing NMDA receptors as a previously unexplored therapeutic target for treating heroin addiction.
Keywords: glutamate, field excitatory postsynaptic potential, diolistic neuron labeling, whole-cell recording, self-administration
Drug addiction is characterized by an enduring vulnerability to relapse, and the propensity to relapse has been linked to cognitive impairments in the prefrontal cortex (PFc) (1, 2). Relapse requires memory recall, and akin to other forms of memory processing, relapse relies on synaptic plasticity. The glutamatergic projection from the PFc to the nucleus accumbens (NAc) undergoes long-lasting neuroplasticity following chronic cocaine administration (3–5), including long-term increases in the strength of excitatory synapses on medium spiny neurons (MSNs) in the NAc following chronic cocaine administration (4, 6, 7). Synaptic potentiation is indicated in studies showing increased AMPA relative to NMDA currents in vitro (7), increased field potentials in prefrontal to accumbens synapses in vivo (6), elevated surface expression of the accumbens AMPA glutamate GluR1 receptor subunit (4), and increased density and diameter of dendritic spines on accumbens MSNs (8, 9). Based on these findings, it has been proposed that pathologies in the PFc to NAc synapses may contain novel pharmacotherapeutic targets for treating addiction (3, 4).
Although chronic cocaine administration alters basal excitatory transmission in PFc synapses on accumbens MSNs, understanding of the role played by drug-induced neuroplasticity in relapse is incomplete and may even be misleading because all studies to date examine neuroplasticity produced after a withdrawal period and do not measure the synaptic plasticity initiated by a relapse episode (5, 10). Another potential limitation in our current understanding is that although the PFc projections to NAc are likely involved in relapse to most drugs of abuse (11), the current perception that addiction is associated with synaptic potentiation in the NAc is derived largely from experiments with chronic cocaine administration (12). An indication that this perception may be misleading can be found in measures of dendritic spine morphology. In as much as spine morphology reflects synaptic strength (13, 14), although cocaine increases dendritic spine density in MSNs, chronic μ-opioid administration reduces spine density (8, 15, 16). However, glutamate release from prefrontal synapses into the core subcompartment of the NAc (NAcore) is required for reinstating both cocaine and heroin-seeking (17, 18).
Given that glutamate release induces synaptic plasticity (19), substantial changes in synaptic strength may occur during relapse. Here we sought to determine if neuroplasticity is induced at glutamatergic synapses in the NAcore by a heroin relapse episode. Moreover, we asked if such synaptic plasticity is required for relapse and endeavored to target the molecular mediators of relapse-associated plasticity to devise a means to suppress relapse. Our results reveal that heroin relapse requires the induction of long-term potentiation (LTP)-like neuroplasticity in the PFc to accumbens synapses, and that both relapse and the associated synaptic plasticity require stimulation of NMDA receptors containing an NMDA2b-containing receptor (NR2B) subunit.
Results
Synaptic Potentiation by Heroin Priming.
We examined the role of neuroplasticity in heroin relapse using the reinstatement model of heroin-seeking (Fig. 1A; see Fig. S1 for detailed behavior). To compare the effect of withdrawal from heroin to a control without previous experience with heroin, two groups of rats were trained. Rats were trained to either self-administer heroin and heroin delivery was paired with a light/tone cue, or were yoked-saline controls (rats receiving intravenous saline when a heroin-trained animal self-administered a heroin infusion). Animals were then heroin-extinguished for 2 to 3 wk, after which electrophysiological and morphological measures of neuroplasticity were made in the NAcore.
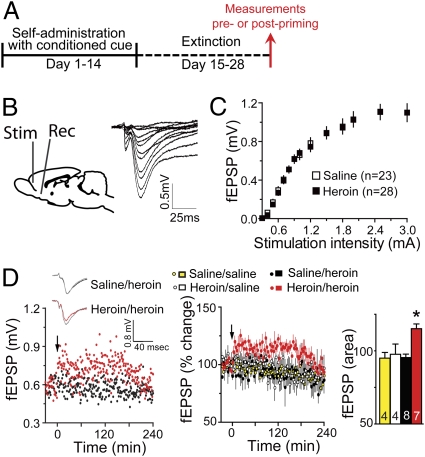
Fig. 1.
