Abstract
Interaction between the mammalian cell polarity proteins mInsc (mammalian homologue of Inscuteable) and Leu-Gly-Asn repeat-enriched protein (LGN), as well as that between their respective Drosophila homologues Inscuteable and Partner of Inscuteable (Pins), plays crucial roles in mitotic spindle orientation, a process contributing to asymmetric cell division. Here, we report a crystal structure of the LGN-binding domain (LBD) of human mInsc complexed with the N-terminal tetratricopeptide repeat (TPR) motifs of human LGN at 2.6-Å resolution. In the complex, mInsc-LBD adopts an elongated structure with three binding modules—an α-helix, an extended region, and a β-sheet connected with a loop—that runs antiparallel to LGN along the concave surface of the superhelix formed by the TPRs. Structural analysis and structure-based mutagenesis define residues that are critical for mInsc–LGN association, and reveal that the activator of G-protein signaling 3 (AGS3)-binding protein Frmpd1 [4.1/ezrin/radixin/moesin (FERM) and PSD-95/Dlg/ZO-1 (PDZ) domain-containing protein 1] and its relative Frmpd4 interact with LGN via a region homologous to a part of mInsc-LBD, whereas nuclear mitotic apparatus protein (NuMA) and the C terminus of LGN recognize the TPR domain in a manner different from that by mInsc. mInsc binds to LGN with the highest affinity (KD ≈ 2.4 nM) and effectively replaces the Frmpd proteins, NuMA, and the LGN C terminus, suggesting the priority of mInsc in binding to LGN. We also demonstrate, using mutant proteins, that mInsc–LGN interaction is vital for stabilization of LGN and for intracellular localization of mInsc.
Keywords: cell signaling, X-ray crystallography
Cell polarization plays an essential role in asymmetric cell division, cell migration, and the proper function of various differentiated cell types (1). In the fruit fly Drosophila melanogaster, neurons and glial cells are produced by asymmetric division of neuroblasts that delaminate as single cells from apical-basal polarized neuroectoderm (2). The asymmetric division requires establishment of apical-basal polarity in neuroblasts; the Bazooka-containing protein complex is specifically localized to the apical cortex for polarity establishment. The mitotic spindle poles, normally arranged in parallel to the plane of the neuroectoderm, are also needed to be oriented along the apical-basal axis, which orientation is driven by another evolutionarily conserved protein complex containing Partner of Inscuteable (Pins), Mud, and the Gαi subunit of trimeric Gi proteins. The adaptor protein Inscuteable (Insc) simultaneously binds to Bazooka and Pins to provide a physical link between the two complexes; the link thereby couples cortical cell polarity and spindle orientation for asymmetric cell division.
Homologues of both Insc and Pins are known to exist exclusively in animals (from insects to mammals). The mammalian homologue of Insc (mInsc) also appears to contribute to mitotic spindle orientation and asymmetric cell division. Like Drosophila Insc, mInsc is capable of simultaneously interacting with the mammalian Bazooka-homologue Par3 and the mammalian Pins-related protein LGN [or activator of G-protein signaling 3 (AGS3)] (3–5). In symmetrically dividing cells, mInsc is distributed to the cortical area overlying the two mitotic spindle poles (4); LGN also localizes to the two cortical regions during mitosis (6, 7). On the other hand, in mouse embryo, mInsc and LGN colocalize at the apical cortex of epidermal progenitors that are dividing asymmetrically along their apical-basal axis (3), and mInsc modifies spindle orientation in the developing rat retina (4). Ectopic expression of mInsc facilitates LGN localization to the apical cortex and induces apical-basal spindle orientation in several epithelial cell types of mice (8–10), leading to an increase in asymmetric cell division (9, 10). Thus, the Insc protein appears to function together with its partner proteins in a manner conserved between Drosophila and mammals.
Pins and its related mammalian proteins LGN and AGS3 consist of the N-terminal domain comprising eight tetratricopeptide repeat (TPR) motifs and the C-terminal region harboring three or four Gαi/o–Loco interaction (GoLoco)/G-protein regulatory (GPR) motifs, each capable of binding to the Gαi subunit of trimeric G proteins (7, 11–13); it is noteworthy that the first TPR (TPR1) among the eight motifs has been often ignored in previous reports because TPR1 does not fit well to the TPR consensus. The TPR motif comprises 34 amino acid residues that adopt a helix-turn-helix structure; TPRs are generally packed into a superhelical domain to mediate protein–protein interactions (14–16). LGN binding to the Mud-related protein nuclear mitotic apparatus protein (NuMA) via the TPR domain is also considered to participate in mitotic spindle orientation (7). In addition, a mutation in the human LGN gene has been identified as cause for nonsyndromic deafness (17); ablation of AGS3 in mice leads to alterations in metabolic function (18) and impaired renal tubular regeneration after acute kidney injury (19). The mammalian Pins-related proteins thus contribute to a variety of biological events.
