Abstract
Specialized proteins in the plasma membrane, endoplasmic reticulum (ER), and mitochondria tightly regulate intracellular calcium. A unique mechanism called store-operated calcium entry is activated when ER calcium is depleted, serving to restore intra-ER calcium levels. An ER calcium sensor, stromal interaction molecule 1 (STIM1), translocates within the ER membrane upon store depletion to the juxtaplasma membrane domain, where it interacts with intracellular domains of a highly calcium-selective plasma membrane ion channel, Orai1. STIM1 gates Orai1, allowing calcium to enter the cytoplasm, where it repletes the ER store via calcium-ATPases pumps. Here, we performed affinity purification of Orai1 from Jurkat cells to identify partner of STIM1 (POST), a 10-transmembrane–spanning segment protein of unknown function. The protein is located in the plasma membrane and ER. POST-Orai1 binding is store depletion-independent. On store depletion, the protein binds STIM1 and moves within the ER to localize near the cell membrane. This protein, TMEM20 (POST), does not affect store-operated calcium entry but does reduce plasma membrane Ca2+ pump activity. Store depletion promotes STIM1–POST complex binding to smooth ER and plasma membrane Ca2+ ATPases (SERCAs and PMCAs, respectively), Na/K-ATPase, as well as to the nuclear transporters, importins-β and exportins.
Keywords: Ca2+ release-activated Ca2+, calcium signaling, immunity
Calcium ions trigger hundreds of biological processes ranging from transcription to apoptosis. Cells maintain a large concentration gradient between the cytoplasm and surrounding compartments to form a calcium battery, enabling rapid increases in cytoplasmic calcium by the opening of ion channels in the plasma membrane (e.g., Orai1 channel) or endoplasmic reticulum [ER; e.g., inositol (1, 4, 5) trisphosphate receptor channel (IP3R)]. This calcium battery is recharged by calcium-ATPases across the smooth ER (SERCA) pumps and plasma membrane Ca2+ (PMCA) pumps. G-protein and tyrosine kinase receptors activate phospholipase C to hydrolyze plasma membrane-specific phosphatidylinositol 4,5-bisphosphate (PIP2) to release soluble inositol triphosphate (IP3) (1). Within seconds, IP3 gates the ER IP3R channel to increase cytoplasmic Ca2+. Over the next few minutes, a plasma membrane Ca2+ entry mechanism [or store-operated Ca2+ entry (SOCE)] is activated via a message from the calcium-depleted ER. SOCE is mediated by the triggered activity of highly selective Orai1 Ca2+ channels [also called Ca2+ release-activated Ca2+ (CRAC) channels]. Most importantly, declining ER [Ca2+] but not increasing cytoplasmic [Ca2+] triggers the activity of the Orai1 channels. This is a crucial distinction, separating it from Ca2+-activated transient receptor potential (TRP) and K+ channels (2, 3).
Stromal interaction molecule 1 (STIM1), a single transmembrane-spanning domain protein primarily residing in the ER, is essential for SOCE activation (4). STIM1's N terminus sits inside the ER, where it senses luminal Ca2+ concentration; its C-terminal protein interaction domain is cytoplasmic. When ER Ca2+ falls, STIM1's luminal E, F hand-sterile alpha motif (EF-SAM) motif likely unfolds (5). STIM1 diffuses within the ER to regions where it can closely approximate the plasma membrane (6–8), where it interacts with Orai1 (9–13). The interaction requires an ∼110-aa cytoplasmic domain of STIM1 [the CRAC activation domain (CAD) (14) or STIM Orai activating region (15)], consisting of a putative coiled-coil and roughly half of an ezrin, radixin, moesin domain. Purified CAD is sufficient to activate Orai1 channels in the absence of store depletion, whereas channel clustering alone is insufficient to activate Orai (14). Presumably, tetrameric Orai1 drifts into the high-avidity STIM1 clusters, where it is bound at both its C and peri-N termini. Binding triggers gating of the Orai1 channel, increasing cytoplasmic calcium. SERCA pumps then recharge the ER Ca2+ battery (16).
A number of proteins have been proposed to interact with STIM1 or Orai, including actin (17), calnexin, exportin-1, transportin-1 (18), EB1 and SERCA2 (19), SERCA3 (20), CaV L type channels (14), P100, a fragment of polycystin 1 (21), Golli, a myelin basic protein (22), CRAC receptor 2A, a cytoplasmic Ca2+ sensor (23), and TRPC channels (e.g., 24, 25). Here, we used tandem affinity purification (TAP) and mass spectrometry (MS) to identify a unique protein [TMEM20; partner of STIM1 (POST)] interacting with STIM1 that enables STIM1 binding to multiple transporters. We propose that after store depletion, high cytosolic Ca2+ is sustained by activation of Orai1 as well as by inhibition of PMCA activity by the STIM1–POST complex.
