Abstract
The contributions of the host microenvironment to the pathogenesis of multiple myeloma, including progression from the non-malignant disorder monoclonal gammopathy of undetermined significance, are poorly understood. In the present study, microarray analysis of a murine model requiring a unique host microenvironment for myeloma development identified decreased host-derived adiponectin compared with normal mice. In support, clinical analysis revealed decreased serum adiponectin concentrations in monoclonal gammopathy of undetermined significance patients who subsequently progressed to myeloma. We investigated the role of adiponectin in myeloma pathogenesis and as a treatment approach, using both mice deficient in adiponectin and pharmacologic enhancement of circulating adiponectin. Increased tumor burden and bone disease were observed in myeloma-bearing adiponectin-deficient mice, and adiponectin was found to induce myeloma cell apoptosis. The apolipoprotein peptide mimetic L-4F was used for pharmacologic enhancement of adiponectin. L-4F reduced tumor burden, increased survival of myeloma-bearing mice, and prevented myeloma bone disease. Collectively, our studies have identified a novel mechanism whereby decreased host-derived adiponectin promotes myeloma tumor growth and osteolysis. Furthermore, we have established the potential therapeutic benefit of increasing adiponectin for the treatment of myeloma and the associated bone disease.
Introduction
Multiple myeloma is the second most common hematologic malignancy with an estimated 20 180 new diagnoses and 10 650 deaths in 2010 in the United States alone.1 Myeloma is characterized by the uncontrolled clonal expansion of malignant plasma cells within the bone marrow and the development of a destructive osteolytic bone disease. Despite many advances, treatment is limited and mostly palliative, leaving myeloma as an incurable and fatal malignancy with a median survival of only ∼ 3 to 5 years. Therefore, there is a need to elucidate the underlying mechanisms that mediate myeloma pathogenesis and identify new and effective therapeutic approaches to halt myeloma progression and prevent tumor growth and osteolytic bone destruction.
Known and unknown elements within the bone marrow can support tumor growth and osteolytic bone disease in myeloma.2 However, the contributions of the host microenvironment during the initial stages of myeloma development are poorly understood. Many features of human myeloma are recapitulated in the well-characterized 5T Radl myeloma model, where transplantation of 5T myeloma cells into mice of the C57Bl/KaLwRijHsd (KaLwRij) substrain results in propagation of myeloma.3,4 Importantly, 5T myeloma cells will not grow in the closely related C57Bl6 strain of mice.4–6 This suggests a critical role for the host microenvironment in the early stages of myeloma, with specific modifications in the bone marrow microenvironment that allow for myeloma development and progression. Moreover, the myeloma-permissive KaLwRij mice may provide valuable insight into the factor(s) that permit tumor establishment and growth.
Adiponectin is an adipokine originally identified as secreted by adipose tissue into the bloodstream. More recently, studies have shown adiponectin to be expressed by other cell types, including bone marrow–derived osteoblasts and fibroblasts.7 The precise physiologic function of adiponectin and its receptors remains unclear, but adiponectin deficiency (hypoadiponectinemia) is thought to play a role in obesity, cardiovascular disease, and diabetes. Interestingly, hypoadiponectinemia has been associated with a high incidence of obesity-related cancer, including endometrial, breast, prostate, and gastric cancers. Decreased adiponectin levels also represent an independent risk factor for breast cancer and are associated with more aggressive phenotypes.8,9 Adiponectin has direct growth inhibitory effects in breast cancer; however, in vivo studies show conflicting results on tumor growth, angiogenesis, and metastases, suggesting a complex role for adiponectin in mammary tumor development.10–13 The role of adiponectin in myeloma has not been investigated.
In the present study, we identified adiponectin as decreased in host environments permissive for myeloma in both mice and humans. We subsequently investigated the role of adiponectin in myeloma pathogenesis and the potential of adiponectin as a therapeutic target, using mice deficient in adiponectin and pharmacologic enhancement of circulating adiponectin levels.
Methods
Bone marrow comparison by microarray
Bone marrow was flushed from age- and sex-matched C57Bl6 and KaLwRij mice (n = 3). Red blood cells were eliminated and RNA isolated from the remaining cells using a monophasic isolation reagent (TRIzol). Pooled RNA samples from each strain were submitted to the Vanderbilt Functional Genomics Shared Resource for hybridization using the Affymetrix GeneChip exon expression array. Differentially expressed genes were based on changes of 2-fold or more between C57Bl6 and KaLwRij bone marrow. All microarray data are available at the Gene Expression Omnibus under accession number GSE31532.