A heroin priming injection produces LTP-like changes in fEPSP in the NAcore of heroin-extinguished rats. (A) Heroin treatment protocol used for data in all figures. (B) (Left) Diagram showing the protocol of fEPSP recording in PFc-NAcore excitatory pathway (Rec, recording site; Stim, stimulation site). (Right) Examples of increasing fEPSP amplitude measured in the NAcore of a heroin-extinguished rat in response to increasing stimulation currents in the PFc. (C) Heroin did not alter the input–output relationship between stimulation strength in the cortex and fEPSP amplitude in the NAcore. (D) Acute heroin (0.25 mg/kg, s.c.) induced an increase in fEPSP amplitude for up to 180 min after injection only in heroin-extinguished animals. (Left) Examples of heroin-induced increase in fEPSP amplitude in heroin-extinguished rat (heroin/heroin), but not in saline-yoked control (saline/heroin). (Inset) Sample traces of fEPSP before and after (gray) heroin priming. (Center) Mean ± SEM for all animals. (Right) Area under the curve for 3 h after acute heroin administration. Arrow indicates heroin or saline administration. *P < 0.01, comparing after heroin priming injection in heroin-extinguished animals (heroin/heroin) to heroin in saline-yoked (saline/heroin), saline in heroin-extinguished (heroin/saline), and saline in saline-yoked (saline/saline) using Bonferroni post hoc.
We first recorded in vivo field excitatory postsynaptic potentials (fEPSP) in the NAcore evoked by stimulating electrodes in the PFc (Fig. 1B). Although no difference between heroin-extinguished and yoked-saline animals was observed in the input–output relationship between current stimulus intensity delivered to the PFc and fEPSP amplitude in the NAcore (Fig. 1C), acute heroin administration increased fEPSP amplitude within 5 min in heroin-extinguished but not yoked-saline rats. The elevation persisted for ∼3 h. A saline injection had no effect in either chronic treatment group on fEPSPs [two-way ANOVA, effect of treatment F(89,1153) = 6.97, P < 0.001 for time course; two-way ANOVA, interaction F(1,19) = 4.67, P = 0.043, for area under the curve, 0–240 min] (Fig. 1D).
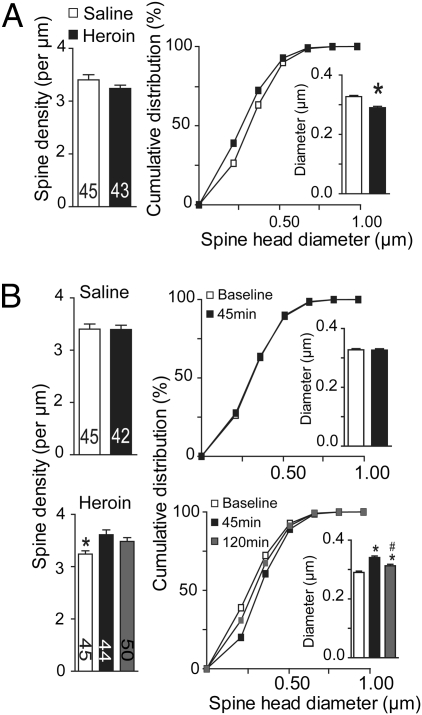
We next determined if the increased fEPSP amplitude by an acute heroin injection was associated with spine remodeling. Spine density and head diameter were quantified in MSNs in the NAcore following diolistic labeling with DiI (see Fig. S2 for examples of DiI-labeled neurons and Table S1 for quantification of spine head diameter and spine frequency for each dendritic segment) (9). Compared with yoked-saline subjects, the heroin-extinguished animals had a reduced spine head diameter [t(89) = 5.89, P < 0.001], but no alteration in spine density (Fig. 2A). We next determined if, akin to fEPSP amplitude (Fig. 1D), initiating heroin-seeking would unmask neuroplasticity that may be necessary for relapse to occur. An acute injection of heroin (0.25 mg/kg s.c.) was administered to reinstate lever pressing in animals extinguished from heroin self-administration (17). By 45 min after an injection of heroin into heroin-extinguished animals, spine density was increased and the density was returning to preinjection baseline by 120 min [F(2, 135) = 5.78, P = 0.004] (Fig. 2B). In contrast, heroin produced no change in yoked-saline rats at 45 min after heroin administration (Fig. 2B). Parallel changes were measured in spine head diameter, where the acute heroin injection increased diameter in heroin [F(2, 135) = 24.70, P < 0.001] but not yoked-saline animals at 45 min after injection (Fig. 2B).
Fig. 2.