Results
TAP of Orai1 from Jurkat Cells.
Human Orai1 N-terminally tagged with Protein A (PrA) and calmodulin-binding peptide (CBP) was stably transfected into Jurkat cells under a tetracycline-inducible promoter. To purify proteins in complex after ER calcium depletion, cells were treated with thapsigargin (1 μM) in Ca2+-free Ringer's solution and the tagged protein was affinity-purified. MS analysis of Orai1-copurified proteins identified TMEM20 (NP_001128130), an unknown hydrophobic protein with 10 putative transmembrane-spanning segments but no identified functional domains. TMEM20 may be a member of the drug/metabolite transporter superfamily (EamA, DUF6), a large group of proteins about which little is known. TMEM20's protein sequence is well conserved among vertebrates; a distant homolog was found in Drosophila (Fig. S1A). TMEM20 RNA is ubiquitously expressed in human tissues (Fig. S1B). For reasons we describe below, we named this protein POST.
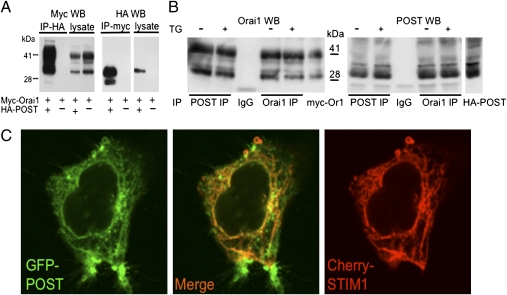
To verify the POST-Orai1 interaction, we expressed epitope-tagged POST and Orai1 in HEK 293 cells and immunoprecipitated proteins using well-characterized antitag antibodies. POST specifically coimmunoprecipitated Orai1, and Orai1 specifically coimmunoprecipitated POST, confirming that these two proteins can form molecular complexes (Fig. 1A and Fig. S2). To characterize endogenous Orai1 and POST proteins, anti-Orai1 and anti-POST antibodies were generated in rabbits. These antibodies specifically immunoprecipitated their target proteins and identified Orai1 [35- and 42- kDa bands (glycosylated), 32.6 kDa predicted] and POST (35-kDa band, 39.8 kDa predicted) on Western blots (Fig. 1B and Fig. S3) but were not suitable for immunofluorescent staining of native proteins. Immunoprecipitation (IP) of endogenous Jurkat Orai1 and POST confirmed that these proteins are components of a molecular complex and revealed that POST-Orai1 binding did not depend on ER Ca2+ content (Fig. 1B).
Fig. 1.
POST binds Orai1 and is expressed in the ER and plasma membrane. (A) HA epitope-tagged POST specifically binds myc-tagged Orai1 coexpressed in HEK 293 cells. Cell lysates were immunoprecipitated with HA-agarose and stained on Western blot (WB) with myc- or HA-antibody conjugated to HRP. (B) Endogenous POST binds Orai1. Jurkat cell lysates were immunoprecipitated with Orai1, POST antibody, or preimmune rabbit IgG, and probed on WB with the indicated antibody (TrueBlot). Lysates of HEK 293 expressing myc-Orai1 (labeled as myc-Or1) and HA-POST were used as markers. Note that store depletion (cells treated with 1 μM thapsigargin for 10 min in Ca2+-free Ringer's solution) did not affect POST-Orai1 interaction. (C) Live confocal fluorescence images of HEK 293 cells coexpressing POST-GFP and Cherry-STIM1. POST was localized in the cell periphery and intracellular membrane network, where it precisely colocalized with Cherry STIM1 (ER). Note the fraction of POST that is expressed in the cell periphery, where STIM1 is not expressed.
On Store Depletion, POST Binds STIM1 and Moves to the Plasma Membrane.
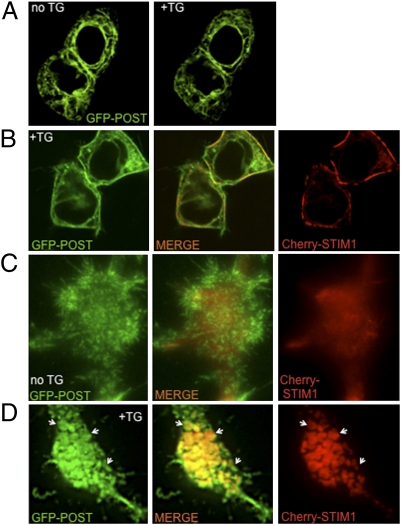
GFP-POST expressed in HEK 293 cells localized to an intracellular membrane network, where it precisely colocalized with Cherry-STIM1 (Fig. 1C and Movie S1), an ER protein. Calcium depletion of stores by thapsigargin treatment did not alter the expression pattern of GFP-POST when expressed alone in HEK 293 cells (Fig. 2A). However, in HEK 293 cells coexpressing GFP-POST and Cherry-STIM1, store depletion resulted in translocation of both proteins to the cell periphery within 4–8 min after thapsigargin application (Movie S2). Confocal images of the two proteins indicate partial overlap at the cell periphery (Fig. 2B and Movie S3), as well as POST colocalization with Orai1 (Fig. S4). Simultaneous total internal reflectance (TIRF) imaging of fluorescent POST and STIM1 clearly demonstrates that POST forms juxtamembrane clusters that precisely colocalized with STIM1 clusters after store depletion (Fig. 2 C and D).
