Abstract
Extensive work in humans using magneto- and electroencephalography strongly suggests that decreased oscillatory α-activity (8–14 Hz) facilitates processing in a given region, whereas increased α-activity serves to actively suppress irrelevant or interfering processing. However, little work has been done to understand how α-activity is linked to neuronal firing. Here, we simultaneously recorded local field potentials and spikes from somatosensory, premotor, and motor regions while a trained monkey performed a vibrotactile discrimination task. In the local field potentials we observed strong activity in the α-band, which decreased in the sensorimotor regions during the discrimination task. This α-power decrease predicted better discrimination performance. Furthermore, the α-oscillations demonstrated a rhythmic relation with the spiking, such that firing was highest at the trough of the α-cycle. Firing rates increased with a decrease in α-power. These findings suggest that α-oscillations exercise a strong inhibitory influence on both spike timing and firing rate. Thus, the pulsed inhibition by α-oscillations plays an important functional role in the extended sensorimotor system.
The prominent posterior α-rhythm (8–14 Hz) was first described by Hans Berger (1) and long considered to reflect cortical idling (2, 3). To a large extent, the α-rhythm has been ignored by animal neurophysiologists (but see ref. 4) and considered to be of little functional relevance. Thus, it remains largely unknown how ongoing α-oscillations relate to neuronal firing.
In contrast to the idling hypothesis, converging electrophysiological evidence in humans suggests that α-oscillations play an important functional role in cognitive processing (5–7). In particular, α-activity might serve to shape the state of sensory brain regions to direct the flow of information and optimize performance (8). In support of this idea, several studies on visual perception have shown that anticipatory α-activity reflects the orienting of attention (9–14) and influences detection performance (15–17). Recently, it was shown that the functionality of α-oscillations can be generalized to the somatosensory system (18–21). Furthermore, α-activity has been implicated in visual (22–25), auditory (26), and somatosensory working-memory performance (27).
These studies strongly suggest that decreased α-activity facilitates processing in task-relevant brain regions, whereas increased α-activity functions to suppress distracting input in task-irrelevant regions. However, given the strong oscillatory nature of the α-activity, it is less clear how it influences processing in a phasic manner. It has been suggested that α-oscillations serve to depress processing every ∼100 ms by a mechanism of pulsed inhibition (5, 28–30). In support of this notion, it has recently been demonstrated that perception is modulated by the prestimulus phase of the α-rhythm (31, 32). Likewise, it was recently shown that the magnitude of the blood-oxygen level-dependent signal in response to a visual stimulus is dependent on the α-phase at stimulus onset (33). The key to understanding the phasic role of α-activity is to relate neuronal firing to both the phase and the magnitude of α-oscillations. Only recently this has become a topic of investigation in intracranial monkey studies focusing on visual areas (34–37). The main issues to be uncovered are the following: (i) Does α-activity exercise an inhibitory or excitatory influence on local neuronal spiking? (ii) Do α-oscillations modulate firing in a phasic manner? (iii) Does the α-modulation on spikes have behavioral consequences? and (iv) Does α-activity play a (similar) functional role beyond the sensory system?
To address these questions, we recorded neuronal activity simultaneously across somatosensory, premotor, and motor cortices in a behaving monkey (38). Both spikes and local field potentials (LFPs) were acquired while the monkey performed a vibrotactile discrimination task (39). Previous studies on this perceptual decision-making paradigm have extensively reported on the role of spikes in relation to various task components (40–42). It is clear that spikes carry the representational code and are responsible for neuronal computations (42). In addition, strong oscillatory activity is present in the LFPs, which reflects synchronized population activity. Thus, these data allowed us to uncover the interaction between oscillatory activity and neuronal spiking and to relate it to the task used here.
Results
We simultaneously recorded LFPs and spikes from primary somatosensory cortex (S1), secondary somatosensory cortex (S2), dorsal premotor cortex (DPC), medial premotor cortex (MPC), and primary motor cortex (M1) while a monkey performed a vibrotactile discrimination task (38) (Fig. 1). Here, we explored the role of oscillatory activity in the α-band (8–14 Hz) and its relation to task performance and neuronal processing. (For discussion of oscillations in other frequency bands involved in this paradigm, see ref. 43.)
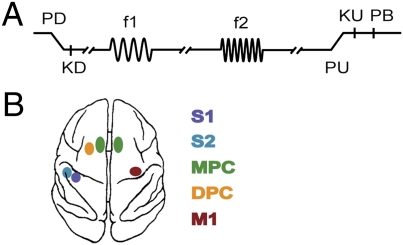
Fig. 1.