A heroin priming injection induces MSN spine remodeling in the NAcore of heroin-extinguished rats. (A) (Left) Mean spine density in yoked-saline or heroin-extinguished animals; (Right) Reduction in spine head diameter in heroin-extinguished animals shown as cumulative diameter distribution (Inset, *P < 0.001, unpaired Student t test). (B) (Left) A heroin priming injection (0.25 mg/kg, s.c.) increased spine head density by 45 min after injection in heroin-extinguished (Heroin), but not yoked-saline animals (Saline); (Right) Heroin increased spine head diameter in heroin-extinguished, but not control animals. *P < 0.01, comparing value before heroin priming injection to 45 and 120 min after injection; #P < 0.01, comparing 120 min to 45 min after heroin priming using Bonferroni post hoc.
Taken together, the data in Figs. 1 and 2 reveal that although heroin self-administration/extinction did not induce a long-lasting change in basal field strength, acute heroin administration in previously heroin-extinguished rats produced an LTP-like increase in fEPSPs that was associated with increases in spine density and head diameter.
Heroin Self-Administration Increases Surface Expression of NR2B.
Because the thinner spines in the NAcore of heroin-extinguished animals were not associated with decreased synaptic strength in the PFc to NAcore projection, whole-cell patch recordings were made from MSNs in NAcore tissue slices after extinction from heroin or yoked-saline to more directly evaluate possible postsynaptic adaptations (see Fig. 1A for treatment protocol). Glutamatergic afferents were electrically stimulated and the AMPA and NMDA currents were pharmacologically isolated by bath applying the NMDAR antagonist D-AP5 (50 μM) at +40-mV clamping voltage to determine the AMPA:NMDA ratio as a surrogate measure of synaptic plasticity (20). When channel current was estimated by total area, a significant decrease in the AMPA:NMDA ratio was observed [t(27) = 2.17, P = 0.039] (Fig. 3A). Consistent with current area revealing a decrease in AMPA:NMDA, the time of NMDA current decay was increased in heroin-extinguished animals when quantified by decay time constant (τ) fitted to a single exponential (21) [t(34) = 2.11, P = 0.043], as well as the time to 37% decay [t(34) = 2.07, P = 0.047] (Fig. 3B).
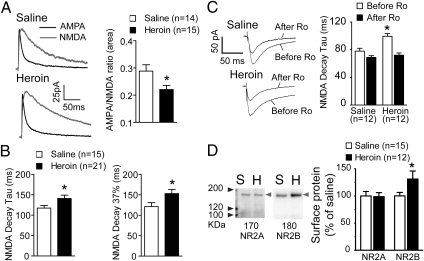
Fig. 3.
Heroin self-administration plus extinction training reduces AMPA:NMDA ratios because of an increased half-life of the NMDA current. (A) (Left) Examples of pharmacologically isolated AMPA and NMDA currents from MSNs in heroin extinguished and yoked-saline animals; (Right) Heroin-extinguished animals have reduced AMPA:NMDA ratio compared with yoked-saline rats when current was estimated by total area. (B) Increase in the decay time (Left: time constant τ; Right: time to 37% decay) for NMDA currents in heroin-extinguished animals. *P < 0.05, comparing heroin to yoked-saline groups using unpaired t test. (C) (Left) Examples of NMDAR EPSC in MSNs from yoked-saline and heroin-extinguished rats in the absence and presence of selective NMDA antagonist Ro-256981 (Ro; 1 μM). (Right) The increased decay constant (τ) of NMDAR EPSC in heroin-extinguished rats was reduced by treatment with Ro-256981. *P < 0.01 comparing heroin-extinguished to yoked-saline group before Ro application using Bonferroni post hoc; there was no difference between these two groups after Ro treatment. (D) (Left) Examples of Western blots from a biotinylation assay showing increased surface expression of NR2B in heroin-extinguished (H) compared with yoked-saline (S) animals. Lanes from left to right show protein size marker, saline, and heroin sample, respectively. Left-pointing light-gray arrowheads indicate the position of the proteins. (Right) Pooled data were normalized to the saline values and shown as mean ± SEM *P < 0.05, comparing heroin-extinguished to yoked-saline groups using unpaired t test.