Fig. 2.
STIM1 translocates with POST to the periplasma membrane region on store depletion. (A) Live confocal image of GFP-POST expressing HEK 293 cells before and after store depletion [1 μM thapsigargin (TG) for 10 min in Ca2+-free Ringer's solution]. (B) Live confocal image of store-depleted HEK 293 cells coexpressing GFP-POST and Cherry-STIM1. (C) TIRF image of HEK 293 cell coexpressing GFP-POST and Cherry-STIM1 before store depletion. (D) TIRF image of store-depleted HEK 293 cells coexpressing GFP-POST and Cherry-STIM1. POST and STIM1 cocluster in proximity to the plasma membrane. Arrows indicate same clusters in all three images.
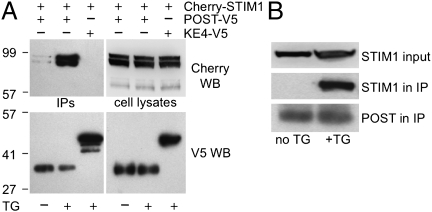
Epitope-tagged STIM1 and POST were coexpressed in HEK 293 cells. POST was immunoprecipitated from cells before and after store depletion. From cells with full Ca2+ stores, V5-tagged POST coimmunoprecipitated a barely detectable amount of Cherry-STIM1 (Fig. 3A). Store depletion dramatically augmented STIM1 co-IP with POST. Under identical conditions, a V5-tagged unrelated membrane protein, KE4, did not bind STIM1, demonstrating specificity of the POST-STIM1 interaction. IP of endogenous POST from Jurkat cells recovered the STIM1–POST molecular complex only under store-depleted conditions (Fig. 3B).
Fig. 3.
POST binds STIM1 on store depletion. (A) Store depletion [cells treated with 1 μM thapsigargin (TG) for 10 min in Ca2+-free Ringer's solution] promotes POST binding to STIM1. Lysates of HEK 293 cells coexpressing Cherry-STIM1 and POST-V5 or KE4-V5 (zinc transporter serving as negative controls) were immunoprecipitated with anti–V5-agarose and stained on Western blot (WB) with RFP (Cherry) antibody. (B) Endogenous Jurkat STIM1 and POST form a molecular complex only on store depletion (POST IP and WB conditions as in Fig. 1B). STIM1 was detected in lysates with rabbit anti-STIM1 antibody and in immunoprecipitates with mouse anti-STIM1 antibody.
POST, Like STIM1, Is also Present in the Plasma Membrane.
STIM1 was initially identified as a plasma membrane protein, but most STIM1 is found in the ER. Because we originally found POST binding to the endogenous plasma membrane channel, Orai1, we wondered whether POST might also be present on the plasma membrane. Indeed, simultaneous TIRF microscopy of GFP-POST and Cherry-STIM1 in cells with full Ca2+ stores revealed that POST could be seen in thin appendages lacking Cherry-STIM1 (Figs. 1C and 2C and Movie S1). Finally, surface biotinylation of HEK 293 proteins clearly demonstrated the presence of endogenous transmembrane POST protein in the plasma membrane (Fig. S5). Quantification of biotinylation indicates that 5–10% of POST is located in the plasma membrane. Thus, like STIM1, POST is both an ER protein and a plasma membrane protein.
POST Overexpression or Down-regulation Does Not Substantially Affect Calcium Entry via Orai1.