Somatosensory discrimination task. (A) Sequence of events during discrimination trials is shown. The mechanical probe is lowered, indenting the glabrous skin of one digit of the restrained hand (PD); the monkey places its free hand on an immovable key (KD); the probe oscillates vertically at the base frequency (f1); after a fixed delay (3 s), a second mechanical vibration is delivered at the comparison frequency (f2); and, after another fixed delay (3 s) between the end of f2 and probe up (PU), the monkey releases the key (KU) and presses either a lateral or medial push button (PB) to indicate whether the comparison frequency (f2) was higher or lower than the base (f1). (B) Overview of recording sites. During each recording session, up to seven electrodes were individually placed in each of the five cortical regions: primary somatosensory cortex (S1), secondary somatosensory cortex (S2), dorsal premotor cortex (DPC), medial premotor cortex (MPC), and primary motor cortex (M1). Both spikes and LFPs were obtained simultaneously through the same microelectrode.
α-Decrease During Somatosensory Discrimination.
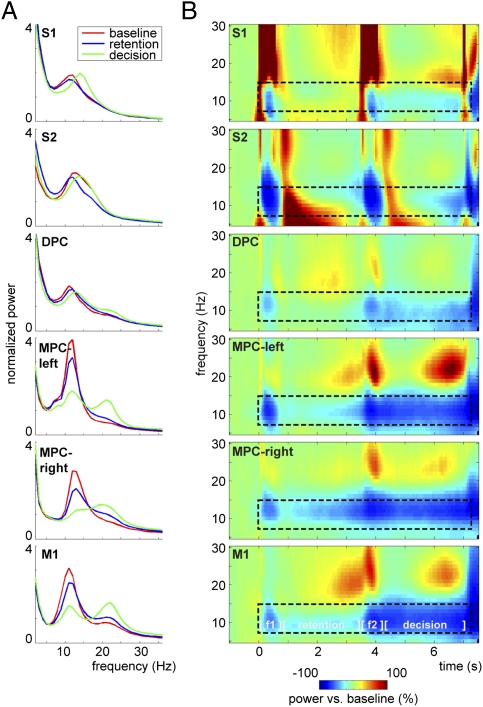
First, we calculated the power spectra of the LFPs for the baseline, retention, and decision intervals (Fig. 2A). For comparison reasons, the spectra were normalized with average power (2–36 Hz). Both correct and incorrect response trials were included in this analysis. The spectra revealed that α-power was dominant in all of the recorded regions during the baseline interval. The α-power was reduced during the execution of the task, but not abolished.
Fig. 2.
α-Power decrease during the somatosensory discrimination task. (A) LFP power spectra for the baseline (t = −1 to 0 s; red), retention (t = 0.5–3.5 s; blue), and decision-delay (t = 4–7 s; green) windows. Spectra were normalized with the average power in the spectrum (2–36 Hz) and show a clear peak in the α-band (8–14 Hz) in all regions, which is most pronounced during the baseline period. S1, S2, DPC, and MPC-left were recorded contralaterally to the stimulated hand; MPC-right and M1 were recorded contralaterally to the response hand. (B) Time-frequency representations showing decrease of power in the α-band during the discrimination task compared with baseline activity. Task components include the presentation of first stimulus (f1, t = 0–0.5 s), retention period (t = 0.5–3.5 s), presentation of second stimulus (f2, t = 3.5–4 s), and decision period (t = 4–7 s) followed by the delayed motor response. α-Band (8–14 Hz) decreases during the task (t = 0–7 s)—as marked by the dashed window—were significant in each of the regions, as shown by cluster-based randomization statistics vs. baseline activity [P < 0.05, corrected for multiple comparisions (Methods)].
To study the temporal development of the α-activity, we calculated the (relative) baseline-corrected time-frequency representations (TFRs) of power (Fig. 2B). Generally, α-power decreased in response to the task compared with baseline. In the somatosensory regions, the main modulation was during stimulus presentation, whereas in the premotor and motor regions an α-decrease could be observed throughout the task. The decrease was strongest during the decision-delay period. A cluster-based randomization test comparing the task-related activity (t = 0–7 s) with the baseline activity (t = −1 − −0.5 s) confirmed that the α-band decreases were statistically significant (cluster-corrected P < 0.05). In short, the LFP signals in all of the measured regions were dominated by band-limited power in the α-band that decreased with task demands.
α-Decrease Influences Performance.
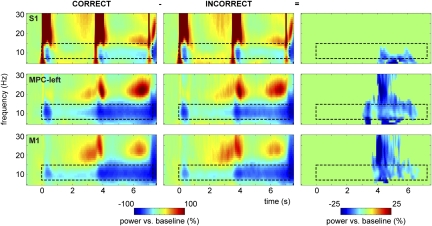
To assess the influence of the LFP α-power modulation on the monkey's performance, we compared the TFRs of trials with correct vs. incorrect responses (Fig. 3). To normalize the sessions, we applied a relative baseline correction to the time-frequency spectra. In S1, MPC-left, and M1, the α-power decrease during the decision period was stronger for correct than for incorrect trials (cluster-corrected P < 0.001). In MPC-left and M1, we also identified a difference in the β-band, but it was not as sustained as the effect in the α-band. No significant effects were identified in the other regions.