Although the amino acid sequence of LGN is about 50% identical to that of Pins, mInsc exhibits only about 20% identity to Drosophila Insc (3–5). Human mInsc contains 532 amino acid residues (5) with an N-terminal region highly homologous to a central domain—the asymmetry (or asymmetric localization) domain—of the fruit fly homologue (20, 21). Drosophila Insc of 859 residues has a long N-terminal extension that is dispensable for the functions of this protein (20, 21) and absent from the mammalian mInsc proteins. The central asymmetry domain of Drosophila Insc is responsible not only for apical cortical localization and mitotic spindle orientation along the apical-basal axis (20, 21) but also for interacting with Pins (12). In the interaction Drosophila Pins uses its N-terminal domain comprising TPRs. Intriguingly, mouse LGN also can bind to Drosophila Insc via the TPR domain, especially via TPRs 4–8, and functionally substitute for Pins in fruit fly neuroblasts (13). In the absence of Pins, Insc does not localize to the apical cortex of neuroblasts and thus fails to orient mitotic spindles (12), indicating that Insc–Pins interaction plays a crucial role in asymmetric cell division. However, the structural basis of the evolutionarily-conserved interaction has remained to be elucidated.
Here, we report the three-dimensional structure of the LGN-binding domain (LBD) of human mInsc (mInsc-LBD, residues 23–69), which is included in the region corresponding to the asymmetric localization module, in complex with the N-terminal TPR domain of human LGN (LGN-N, residues 13–414), determined by X-ray crystallography at 2.6-Å resolution. In the evolutionarily-conserved complex, mInsc-LBD adopts a structure containing an α-helix and an antiparallel β-sheet linked by an extended region. By structure-based mutagenesis, we define residues in mInsc and LGN that are critical for mInsc–LGN association. The AGS3-binding protein Frmpd1 [4.1/ezrin/radixin/moesin (FERM) and PSD-95/Dlg/ZO-1 (PDZ) domain-containing protein 1] (22) and its relative Frmpd4 bind to LGN via a region homologous to a part of mInsc-LBD, whereas NuMA and the C terminus of LGN recognize the TPR domain differently from mInsc. mInsc binds to LGN with the highest affinity (KD ≈ 2.4 nM) and effectively replaces not only the Frmpd proteins but also NuMA and the LGN C terminus, suggesting overlapping roles for the concave surface of LGN. We further demonstrate that mInsc–LGN interaction is crucial for LGN stabilization by mInsc and for mInsc localization to the cell cortex over the mitotic spindle poles in mitotic cells.
Results and Discussion
Overall Structure of the mInsc–LGN Complex.
In the present study, we determined the three-dimensional structure of the N-terminal TPR domain of human LGN (LGN-N, residues 13–414) and the LBD of human mInsc (mInsc-LBD, residues 23–69) (Fig. S1A), the latter of which locates in a region corresponding to the asymmetric localization domain of Drosophila Insc (20, 21). The crystal structure contains three individual complexes per asymmetric unit (Fig. S1 and Table S1). Because the mode of interaction among the three complexes in the asymmetric unit is highly similar except small differences probably due to crystal packing (Fig. S1), we discuss only one representative complex structure here. Although, in the crystal, an LGN-N makes direct contacts with another LGN-N, the interaction appears to be without biological relevance and thus attributed to crystal packing. This is because analytical ultracentrifugation revealed that LGN-N forms a 1∶1 heterodimer with mInsc-LBD in solution (Fig. S1), and because homotypic interaction of the isolated TPR domain or that of full-length LGN was not observed in cells coexpressing mInsc (Fig. S1).
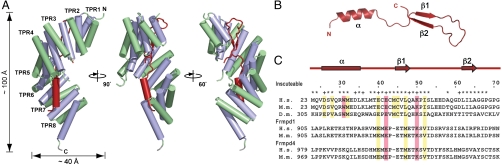
Amino acid sequence analysis by the tool TPRpred (23) predicts the presence of eight TPR motifs in LGN-N, albeit the first and fourth TPRs are atypical ones. In agreement with the prediction, in the mInsc–LGN complex, LGN-N indeed contains eight full TPRs with a C-terminal capping α-helix (Fig. 1A). In general, each TPR motif comprises a pair of antiparallel α-helices, designated as Aα and Bα; successive Aα and Bα helices form a right-handed superhelix with Aα-helices lining the inner concave groove and Bα-helices forming the outer convex surface (14–16). In LGN complexed with mInsc, the eight TPRs together with the capping helix (17 antiparallel α-helices in total) adopt a solenoid-like superhelix structure measuring about 100 Å in length and about 40 Å in diameter (Fig. 1A and Fig. S2). The α-helices in TPR2–8 are composed of 17 residues except those in TPR4 (Aα of 20 and Bα of 23 residues), although Aα and Bα of TPR1 compromise 13 and 14 residues, respectively, the lengths of which correspond to those of canonical TPR motifs.
Fig. 1.
Overall structure of the mInsc–LGN complex. (A) Cartoon representation of mInsc-LBD in complex with LGN-N. Three views of the complex are related by a 90° and 150° rotation about the LGN superhelical axis. mInsc-LBD is colored in red, Aα helices in LGN TPR in purple, and Bα helices in LGN TPR in green. (B) Ribbon diagram of mInsc-LBD from the complex structure. (C) Sequence alignment of mInsc-related proteins: human (H. s.) mInsc (AB236158) and mouse (M. m.) mInsc (DQ205645), and Drosophila (D. m.) Insc (CAA58175); human (AB023184) and mouse (AAI58061) Frmpd1; and human (AB002314) and mouse (AAI39430) Frmpd4. Secondary structure elements on mInsc-LBD are shown in red. Asterisks (*) indicate the residues located in the buried surface; plus characters (+) mark the residues with a side chain involved in a potential intermolecular salt bridge and/or a hydrogen bond with LGN residues. Residues boxed in magenta are ones crucial for the interaction with LGN, as determined by mutagenesis studies, and conserved residues are boxed in yellow.
