Abstract
CD4 T cells that acquire cytotoxic phenotype and function have been repeatedly identified in humans, mice, and other species in response to many diverse pathogens. Since CD4 cytotoxic T cells are able to recognize antigenic determinants unique from those recognized by the parallel CD8 cytotoxic T cells, they can potentially contribute additional immune surveillance and direct effector function by lysing infected or malignant cells. Here, we briefly review much of what is known about the generation of cytotoxic CD4 T cells and describe our current understanding of their role in antiviral immunity. Furthering our understanding of the many roles of CD4 T cells during an anti-viral response is important for developing effective vaccine strategies that promote long-lasting protective immunity.
1. Introduction
CD4 T cells are well known for their helper roles including those that promote antibody class switching, enhancing the development of cytotoxic T lymphocyte (CTL) activity of CD8 T cells and their ability to be functional memory cells, as well as inducing the phagocytic activity of innate immune cells to name a few (Figure 1). To perform these important roles, CD4 T cells differentiate into unique effector helper subsets characterized by their expression of specific cytokines and transcription factors as outlined in Figure 2. A lesserknown role for CD4 T cells, however, is their ability to acquire cytotoxic activity and directly kill infected, transformed, or allogeneic MHC class II+ (class II) cells. Cytotoxic CD4 T cells (ThCTL) identified by cytotoxic phenotype and/or function have been repeatedly identified over the past three decades and shown to recognize a diversity of pathogens. ThCTL were once thought to be an anomaly associated with long-term in-vitro culturing of CD4 T cell lines and clones generated from both humans [1–4] and mice [5, 6]. However, ThCTL have also been identified in the peripheral blood mononuclear cells (PBMCs) of humans seropositive for chronic viral infections including human cytomegalovirus (HCMV) [7–10], hepatitis viruses [11], and human immunodeficiency virus 1 (HIV-1) [7, 12, 13]. ThCTL have also been identified in mice infected with chronic viruses including lymphocytic choriomeningitis virus (LCMV) [14] and gamma-herpes virus [15]. The generation of ThCTL, however, is not just restricted to conditions of chronic antigen stimulation or chronic viral stimulation, as we have also identified ThCTL in the lungs of mice 7 days following primary infection with influenza virus A (unpublished results). Despite these observations, there is still much we do not know about ThCTL, including the specific events that occur during infection that induce the acquisition of cytotoxic function, and whether ThCTL can play a significant protective role during an antiviral immune response. In particular, we can postulate that ThCTL could aid in viral clearance and the fact that they and CD8 T cells recognize distinct epitopes could make selection of viral variants much less likely. Moreover, ThCTL may have different properties that make them less inflammatory including a faster contraction and secretion of cytokines and chemokines that promote repair, although this has not been established. As we analyze the potential of ThCTL to enhance antiviral immunity, we will want to evaluate these possibilities.
Figure 1.
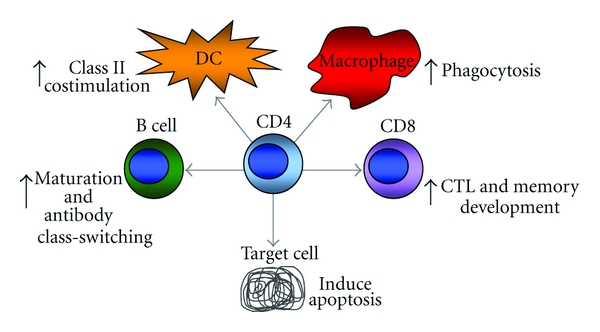
The many roles of CD4 T cells in promoting antiviral immunity multiple direct and indirect cellular interactions with CD4 T cells promotes antiviral immunity. CD4 T cells can promote affinity maturation and antibody class switching by B cells, enhance antigen presentation and costimulation of dendritic cells (DC), induce the phagocytic activity of macrophages, and promote the development of CD8 T cells into cytotoxic T lymphocytes (CTLs) and functional memory cells. A lesser known role for CD4 T cells is the development of cytotoxic activity directed against infected target cells.
Figure 2.
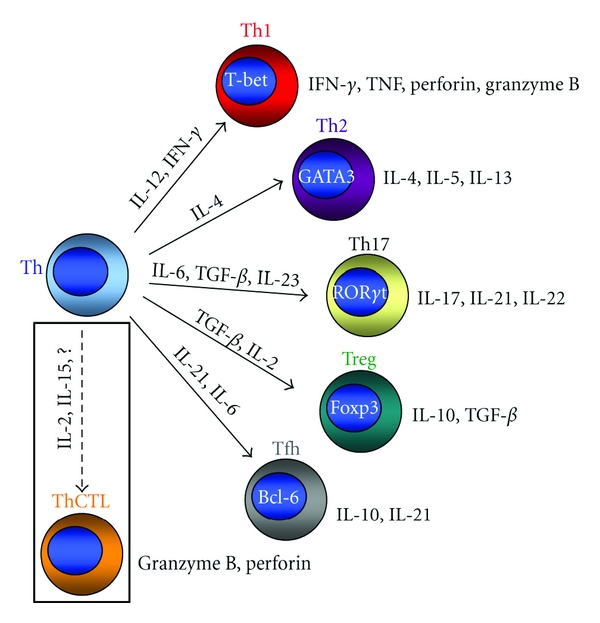
CD4 T cell effector subsets A CD4 T cell (Th) can differentiate into unique effector subsets determined in part by the cytokine milieu that is present when the cell encounters antigen. Effector subsets are classified by the dominant transcription factor in concert with the cytokines that they express. Th cells that develop optimal cytotoxic activity (ThCTL) may be distinct from the other identified effector subtypes.