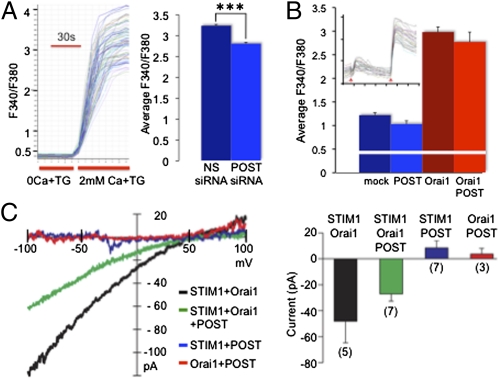
POST binding to Orai1, as well as store depletion-stimulated POST binding to STIM1 followed by POST-STIM1 translocation to the previously well-characterized juxtamembrane STIM1 clusters (6–8), suggests that POST might modulate Orai1 activity. To test this possibility, we knocked down POST mRNA in Jurkat cells with siRNA (Fig. S6) and measured store-operated Ca2+ influx via Orai1. Despite a fourfold decrease in POST mRNA, thapsigargin-induced maximal Ca2+ levels in Jurkat cells were only slightly reduced (Fig. 4A). Similarly, POST overexpression in HEK 293 cells expressing STIM1 and Orai1 did not change basal Ca2+ levels and did not affect store-operated Ca2+ influx (Fig. 4B). Finally, patch-clamp recordings from STIM1/Orai/POST-expressing HEK 293 cells revealed no novel Ca2+ current or significantly modulated CRAC current in the cells overexpressing POST (Fig. 4C). We conclude that POST is not critical for store-operated STIM1-dependent Orai1 activation (CRAC).
Fig. 4.
POST abundance does not substantially affect store-operated Ca2+ influx via Orai1. (A) Store depletion-induced Ca2+ influx in Fura-2–loaded Jurkat cells. siRNA-mediated POST down-regulation (Fig. S6) caused a minor but statistically significant change in Ca2+ influx. (Left) Example and protocol for Fura-2 fluorescence recording. (Right) Average maximal response ± SEM [total of 270 cells for each nonsilencing (NS) and POST siRNA; 5 samples, 2 independent experiments]. ***P < 0.001 (Student's t test). (B) POST overexpression in HEK 293 cells stably transfected with STIM1 did not induce Ca2+ influx and did not modulate store-operated Ca2+ influx via Orai1. Cytosolic Ca2+ changes were recorded using Fura-2. (Inset) Example and protocol of Fura-2 fluorescence recording. The left arrow indicates perfusion with Ca2+-free Ringer's solution plus 1 μM thapsigargin (TG); the right arrow indicates perfusion with Ringer's solution containing 2 mM Ca2+ and 1 μM TG. Bars represent average values of maximal response ± SD to 2 mM Ca2+ (for each condition, 70–80 cells recorded; 3 experiments). The white line indicates average F340/F380 ratio for Ca2+-free Ringer's solution. (C) Representative current-voltage traces (Left) and summary of inward Ca2+ currents measured at −100 mV (Right) from HEK 293T cells overexpressing STIM1, Orai1, and POST. Cells labeled as STIM1-expressing are stable STIM1 transfectants. Currents were measured with 20 mM external [Ca2+] following passive store depletion with 10 mM BAPTA in the pipette. Background currents subtracted for the representative traces and averages (±SEM) are shown in the bar graph.
Store Depletion Promotes POST-Dependent STIM1 Binding to SERCA2, PMCA, Na/K-ATPase, and the Nuclear Transporters Importin-β1 and Exportin-1.
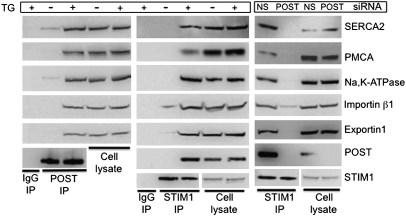
To gain further insight into POST function, we performed a second round of TAP, this time using epitope-tagged POST as bait (Table S1). Human POST was affinity-purified from HEK 293 cells in which stores had been depleted by thapsigargin in Ca2+-free Ringer's solution. To our surprise, MS/MS analysis of POST-copurified proteins identified SERCA2 (recovered peptides belonged to 3 isoforms of SERCA2), the Na/K-ATPase α1-subunit (NP_000692), and two PMCAs (ATP2B1; NP_001001323 and ATP2B4; NP_001001396) as well as multiple isoforms of the nuclear transport receptors importin and exportin. To verify these interactions, we immunoprecipitated endogenous POST from HEK 293 and Jurkat cells. POST specifically coimmunoprecipitated SERCA2, PMCAs, the Na/K-ATPase α-subunit, and the nuclear carrier proteins, importin-β1 and exportin-1 binding was substantially enhanced in samples obtained from cells with Ca2+-depleted stores (Fig. 5, Left, and Fig. S7A).
Fig. 5.
Store depletion stimulates POST-dependent STIM1 binding to multiple transporters. (Left) POST binds SERCA2, PMCA, and Na/K-ATPase on store depletion. The POST immunoprecipitate from HEK 293 cells was probed with antibodies to the indicated proteins. Store depletion conditions were as described in Fig. 1. (Center) STIM1 binds POST targets on store depletion. HEK 705 (not induced with tetracycline) cell lysates were immunoprecipitated with rabbit STIM1 antibody and probed with antibody to the indicated proteins. (Right) POST is required for store depletion-dependent STIM1 binding to SERCA2, PMCA, Na/K-ATPase, importin-β1, and exportin-1. HEK 705 cells were transfected with nonsilencing (NS) or POST siRNA; 4 d after transfection, cells were treated with thapsigargin (TG) and cell lysates were immunoprecipitated with anti-STIM1 rabbit antibody and probed with the indicated antibody.