A slower NMDA current decay may indicate elevated surface expression of NMDA receptors containing NR2B subunits (22). To examine this possibility, NMDAR-mediated excitatory postsynaptic current (EPSCs) were isolated using the AMPAR antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μM) at −40-mV clamping voltage, and NR2B-containing NMDA channels were antagonized by Ro-256981 (1 μM). Compared with yoked-saline, heroin-extinguished rats exhibited a slower NMDA current decay, which was sensitive to Ro-256981 [two-way ANOVA, repeated measures, interaction F(1,22) = 7.60, P = 0.021] (Fig. 3C). Next, a biotinylation strategy was used on NAcore tissue slices to directly quantify NR2A and NR2B surface expression in heroin-extinguished subjects. Heroin-extinguished rats showed an increase in NR2B surface expression compared with yoked-saline animals [t(25) = 2.07, P = 0.049] (Fig. 3D), but no change in NR2A expression. An increase in NR2B expression has been previously associated with a decrease in spine diameter (23), akin to the reduced spine diameter in heroin-extinguished rats (Fig. 2G). In addition, the parallel increase in NR2B expression and NMDA current after heroin is consistent with the reduced AMPA:NMDA ratio and the lack of difference between treatment groups in the surface expression of the GluR1 and GluR2 AMPA receptor subunits (Fig. S3A). Protein levels in whole-cell lysates were not different between the heroin-extinguished and yoked-saline groups for any protein measured (Fig. S3B).
NR2B Mediates Relapse-Associated Synaptic Potentiation.
The next set of experiments sought to link the LTP-like changes in spine morphology and field-potential amplitude (Figs. 1 and 2) with the capacity of self-administered heroin to produce an enduring up-regulation of NR2B (Fig. 3). Ifenprodil is a selective antagonist of NR2B-containing NMDA receptors (24), and microinjecting ifenprodil (8 ρmol; 10 μM over 8 min) into the NAcore before administering a heroin challenge injection abolished the increase in fEPSP amplitude induced by heroin in heroin-extinguished animals [two-way ANOVA, interaction between treatment and time F(39,234) = 2.33, P < 0.001] (Fig. 4A; see Fig. S4 for examples of recordings). However, intra-NAcore microinjections of ifenprodil up to 20 ρmol did not alter field-potential amplitude in yoked-saline control subjects (Fig. S5). Supporting equivalent access of drug to the site of recording, in contrast to differential effects of ifenpodil in the yoked-saline versus heroin-extinguished treatment groups, inhibition of AMPA/kainate receptors with CNQX (10 μM) reduced fEPSP amplitude in all treatment groups (Fig. 4A and Fig. S5).
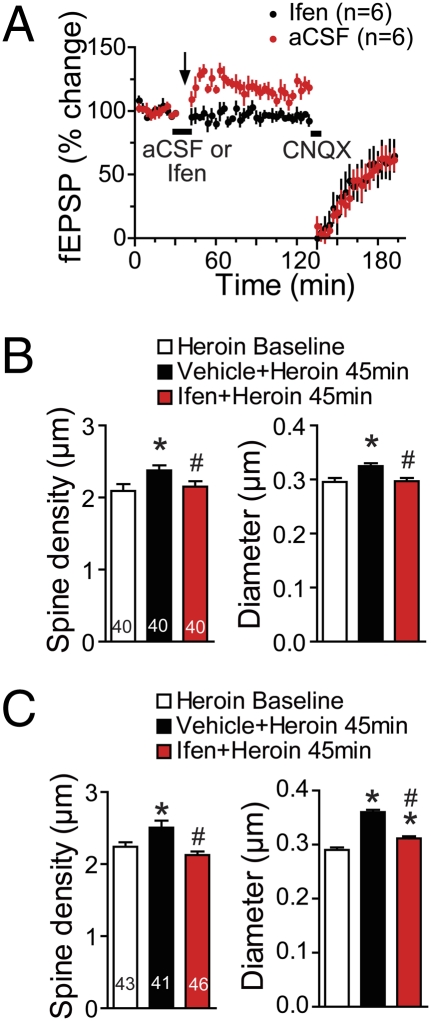
Fig. 4.
The up-regulation of NR2B after extinction from heroin self-administration mediates the neuroplasticity initiated by acute heroin administration. (A) The LTP-like increase in fEPSP amplitude elicited by heroin (0.25 mg/kg, s.c.) in heroin-extinguished animals was prevented by pretreating the NAcore with the selective NR2B antagonist ifenpridil (Ifen; 8 ρmol in 0.8 μL over 8 min). Data are shown as mean ± SEM. Bar indicates the aCSF or Ifen infusion, and arrow indicates acute heroin administration. (B) The increases in spine density and head diameter elicited by a heroin priming injection in heroin-extinguished subjects were prevented by Ifen (8 ρmol) microinjected directly into the NAcore. (C) The increases in spine density and spine head diameter were also prevented by systemic Ifen (3.0 mg/kg, i.p.). Number of neurons quantified is shown in the bar. *P < 0.05, compared with diameter and density before administering the heroin priming injection, #P < 0.05, comparing Ifen-pretreated group to vehicle group using Bonferroni post hoc.