2. Class II-Restricted Cytotoxic Activity
Unlike CD8 CTL that recognize cognate antigen in the context of ubiquitously expressed MHC class I molecules, the cytotoxic function of ThCTL is restricted to class II antigen-presenting cells (APCs) such as the professional APCs that include dendritic cells, macrophages, and B cells, as well as a number of infected tissue types. One of the most extensively studied ThCTL subsets in humans are those generated against Epstein Barr Virus (EBV), a herpes virus typically harbored in latent form by B cells. ThCTL have been found to recognize both lytic and latent EBV class II antigens presented by conventional and transformed B cells [16–19]. ThCTL have also been identified in HIV-1 seropositive individuals [7, 12], a lentivirus that infects professional APCs and CD4 T cells that can also express class II upon activation in humans but not in mice [20–22]. CD4 T cell clones from macaques infected with another lentivirus, Simian immunodeficiency virus (SIV), were found to eliminate SIV-infected macrophages but not infected CD4 T cells in-vitro [23]. Also, CD4 T cells from the PBMCs of individuals vaccinated against poliovirus were shown to kill poliovirus-infected macrophages and dendritic cells [24]. Other nonprofessional APCs that harbor viruses and support their replication can also express class II such as the epithelial cells that line the respiratory tract that are infected by viruses such as influenza and parainfluenza, as well as endothelial cells and glial cells which can harbor CMV. ThCTL clones derived from HCMV-seropositive individuals were shown to kill glial cells expressing HCMV glycoprotein B [25] and lympoblastoid cell lines expressing CMV antigen [26].
In-vivo class II-restricted killing has also been demonstrated in virally infected mice. First shown by Jellison et al., splenocytes pulsed with LCMV GP61 class II peptide and not irrelevant peptide were specifically eliminated in LCMV-infected mice [14]. This was also shown for spleen cells pulsed with influenza HA126 peptide in influenza-infected mice [27], and spleen cells pulsed with West Nile Virus (WNV) NS31616 peptide in WNV-infected mice [28]. Taken together, the majority of studies that have measured the cytotoxic activity of ThCTL have either used peptide-pulsed transformed B cells or splenocytes as target cells, hence we still know very little about the diversity of target cell types that human or mouse ThCTL can recognize and kill. Also unknown is whether there are key attributes of infected target cells that promote ThCTL generation and what host and viral factors favor their induction.
3. Role for ThCTL When CD8 CTL Are Impaired
One of the challenges of identifying ThCTL in situ is that they are overshadowed and seemingly inhibited by the presence of CD8 CTL which outnumber them [29, 30]. Thus it is not surprising that ThCTL have also been identified in virally infected mice deficient in, or depleted of, CD8 T cells. Stuller and Flaño showed specific killing of spleen cells pulsed with γHV68 in gamma herpes virus-infected mice depleted of CD8 T cells [15]. In beta-2 microglobulin KO (β2m−/−) mice that lack most class I MHC expression and hence have very few CD8 T cells and NK cells, ThCTL were identified in the respiratory tract during Sendai parainfluenza virus infection [31], and also in β2m−/− mice infected with LCMV [32, 33]. ThCTL can also arise from naïve TCR transgenic CD4 T cells transferred to a lymphopenic host and kill adoptively transferred splenocytes presenting cognate pepide (unpublished results). Moreover, lymphopenia-induced ThCTL have been shown to eradicate melanoma tumors in mice [34, 35], highlighting a potential therapeutic role for ThCTL in antitumor immunity.
ThCTL could also play a role in compensating for age-related decline in CD8 CTL activity or when CD8 CTL activity is impaired. Interestingly, Zhou and McElhaney found that the cytolytic function of influenza-specific CD4 T cells was preserved in aged individuals at least 10 weeks postvaccination, however, the CD8 CTL response was significantly impaired within 4 weeks postvaccination compared to younger individuals [36]. Moreover, ThCTL may be found to play a prominent role in antiviral responses to pathogens that have evolved mechanisms to evade detection by CD8 CTL. Many viruses including HIV-1, HSV, and HCMV employ mechanisms that inhibit normal MHC class I antigen processing and presentation [37, 38], enhancing their ability to evade detection by CD8 CTL. During the latent stage of infection, EBV expresses a limited number of proteins including Epstein-Barr Nuclear Antigen 1 (EBNA1) that is expressed in B cells as well as the EBV-associated malignancy, Burkitt's lymphoma (BL). EBNA1 inhibits its own degradation and translation thus preventing presentation of EBNA1 antigens on MHC class I molecules [39, 40]. Interestingly, EBNA1 does gain access to endogenous MHC class II processing by the process of autophagy [16, 41]. EBNA1 contains a dominant EBV CD4 T cell epitope, and EBNA1-specific ThCTL have been shown to kill BL cells [42, 43]. As ThCTL have been repeatedly described in EBV carriers, one may speculate they play a role in controlling EBV infection, or control the development of EBV-associated malignancies. Interestingly, the loss of EBV-specific functional CD4 T cells has been found to correlate with the development of EBV-associated lymphomas in some individuals [44–46].