Because we had found that POST bound STIM1 on store depletion, we tested whether STIM1 also interacts with POST targets. Fig. 5 (Center) shows that STIM1 binds POST target proteins after STIM1 activation by Ca2+ store depletion. Store depletion-dependent STIM1 binding to PMCA was also detected in Jurkat cells (Fig. S7B). siRNA-mediated POST protein knockdown completely eliminated STIM1 binding to SERCA2, PMCA, Na/K-ATPase, and exportin-1 and substantially decreased binding to importin-β1 (Fig. 5, Right), indicating that POST is critical for binding the store depletion-activated STIM1 with these transporters.
POST attenuates PMCA activity in store-depleted cells.
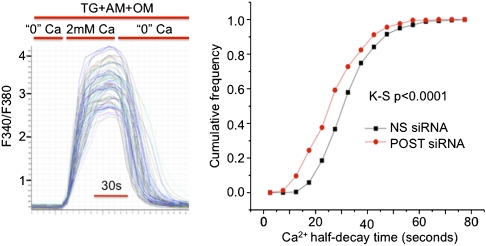
So far, our evidence indicates that activated STIM1 binds and translocates POST to the plasma membrane and that this complex binds several transporters. As shown above, we found no evidence for substantial POST regulation of Orai1 conductance. We next tested whether POST affected PMCA activity by studying the effect of siRNA-mediated POST knockdown on PMCA activity in Jurkat cells. The removal of extracellular Ca2+ after store depletion-induced Ca2+ influx results in a rapid decline of cytosolic calcium. The rapid decline in cytosolic [Ca2+] could be mediated by Ca2+ extrusion via SERCA, PMCAs, and uptake by mitochondria (26). When SERCA was inhibited by thapsigargin and mitochondria by antimycin and oligomycin, the cytosolic [Ca2+] decrease in Jurkat cells was mediated almost exclusively by PMCA activity (26). We used the rate of cytosolic Ca2+ decline as a measure of PMCA activity in Jurkat cells (Fig. 6, Left). As shown in Fig. 6 (Right), POST knockdown increased PMCA-mediated Ca2+ extrusion rates. PMCA activation explains the small decrease of store depletion-dependent Ca2+ influx in Jurkat cells in which POST had been down-regulated (Fig. 4A). We conclude that a PMCA complex containing POST has lower Ca2+ pump activity. This result is consistent with a model in which, after store depletion, high cytosolic Ca2+ is sustained by activation of Orai1 as well as by inhibition of PMCA activity by the STIM1–POST complex.
Fig. 6.
POST inhibits PMCA activity in store-depleted cells. Four days after siRNA transfection, Jurkat cells were loaded with Fura-2 and stores were depleted in Ca2+-free Ringer's solution containing 1 μM thapsigargin (TG) for 10 min before imaging. (Left) Traces of Fura-2 fluorescence recordings from multiple cells in a single sample. During the experiment, all solutions contained 1 μM TG, 2 μM antimycin A (AM), and 1 μM oligomycin (OM). The half-time (T1/2) of the F340/F380 decay was calculated for each trace. (Right) Cumulative frequency of T1/2s for the cell population in two independent experiments for each condition [275 cells for nonsilencing (NS) RNA and 259 cells for POST siRNA]. K-S P < 0.0001, Kolmogorov–Smirnov probability calculation.
Discussion
We provide strong evidence that a previously unrecognized ER protein, POST, can associate with STIM1 and PMCAs, SERCAs, Na/K-ATPases, and nuclear transporters (importins-β and exportins). POST interaction with these molecules depends on store depletion. On store depletion, POST becomes strongly bound to STIM1 and translocates to clusters in proximity to the plasma membrane and also to the nuclear envelope (Fig. S4). At present, we cannot distinguish between the possibility that STIM1 is required only for POST translocation or whether it is also obligatory for POST binding to its targets. Finally, a minority of POST molecules are expressed in the plasma membrane, where it binds the Orai1 channel. POST association with Orai1 does not depend on store depletion. POST down-regulation or overexpression did not substantially affect the store depletion-regulated Orai1 Ca2+ conductance (CRAC). This raises the possibility that POST could modulate Orai1 activity in response to other physiological stimuli, independent of store depletion.