In the mInsc–LGN complex, mInsc-LBD forms an elongated structure that runs antiparallel along the TPR groove, burying approximately 4,200 Å2 of the surface of LGN. mInsc-LBD can be roughly divided into three binding modules: an α-helix (residues 26–35), an extended region (residues 36–44), and an antiparallel β-sheet—the strands β1 (residues 45–48) and β2 (residues 62–65) are connected with a 13-residue loop (Fig. 1). These well-ordered modules form extensive hydrophobic and electrostatic interactions with the eight TPRs of LGN: Residues 26–35 of mInsc-LBD hold into the α-helix and travel in the groove, packing on the Aα helices of TPRs 6–8 of LGN; residues 36–44 continue to travel along the groove made up of the Aα helices of TPRs 4 and 5; and the antiparallel β-sheet and the loop between the β-strands fit into the groove formed by TPRs 1–3.
Interactions Between LGN and the α-Helix of mInsc-LBD.
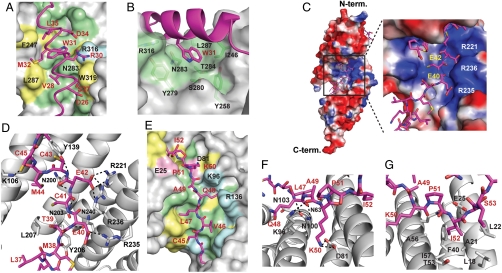
The N-terminal region of mInsc-LBD forms an α-helix spanning residues 26 to 35 (Fig. 2A). Trp31, an invariant residue (completely conserved from insects to mammals), and three hydrophobic residues in the α-helix, Val28, Met32, and Leu35, form a surface complementary to the LGN groove formed by TPRs 6–8 (Fig. 2A), which may stabilize the α-helical structure. Although Leu35 is replaced with glutamine in Drosophila Insc, both Val28 and Met32 are conserved between human mInsc and its Drosophila homologue: Val28 interacts with the invariant residues Asn283, Thr286, and Leu287 on TPR7; and Met32 with the conserved residues Ile246 and Phe247 on TPR6. Intriguingly, Trp31 in mInsc is encircled by many residues on LGN TPRs 6–8 (Ile246, Tyr258, Tyr279, Ser280, Asn283, Thr284, and Arg316), thereby making extensive interactions with them (Fig. 2A and Fig. S3). These residues are all conserved between mammalian and Drosophila Pins-related proteins (Fig. S2). Among them, LGN Asn283 may be particularly important because it forms many direct contacts with mInsc Trp31 (Fig. 2B). The N-terminal residue of the mInsc-LBD α-helix, Asp26, forms contacts with the side chains of Trp319 and Asn323 on TPR8 (Fig. 2A); Drosophila Insc also has an acidic residue (i.e., Glu) for the position of Asp26. Thus, the interaction via the α-helical region is likely conserved from Drosophila to mammals, in which the invariant tryptophan of Insc-related protein likely plays a major role.
Fig. 2.
Interactions between mInsc-LBD and LGN-N. (A) Surface representation showing the binding interface between LGN and the α-helix of mInsc-LBD. Residues in mInsc-LBD are labeled in red, and LGN residues in black. (B) Trp31 on the α-helix of mInsc-LBD (magenta) is encircled with many residues of LGN (green). (C) Electrostatic surface potentials of LGN are shaded blue in positively charged regions and red in negatively charged regions. Residues in the extended region of mInsc-LBD are labeled in yellow, and LGN residues in white. (D) Hydrogen bonds between the mInsc extended region and LGN are shown as dashed lines. (E) Surface representation showing the binding interface between LGN and a part of the mInsc β-loop-β module (from Cys45 to Ile52). (F) Hydrogen bonds between the mInsc β-loop-β module and LGN are shown as dashed lines. (G) Ile52 of mInsc is encircled by hydrophobic residues of LGN. Residues in mInsc-LBD and LGN are labeled in red and black, respectively, except in C. In A and E, LGN residues completely conserved among Pins-related proteins (Fig. S2) are colored as follows: the acidic residues in pink, basic residues in cyan, hydrophobic residues in yellow, or uncharged polar residues in green.
Interactions Between LGN and the Extended Region of mInsc-LBD.