4. ThCTL Cytotoxic Effector Mechanisms
The cytotoxic effector mechanisms reported thus far to be utilized by ThCTL are shared with those used by CD8 CTL including cytotoxic granule exocytosis and Fas/Fas ligand- (FasL-) mediated apoptosis. The cytotoxic mechanism considered the dominant mechanism utilized by ThCTL is the directed exocytosis of cytotoxic granules into a target cell to induce apoptosis. Contained within these granules are granzymes that are serine esterases that target various substrates to initiate and mediate cellular apoptosis [47]. The granzymes gain intracellular access into target cells with the help of the protein perforin that polymerizes to form channel pores through the plasma membrane [48]. Perforin-mediated cytolytic activity has been suggested for virus-specific ThCTL clones that recognized peptides derived from EBV latent membrane proteins [49], HIV-1 gag protein [50], poliovirus [24], and dengue virus capsid protein [51]. Granulysin, an enzyme with both cytolytic and antimicrobial properties, is also found in cytotoxic granules in NK cells and activated T cells in humans [52]. Granulysin was found to play an important role in the killing of the yeast Cryptococcus neoformans by ThCTL [53]. No homolog for granulysin has been identified in mouse. The binding of Fas receptor on target cells to FasL expressed on effector cells initiates the formation of a death-inducing signaling complex and induction of target cell apoptosis [54]. Expression of Fas on target cells has been shown to lend them susceptible to lysis by ThCTL [55]. Furthermore, a lack of Fas expression on target cells impairs killing by ThCTL generated in mice infected with LCMV [14, 33] and WNV [28]. A third cytotoxic mechanism we should mention is the induction of apoptosis via the binding of TNF-related apoptosis-inducing ligand (TRAIL) to death receptors DR4 and DR5 expressed on target cells. T cells have been shown to induce apoptosis of bystander cells via this pathway, however, the relevance of this apoptotic pathway for class II-mediated killing is not yet known.
Differential regulation of cytotoxic gene expression in CD8 T cells compared to CD4 T cells has been described. Unlike CD4 T cells, single positive CD8 thymocytes express perforin mRNA transcript [56] and likely maintain this expression once they emigrate out into the periphery, while CD4 T cells require activation through their T cell receptor to induce perforin expression [57, 58]. CD4 T cells also require TCR stimulation to upregulate their expression of granzyme B [59]. We also found that naïve mouse CD8 T cells cultured with IL-2 for 72 hours expressed increased granzyme B protein, however, CD4 T cells required the addition of TCR activation (N. B. Marshall, S. L. Swain; unpublished observations). Thus it is possible that CD8 T cells are better primed for the immediate development of cytotoxic function when in a naïve state, while further stimuli are necessary to convert CD4 T cells into killers. Whether the CTL program in CD4 T cells once activated is maintained into memory has not to our knowledge been reported, but this is clearly of interest. If it is, it would support the concept that the CD4 T cells that become cytotoxic represent a separate polarized functional subset.
5. ThCTL Phenotype
Degranulation is required for perforin- and granzyme-mediated killing of target cells. The core of the cytotoxic granules contains the effector proteins granzymes and perforin; the membrane of the cytotoxic granules contains lysosomal-associated membrane glycoproteins (LAMPs) including CD107a (LAMP-1) and CD107b (LAMP-2) [60]. During degranulation, the granule membranes merge with the outer cellular membrane, thus the transient transfer of LAMP proteins onto the surface of a cell is currently considered to be a marker of degranulation. Nemes et al. found that approximately half of HIV-1 gag-specific CD4 T cells in infected individuals expressed CD107a [61]. Casazza et al. identified CD107a+ perforin+ granzyme+ CD4 T cells in HCMV-seropositive individuals with cytolytic activity [62]. CD107a+ ThCTL have also been identified in mice infected with gamma herpes virus [63] and in mice infected with influenza virus A (unpublished results). ThCTL are thus currently identified by the coexpression of perforin, granzyme B, and surface expression of CD107a. One of the challenges of identifying ThCTL in mice, however, is the lack of a reliable antibody that can be used to stain for perforin expression in mouse cells [58].
In individuals with chronic viral infections, ThCTL have a phenotype consistent with that of differentiated effector T cells including the loss of expression of the TNF receptor family member, CD27, and the costimulatory molecule, CD28. However, CD28neg CD27neg perforin+ ThCTL were identified in the PBMCs of HIV-1 seropositive individuals [12], and in individuals with viral hepatitis [11]. In HCMV-seropositive individuals, ThCTL that expressed granzyme B, perforin, and CD107a were CD27neg and CD28neg [8, 62], and they were further identified to coexpress the killer lectin-like receptor (KLR) NKG2D [9]. Engagement of NKG2D has been shown to activate the cytolytic activity of NK cells and gamma delta T cells [64–66]. A role for NKG2D or other KLRs in ThCTL cytotoxic activity is not yet known. Consistent with the phenotype of ThCTL in humans, ThCTL in mice infected with gamma herpes virus were also found to be CD27neg [15]. EBV-specific CD4 T cells, however, were both CD27+ and CD28+ in EBV-seropositive individuals [67]. We have also found that ThCTL generated in the lungs of mice during primary influenza virus A infection are CD27+/- [68] and CD28+ (unpublished results). Thus the expression of CD27 and CD28 by virus-specific ThCTL is determined by the particular viral response which is also true for CD8 T cells [69]. Also, the anatomical site in which ThCTL are generated may help to determine their unique signature phenotype as is the case for other types of effector CD4 and CD8 T cells. For example, ThCTL generated in the lungs of influenza-infected mice express high levels of CD103, a receptor for epithelial cadherin, which is not expressed by the CD4 T cells found in the spleen and draining lymph nodes (unpublished results). Thus phenotypic variation of ThCTL across different anatomical sites and pathogenic infections is expected.