Akin to the capacity of ifenprodil to prevent the potentiation of field potentials by an acute heroin injection in heroin-extinguished subjects, ifenprodil pretreatment either into the NAcore (8 ρmol) (Fig. 4B) or systemically (3 mg/kg, i.p.) (Fig. 4C) prevented the increase in spine density [systemic F(2, 128) = 7.35, P < 0.001; intra-NAcore F(2, 119) = 3.41, P = 0.035] and spine head diameter [systemic F(2, 119) = 7.03, P < 0.001] elicited 45 min after an acute heroin injection.
Relapse Is Suppressed by NR2B Inhibition.
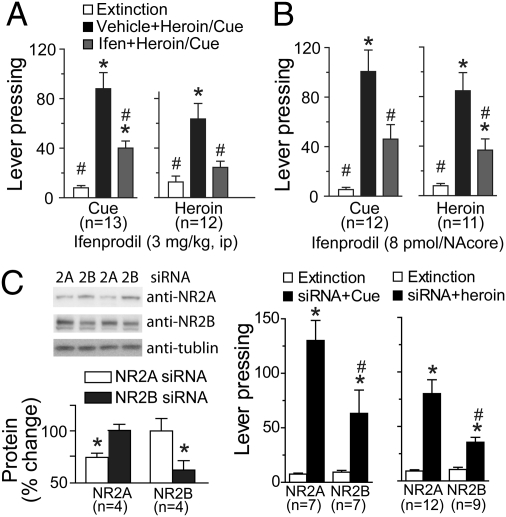
In the final experiments we sought to link NR2B-mediated synaptic potentiation with heroin-induced relapse. Both heroin and heroin-conditioned cues significantly reinstated active lever pressing in the rats extinguished from heroin self-administration (Fig. 5). Animals pretreated with saline, intraperitoneally, or intra-NAcore artificial cerebrospinal fluid (aCSF) showed significant reinstatement of lever pressing following heroin injection or presentation of heroin cues (Fig. 5 A and B). Pretreatment with systemic ifenprodil inhibited both heroin- [F(2, 22) = 16.01, P < 0.001] and cue-induced [F(2, 24)= 28.16, P < 0.001] reinstatement of lever pressing (Fig. 5A). Similarly, ifenprodil microinjected directly into the NAcore prevented cue- [F(2, 22) = 13.43, P < 0.001] and heroin-induced [F(2, 20) = 8.91, P < 0.001] reinstatement of lever pressing (Fig. 5B). Although the doses of ifenprodil used are thought to be relatively selective for NR2B, to control for the possible nonspecific blockade of NR2A receptors by ifenprodil, daily siRNA microinjections were made into the NAcore over the last 3 d of extinction training to reduce either NR2A or NR2B synthesis. Each construct produced ∼30% reduction in the surface expression of their targeted proteins [NR2A, t(6) = 2.88, P = 0.028; NR2B, t(6) = 2.61, P = 0.040] (Fig. 5C). The siRNA-NR2B significantly inhibited cue- or heroin-induced reinstatement of lever pressing compared with rats microinjected with siRNA-NR2A [two-way ANOVA, cue-interaction F(1,12) = 5.78, P = 0.033; heroin-interaction F(1,19) =9.11, P = 0.007] (Fig. 5C). Supporting the relative selectivity of ifenprodil on reinstated behavior, this dose of ifenprodil (3 mg/kg, i.p.) did not alter locomotor activity in a novel open field, nor did ifenprodil alone (3 mg/kg, i.p., or 8 pmol into the NAcore) alter extinction levels of lever pressing (Fig. S6).
Fig. 5.
Blockade of NR2B prevents reinstated heroin seeking. (A) The reinstatement of lever pressing by heroin or a light/tone cue previously associated with heroin infusion was reduced by pretreatment with Ifen (3.0 mg/kg, i.p.). (B) Ifen (8 ρmol) administration into the NAcore prevented heroin- or cue-induced reinstatement of active lever pressing. *P < 0.01, compared with exctinction lever pressing, #P < 0.01, compared with heroin or cue induced levels of pressing using Bonferroni post hoc. (C) Treatment with siRNA directed against NR2A or NR2B significantly lowered the content of the targeted protein (*P < 0.05, comparing siRNA NR2A to siRNA NR2B), but only treatment with NR2B siRNA significantly reduced cue- or heroin- induced reinstatement comparing with NR2A siRNA (*P < 0.01, compared with exctinction lever pressing, #P < 0.01, comparing siRNA NR2A to siRNA2B using Bonferroni post hoc.).