The extended region of mInsc-LBD comprising residues Lys35 through Met44 interacts with the positively charged region on the concave surface of the LGN TPR superhelix (Fig. 2C). The main chains of mInsc Glu40, Glu42, and Cys43 form hydrogen bonds with the side chains of the LGN conserved residues Asn203, Asn200, and Tyr139, respectively (Fig. 2D); these interactions appear to stabilize the conformation of the mInsc extended region. The carboxy side chain of mInsc Glu42 makes salt bridges with both Arg221 on TPR5 and Arg236 on TPR6, whereas the side chain of Glu40 coordinates with that of Arg235 on TPR6 (Fig. 2D). Both the acidic residues of mInsc are completely conserved in LGN/Pins-binding regions of Insc-related proteins as well as in the AGS3-binding domain of Frmpd1 and its corresponding region of Frmpd4 (Fig. 1C). Consistent with this, the three basic residues Arg221, Arg235, and Arg236 are completely conserved among the mammalian Pins-related proteins LGN and AGS3, and Drosophila Pins (Fig. S2). These findings suggest that the extended region of Insc-related proteins recognizes their partners mainly via ionic interactions in an evolutionarily conserved manner.
Interactions Between LGN and the β-Loop-β Module of mInsc-LBD.
The β-sheet formed between the strands β1 (residues 45–48) and β2 (residues 62–65) is connected with a loop of residues 49–61. In the loop, residues 49–52 make contacts with TPRs 1–3 (Fig. 2E), although the subsequent region (residues 53–61) is solvent exposed. In particular, the ε amino group of the invariant residue Lys50 coordinates with the carboxy group of LGN Asp81 (Fig. 2F), which is conserved during evolution in Pins-related proteins. On the other hand, Ile52, an invariant residue among Insc-related proteins, is buried in a hydrophobic pocket formed by TPRs 1 and 2 (Fig. 2G); the isoleucine is replaced by valine in Frmpd1 and Frmpd4 (Fig. 1C). Residues in the region C-terminal to Ile52, including the strand β2, are not conserved in Frmpd1 or Frmpd4.
Mutational Analysis of mInsc–LGN Interaction.
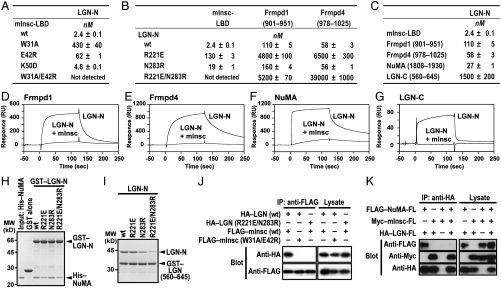
Key features of the mInsc-LBD–LGN-N structure appear to be maintained throughout evolution of animals (from insects to mammals): Residues involved in mInsc–LGN-N interaction are almost completely conserved (Fig. 1C and Fig. S2). This probably explains the reason why mouse LGN directly binds to Drosophila Pins and functionally replaces Pins in asymmetric cell division of Drosophila embryonic neuroblasts (13). Wild-type mInsc bound to LGN with an apparent KD value of approximately 2.4 nM (Fig. 3A and Fig. S4). Substitution of alanine for mInsc Trp31, an invariant residue in mInsc-related proteins, resulted in about 200-fold decrease in the affinity for LGN (KD of approximately 430 nM). Replacement of mInsc Glu42, another invariant residue, by arginine led to a slight but significant decrease in interaction with LGN (KD of approximately 60 nM). The combined substitutions for Trp31 and Glu42 completely abrogated the interaction (Fig. S5A). Thus, Trp31 in the α-helix and Glu42 in the extended region both play a crucial role in binding to LGN. Although the K50D substitution marginally affected mInsc–LGN interaction (Fig. 3A), the E42R/K50D double substitution more effectively inhibited the interaction than the E42R single substitution (Fig. S5B). Thus, the invariant residue Lys50 in the mInsc β-loop-β module appears to substantially contribute to the interaction with LGN.
Fig. 3.
Mutational analysis of mInsc–LGN interaction. (A–C) The KD values obtained from surface plasmon resonance analyses of LGN-N binding to the indicated protein. (D–G) Responses of a biosensor surface coupled with Frmpd1 (D), Frmpd4 (E), NuMA (F), or LGN-C (G) upon injection of LGN-N with or without 1.25 equivalent molar concentration of mInsc-LBD. (H) GST–LGN-N [wild-type (wt), R221E, N283R, or R221E/N283R] was incubated with His–NuMA-(1808–1930), and pulled down with glutathione-Sepharose beads. The precipitated proteins were subjected to SDS-PAGE and stained with Coomassie Brilliant Blue. Positions for marker proteins are indicated in kD. (I) GST–LGN-(560–645) was incubated with LGN-N (wt, R221E, N283R, or R221E/N283R), and analyzed as in H. (J and K) FLAG-tagged full-length mInsc was coexpressed with HA-tagged full-length LGN (J) in COS-7 cells, or FLAG-tagged full-length NuMA and HA-tagged full-length LGN were expressed with or without Myc-tagged full-length mInsc in COS-7 cells (K). Proteins of the cell lysates were immunoprecipitated (IP) with the indicated antibody, followed by immunoblot analysis (Blot) with the indicated antibody.
We next tested the role of LGN Arg221 on TPR5 and Asn283 on TPR7, which make direct contacts with the crucial residues Glu42 and Trp31 in mInsc, respectively. The R221E/N283R substitution abrogated LGN interaction with mInsc, although both of the single substitutions slightly affected the interaction (Fig. 3B and Fig. S5C). The significant role of these residues on TPR5 and TPR7 is also in agreement with the observation that TPRs 5–8 of LGN, but not TPRs 1–4, mainly contributes to the interaction with mInsc (Fig. S6A).