6. ThCTL Generation
Although CD4 T cells with cytotoxic activity have been identified within Th0- [70–72], Th2- [73, 74], and Treg- [75] effector T cell subsets, ThCTL are most often described as being Th1-like, characterized by the production of IFN-γ or as polyfunctional with production of some combination of the cytokines IFN-γ, TNF-alpha, and IL-2 [8, 12, 28, 49, 51, 62, 76]. Th1-polarized or Th1-like CD4 T cells are known to demonstrate cytotoxic activity [27, 43, 72, 77], however, the expression of these cytokines is not required for their cytotoxic function [14, 27, 31, 63]. Th1-polarizing conditions include the presence of IL-12 and neutralizing anti-IL-4 antibody which, respectively, have been shown to enhance (IL-12) and inhibit (IL-4) cytotoxic activity. IL-12 enhances cytotoxic activity by increasing expression of perforin and granzymes through the activation of STAT4 [78–81]. Conversely, IL-4, which activates STAT6, has been shown to inhibit the cytotoxic activity of in-vitro generated ThCTL [72].
Th1-polarizing conditions, however, are not the optimal conditions for generating ThCTL. CD4 T cells differentiated under Th1-polarizing conditions do express perforin, however, the transcript levels are still >20-times less than that of activated CD8 T cells [82]. The cytotoxic activity of CD4 T cells is further enhanced when polarized under Th0 conditions in the presence of IL-2 [72]. For CD8 CTL, IL-2 has been shown to increase the expression of perforin and granzymes and enhance cytotoxic activity [83, 84]. The activation of STAT5 by IL-2 has been shown to enhance perforin expression in CD8 CTL [85], and also in EBV-specific ThCTL clones [57]. IL-2 also strongly induces the expression of the transcription factor, Eomesodermin (Eomes) [86]. The transcription factors Runx3 and Eomes promote CTL development and are sufficient to induce IFN-gamma, perforin, and granzyme B expression [87, 88]. Eomes was required for the upregulation of granzyme B expression in cytotoxic Th1 cells responding to staphylococcal enterotoxin A in the presence of agonist antibodies to CD134 (OX40) and CD137 (4-1BB) [89]. The requirement for Eomes in ThCTL development and cytotoxic activity is still not yet well established. IL-15, a cytokine structurally similar to IL-2, binds the IL-2/IL-15 beta chain and common gamma chain also activating STAT5 and was recently shown to enhance the expression of granzyme B and perforin in human CD28neg CD4 T cells [90].
By analogy to conditions inducing other T cell subsets, it is likely that in addition to IL-2, other factors will be found to act in concert to promote the development of ThCTL. For example, heat shock protein 70 was shown to induce the cytotoxic activity of CD4 T cells that was further enhanced in the presence of IL-15, as well as IL-12 or IL-7 [91]. Costimulatory pathways may also play a role in inducing optimal ThCTL. Costimulation through OX-40 and 4-1BB was recently found to enhance the cytotoxic activity of Th1 cells [89]. Antigen dose and persistence may also affect ThCTL generation. In influenza-infected mice, ThCTL are only found in the lung suggesting maximum or repeated antigen stimulation may be necessary. We have also found that low antigen dose favors in-vitro ThCTL generation, but with the addition of exogenous IL-2 [72].
7. Role of ThCTL in Antiviral Activities
There are few studies of the contribution of ThCTL in immune protection. We found that perforin-dependent cytotoxicity played a key role when in-vitro-generated Th1 effectors were introduced into unprimed mice infected with high-dose influenza virus [27]. Moreover, when we analyze the protective mechanisms used by Th1 memory cells, we find that monoclonal memory cells drive the selection of virus variants and that this mechanism is perforin-dependent (T. M. Strutt, K. K. McKinstry, S. L. Swain, unpublished results). Thus CD4-mediated cytotoxicity seems to be one of several key mechanisms that contribute to viral control and elimination. A key complication of the multipronged strategy that CD4 T cells use to combat pathogens is that individual mechanisms may be redundant except when virus titers rise to very high levels. Thus, further studies are needed to define the role of ThCTL in response to different pathogens.
8. Summary
A subset of CD4 T cells can acquire cytotoxic activity during an antiviral immune response and kill target cells presenting cognate viral antigen. A role for ThCTL is highlighted when the generation and/or immune surveillance by CD8 CTL is impaired, which can occur when immune evasion mechanisms are employed by the virus. Furthermore, ThCTL may also play a role in controlling the development of viral-associated malignancies including EBV-associated lymphomas. ThCTL are identified phenotypically by their expression of markers of cytotoxic granule exocytosis including perforin, granzyme B, and surface expression of degranulation marker, CD107a. We still know little about the factors necessary to generate ThCTL, although TCR activation is required for the upregulation of granzyme B and perforin expression. The expression of cytotoxic mediators is further enhanced by the addition of cytokines including IL-12, IL-2, and IL-15. Although ThCTL often share characteristics with Th1 cells, there is evidence that Th1-polarizing conditions do not produce optimal killers, thus ThCTL may represent a distinct CD4 T cell effector subset. We propose that CD4 T cell-mediated cytotoxicity is one component of the antiviral arsenal of T cells and will in time be seen as a valuable player in antiviral responses where destruction of target cells is an important aspect of protective immunity.
Acknowledgment
This work was supported by the National Institutes of Health Grant no. P01AI46530.