On store depletion, STIM1 becomes strongly bound to the POST-targeted molecules SERCA, PMCA, and Na/K-ATPase as well as to the nuclear transporters, importins-β and exportins. Store depletion-dependent STIM1 binding to SERCA2 (19) and some karyopherins (18) has been reported previously. Here, we demonstrate that STIM1 binding to all these molecules requires POST. Thus, in the simplest interpretation, POST is a scaffolding molecule.
We demonstrated that in store-depleted cells in which the STIM1–POST complex is bound to PMCA, POST knockdown resulted in an increase in PMCA activity. This suggests that formation of the STIM1–POST complex with PMCA attenuates Ca2+ pump activity and may sustain cytosolic Ca2+ elevation. Estimation of POST's regulation of PMCA activity depends on whether PMCA is evenly distributed in the plasma membrane or, like Orai1 (11), clusters in junctional areas on STIM1 activation. Because the ER-PM junction comprises only ∼5% of the cell surface area in Jurkat cells (6–8), the actual shift of PMCA activity may be much larger. We did not determine whether STIM1-POST regulates SERCA or Na/K-ATPase activities. Both of these latter transporter families regulate intracellular Ca2+; Na/K-ATPases do so indirectly by providing the voltage gradient that drives Ca2+ entry and through homeostatic mechanisms. It is intriguing that POST has the putative architecture of a transporter. Whether this allows it to complex with other transporters, or whether it is itself a transporter, is an important question for future studies. In any case, we hypothesize that POST, or a POST–STIM1 complex, regulates the activity of the targeted transporters that coordinate cytosolic Ca2+ through modulation of pump activities.
Currently, the most puzzling finding of our experiments is the binding of STIM1-POST to karyopherin complexes. Because ER proteins occupy the space between inner and outer nuclear membranes, they may be accessible to nuclear pore complex proteins. By raising cytosolic Ca2+, store depletion regulates nuclear factor of activated T-cell (NFAT) translocation (27). A more direct interaction of the STIM1–POST complex with nuclear gate proteins raises the fascinating possibility that nuclear import/export is directly modulated upon store depletion.
Materials and Methods
cDNA Constructs.
The protein A-TEV protease-CBP tag (ATC)-TAP vector was made by subcloning the Kozak-PrA-TEV-CBP sequence [PCR-amplified from pBS1479 (28) into pcDNA4TO; Invitrogen]. In-frame subcloning of the human Orai1 coding sequence (NM_032790; Origene TC124465) into the ATC-TAP vector generated the N-terminal TAP-Orai1 cDNA. Human Orai1 coding sequence was subcloned into a modified pEGFP-C1 in which the EGFP sequence was replaced by mCherry (AY678264, generous gift of R. Tsien, University of California, San Diego, CA). HA-Orai1 was made in pcDNA6 (Invitrogen).
The POST coding sequence was PCR-amplified from the TMEM20 cDNA clone IMAGE:8143817 (Open Biosystems clone 8143817). C-terminal fusion TAP vector [CTAP-HS, containing the sequence encoding an HA-epitope tag–streptavidin-binding peptide (HS) followed by internal ribosome entry site (IRES) and EGFP] was made by sequential subcloning of the PCR-amplified streptavidin binding peptide with-Stop codon and IRES-EGFP sequences from pCEMM-CTAP [EF467048 (29)] into a modified pcDNA4TO containing the HA-tag sequence (CTAP-HS plasmid map and sequence provided on request). C-terminal POST-TAP cDNA was made by in-frame subcloning of the human POST coding sequence into a TAP-HS vector. Additional tagged POST constructs included POST-EGFP in pEGFPN2 (Clontech), POST-V5/Red-pTracer-V5 (Invitrogen pTracer-CMV2 backbone in which the CMV promoter was replaced with a CAG promoter, the V5 epitope tag sequence was introduced, and EGFP was replaced by mCherry). The Cherry-STIM1 construct was made in pcDNA6 by insertion of the mCherry sequence after aa 22 of human STIM1. The KE4/SLC39A7-V5 construct was a generous gift from K. Taylor (Cardiff University, Cardiff, United Kingdom).
Cell Lines and Transfection.