Fig. 3.
Stronger α-decrease during the decision interval for correct vs. incorrect responses. Time-frequency representations showing a decrease of power in the α-band during the discrimination task compared with baseline activity, separately for correct trials (Left) and incorrect trials (Center). α-Power decrease during the decision delay was significantly stronger for correct vs. incorrect response trials (Right), showing significant time-frequency samples only (P < 0.001) in S1, MPC-left, and M1.
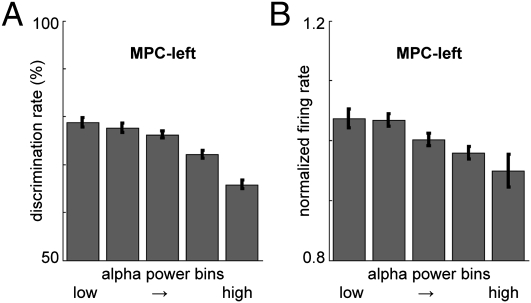
To further investigate the relationship between the α-decrease and performance, we sorted the single trials according to α-power in the decision-time window (t = 4–7 s). Trials were divided in five equal-sized bins, separately for each region, on the basis of α-power. Then we computed the average performance (discrimination rate) for the trials within each α-power bin. This measure has the following advantages over the correct vs. incorrect TFR approach: (i) it is more sensitive as α-power is averaged over the 3-s decision window; (ii) no baseline correction is required, so effects being introduced due to baseline differences can be ruled out; and (iii) trial numbers are equal for all bins, thus precluding unequal sample sizes. We found a significant relation between α-power and performance for S1, S2, DPC, MPC-left, and M1: the discrimination rate decreased systematically with increasing α-power (Fig. 4A, Fig. S1A, and Table S1). In MPC-right, α-power and discrimination rate did not correlate. This confirms the findings in Fig. 3: lower α-power during the decision delay resulted in better performance.
Fig. 4.
α-Power predicts task performance and firing rate in MPC-left. (A) For each recording site (per session), trials were divided into five equal-sized bins on the basis of α-power level during the decision-delay period (t = 4–7 s), and average performance was computed per bin. Discrimination rate decreased with increasing α-power (P < 0.001), shown here for MPC-left. Error bars indicate the SEM. (B) Similarly, firing rate (normalized with average firing rate per recording site) decreased with increasing α-power (P < 0.001).
α-Power Correlates with Spike-Firing Rate.
Next, we computed the average spike-firing rate during the decision delay for the trials within each of the α-power bins. For S2, MPC-left, MPC-right, and M1, lower α-power was associated with higher firing rates, whereas for S1 firing rate was the lowest for medium α-bins and increased for high α-bins (Fig. 4B, Fig. S1B, and Table S1).
α-Phase Predicts Spiking.
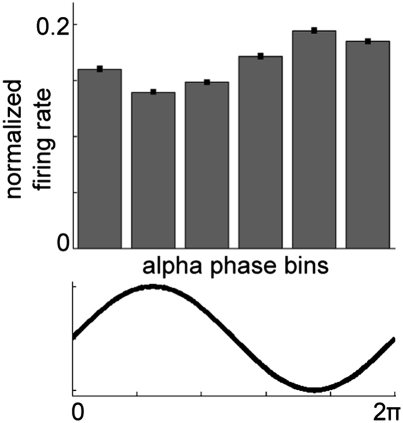
According to the pulsed-inhibition hypothesis, neuronal processing is not only affected by α-power, but also limited to certain parts of the α-cycle. To assess how α-phase relates to spike activity, we divided the α-cycle in six bins (on the basis of the phase) and computed the relative firing rate within each phase bin (Fig. 5). Here, we show that during the down-going phase and trough of the α-cycle, the spike-firing rate was high, whereas it was low during the peak of the α-cycle (one-way ANOVA, all regions combined: F(5,3348) = 292.650, P < 0.001; post hoc tests showed that all bins differed significantly from each other). As the patterns were virtually similar for the different regions, we here combined all recording sites; further testing confirmed these results per region (Fig. S2) (one-way ANOVA per region, all P < 0.001). Thus, spike-firing rate seems to be modulated by the phase of α-oscillations. The strongest firing is observed during the trough of the α-oscillations measured in the LFPs.
Fig. 5.