References
- 1.Wagner H, Starzinski-Powitz A, Jung H, Roellinghoff M. Induction of I region-restricted hapten-specific cytotoxic T lymphocytes. Journal of Immunology. 1977;119(4):1365–1368. [PubMed] [Google Scholar]
- 2.Feighery C, Stastny P. HLA-D region-associated determinants serve as targets for human cell-mediated lysis. Journal of Experimental Medicine. 1979;149(2):485–494. doi: 10.1084/jem.149.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleischer B. Acquisition of specific cytotoxic activity by human T4+ T lymphocytes in culture. Nature. 1984;308(5957):365–367. doi: 10.1038/308365a0. [DOI] [PubMed] [Google Scholar]
- 4.Tite JP, Janeway CA., Jr Cloned helper T cells can kill B lymphoma cells in the presence of specific antigen: Ia restriction and cognate vs. noncognate interactions in cytolysis. European Journal of Immunology. 1984;14(10):878–886. doi: 10.1002/eji.1830141004. [DOI] [PubMed] [Google Scholar]
- 5.Maimone MM, Morrison LA, Braciale VL, Braciale TJ. Features of target cell lysis by class I and class II MHC-restricted cytolytic T lymphocytes. Journal of Immunology. 1986;137(11):3639–3643. [PubMed] [Google Scholar]
- 6.Lukacher AE, Morrison LA, Braciale VL. Malissen B and braciale TJ: expression of specific cytolytic activity by H-21 region-restricted, influenza virus-specific T lymphocyte clones. Journal of Experimental Medicine. 1985;162(1):171–187. doi: 10.1084/jem.162.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaunders JJ, Dyer WB, Wang B, et al. Identification of circulating antigen-specific CD4+ T lymphocytes with a CCR5+, cytotoxic phenotype in an HIV-1 long-term nonprogressor and in CMV infection. Blood. 2004;103(6):2238–2247. doi: 10.1182/blood-2003-08-2765. [DOI] [PubMed] [Google Scholar]
- 8.van Leeuwen EM, Remmerswaal EB, Vossen MT, et al. Emergence of a CD4+CD28− granzyme B+, cytomegalovirus-specific T cell subset after recovery of primary cytomegalovirus infection. Journal of Immunology. 2004;173(3):1834–1841. doi: 10.4049/jimmunol.173.3.1834. [DOI] [PubMed] [Google Scholar]
- 9.Sáez-Borderías A, Gumá M, Angulo A, Bellosillo B, Pende D, López-Botet M. Expression and function of NKG2D in CD4+ T cells specific for human cytomegalovirus. European Journal of Immunology. 2006;36(12):3198–3206. doi: 10.1002/eji.200636682. [DOI] [PubMed] [Google Scholar]
- 10.Suni MA, Ghanekar SA, Houck DW, et al. CD4+CD8dim T lymphocytes exhibit enhanced cytokine expression, proliferation and cytotoxic activity in response to HCMV and HIV-1 antigens. European Journal of Immunology. 2001;31(8):2512–2520. doi: 10.1002/1521-4141(200108)31:8<2512::aid-immu2512>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Aslan N, Yurdaydin C, Wiegand J, et al. Cytotoxic CD4+ T cells in viral hepatitis. Journal of Viral Hepatitis. 2006;13(8):505–514. doi: 10.1111/j.1365-2893.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 12.Appay V, Zaunders JJ, Papagno L, et al. Characterization of CD4+ CTLs ex vivo. Journal of Immunology. 2002;168(11):5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 13.Norris PJ, Moffett HF, Yang OO, et al. Beyond help: direct effector functions of human immunodeficiency virus type 1-specific CD4+ T cells. Journal of Virology. 2004;78(16):8844–8851. doi: 10.1128/JVI.78.16.8844-8851.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jellison ER, Kim SK, Welsh RM. Cutting edge: MHC class II-restricted killing in vivo during viral infection. Journal of Immunology. 2005;174(2):614–618. doi: 10.4049/jimmunol.174.2.614. [DOI] [PubMed] [Google Scholar]
- 15.Stuller KA, Flaño E. CD4 T cells mediate killing during persistent gammaherpesvirus 68 infection. Journal of Virology. 2009;83(9):4700–4703. doi: 10.1128/JVI.02240-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna R, Burrows SR, Steigerwald-Mullen PM, Moss DJ, Kurilla MG, Cooper L. Targeting Epstein-barr virus nuclear antigen 1 (EBNA1) through the class II pathway restores immune recognition by EBNA1-specific cytotoxic T lymphocytes: evidence for HLA-DM-independent processing. International Immunology. 1997;9(10):1537–1543. doi: 10.1093/intimm/9.10.1537. [DOI] [PubMed] [Google Scholar]
- 17.Adhikary D, Behrends U, Moosmann A, Witter K, Bornkamm GW, Mautner J. Control of Epstein-barr virus infection in vitro by T helper cells specific for virion glycoproteins. Journal of Experimental Medicine. 2006;203(4):995–1006. doi: 10.1084/jem.20051287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landais E, Saulquin X, Scotet E, et al. Direct killing of Epstein-barr virus (EBV)-infected B cells by CD4 T cells directed against the EBV lytic protein BHRF1. Blood. 2004;103(4):1408–1416. doi: 10.1182/blood-2003-03-0930. [DOI] [PubMed] [Google Scholar]
- 19.Long HM, Haigh TA, Gudgeon NH, et al. CD4+ T-cell responses to Epstein-barr virus (EBV) latent-cycle antigens and the recognition of EBV-transformed lymphoblastoid cell lines. Journal of Virology. 2005;79(8):4896–4907. doi: 10.1128/JVI.79.8.4896-4907.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holling TM, van der Stoep N, Quinten E, van den Elsen PJ. Activated human T cells accomplish MHC class II expression through T cell-specific occupation of class II transactivator promoter III. Journal of Immunology. 2002;168(2):763–770. doi: 10.4049/jimmunol.168.2.763. [DOI] [PubMed] [Google Scholar]