In addition, in vivo interaction between full-length mInsc and full-length LGN was disrupted by the W31A/E42R substitution in mInsc or by the R221E/N283R substitution in LGN (Fig. 3J). It is known that mInsc also binds to AGS3 (5), a protein closely related to LGN. Indeed, full-length mInsc interacted not only with ectopically expressed full-length AGS3 (Fig. S5D) but also with endogenous AGS3 (Fig. S5E). In both cases, the W31A/E42R substitution in mInsc led to a loss of the interaction, suggesting that mInsc recognizes AGS3 in a way similar to that of LGN.
Comparison of Binding Mode of mInsc with Those of Other LGN-Binding Proteins.
As shown in Fig. 1C, a part of mInsc-LBD exhibits a significant homology to a part of the AGS3-binding region of Frmpd1, a protein that may be involved in G-protein signaling by directly interacting with the AGS3 TPR domain (22), and also to a corresponding region of Frmpd4, an Frmpd1-related protein that is proposed to participate in dendritic spine morphogenesis (24). Indeed the mInsc-LBD–homologous regions of the Frmpd proteins bound to LGN but with lower affinities than mInsc (Fig. 3C and Fig. S4), and were effectively replaced with mInsc (Fig. 3 D and E). The R221E substitution in LGN almost completely abrogated the interaction with Frmpd1 or Frmpd4, whereas the N283R substitution did not affect the interaction (Fig. 3B and Fig. S5). This is in contrast with LGN interaction with mInsc, a severe disruption of which requires simultaneous substitution for Arg221 and Asn283 (Fig. 3B). This may be explicable by the fact that Frmpd1 and Frmpd4 lack a region corresponding to the mInsc-LBD α-helix, which contributes to interaction of mInsc with the C-terminal TPR domain of LGN. On the other hand, dual substitution of arginine for Glu922 and Glu924 in Frmpd1 resulted in a loss of the interaction with LGN, supporting the idea that Frmpd1 recognizes LGN at least partly in a manner similar to that by mInsc (Fig. S5H). Less inhibition was observed in the corresponding substitution in mInsc: The mutant mInsc carrying the E40R/E42R substitution in the extended region is still capable of strongly interacting with LGN probably via the N-terminal α-helix. In addition, the first β-strand and the subsequent four residues of mInsc, which make contacts with TPRs 1–3 of LGN, are conserved in Frmpd1 and Frmpd4 (Fig. 1C). These findings may explain why neither TPRs 1–4 nor TPRs 5–8 fully interacts with Frmpd1 and Frmpd4 (Fig. S6).
We next compared LGN-binding modes between mInsc and NuMA. The amino acid sequence of NuMA does not show any similarity to that of mInsc-LBD. The C-terminal region of NuMA (residues 1808–1930) bound to LGN-N with a KD of approximately 27 nM, a value of which is about tenfold higher than that of mInsc (Fig. 3C and Fig. S4). LGN TPRs 1–4 also interacted with NuMA, but to a lesser extent than TPRs 1–8; on the other hand, NuMA did not interact with TPRs 5–8 (Fig. S6D), which is in contrast to the finding that LGN binding to mInsc is largely dependent on TPRs 5–8. In addition, the R221E/N283R substitution in LGN only slightly attenuated NuMA–LGN interaction (Fig. 3H), although the mutation led to a complete loss of LGN binding to mInsc, Frmpd1, and Frmpd4. Thus NuMA appears to recognize LGN in a manner distinct from that by mInsc. On the other hand, interaction of NuMA with LGN was inhibited by mInsc both in vivo and in vitro (Fig. 3 F and K), indicating that the bindings of NuMA and mInsc to LGN are mutually exclusive.
The TPR domain of LGN has been reported to interact intramolecularly with the Goloco-motif-containing C-terminal region, which contributes to regulation of LGN (7). The intramolecular interaction was abrogated by the presence of mInsc (Fig. 3G) or by deletion of either TPRs 1–4 or TPRs 5–8 (Fig. S6). These properties are similar to those observed in LGN binding to Frmpd1 and Frmpd4. However, although binding to the Frmpd proteins was almost completely lost by the R221E substitution in LGN (Fig. 3B and Fig. S5), the same substitution did not affect the intramolecular interaction of LGN (Fig. 3I). Taken together, the TPR domain of LGN differently recognizes mInsc, NuMA, and the C-terminal region of LGN, whereas Frmpd1 and Frmpd4 interact with LGN via a region that exhibits homology to a part of mInsc-LBD.
Role of mInsc–LGN Interaction in Stabilization of LGN.