Firing in relation to the α-cycle. For each recording site, α-cycles were extracted from the data (retention and decision-delay periods) and divided into six phase bins. For each bin, the normalized firing rate was computed (relative to the average firing rate during that particular cycle). Firing rate was highest at the α-trough and lowest at the α-peak (P < 0.001). Here we show the combined grand average over all sites; similar patterns were observed in each of the individual regions (Fig. S2).
Spike-Field Coherence.
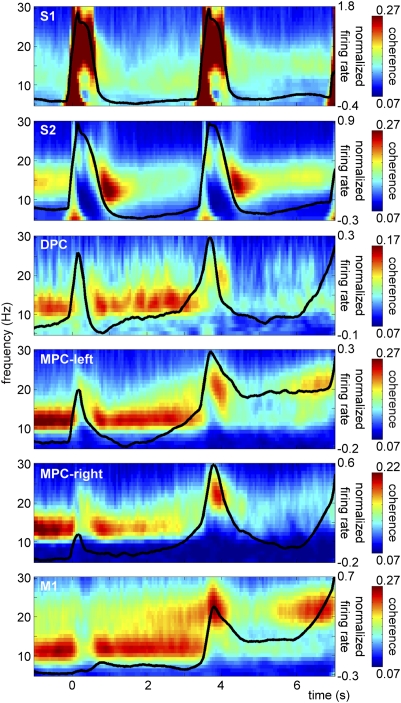
To further study the interaction between the α-oscillations and neuronal processing, we computed coherence between the spikes and LFPs [i.e., spike-field coherence (SFC)] within each region (Fig. 6). SFC captures both amplitude and phase effects. We observed strong sustained SFC limited to the α-band; in the motor areas, it included the β-band as well. In most regions, the SFC in the α-band was highest during baseline and the retention period (especially in premotor and motor regions) and dropped during stimulus presentation and the decision-delay period. The SFC patterns resembled the LFP power effects. The sensory regions differed from the (pre)motor regions in that S1 showed a strong evoked response reflecting the stimulus frequency (range 10–34 Hz), which is hard to dissociate from any ongoing (α) SFC, and in S2 there was an additional transient increase of (α/β) SFC right after the stimulus presentation.
Fig. 6.
Spike-field coherence. Time-frequency representations showing spike-field coherence within each of the recorded regions (showing significant time-frequency samples only). Significant SFC was observed in the α-band in all regions, as revealed by cluster-based randomization statistics vs. shuffled data [P < 0.001, corrected for multiple comparisons (Methods)]. Normalized firing rate is plotted on top (black line) (in arbitrary units, averaged over all sessions). Coherence in the α-band was strongest during baseline (t = -1–0 s) and the retention period (t = 0.5–3.5 s), whereas it dropped off during the stimulus (f1, t = 0–0.5 s; f2, t = 3.5–4 s) and decision periods (t = 4–7 s). Strong α-band coherence was accompanied by a low spike-firing rate.
To statistically test the SFC effects, we computed SFC with the same data but with the trials shuffled between spikes and LFP recordings (i.e., to destroy existing temporal structure between spikes and LFPs within a trial). A cluster-based randomization test comparing the observed SFC with the shuffled SFC estimate confirmed statistical significance of the reported effects (cluster-corrected P < 0.001). The general picture emerging is that neural firing is locked to the phase of the ongoing α-activity. This locking is particularly strong when α-activity is high.
Next, we calculated the average firing rate over time (see traces plotted on top of the SFC in Fig. 6). We observed that in most regions the firing rate increased from the baseline to the retention interval and then during the decision-interval periods. High firing rate was accompanied by lower SFC; this effect was particularly apparent in MPC and M1. This is in line with the hypothesis that the α-rhythm reflects a mechanism of functional disengagement: when α is high, processing (here reflected by firing rate) is inhibited, whereas an α-decrease enables processing and facilitates performance.
Discussion
In the current study we explored the functional role of oscillatory α-band activity in a somatosensory discrimination task and the interaction between α-oscillations and spikes. The LFPs in somatosensory, premotor, and motor regions were dominated by oscillations in the α-band. Furthermore, the neuronal spiking was locked to the trough of the ongoing α-oscillations. We observed a general α-power decrease during the somatosensory discrimination task. This effect was visible in the spike-field coherence as well. The decrease was most prominent during stimulus presentation and, in premotor and motor regions, during the decision delay. The systematic decrease in α-power and spike-field coherence was less pronounced in the sensory regions. Importantly, the α-power decrease was associated with better task performance and increased spike activity. These findings support the idea that the α-rhythm reflects a local mechanism in which neuronal firing is inhibited in a rhythmic manner: the stronger the α-activity, the stronger the inhibition. In this view, when α-activity is (too) high in task-relevant areas, processing capabilities are reduced, resulting in diminished performance.