HEK 293 cells were transfected using Lipofectamine 2000 with 0.4–0.5 μg of DNA per 2 × 106 cells for imaging experiments or 10 μg of DNA per 107 cells for IP experiments. For siRNA-mediated POST knockdown, HEK 293 and HEK 705 cells were transfected with 20 nM siRNA using HiPerfect (Roche). A total of 3 × 106 Jurkat cells were nucleofected with 50 pmol of siRNA and Amaxa V solution (Lonza) using the S18 nucleofection protocol. HEK 293 and Jurkat cells stably expressing a tetracycline-dependent repressor (TR) were selected with blasticidin from cells transfected with pcDNA6TR (Invitrogen), and clonal cells with high TetR expression were further selected (HEK-TR and Jurkat-TR clones). HEK 293 cells stably overexpressing STIM1 were the generous gift of Donald Gill (Temple University, Philadelphia, PA). Jurkat cells stably expressing N-terminal tandem affinity purification (NTAP-Orai1) NTAP-Orai1 were obtained after transfection of Jurkat-TR cells with Orai1/ATC-TAP cDNA, selection of stable cells with 0.5 mg/mL Zeocin (invitrogen), and further selection of clonal cells expressing minimal background TAP-Orai1 and substantial tetracycline-induced expression. HEK 293-TR cells stably expressing POST-CTAP (HEK 705) were selected with 0.5 mg/mL Zeocin; after induction of protein expression with tetracycline, they were sorted by FACS for cells expressing GFP. TAP-Orai1 and POST-TAP protein expression was induced with 1 μg/mL tetracycline for 8–14 h.
TAP and MS.
A total of 109 Jurkat cells stably expressing NTAP-Orai1 were treated for 10 min at room temperature with 1 μM thapsigargin in Ca2+-free Ringer's solution (155 mM NaCl, 4.5 mM KCl, 3 mM MgCl2, 10 mM d-glucose, 5 mM Hepes, 1 mM EGTA) and lysed in buffer containing 10 mM Hepes, 150 mM NaCl, 1% Triton X-100, and protease inhibitor mixture (Pierce) at pH 7.5. TAP-Orai1 was purified by binding to immobilized IgG (Pierce) and eluted with TEV protease, followed by binding to immobilized calmodulin (Stratagene). The purified Orai1 complex eluted with EDTA in a final volume of 300 μL. A total of 3 × 108 POST-CTAP–expressing HEK 705 cells were store-depleted and lysed as described above for Jurkat cells. POST-CTAP protein was bound to immobilized HA mAb (Roche), and after vigorous washing with lysis buffer, bound proteins were eluted with HA peptide (1 mg/mL, 3 × 100 μL for 30 min at 37 °C). Eluted proteins were bound to immobilized streptavidin (Pierce) and extensively washed, and POST–TAP complexes were eluted with 3 × 100 μL of 3 mM biotin. Proteins of purified Orai1 and POST complexes were separated on SDS/PAGE, and protein bands were excised and in-gel–digested with trypsin. Dried peptides were dissolved in-matrix and subjected to MALDI-TOF MS and MS/MS analyses using a 4700 Proteomics analyzer and GPS software (Applied Biosystems). A combination of peptide mass fingerprinting and MS/MS analysis was performed using the Mascot search engine (Matrix Science). Collision-induced dissociation spectra were submitted for protein identification with a precursor precision tolerance of 1 Da and an MS/MS fragment tolerance of 0.5 Da.
Antibodies and IP.
Transfected cells were solubilized in lysis buffer [50 mM Tris-Cl (pH 8.0), 150 mM NaCl, 1% Triton X-100] supplemented with protease inhibitor mixture, immunoprecipitated with the specified antibody, and washed with lysis buffer. The rabbit TrueBlot system (eBiosciences) was used for endogenous Jurkat POST-Orai1 co-IP; POST and Orai1 antibody was generated in rabbits to human POST (aa 1–68) or human Orai1 (aa 1–62) fused to GST. Immune serum was depleted of anti-GST antibody with immobilized GST and antibody affinity-purified over immobilized POST (aa 1–68)-GST and Orai1 (aa 1–62)-GST fusion proteins, respectively. Anti-STIM1 antibody was generated in rabbits against a peptide corresponding to aa 657–685 of human STIM1 [characterized by Zhang et al. (13)] and affinity-purified. Commercially available antibodies were mouse STIM1 (BD Pharmingen); HA-HRP and HA affinity matrix (Roche); mouse V5 and V5-HRP (Invitrogen); RFP (Millipore AB3216); mouse SERCA2 mAb sc-53010; mouse Karyopherin-β1 (importin-β1) mAb sc-137016; goat CRM1 (exportin-1) sc-53010 (Santa Cruz); mouse Na/K-ATPase mAb (Pierce MA3-928); and pan-PMCA mouse mAb (Abcam ab2825).
Fluorescence Microscopy.