- 21.Ko HS, Fu SM, Winchester RJ, Yu DT, Kunkel HG. Ia determinants on stimulated human T lymphocytes. Occurrence of mitogen- and antigen-activated T cells. Journal of Experimental Medicine. 1979;150(2):246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang CH, Hong SC, Hughes CC, Janeway CA, Jr, Flavell RA. CIITA activates the expression of MHC class II genes in mouse T cells. International Immunology. 1995;7(9):1515–1518. doi: 10.1093/intimm/7.9.1515. [DOI] [PubMed] [Google Scholar]
- 23.Sacha JB, Giraldo-Vela JP, Buechler MB, et al. Gag- and Nef-specific CD4+ T cells recognize and inhibit SIV replication in infected macrophages early after infection. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(24):9791–9796. doi: 10.1073/pnas.0813106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahid R, Cannon MJ, Chow M. Virus-specific CD4+ and CD8+ cytotoxic T-cell responses and long-term T-cell memory in individuals vaccinated against polio. Journal of Virology. 2005;79(10):5988–5995. doi: 10.1128/JVI.79.10.5988-5995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hegde NR, Dunn C, Lewinsohn DM, Jarvis MA, Nelson JA, Johnson DC. Endogenous human cytomegalovirus gB is presented efficiently by MHC class II molecules to CD4+ CTL. Journal of Experimental Medicine. 2005;202(8):1109–1119. doi: 10.1084/jem.20050162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Leeuwen EMM, Remmerswaal EBM, Heemskerk MHM, Ten Berge IJM, van Lier RAW. Strong selection of virus-specific cytotoxic CD4+ T-cell clones during primary human cytomegalovirus infection. Blood. 2006;108(9):3121–3127. doi: 10.1182/blood-2006-03-006809. [DOI] [PubMed] [Google Scholar]
- 27.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perform and antibody-mediated mechanisms give a one-two punch. Journal of Immunology. 2006;177(5):2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 28.Brien JD, Uhrlaub JL, Nikolich-Zǔgich J. West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. Journal of Immunology. 2008;181(12):8568–8575. doi: 10.4049/jimmunol.181.12.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams NS, Engelhard VH. Perforin-dependent cytotoxic activity and lymphokine secretion by CD4+ T cells are regulated by CD8+ T cells. Journal of Immunology. 1997;159(5):2091–2099. [PubMed] [Google Scholar]
- 30.Bourgault I, Gomez A, Gomrad E, Picard F, Levy JP. A virus-specific CD4+ cell-mediated cytolytic activity revealed by CD8+ cell elimination regularly develops in uncloned human antiviral cell lines. Journal of Immunology. 1989;142(1):252–256. [PubMed] [Google Scholar]
- 31.Hou S, Fishman M, Murti KG, Doherty PC. Divergence between cytotoxic effector function and tumor necrosis factor α production for inflammatory CD4+ T cells from mice with Sendai virus pneumonia. Journal of Virology. 1993;67(10):6299–6302. doi: 10.1128/jvi.67.10.6299-6302.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller D, Koller BH, Whitton JL, LaPan KE, Brigman KK, Frelinger JA. LCMV-specific, class II-restricted cytotoxic T cells in β 2-microglobulin-deficient mice. Science. 1992;255(5051):1576–1578. doi: 10.1126/science.1347959. [DOI] [PubMed] [Google Scholar]
- 33.Zajac AJ, Quinn DG, Cohen PL, Frelinger JA. Fas-dependent CD4+ cytotoxic T-cell-mediated pathogenesis during virus infection. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(25):14730–14735. doi: 10.1073/pnas.93.25.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quezada SA, Simpson TR, Peggs KS, et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. Journal of Experimental Medicine. 2010;207(3):637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie Y, Akpinarli A, Maris C, et al. Naive tumor-specific CD4+ T cells differentiated in vivo eradicate established melanoma. Journal of Experimental Medicine. 2010;207(3):651–667. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou X, McElhaney JE. Age-related changes in memory and effector T cells responding to influenza A/H3N2 and pandemic A/H1N1 strains in humans. Vaccine. 2011;29(11):2169–2177. doi: 10.1016/j.vaccine.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alcami A, Koszinowski UH. Viral mechanisms of immune evasion. Immunology Today. 2000;21(9):447–455. doi: 10.1016/S0167-5699(00)01699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen JL, Morris CR, Solheim JC. Virus evasion of MHC class I molecule presentation. Journal of Immunology. 2003;171(9):4473–4478. doi: 10.4049/jimmunol.171.9.4473. [DOI] [PubMed] [Google Scholar]
- 39.Levitskaya J, Coram M, Levitsky V, et al. Inhibition of antigen processing by the internal repeat region of the Epstein-barr virus nuclear antigen-1. Nature. 1995;375(6533):685–688. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- 40.Yin Y, Manoury B, Fåhraeus R. Self-inhibition of synthesis and antigen presentation by Epstein-barr virus-encoded EBNA1. Science. 2003;301(5638):1371–1374. doi: 10.1126/science.1088902. [DOI] [PubMed] [Google Scholar]
- 41.Paludan C, Schmid D, Landthaler M, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307(5709):593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 42.Münz C, Bickham KL, Subklewe M, et al. Human CD4+ T lymphocytes consistently respond to the latent Epstein-barr virus nuclear antigen EBNA1. Journal of Experimental Medicine. 2000;191(10):1649–1660. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paludan C, Bickham K, Nikiforow S, et al. Epstein-barr nuclear antigen 1-specific CD4+ Th1 cells kill Burkitt’s lymphoma cells. Journal of Immunology. 2002;169(3):1593–1603. doi: 10.4049/jimmunol.169.3.1593. [DOI] [PubMed] [Google Scholar]
- 44.Piriou E, van Dort K, Nanlohy NM, van Oers MHJ, Miedema F, van Baarle D. Loss of EBNA1-specific memory CD4+ and CD8+ T cells in HIV-infected patients progressing to AIDS-related non-Hodgkin lymphoma. Blood. 2005;106(9):3166–3174. doi: 10.1182/blood-2005-01-0432. [DOI] [PubMed] [Google Scholar]