Functional Role of α-Activity Extends Beyond Sensory Regions.
We found that decreases in α-activity during the decision period correlated with task performance in S1, S2, DPC, MPC-left, and M1. The current findings are consistent with previous work showing that somatosensory α-activity influences discrimination performance (18, 27) and with the visuospatial attention literature linking α-activity to detection performance (15–17). These previous findings have resulted in the notion that α-activity is involved in sensory gating. Importantly, we show here that α-activity in premotor and motor regions influences performance in a similar way as in sensory regions. This shows that oscillatory α-activity does not only play an important functional role in sensory regions, but also is intimately involved in neuronal processing in the extended motor system.
Case for Pulsed Inhibition by Oscillatory α-Activity.
From previous studies using a task similar to the one used here, it is clear that spikes carry the representational code and are responsible for neuronal computations (40–42). Here, we provide insight into how this spiking interacts with ongoing oscillations. We demonstrated that spikes and α-oscillations were phase-synchronized, especially in motor and premotor regions during the baseline and retention interval when α-power was strong and the firing rate was low. Furthermore, we showed that decreasing α-power was associated with an increase in firing rate. In particular, spiking was more likely to occur at the trough than at the peak of the α-cycle. These findings are consistent with the pulsed-inhibition hypothesis (5, 28, 29), which states that the α-oscillations are a consequence of bouts of inhibition that prevent firing and thus reduce neuronal processing.
In this view, α-oscillations modulate neuronal processing in a phasic manner (44), with direct implications for subsequent performance. The stronger the α-power, the stronger the functional inhibition that then reduces the spike activity. It is important to note that, in this framework, the α-oscillations inhibit firing in each cycle but do not necessarily induce rhythmic spiking of individual neurons at the α-frequency. Spikes reflect neuronal processing and hence are informative on task aspects such as stimulus coding and comparison (40–42). Oscillations serve to either facilitate or inhibit processing by modulating the timing of spike firing. Because α-oscillations reflect the state of the system, they predict the overall performance, whereas spikes reveal the contribution of single cells to various task aspects.
To this date, only a few intracranial studies have looked specifically into how the α-rhythm relates to spiking and performance (34–37). Recent work using an intermodal attention paradigm where monkeys had to selectively attend to either a visual or an auditory stimulus has reported that increased α-activity in V2 and V4 resulted in faster auditory stimulus detection (36), and a decrease of α-activity in V1 was associated with visual attention (34). Furthermore, in V1, V2, and V4, coherence between α-band oscillations and (multiunit) spikes that decreased with visual attention was demonstrated (34, 37). These findings are in line with our current findings and the inhibition hypothesis.
However, in the inferotemporal cortex (IT), which is involved in visual processing, decreased α-activity leads to faster auditory detection (36). Furthermore, prestimulus α-activity in IT increased with visual attention and was associated with an increase in multiunit firing (35). Although the findings in the early visual cortex are in line with our results in the sensorimotor system and support the inhibition hypothesis, these findings from IT are at odds with the inhibition hypothesis. Whether α-activity indeed plays a different functional role in IT remains to be seen. Buffalo et al. (37) recently reported different modulation of α-band SFC in superficial vs. deep laminar layers. Further research into the underlying neurophysiological mechanisms, taking the different cortical layers into account, is warranted.
Conclusion
By simultaneously recording LFPs and spikes in a monkey performing a somatosensory discrimination task, we demonstrated that a decrease of α-power across sensorimotor cortices correlated with better discrimination performance. Furthermore, we showed that the α-rhythm interacts with spike activity: firing rate goes up when α-power goes down. Importantly, the neuronal firing is strongly related to the phase of ongoing α-oscillations. Previously, α-power has been shown to play a functional role in sensory regions. Our findings suggest that the α-rhythm plays a similar role in motor and prefrontal regions and reflects a general mechanism for setting the state of cortical networks.
Methods
One monkey (Macaca mulatta) was trained to perform a somatosensory discrimination task in which it had to discriminate the difference in frequency between two mechanical vibrations delivered sequentially to the right hand (Fig. 1A). Responses were made with the left hand. Both spikes and LFPs were recorded simultaneously from five locations in the somatosensory, premotor, and motor cortex (see ref. 38 for details) (Fig. 1B). The animal was handled in accordance with the standards of the National Institutes of Health and the Society for Neuroscience.
Experimental Paradigm.