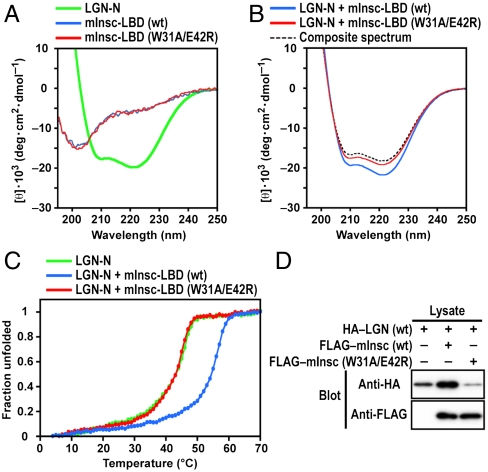
We next analyzed the structural effects of mInsc–LGN interaction using circular dichroism (CD) spectroscopy. As with other TPR proteins, LGN-N does show the potential to independently adopt α-helical structure (Fig. 4A). However, relative to either protein alone, the mInsc–LGN complex showed increased helical content (Fig. 4B). Similar CD spectra were observed in stabilization of the APC6 TPR domain, comprising eight TPR helices and a capping α-helix, by the CDC26 N-terminal region of 29 amino acid residues (25). Furthermore, thermal-denaturation profiles revealed that mInsc-LBD increases in denaturing temperature of LGN-N (Fig. 4C). The stabilizing effect was abrogated by the W31A/E42R substitution in mInsc, a mutation that leads to a loss of binding to LGN. Thus, mInsc-LBD probably confers structural stability upon LGN-N. Our crystallization trials of mInsc-unbound LGN-N were unsuccessful, which may be due to the flexible nature of this TPR domain in solution. CD spectra of the isolated mInsc-LBD suggest a random-coil conformation (Fig. 4A), which might be expected for a protein/domain that interacts with its binding partner in an elongated conformation.
Fig. 4.
Requirement of mInsc–LGN interaction for stabilization of LGN. (A) CD spectra for the N-terminal domain of LGN (green), wild-type mInsc-LBD (blue), or mInsc-LBD with the W31A/E42R substitution (red). (B) CD spectra for the mixture of LGN-N and mInsc-LBD (wt) (blue) and the mixture of LGN-N and mInsc-LBD (W31A/E42R) (red). The theoretical curve assuming no interaction between the two proteins is shown as dashed line (black). (C) Thermal-denaturation profiles for LGN-N (green), the mixture of LGN-N and mInsc-LBD (wt) (blue), and the mixture of LGN-N and mInsc-LBD (W31A/E42R) (red). (D) mInsc–LGN interaction stabilizes LGN in COS-7 cells. HA-tagged full-length LGN was coexpressed with or without FLAG-tagged full-length mInsc (wt) or its mutant protein (W31A/E42R) in COS-7 cells. Proteins of the cell lysates (Lysate) were subjected to immunoblot analysis (Blot) with the anti-HA or anti-FLAG antibody.
Coexpression of mInsc drastically enhanced the content of LGN at the protein level in COS-7 cells (Fig. 4D), suggestive of mInsc-mediated stabilization of LGN. On the other hand, a mutant mInsc (W31A/E42R), incapable of binding to LGN, failed to stabilize the LGN protein. These findings indicate that mInsc stabilizes LGN in vivo via the direct interaction.
Role of mInsc–LGN Interaction in mInsc Localization in Mammalian Cells.
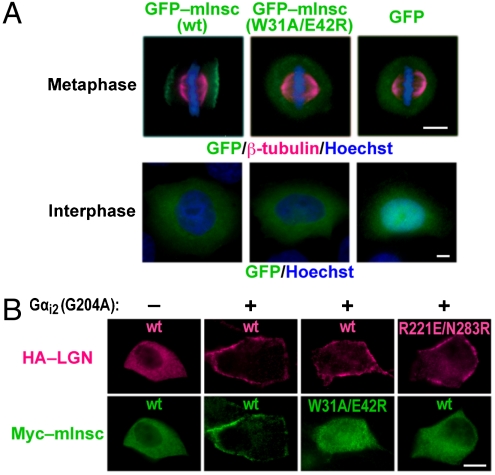
Although mInsc is reported to localize during asymmetric cell division to the apical cortex of several cell types in mammalian embryos (8–10), it is also known that mInsc is recruited to the cortex of symmetrically dividing cells in culture (4). To analyze the subcellular localization of mInsc during mitosis, we transfected HeLa cells with a functional GFP-tagged form of human mInsc that retained the ability to bind to endogenous LGN (Fig. S5I). Although GFP–mInsc was uniformly cytoplasmic in interphase cells, the protein translocated during metaphase to the plasma membrane and concentrated in the cortical area overlying the two spindle poles (Fig. 5A), which distribution is consistent with that observed in NIH/3T3 mouse fibroblasts (4). mInsc (W31A/E42R), defective in binding to LGN, failed to move to the cortical area, indicating a crucial role of mInsc–LGN interaction.
Fig. 5.
Requirement of mInsc–LGN interaction for localization of mInsc. (A) HeLa cells expressing GFP alone, GFP–mInsc (wt), or GFP–mInsc (W31A/E42R) were fixed and stained using the anti-β-tubulin antibody (magenta), Hoechst (blue), and GFP fluorescence (green). Scale bar, 5 μm. (B) MDCK cells were transfected with the expression plasmids for HA-LGN (wt or R221E/N283R), Myc–mInsc (wt or W31A/E42R), and/or Gαi2 (G204A). The cells were fixed and stained with the anti-HA and anti-Myc antibodies. Scale bar, 10 μm.