Discussion
Our study reveals that for heroin relapse to occur, prefrontal afferents making synapses onto MSNs in the NAcore undergo an LTP-like increase in synaptic strength, measured as an increase in both field-potential amplitude and dendritic spine density and diameter. Importantly, the ability for heroin-induced relapse to increase these surrogate measures of synaptic strength depended on the fact that heroin self-administration/extinction caused an enduring recruitment of NR2B containing NMDA receptors to the cell surface.
The contribution of NR2B recruitment to the cell surface in neuroplasticity has been debated (25), but it is generally thought that NR2B surface expression is associated with thin, less stable dendritic spines (23, 26). This association is congruent with our findings that MSNs of rats trained to self-administer heroin have both increased NR2B surface expression and decreased spine head diameter. Interestingly, NR2B expression is associated with an increase in silent synapses (27, 28), and NR2B-containing silent synapses have been proposed to provide a mechanism whereby synapses can rapidly potentiate to induce LTP-like changes (29, 30). Correspondingly, we find that the PFc to NAcore synapses rapidly develop an LTP-like increase in field strength only in heroin-extinguished animals with up-regulated NR2B.
Two recent studies reveal that although psychostimulant administration produces changes in NAc NR2B using cocaine- or amphetamine-induced behavioral sensitization. However, the profile of changes differ from what we observed using heroin in the self-administration/extinction model, pointing to interpretational caveats for the role of NR2B between studies. Chronic noncontingent cocaine administration produces a short-lived increase in NR2B in the shell of the accumbens (27), but NR2B is down-regulated for up to 60 d following daily amphetamine injections (31). In addition, inhibition of NR2B during daily cocaine administration prevented the development of sensitization (32), but acute blockade of NR2B produced a sensitized response to amphetamine in control animals (31). The apparent distinctions in NR2B plasticity could be because heroin and cocaine are different classes of addictive drug or the fact that psychostimulant-induced changes in NR2B function in accumbens MSNs were observed in a noncontingent drug administration model (3, 33–36).
A role for MSN spine morphology in drug addiction has been questioned because chronic psychostimulants increase (8) and chronic heroin decreases spine density or head diameter (Fig. 2) (15, 16). However, a challenge injection with heroin (present study) or cocaine (in rats withdrawn from chronic cocaine) (9) elicits a transient increase in spine head diameter. Thus, rather than the changes in the steady-state morphology of MSN dendritic spines after chronic heroin or cocaine contributing to reinstated drug-seeking or behavioral sensitization, perhaps it is the shared LTP-like changes in spine morphology produced by a heroin or cocaine challenge injection that more directly underpin the augmented behavioral responses elicited by repeated.
Finally, it should be noted that NR2B outside of the NAcore may contribute to reinstated heroin-seeking because inhibiting NR2B in the NAcore only partially reduced heroin-seeking compared with systemic ifenprodil. Furthermore, either an mGluR2/3 agonist or D1 antagonist inhibits context-induced heroin-seeking selectively in the shell subcompartment of the NAc and not the NAcore (37, 38), indicating that other receptors and brain areas may contribute non-NR2B mechanisms (39).
In summary, our data demonstrate the importance of relapse-induced potentiation of PFc to NAcore synapses in mediating heroin relapse, thereby supporting the postulated contribution of PFc-based cognitive neuropathologies in relapse episodes. The reinstated heroin seeking and synaptic potentiation measured as both increased fEPSP and dendritic spine enlargement that occurs during a relapse episode required up-regulation and stimulation of NR2B-containing NMDA receptors. These data support the potential use of NR2B antagonists to inhibit relapse in heroin addiction.
Experimental Procedures
See SI Experimental Procedures for more detailed description of procedures.
Animal Housing and Surgery.
All procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Assessment and Accreditation of Laboratory Animal Care. Male Sprague–Dawley rats (250 g on arrival; Charles River Laboratories) were individually housed in a temperature- and humidity-controlled environment with a 12-h dark/light cycle (6:00 PM, lights on). The surgical details have been described in a previous study (17). Intracranial guide cannulas (30-guage) were stereotaxically implanted bilaterally into the NAcore.
Heroin Self-Administration Procedures.