Vibrotactile stimuli were delivered to one of the fingers of the right, restrained hand. Stimuli consisted of 500-ms pulse trains with frequencies of 10–34 Hz. Each of these pulses consisted of a single-cycle sinusoid lasting 20 ms. Stimulus amplitudes were adjusted to equal subjective intensity [i.e., lower amplitudes for higher frequencies (39)]. After presentation of the first stimulus (f1), a 3-s retention period was followed by the presentation of the second stimulus (f2). The monkey's task was to indicate whether f2 was of lower or higher frequency than f1 by means of a left-hand button press after a 3-s forced-delay period. The animal was rewarded for correct discrimination performance by a drop of liquid.
Data Acquisition.
Here, we report data from 47 recording sessions during which both spikes and LFPs were obtained from up to 240 trials per session. Neuronal recordings were acquired with an array of seven independent, movable microelectrodes inserted in each of five areas simultaneously (38). These areas included S1 (47 sessions), S2 (47 sessions), DPC (34 sessions), and MPC (33 sessions) in the hemisphere contralateral to the stimulated hand and MPC (13 sessions) and M1 (39 sessions) in the hemisphere contralateral to the response hand. Neurons from S1 and S2 were selected on the basis of cutaneous receptive field properties. Neurons of the frontal cortex were selected if they responded to any of the different components of the discrimination task. Cortical areas were identified on the basis of cortical landmarks. The neuronal signal of each microelectrode was sampled at 30 kHz, and spikes were sorted online. Simultaneously, the LFPs were obtained by using a 250-Hz low-pass filter and stored at 2 kHz for offline analysis. A more extensive description of the task and recording procedures can be found in previous publications (38, 39).
Data Analysis.
For data analysis we used custom-build Matlab code and the FieldTrip toolbox (45). The data were down-sampled offline to a sampling frequency of 1 kHz. For each recording session, all trials were first cleaned from artifacts. A band-stop filter was applied to remove line noise (60 Hz and harmonics) caused by the power net. To remove further recording artifacts, the data were rereferenced per cortical region: for each recording site (within each session), the average signal from electrodes in that same region was subtracted per time point. Trials containing the remaining artifacts (e.g., due to movement or electronic interference) were removed on the basis of visual inspection of the data.
Spectral Analysis.
Power spectra (2–36 Hz) were computed using a fast Fourier transform approach. Trials were segmented into 1-s epochs and multiplied with a Hanning taper to improve the spectral estimation. Furthermore, to inspect the time course of the frequency effects, we computed the TFRs of power using an adaptive sliding-time window of five cycles in length (Δt = 5/f) multiplied with a Hanning taper. Per condition of interest, the power was averaged over trials within each recording session. TFRs were normalized by a relative baseline correction (t = −1 – −0.5 s) and then averaged over electrodes within the same region (per session). This procedure gives average power spectra per region for each session, which were used in the statistical analysis. Subsequently, a grand average was computed over recording sessions (per region) for visualization purposes.
SFC was calculated using the time-resolved spectra Sx(f) and Sy(f) of the fields and spikes, respectively, and their cross spectrum Syx(f). The spike signal was represented as the firing rate per 1-ms bins, and spectra were calculated in the same way as for the LFPs. SFC is given by the following equation:
and ranges between 0, which indicates no phase relationship, and 1, which indicates that the two signals are fully coherent (46).
In addition, to establish the relation between the phase of the α-cycle and the firing rate, we band-pass filtered (8–14 Hz) the time domain data and, for each trial, cut out the α-cycles (on the basis of zero crossings) from the retention and decision delays. Each single α-cycle was divided into six equal-length phase bins, and for each bin the number of spikes was counted. Doing this for each of the α-cycle segments, for all trials and recording sites, allowed us to assess whether there was a systematic modulation of firing rate during the α-phase. Per region, we applied a one-way ANOVA to test whether there were significant differences in normalized firing rate between the different α-phase bins.
In the spectral analysis, the following numbers of unique LFP recording sites were included: S1, 258 sites; S2, 252 sites; DPC, 151 sites; MPC-left, 133 sites; MPC-right, 71 sites; and M1, 132 sites. In addition, for the SFC analysis, the following numbers of single-unit spike recordings were included: S1, 129 units; S2, 308 units; DPC, 115 units; MPC-left, 137 units; MPC-right, 127 units; and M1, 246 units. The following numbers of individual recording sites for which data for both LFP and spike activity were available were included in the α-power/phase to firing rate analysis: S1, 93 sites; S2, 170 sites; DPC, 61 sites; MPC-left, 79 sites; MPC-right, 53 sites; and M1, 103 sites.
Statistical Analysis.