It is also known that overexpression of the Gα subunit of the Gi-family trimeric G proteins in Madin–Darby canine kidney (MDCK) cells leads to a recruitment of LGN to the cell cortex even at interphase (7). This is because LGN directly binds via the C-terminal GoLoco/GPR motifs to the plasma-membrane-associated protein Gαi in a GDP-bound form. Consistent with this, in cells expressing Gαi2 (G204A), a mutant protein in a constitutively GDP-bound form (26), LGN and its binding partner mInsc were both present at the cell cortex (Fig. 5B). The localization of mInsc was lost when mInsc–LGN interaction was disrupted by the W31A/E42R substitution in mInsc or by the R221E/N283R substitution in LGN. Thus, correct localization of mInsc requires its interaction with LGN.
Concluding Remarks
Here, we report a crystal structure of the mInsc–LGN complex at 2.6-Å resolution. In the evolutionarily conserved complex, mInsc-LBD runs along the groove formed by the LGN TPR motifs, adopting an elongated structure comprising three distinct modules: an α-helix, an extended region, and an antiparallel β-sheet connected by a loop. Thus, mInsc-LBD, albeit intrinsically disordered, becomes folded into a highly ordered conformation with both α-helix and β-sheet, a structure that is not found in other targets on the concave surface of TPR domains. Structural and mutational analyses define residues critical for mInsc–LGN association: Residues in the α-helix and the extended region play more important roles than those in the β-loop-β module. AGS3 appears to bind to mInsc in a manner similar to that by LGN, which is consistent with the almost complete conservation of residues involved in the interaction with mInsc.
The present study also reveals that the LGN TPR domain directly binds to Frmpd1 and its related protein Frmpd4 in a manner similar to but distinct from that by mInsc. The Frmpd proteins lack the α-helix of mInsc-LBD but interact with LGN via a region that is homologous to the extended region and a part of the β-loop-β module, consisting of the first β-strand and its C-terminal flanking region. Experiments using mutant proteins suggest that NuMA and the C-terminal region of LGN bind to the LGN TPR domain in a fashion different from that by mInsc and Frmpd proteins. Because mInsc replaces the LGN C terminus in binding to the TPRs, the intramolecular interaction in LGN is not expected to prevent TPR binding to mInsc; indeed, full-length LGN is capable of associating with mInsc. It is considered that mInsc tethers the two evolutionarily conserved complexes, one comprising Par3, Par6, and aPKC and the other containing LGN and Gαi (Fig. S6F), which agrees with the present finding that membrane-bound Gαi recruits mInsc to the membrane in an LGN-dependent manner. Entry of NuMA, a protein crucial for spindle orientation, into the LGN–Gαi complex may lead to their release from the mInsc–Par network, because bindings of NuMA and mInsc to LGN are mutually exclusive. The competition may regulate spindle orientation during mitosis.
Furthermore, we show that mInsc–LGN interaction increases heat stability of the LGN TPR domain, and it also stabilizes LGN at the cellular level. It is known that, in symmetrically dividing cells, mInsc is recruited to the cell cortex overlying the two mitotic spindle poles. The recruitment requires mInsc binding to LGN, as clearly shown in the present study. Future studies should be addressed to clarify the role of mInsc–LGN interaction in other mInsc-mediated biological events thus-far unidentified.
Materials and Methods
Crystallization and structural determination of the mInsc-LBD–LGN-N complex were performed according to protocols used for other proteins as previously described (27, 28) with minor modifications. The coordinates and structure factors have been deposited in the Protein Data Bank (PDB) with the PDB ID code 3SF4. Detailed description of crystallization, structural determination, and other experimental procedures are provided in the SI Materials and Methods.
Note Added in Proof.
While this paper was under revision, the paper by Zhu et al. (29) was published.
Supplementary Material
Acknowledgments.
We thank D. Kohda (Kyushu University) for helpful advice and discussion; M. Horiuchi and F. Inagaki (Hokkaido University) for technical advice; D.A. Compton (Dartmouth Medical School) for generously providing the cDNA for human NuMA; N. Morinaga, N. Kubo, and Y. Kage, and M. Nishinou for technical and secretarial assistance. The experiments at the Photon Factory were approved by the High Energy Accelerator Research Organization as proposal 2010G662. This work was supported in part by Grants-in-Aid for Scientific Research and Targeted Proteins Research Program from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110951108/-/DCSupplemental.
Data deposition: The atomic coordinates and structure factors of the mInsc–LGN complex have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3SF4).