- 45.Gasser O, Bihl FK, Wolbers M, et al. HIV patients developing primary CNS lymphoma lack EBV-specific CD4+ T cell function irrespective of absolute CD4+ T cell counts. PLoS Medicine. 2007;4(3):p. e96. doi: 10.1371/journal.pmed.0040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heller KN, Arrey F, Steinherz P, et al. Patients with Epstein-barr virus-positive lymphomas have decreased CD4+ T-cell responses to the viral nuclear antigen 1. International Journal of Cancer. 2008;123(12):2824–2831. doi: 10.1002/ijc.23845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chowdhury D, Lieberman J. Death by a thousand cuts: granzyme pathways of programmed cell death. Annual Review of Immunology. 2008;26:389–420. doi: 10.1146/annurev.immunol.26.021607.090404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pipkin ME, Lieberman J. Delivering the kiss of death: progress on understanding how perforin works. Current Opinion in Immunology. 2007;19(3):301–308. doi: 10.1016/j.coi.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haigh TA, Lin X, Jia H, et al. EBV latent membrane proteins (LMPs) 1 and 2 as immunotherapeutic targets: LMP-specific CD4+ cytotoxic T cell recognition of EBV-transformed B cell lines. Journal of Immunology. 2008;180(3):1643–1654. doi: 10.4049/jimmunol.180.3.1643. [DOI] [PubMed] [Google Scholar]
- 50.Norris PJ, Sumaroka M, Brander C, et al. Multiple effector functions mediated by human immunodeficiency virus-specific CD4+ T-cell clones. Journal of Virology. 2001;75(20):9771–9779. doi: 10.1128/JVI.75.20.9771-9779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gagnon SJ, Ennis FA, Rothman AL. Bystander target cell lysis and cytokine production by dengue virus- specific human CD4+ cytotoxic T-lymphocyte clones. Journal of Virology. 1999;73(5):3623–3629. doi: 10.1128/jvi.73.5.3623-3629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clayberger C, Krensky AM. Granulysin. Current Opinion in Immunology. 2003;15(5):560–565. doi: 10.1016/s0952-7915(03)00097-9. [DOI] [PubMed] [Google Scholar]
- 53.Zheng CF, Ma LL, Jones GJ, et al. Cytotoxic CD4+ T cells use granulysin to kill Cryptococcus neoformans, and activation of this pathway is defective in HIV patients. Blood. 2007;109(5):2049–2057. doi: 10.1182/blood-2006-03-009720. [DOI] [PubMed] [Google Scholar]
- 54.Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death and Differentiation. 2003;10(1):26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 55.Stalder T, Hahn S, Erb P. Fas antigen is the major target molecule for CD4+ T cell-mediated cytotoxicity. Journal of Immunology. 1994;152(3):1127–1133. [PubMed] [Google Scholar]
- 56.Liu X, Taylor BJ, Sun G, Bosselut R. Analyzing expression of perforin, Runx3, and Thpok genes during positive selection reveals activation of CD8-differentiation programs by MHC II-signaled thymocytes. Journal of Immunology. 2005;175(7):4465–4474. doi: 10.4049/jimmunol.175.7.4465. [DOI] [PubMed] [Google Scholar]
- 57.Niiya H, Sakai I, Lei J, et al. Differential regulation of perforin expression in human CD4+ and CD8+ cytotoxic T lymphocytes. Experimental Hematology. 2005;33(7):811–818. doi: 10.1016/j.exphem.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Pipkin ME, Rao A, Lichtenheld MG. The transcriptional control of the perforin locus. Immunological Reviews. 2010;235(1):55–72. doi: 10.1111/j.0105-2896.2010.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104(9):2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- 60.Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods in Cell Biology. 2004;2004(75):497–512. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- 61.Nemes E, Bertoncelli L, Lugli E, et al. Cytotoxic granule release dominates gag-specific CD4+ T-cell response in different phases of HIV infection. AIDS. 2010;24(7):947–957. doi: 10.1097/QAD.0b013e328337b144. [DOI] [PubMed] [Google Scholar]
- 62.Casazza JP, Betts MR, Price DA, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. Journal of Experimental Medicine. 2006;203(13):2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stuller KA, Cush SS, Flano E. Persistent γ-herpesvirus infection induces a CD4 T cell response containing functionally distinct effector populations. Journal of Immunology. 2010;184(7):3850–3856. doi: 10.4049/jimmunol.0902935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress- inducible MICA. Science. 1999;285(5428):727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 65.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nature Immunology. 2000;1(2):119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 66.Cosman D, Müllberg J, Sutherland CL, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14(2):123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 67.Amyes E, Hatton C, Montamat-Sicotte D, et al. Characterization of the CD4+ T cell response to Epstein-barr virus during primary and persistent infection. Journal of Experimental Medicine. 2003;198(6):903–911. doi: 10.1084/jem.20022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown DM. Cytolytic CD4 cells: direct mediators in infectious disease and malignancy. Cellular Immunology. 2010;262(2):89–95. doi: 10.1016/j.cellimm.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nature Medicine. 2002;8(4):379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 70.Kaneko T. Human autoreactive (Th0) CD4+ T-cell clones with cytolytic activity recognizing autologous activated T cells as the target. Human Immunology. 2000;61(8):780–788. doi: 10.1016/s0198-8859(00)00146-4. [DOI] [PubMed] [Google Scholar]