To establish whether the differences in power (as observed in the TFRs) between two conditions (i.e., correct vs. incorrect trials, task vs. baseline activation) were significantly different from 0, a cluster-based nonparametric randomization test was applied within sessions (47). By clustering neighboring samples (i.e., time-frequency points) that show the same effect, this test deals with the multiple comparisons problem while taking into account the dependency of the data. For each sample, a dependent-sample t-value was computed. All samples were selected for which this t-value exceeded an a priori threshold (uncorrected P < 0.05), and these were subsequently clustered on the basis of temporal–spectral adjacency. The sum of the t values within a cluster was used as the cluster-level statistic. The cluster with the maximum sum was subsequently used as a test statistic. This was done within sessions and separately per region. By randomizing the data across the two conditions and recalculating the test statistic 1,000 times, we obtained a reference distribution of maximum cluster t values to evaluate the statistic of the actual data. A similar approach was applied to compare the observed SFC with a shuffled SFC estimate.
Supplementary Material
Acknowledgments
We thank Adrián Hernández, Antonio Zainos, and Manuel Alvarez for technical assistance. S.H. was supported by the Division for the Earth and Life Sciences Open Competition Grant 817.02.010 from The Netherlands Organization for Scientific Research; O.J. was supported by Vici Grant 453.09.002 from The Netherlands Organization for Scientific Research; S.H. and O.J. were both supported by the BrainGain Smart Mix Programme of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture and Science; R.R. was supported by an International Research Scholars Award from the Howard Hughes Medical Institute and by grants from the Consejo Nacional de Ciencia y Tecnología and Dirección del Personal Académico de la Universidad Nacional Autónoma de México.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117190108/-/DCSupplemental.
References
- 1.Berger H. Uber das elektroenkephalogramm des Menschen [On the human electroencephalogram] Arch Psychiatr Nervenkr. 1929;87:527–570. German. [Google Scholar]
- 2.Adrian ED, Matthews BHC. The Berger rhythm: Potential changes from the occipital lobes in man. Brain. 1934;57:355–385. doi: 10.1093/brain/awp324. [DOI] [PubMed] [Google Scholar]
- 3.Pfurtscheller G, Stancák A, Jr, Neuper C. Event-related synchronization (ERS) in the alpha band—an electrophysiological correlate of cortical idling: A review. Int J Psychophysiol. 1996;24(1-2):39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- 4.Lopes Da Silva FH, Storm Van Leeuwen W. The cortical source of the alpha rhythm. Neurosci Lett. 1977;6:237–241. doi: 10.1016/0304-3940(77)90024-6. [DOI] [PubMed] [Google Scholar]
- 5.Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Res Brain Res Rev. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30(4):150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Cooper NR, Croft RJ, Dominey SJJ, Burgess AP, Gruzelier JH. Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. Int J Psychophysiol. 2003;47(1):65–74. doi: 10.1016/s0167-8760(02)00107-1. [DOI] [PubMed] [Google Scholar]
- 8.Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front Hum Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foxe JJ, Simpson GV, Ahlfors SP. Parieto-occipital ∼10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport. 1998;9:3929–3933. doi: 10.1097/00001756-199812010-00030. [DOI] [PubMed] [Google Scholar]
- 10.Fu KMG, et al. Attention-dependent suppression of distracter visual input can be cross-modally cued as indexed by anticipatory parieto-occipital alpha-band oscillations. Brain Res Cogn Brain Res. 2001;12(1):145–152. doi: 10.1016/s0926-6410(01)00034-9. [DOI] [PubMed] [Google Scholar]
- 11.Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol. 2006;95:3844–3851. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- 12.Sauseng P, et al. A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur J Neurosci. 2005;22:2917–2926. doi: 10.1111/j.1460-9568.2005.04482.x. [DOI] [PubMed] [Google Scholar]
- 13.Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20(6):RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rihs TA, Michel CM, Thut G. A bias for posterior alpha-band power suppression versus enhancement during shifting versus maintenance of spatial attention. Neuroimage. 2009;44(1):190–199. doi: 10.1016/j.neuroimage.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Händel BF, Haarmeier T, Jensen O. Alpha oscillations correlate with the successful inhibition of unattended stimuli. J Cogn Neurosci. 2011;23:2494–2502. doi: 10.1162/jocn.2010.21557. [DOI] [PubMed] [Google Scholar]