References
- 1.St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: Parallels and diversity. Cell. 2010;141:757–774. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Siller KH, Doe CQ. Spindle orientation during asymmetric cell division. Nat Cell Biol. 2009;11:365–374. doi: 10.1038/ncb0409-365. [DOI] [PubMed] [Google Scholar]
- 3.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Žigman M, et al. Mammalian Inscuteable regulates spindle orientation and cell fate in the developing retina. Neuron. 2005;48:539–545. doi: 10.1016/j.neuron.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 5.Izaki T, Kamakura S, Kohjima M, Sumimoto H. Two forms of human Inscuteable-related protein that links Par3 to the Pins homologues LGN and AGS3. Biochem Biophys Res Commun. 2006;341:1001–1006. doi: 10.1016/j.bbrc.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 6.Kaushik R, Yu F, Chia W, Yang X, Bahri S. Subcellular localization of LGN during mitosis: Evidence for its cortical localization in mitotic cell culture systems and its requirement for normal cell cycle progression. Mol Biol Cell. 2003;14:3144–3155. doi: 10.1091/mbc.E03-04-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du Q, Macara IG. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell. 2004;119:503–516. doi: 10.1016/j.cell.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 8.Konno D, et al. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- 9.Poulson ND, Lechler T. Robust control of mitotic spindle orientation in the developing epidermis. J Cell Biol. 2010;191:915–922. doi: 10.1083/jcb.201008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams SE, Beronja S, Pasolli HA, Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takesono A, et al. Receptor-independent activators of heterotrimeric G-protein signaling pathways. J Biol Chem. 1999;274:33202–33205. doi: 10.1074/jbc.274.47.33202. [DOI] [PubMed] [Google Scholar]
- 12.Yu F, Morin X, Cai Y, Yang X, Chia W. Analysis of partner of inscuteable, a novel player of Drosophila asymmetric divisions, reveals two distinct steps in inscuteable apical localization. Cell. 2000;100:399–409. doi: 10.1016/s0092-8674(00)80676-5. [DOI] [PubMed] [Google Scholar]
- 13.Yu F, et al. A mouse homologue of Drosophila pins can asymmetrically localize and substitute for pins function in Drosophila neuroblasts. J Cell Sci. 2003;116:887–896. doi: 10.1242/jcs.00297. [DOI] [PubMed] [Google Scholar]
- 14.Das AK, Cohen PW, Barford D. The structure of the tetratricopeptide repeats of protein phosphatase 5: Implications for TPR-mediated protein-protein interactions. EMBO J. 1998;17:1192–1199. doi: 10.1093/emboj/17.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Andrea LD, Regan L. TPR proteins: The versatile helix. Trends Biochem Sci. 2003;28:655–662. doi: 10.1016/j.tibs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Kulkarni K, Hanrahan SJ, Thompson AJ, Barford D. The APC/C subunit Cdc16/Cut9 is a contiguous tetratricopeptide repeat superhelix with a homo-dimer interface similar to Cdc27. EMBO J. 2010;29:3733–3744. doi: 10.1038/emboj.2010.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh T, et al. Whole exome sequencing and homozygosity mapping identify mutation in the cell polarity protein GPSM2 as the cause of nonsyndromic hearing loss DFNB82. Am J Hum Genet. 2010;87:90–94. doi: 10.1016/j.ajhg.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumer JB, et al. Activator of G protein signaling 3 null mice: I. Unexpected alterations in metabolic and cardiovascular function. Endocrinology. 2008;149:3842–3849. doi: 10.1210/en.2008-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regner KR, et al. Loss of activator of G-protein signaling 3 impairs renal tubular regeneration following acute kidney injury in rodents. FASEB J. 2011;25:1844–1855. doi: 10.1096/fj.10-169797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knoblich JA, Jan LY, Jan YN. Deletion analysis of the Drosophila Inscuteable protein reveals domains for cortical localization and asymmetric localization. Curr Biol. 1999;9:155–158. doi: 10.1016/s0960-9822(99)80070-0. [DOI] [PubMed] [Google Scholar]
- 21.Tio M, Zavortink M, Yang X, Chia W. A functional analysis of inscuteable and its roles during Drosophila asymmetric cell divisions. J Cell Sci. 1999;112:1541–1551. doi: 10.1242/jcs.112.10.1541. [DOI] [PubMed] [Google Scholar]
- 22.An N, Blumer JB, Bernard ML, Lanier SM. The PDZ and band 4.1 containing protein Frmpd1 regulates the subcellular location of activator of G-protein signaling 3 and its interaction with G-proteins. J Biol Chem. 2008;283:24718–24728. doi: 10.1074/jbc.M803497200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karpenahalli MR, Lupas AN, Söding J. TPRpred: A tool for prediction of TPR-, PPR- and SEL1-like repeats from protein sequences. BMC Bioinformatics. 2007;8:2. doi: 10.1186/1471-2105-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HW, et al. Preso, a novel PSD-95-interacting FERM and PDZ domain protein that regulates dendritic spine morphogenesis. J Neurosci. 2008;28:14546–14556. doi: 10.1523/JNEUROSCI.3112-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Dye BT, Rajashankar KR, Kurinov I, Schulman BA. Insights into anaphase promoting complex TPR subdomain assembly from a CDC26-APC6 structure. Nat Struct Mol Biol. 2009;16:987–989. doi: 10.1038/nsmb.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu F, et al. Locomotion defects, together with Pins, regulates heterotrimeric G-protein signaling during Drosophila neuroblast asymmetric divisions. Genes Dev. 2005;19:1341–1353. doi: 10.1101/gad.1295505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuzawa S, et al. Structural basis for activation of the receptor tyrosine kinase KIT by stem cell factor. Cell. 2007;130:323–334. doi: 10.1016/j.cell.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 28.Yuzawa S, et al. A molecular mechanism for autoinhibition of the tandem SH3 domains of p47phox, the regulatory subunit of the phagocyte NADPH oxidase. Genes Cells. 2004;9:443–456. doi: 10.1111/j.1356-9597.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, et al. LGN/mInsc and LGN/NuMA complex structures suggest distinct functions in asymmetric cell division for the Par3/mInsc/LGN and Gαi/LGN/NuMA pathways. Mol Cell. 2011;43:418–431. doi: 10.1016/j.molcel.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.