All self-administration experiments occurred in standard operant chambers with two retractable levers, a house light, a cue light, and tone generator (Med Associates). During 3-h sessions on 14 consecutive days, rats were trained to press the active lever on a fixed ratio 1 schedule with 20-s timeout for an infusion of heroin-hydrochloride (100 μg/infusion for days 1–2, 50 μg per infusion for days 3–4, 25 μg per infusion for days 5–14. Heroin was kindly provided by the National Institute on Drug Abuse, Rockville, MD). Concurrent with the drug infusion, a cue tone (2,900 Hz) and cue light immediately above the active lever turned on. After 14 d of self-administration, rats began extinction training. Active lever presses produced no drug infusion or light/tone cues. For heroin prime-induced reinstatement, rats received a single injection of heroin (0.25 mg/kg, s.c.) before beginning the 3-h session, and the session program was identical to the extinction program. For cue-induced reinstatement, no injection was given, but the session program was identical to the self-administration session program (i.e., cue tone and light turned on with an active lever press). Rats did not receive intravenous drug infusions during either mode of reinstatement trial.
Drug Treatment.
For systemic treatment, ifenprodil (Ascent Scientific) was dissolved in sterile water with 3 mg/mL, and was administered (intraperitoneally) to rats 30 min before heroin or cue priming. For NAcore microinjection, ifenprodil was dissolved in aCSF creating a final concentration of 10 μM ifenprodil, and was administered at a dose of 8 ρmol per side via 33-gauge needle immediately before administering a heroin priming injection. In a separate experiment, siRNA silencing of NR2A or NR2B (31) was microinjected into the NAcore [2 μL siRNA conjugated with a lipid-based siRNA transfection reagent (40) at a final concentration 200 nM] once daily for 3 consecutive days. The rats underwent a cue- or drug-induced reinstatement test 24 h after the last injection.
Imaging Acquisition and Quantification of Dendritic Spine.
The details of diolistic neuron labeling and confocal imaging acquisition have been described in a previous study (9). Before quantitative analysis, imaging data were deconvoluted by Autoquant (Media Cybernetics). Only cells localized to the NAcore were quantified, and spine quantification commenced on dendrites beginning at >75 μm distal to the soma, and after the first branch point. Measurements were made out to a maximum of 200 μm from the soma, and the length of dendrite quantified was 40–55 μm. For each of three to five animals examined at each group, at least 12 neurons were analyzed. A protocol based on Filament module of IMARIS software (Bitplane) was used that quantifies spine density and head diameter (i.e., the diameter of a vertex at the spine terminal point).
Biotinylation Assay.
The NAcore was dissected and sliced into prism-shaped sections (200 μm) with a McIlwain tissue chopper (Vibratome). The tissue was incubated for 30 min in 300 μL PBS containing 1 mg/mL Sulfo-NHS-Biotin (Pierce) at 4 °C with gentle shaking, and reaction was quenched by adding ice-cold 100 mM glycine in PBS. Then, the tissues were homogenized by sonication in 300 μL 1% SDS in RIPA lysis buffer (Pierce) containing protease inhibitor mixture (Roche). After incubating 60 min on ice, the protein-contained solution was centrifuged and the supernatant was added with 100 μL NeutrAvidin agarose resin and incubated for 2 h at 4 °C with gentle rotation. The biotinylated protein was eluted by 50 μL loading buffer (1% SDS and 50 mM DTT) with heating at 80 °C for 5 min. The concerned proteins in biotinylated fraction will be detected with immunoblotting.
Electrophysiology in Vivo.
The rats were anesthetized with urethane (1.5 g/kg, i.p.),and mounted in a stereotaxic apparatus (Narishige). Concentric bipolar stimulating electrodes (Rhodes Medical Instruments) were placed in the prelimbic medial PFc. Recording electrodes were aimed at the dorsomedial region of the NAc. To apply ifenprodil, CNQX, or aCSF locally, a microinjection needle (33-gauge) was inserted into the NAcore slowly (10 μm/30 s) to avoid maiming the local anatomical structure.
Whole-Cell Recordings in Acute Brain Slices.
Rats were anesthetized with ketamine HCl (87.5 mg/kg, i.p.), and perfused transcardially with ice-cold aCSF. Coronal slices (230-μm thick) containing the NAc were used for recording. Picrotoxin (50 μM) was added to block GABAA receptor-mediated inhibitory postsynaptic currents during recording. To assess excitatory synaptic transmission, neurons were voltage clamped at –80 mV using a Multiclamp 700B amplifier (Molecular Devices). Recording electrodes (1.5–2 MΩ) were placed in NAcore, and afferents were stimulated at 0.05 Hz by a bipolar electrode placed about 150 μm dorsomedial to the recorded neurons. AMPAR/NMDAR ratios were calculated from evoked-EPSCs at +40 mV with and without 50 μM D-AP5. To assess the contribution of NR2A/NR2B subunits in MSNs, the NMDAR EPSC was isolated by including CNQX (10 μM) and picrotoxin (50 μM) in aCSF when the voltage was clamped at −40 mV to relieve the voltage-dependent Mg2+ block of NMDA receptor channels, and the NR2A component was isolated by the bath application of selective NR2B antagonist Ro256981 (1 μM). NMDA current decay was quantified by the decay time constant (τ) fitting to a single exponential (21, 41) and the time elapsed from the EPSC peak to 37% peak amplitude.