- 71.Yasukawa M, Ohminami H, Arai J, Kasahara Y, Ishida Y, Fujita S. Granule exocytosis, and not the Fas/Fas ligand system, is the main pathway of cytotoxicity mediated by alloantigen-specific CD4+ as well as CD8+ cytotoxic T lymphocytes in humans. Blood. 2000;95(7):2352–2355. [PubMed] [Google Scholar]
- 72.Brown DM, Kamperschroer C, Dilzer AM, Roberts DM, Swain SL. IL-2 and antigen dose differentially regulate perforin- and FasL-mediated cytolytic activity in antigen specific CD4+ T cells. Cellular Immunology. 2009;257(1-2):69–79. doi: 10.1016/j.cellimm.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McKisic MD, Lancki DW, Fitch FW. Cytolytic activity of murine CD4+ T cell clones correlates with IFN-γ production in mouse strains having a BALB/c background. Journal of Immunology. 1993;150(9):3793–3805. [PubMed] [Google Scholar]
- 74.Lancki DW, Hsieh CS, Fitch FW. Mechanisms of lysis by cytotoxic T lymphocyte clones: lytic activity and gene expression in cloned antigen-specific CD4+ and CD8+ T lymphocytes. Journal of Immunology. 1991;146(9):3242–3249. [PubMed] [Google Scholar]
- 75.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nature Reviews Immunology. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahon BP, Katrak K, Nomoto A, Macadam AJ, Minor PD, Mills KH. Poliovirus-specific CD4+ Th1 clones with both cytotoxic and helper activity mediate protective humoral immunity against a lethal poliovirus infection in transgenic mice expressing the human poliovirus receptor. Journal of Experimental Medicine. 1995;181(4):1285–1292. doi: 10.1084/jem.181.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Erb P, Grogg D, Troxler M, Kennedy M, Fluri M. CD4+ T cell-mediated killing of MHC class II-positive antigen-presenting cells. I. Characterization of target cell recognition by in vivo or in vitro activated CD4+ killer T cells. Journal of Immunology. 1990;144(3):790–795. [PubMed] [Google Scholar]
- 78.Yamamoto K, Shibata F, Miyasaka N, Miura O. The human perforin gene is a direct target of STAT4 activated by IL-12 in NK cells. Biochemical and Biophysical Research Communications. 2002;297(5):1245–1252. doi: 10.1016/s0006-291x(02)02378-1. [DOI] [PubMed] [Google Scholar]
- 79.Salcedo TW, Azzoni L, Wolf SF, Perussia B. Modulation of perforin and granzyme messenger RNA expression in human natural killer cells. Journal of Immunology. 1993;151(5):2511–2520. [PubMed] [Google Scholar]
- 80.Deblaker-Hohe DF, Yamauchi A, Yu CR, Horvath-Arcidiacono JA, Bloom ET. IL-12 synergizes with IL-2 to induce lymphokine-activated cytotoxicity and perforin and granzyme gene expression in fresh human NK cells. Cellular Immunology. 1995;165(1):33–43. doi: 10.1006/cimm.1995.1184. [DOI] [PubMed] [Google Scholar]
- 81.Aste-Amezaga M, D’Andrea A, Kubin M, Trinchieri G. Cooperation of natural killer cell stimulatory factor/interleukin-12 with other stimuli in the induction of cytokines and cytotoxic cell-associated molecules in human T and NK cells. Cellular Immunology. 1994;156(2):480–492. doi: 10.1006/cimm.1994.1192. [DOI] [PubMed] [Google Scholar]
- 82.Pipkin ME, Ljutic B, Cruz-Guilloty F, et al. Chromosome transfer activates and delineates a locus control region for perforin. Immunity. 2007;26(1):29–41. doi: 10.1016/j.immuni.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 83.Janas ML, Groves P, Kienzle N, Kelso A. IL-2 regulates perforin and granzyme gene expression in CD8+ T cells independently of its effects on survival and proliferation. Journal of Immunology. 2005;175(12):8003–8010. doi: 10.4049/jimmunol.175.12.8003. [DOI] [PubMed] [Google Scholar]
- 84.Smyth MJ, Ortaldo JR, Shinkai YI, et al. Interleukin 2 induction of pore-forming protein gene expression in human peripheral blood CD8+ T cells. Journal of Experimental Medicine. 1990;171(4):1269–1281. doi: 10.1084/jem.171.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang J, Scordi I, Smyth MJ, Lichtenheld MG. Interleukin 2 receptor signaling regulates the perforin gene through signal transducer and activator of transcription (Stat)5 activation of two enhancers. Journal of Experimental Medicine. 1999;190(9):1297–1307. doi: 10.1084/jem.190.9.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32(1):79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cruz-Guilloty F, Pipkin ME, Djuretic IM, et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. Journal of Experimental Medicine. 2009;206(1):51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pearce EL, Mullen AC, Martins GA, et al. Control of effector CD8+ T cell function by the transcription factor eomesodermin. Science. 2003;302(5647):1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 89.Qui HZ, Hagymasi AT, Bandyopadhyay S, et al. CD134 plus CD137 dual costimulation induces eomesodermin in CD4 T cells To program cytotoxic Th1 differentiation. Journal of Immunology. 2011;187(7):3555–3564. doi: 10.4049/jimmunol.1101244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alonso-Arias R, Moro-Garcia MA, Vidal-Castineira JR, et al. IL-15 preferentially enhances functional properties and antigen-specific responses of CD4+CD28null compared to CD4+CD28+ T cells. Aging Cell. 2011;10(5):844–852. doi: 10.1111/j.1474-9726.2011.00725.x. [DOI] [PubMed] [Google Scholar]
- 91.Figueiredo C, Wittmann M, Wang D, et al. Heat shock protein 70(HSP70) induces cytotoxicity of T-helper cells. Blood. 2009;113(13):3008–3016. doi: 10.1182/blood-2008-06-162727. [DOI] [PubMed] [Google Scholar]