- 16.Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gould IC, Rushworth MF, Nobre AC. Indexing the graded allocation of visuospatial attention using anticipatory alpha oscillations. J Neurophysiol. 2011;105:1318–1326. doi: 10.1152/jn.00653.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haegens S, Händel BF, Jensen O. Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J Neurosci. 2011;31:5197–5204. doi: 10.1523/JNEUROSCI.5199-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones SR, et al. Cued spatial attention drives functionally relevant modulation of the mu rhythm in primary somatosensory cortex. J Neurosci. 2010;30:13760–13765. doi: 10.1523/JNEUROSCI.2969-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson KL, Ding M. Attentional modulation of the somatosensory mu rhythm. Neuroscience. 2011;180:165–180. doi: 10.1016/j.neuroscience.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 21.van Ede F, de Lange F, Jensen O, Maris E. Orienting attention to an upcoming tactile event involves a spatially and temporally specific modulation of sensorimotor alpha- and beta-band oscillations. J Neurosci. 2011;31:2016–2024. doi: 10.1523/JNEUROSCI.5630-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci. 2007;27:3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuladhar AM, et al. Parieto-occipital sources account for the increase in alpha activity with working memory load. Hum Brain Mapp. 2007;28:785–792. doi: 10.1002/hbm.20306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9-12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex. 2002;12:877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- 25.Medendorp WP, et al. Oscillatory activity in human parietal and occipital cortex shows hemispheric lateralization and memory effects in a delayed double-step saccade task. Cereb Cortex. 2007;17:2364–2374. doi: 10.1093/cercor/bhl145. [DOI] [PubMed] [Google Scholar]
- 26.Krause CM, Lang AH, Laine M, Kuusisto M, Pörn B. Event-related EEG desynchronization and synchronization during an auditory memory task. Electroencephalogr Clin Neurophysiol. 1996;98:319–326. doi: 10.1016/0013-4694(96)00283-0. [DOI] [PubMed] [Google Scholar]
- 27.Haegens S, Osipova D, Oostenveld R, Jensen O. Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network. Hum Brain Mapp. 2010;31(1):26–35. doi: 10.1002/hbm.20842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazaheri A, Jensen O. Rhythmic pulsing: Linking ongoing brain activity with evoked responses. Front Hum Neurosci. 2010;4:177. doi: 10.3389/fnhum.2010.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathewson KE, et al. Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Front Psychol. 2011;2:99. doi: 10.3389/fpsyg.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.VanRullen R, Koch C. Is perception discrete or continuous? Trends Cogn Sci. 2003;7:207–213. doi: 10.1016/s1364-6613(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 31.Busch NA, Dubois J, VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. J Neurosci. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. To see or not to see: Prestimulus alpha phase predicts visual awareness. J Neurosci. 2009;29:2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheeringa R, Mazaheri A, Bojak I, Norris DG, Kleinschmidt A. Modulation of visually evoked cortical FMRI responses by phase of ongoing occipital alpha oscillations. J Neurosci. 2011;31:3813–3820. doi: 10.1523/JNEUROSCI.4697-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bollimunta A, Mo J, Schroeder CE, Ding M. Neuronal mechanisms and attentional modulation of corticothalamic α oscillations. J Neurosci. 2011;31:4935–4943. doi: 10.1523/JNEUROSCI.5580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mo J, Schroeder CE, Ding M. Attentional modulation of alpha oscillations in macaque inferotemporal cortex. J Neurosci. 2011;31:878–882. doi: 10.1523/JNEUROSCI.5295-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bollimunta A, Chen Y, Schroeder CE, Ding M. Neuronal mechanisms of cortical alpha oscillations in awake-behaving macaques. J Neurosci. 2008;28:9976–9988. doi: 10.1523/JNEUROSCI.2699-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. Laminar differences in gamma and alpha coherence in the ventral stream. Proc Natl Acad Sci USA. 2011;108:11262–11267. doi: 10.1073/pnas.1011284108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernández A, et al. Procedure for recording the simultaneous activity of single neurons distributed across cortical areas during sensory discrimination. Proc Natl Acad Sci USA. 2008;105:16785–16790. doi: 10.1073/pnas.0808702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernández A, Salinas E, García R, Romo R. Discrimination in the sense of flutter: New psychophysical measurements in monkeys. J Neurosci. 1997;17:6391–6400. doi: 10.1523/JNEUROSCI.17-16-06391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernández A, et al. Decoding a perceptual decision process across cortex. Neuron. 2010;66:300–314. doi: 10.1016/j.neuron.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 41.Lemus L, et al. Neural correlates of a postponed decision report. Proc Natl Acad Sci USA. 2007;104:17174–17179. doi: 10.1073/pnas.0707961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romo R, Salinas E. Flutter discrimination: Neural codes, perception, memory and decision making. Nat Rev Neurosci. 2003;4:203–218. doi: 10.1038/nrn1058. [DOI] [PubMed] [Google Scholar]
- 43.Haegens S, et al. Beta oscillations in the monkey sensorimotor network reflect somatosensory decision making. Proc Natl Acad Sci USA. 2011;108:10708–10713. doi: 10.1073/pnas.1107297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lőrincz ML, Kékesi KA, Juhász G, Crunelli V, Hughes SW. Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron. 2009;63:683–696. doi: 10.1016/j.neuron.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011 doi: 10.1155/2011/156869. 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J Neurosci. 2008;28:4823–4835. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.