Statistics.
All spine density and diameter data were statistically analyzed after averaging multiple dendrite segment measurement for each neuron. Protein data in the heroin groups were normalized to the values of yoke-saline groups. When two groups were compared, the data were statistically analyzed using a two-tailed unpaired t test. For comparing the multiple measurements in the same experiment the data were analyzed using a one-way or two-way ANOVA. The electrophysiological data were normalized to baseline field amplitude in each group and evaluated using a two-way ANOVA with repeated measures over time. Bonferroni tests were applied for multiple comparisons and P < 0.05 was considered statistically significant. All statistical tests were conducted using SPSS or Graphpad Prism software.
Supplementary Material
Acknowledgments
We thank Charles Thomas, Megan Hensley, Lauren Jamison, and Martijn Huits for help in rat self-administration training, and Arthur Riegel for technical support on whole-cell record. This work was supported by Grants DA015369 and DA003906 (to P.W.K.) from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112052108/-/DCSupplemental.
References
- 1.Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56(Suppl 1):186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 3.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 4.Conrad KL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 6.Moussawi K, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Shen HW, et al. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everitt BJ, et al. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 14.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 15.Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46:271–279. doi: 10.1002/syn.10146. [DOI] [PubMed] [Google Scholar]
- 16.Spiga S, Puddu MC, Pisano M, Diana M. Morphine withdrawal-induced morphological changes in the nucleus accumbens. Eur J Neurosci. 2005;22:2332–2340. doi: 10.1111/j.1460-9568.2005.04416.x. [DOI] [PubMed] [Google Scholar]
- 17.LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards DA, et al. Glutamate induces the rapid formation of spine head protrusions in hippocampal slice cultures. Proc Natl Acad Sci USA. 2005;102:6166–6171. doi: 10.1073/pnas.0501881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Popescu G, Robert A, Howe JR, Auerbach A. Reaction mechanism determines NMDA receptor response to repetitive stimulation. Nature. 2004;430:790–793. doi: 10.1038/nature02775. [DOI] [PubMed] [Google Scholar]
- 22.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004(255):re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 23.Oray S, Majewska A, Sur M. Effects of synaptic activity on dendritic spine motility of developing cortical layer v pyramidal neurons. Cereb Cortex. 2006;16:730–741. doi: 10.1093/cercor/bhj019. [DOI] [PubMed] [Google Scholar]
- 24.de Marchena J, et al. NMDA receptor antagonists reveal age-dependent differences in the properties of visual cortical plasticity. J Neurophysiol. 2008;100:1936–1948. doi: 10.1152/jn.90290.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kash T, Winder D. NMDAR LTP and LTD induction: 2B or Not 2B…is that the question? Debates Neurosci. 2007;1:79–84. [Google Scholar]
- 26.Sobczyk A, Scheuss V, Svoboda K. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J Neurosci. 2005;25:6037–6046. doi: 10.1523/JNEUROSCI.1221-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang YH, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakayama K, Kiyosue K, Taguchi T. Diminished neuronal activity increases neuron-neuron connectivity underlying silent synapse formation and the rapid conversion of silent to functional synapses. J Neurosci. 2005;25:4040–4051. doi: 10.1523/JNEUROSCI.4115-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poncer JC. Hippocampal long term potentiation: Silent synapses and beyond. J Physiol Paris. 2003;97:415–422. doi: 10.1016/j.jphysparis.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Mao LM, et al. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown T, et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011;31:8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelley AE. Memory and addiction: Shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Sutton MA, et al. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- 35.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ovari J, Leri F. Inactivation of the ventromedial prefrontal cortex mimics re-emergence of heroin seeking caused by heroin reconditioning. Neurosci Lett. 2008;444:52–55. doi: 10.1016/j.neulet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salahpour A, Medvedev IO, Beaulieu JM, Gainetdinov RR, Caron MG. Local knockdown of genes in the brain using small interfering RNA: A phenotypic comparison with knockout animals. Biol Psychiatry. 2007;61:65–69. doi: 10.1016/j.biopsych.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Barth AL, Malenka RC. NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci. 2001;4:235–236. doi: 10.1038/85070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.