A custom-built objective-based TIRF microscope contained 488-nm and 532-nm lasers that were focused and transmitted via the rear illumination port of an Olympus IX70 inverted microscope (dual illuminator for laser-based TIRF). Digitally synchronized mechanical shutters controlled exposure times. Emitted fluorescent light was collected via a cooled-CCD camera (Hamamatsu 9100; SlideBook software; Olympus). Confocal images were acquired with a Fluoview-1000 (Olympus). For [Ca2+]I measurements, cells were loaded with 3 μM Fura-2 acetoxymethyl (AM) ester (Molecular Probes) at 22–25 °C for 30 min in culture medium, washed with fresh medium, and attached to poly-l-lysine–treated glass-bottomed Petri dishes. Thirty minutes before imaging, cells were washed with Ringer's solution [155 mM NaCl, 4.5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM d-glucose, 5 mM Hepes (pH 7.4)]. A Xenon light source filtered at 340 nm and 380 nm illuminated cells; emission (λ > 480 nm) was captured by a cooled CCD camera (Hamamatsu C4742-80-12AG; SlideBook software, Olympus). Background-corrected 340/380 ratio images were collected every 4.5 s.
Electrophysiology.
HEK 293T cells expressing STIM1, Orai1, and/or POST were patch-clamped using whole-cell recording methods. Internal solution contained 120 mM aspartic acid, 10 mM Cs4 (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid) BAPTA, 4 mM MgATP, 8 mM NaCl, and 10 mM Hepes (pH adjusted to 7.2 with CsOH). The Cs-Asp internal solution minimized background currents through K+ and Cl− channels present in HEK 293T cells. Internal BAPTA (10 mM) passively depleted Ca2+ stores and activated CRAC currents. The bath solution contained 105 mM sodium gluconate, 1 mM MgCl2, 2 or 20 mM CaCl2, 5 mM KCl, 10 mM glucose, 10 mM Hepes-acid, and 80 mM mannitol (pH 7.4). Currents were measured during 140-ms voltage ramps from −100 to +100 mV in 2-s intervals. Whole-cell access was obtained in the presence of 2 mM external Ca2+. Current traces obtained immediately after rupture were used for background subtraction as significant CRAC had yet to develop. Peak CRAC currents were obtained in 20 mM external Ca2+. Our background subtraction method could underestimate CRAC currents by up to 10% but should be similar across groups.
Supplementary Material
Acknowledgments
We thank Nikolai Skiba for help with MALDI-TOF analysis and Nat Blair for assistance with Ca2+ decay analysis.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117231108/-/DCSupplemental.
References
- 1.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu MM, Luik RM, Lewis RS. Some assembly required: Constructing the elementary units of store-operated Ca2+ entry. Cell Calcium. 2007;42:163–172. doi: 10.1016/j.ceca.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: Local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 10.Prakriya M, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 11.Vig M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeromin AV, et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang SL, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park CY, et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan JP, et al. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clapham DE. A STIMulus Package puts orai calcium channels to work. Cell. 2009;136:814–816. doi: 10.1016/j.cell.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Darbellay B, et al. STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J Biol Chem. 2009;284:5370–5380. doi: 10.1074/jbc.M806726200. [DOI] [PubMed] [Google Scholar]
- 18.Saitoh N, et al. Identification of functional domains and novel binding partners of STIM proteins. J Cell Biochem. 2011;112(1):147–156. doi: 10.1002/jcb.22910. [DOI] [PubMed] [Google Scholar]
- 19.Sampieri A, Zepeda A, Asanov A, Vaca L. Visualizing the store-operated channel complex assembly in real time: Identification of SERCA2 as a new member. Cell Calcium. 2009;45:439–446. doi: 10.1016/j.ceca.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 20.López JJ, et al. STIM1 regulates acidic Ca2+ store refilling by interaction with SERCA3 in human platelets. Biochem Pharmacol. 2008;75:2157–2164. doi: 10.1016/j.bcp.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Woodward OM, et al. Identification of a polycystin-1 cleavage product, P100, that regulates store operated Ca entry through interactions with STIM1. PLoS ONE. 2010;5:e12305. doi: 10.1371/journal.pone.0012305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh CM, Doherty MK, Tepikin AV, Burgoyne RD. Evidence for an interaction between Golli and STIM1 in store-operated calcium entry. Biochem J. 2010;430:453–460. doi: 10.1042/BJ20100650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srikanth S, et al. A novel EF-hand protein, CRACR2A, is a cytosolic Ca2+ sensor that stabilizes CRAC channels in T cells. Nat Cell Biol. 2010;12:436–446. doi: 10.1038/ncb2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang GN, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 25.Liao Y, et al. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci USA. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bautista DM, Hoth M, Lewis RS. Enhancement of calcium signalling dynamics and stability by delayed modulation of the plasma-membrane calcium-ATPase in human T cells. J Physiol. 2002;541:877–894. doi: 10.1113/jphysiol.2001.016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 28.Rigaut G, et al. A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotechnol. 1999;17:1030–1032. doi: 10.1038/13732. [DOI] [PubMed] [Google Scholar]
- 29.Bürckstümmer T, et al. An efficient tandem affinity purification procedure for interaction proteomics in mammalian cells. Nat Methods. 2006;3:1013–1019. doi: 10.1038/nmeth968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.