Human patient samples
Serum samples from monoclonal gammopathy of undetermined significance (MGUS) patients with progression to myeloma, MGUS patients with no progression to myeloma, and the respective age-, sex-, and body mass index (BMI)–matched controls were obtained through collaborations with M.T.D. Samples were obtained with approval from the Institutional Review Board and Biospecimen Protocol Review Group of the Mayo Clinic.
Cell culture
The 5TGM1-GFP myeloma cell line was cultured as previously described.14 For primary bone marrow stromal cells (BMSCs), bone marrow was flushed from the tibia and femur of age- and sex-matched mice. Briefly, cells were plated at 1 × 107/mL in α-MEM supplemented with 10% FCS. Adherent cells were washed and culture media replaced after 3 to 4 days. Cells were cultured for ∼ 3 weeks, until confluent.
RT-PCR
RNA from cell lines and primary BMSCs was isolated using the RNeasy kit (QIAGEN). cDNA was generated using the SuperScript III First Strand Synthesis SuperMix kit (Invitrogen). Mouse adiponectin was detected by real-time PCR using TaqMan Gene Expression assay primer sets (Applied Biosystems). Relative gene expression of adiponectin was normalized to the TaqMan Gene Expression assay for GAPD (Applied Biosystems). Primer sequences are listed in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Animal models
Studies were conducted using age-, sex-, and weight-matched KaLwRij (Harlan Netherlands) or adiponectin-deficient mice, which were kindly provided by Dr Ken Walsh, Boston University and were bred with recombinase-activating gene-2 (RAG-2) immunodeficient mice (C57BL/6 background, Taconic Farms) to generate double-knockout animals. Littermates that were wild-type (WT) for adiponectin and knockout for RAG-2 were used as controls. Studies were approved by the Vanderbilt University Institution of Animal Care and Use Committee.
Adiponectin-deficient mice or WT controls were intravenously inoculated with 106 5TGM1-GFP cells or vehicle control. Sera were assayed for monoclonal mouse IgG2bκ paraprotein as described previously.14 Myeloma bone disease was assessed as described previously15 and outlined in Supplemental data. L-4F (200 μg/100 g daily, intraperitoneally) or vehicle control was administered to 4-week-old KaLwRij mice before tumor cell inoculation for 28 days until treated mice showed an increase in serum adiponectin. Mice were then inoculated with 106 5TGM1-GFP cells or vehicle control by intravenous injection and continued to receive daily treatment with L-4F until killed. Tumor burden and bone disease were assessed as described previously. Identical studies were performed in adiponectin-deficient mice or WT littermate controls. In additional survival studies, KaLwRij mice were treated with L-4F and inoculated with tumor cells as described previously. Treatment was continued until evidence of paraplegia, which was used as a surrogate for survival, at which point mice were killed. A separate cohort of KaLwRij mice was inoculated with 106 5TGM1-GFP myeloma cells by subcutaneous injection. Treatment with L-4F or vehicle control commenced from time of tumor inoculation. Once palpable, tumors were measured daily and tumor volume calculated according to LeBlanc et al.16
Immunoblotting and ELISAs
The different isoforms of adiponectin were detected by immunoblotting (supplemental Methods). An ELISA for high molecular weight (HMW) adiponectin (Millipore) was used according to the manufacturer's instructions. Serum concentrations of testosterone and glucose were measured according to the manufacturer's instructions using commercially available ELISA kits (testosterone, R&D Systems; glucose, Cambridge Biosciences).
Apoptosis
5TGM1 myeloma cells, ST2, C57Bl6, and KaLwRij BMSCs were cultured for 48 hours with 5 to 15 μg/mL recombinant adiponectin (ProSpec). The percentage of viable, apoptotic, and necrotic cells was determined by annexin V and SYTOX AADvanced cell staining for flow cytometric analysis (Invitrogen).
Statistical analysis
Statistical significance was determined using a Mann-Whitney U test for nonparametric data and considered significant given P ≤ .05. One-way ANOVA and Tukey-Kramer method tests were used for analysis of multiple groups in both in vivo and in vitro studies. Two-way ANOVA was used for analysis of effects of L-4F on tumor burden over time. Survival data were analyzed using a log-rank (Mantel-Cox) test.
Results
Adiponectin is decreased in the host microenvironment of mice permissive for multiple myeloma
The KaLwRij strain of mice of the well-established Radl model of myeloma is unique in that 5T myeloma cells will only grow in syngeneic KaLwRij mice, and not in closely related C57Bl6 mice (Figure 1A). This suggests an important role for the KaLwRij host microenvironment in myeloma pathogenesis. The similarities between human myeloma and myeloma observed in the KaLwRij mice led us to hypothesize that differences between a myeloma-permissive and non-permissive microenvironment might provide valuable insights into the development of human myeloma and the associated bone disease.
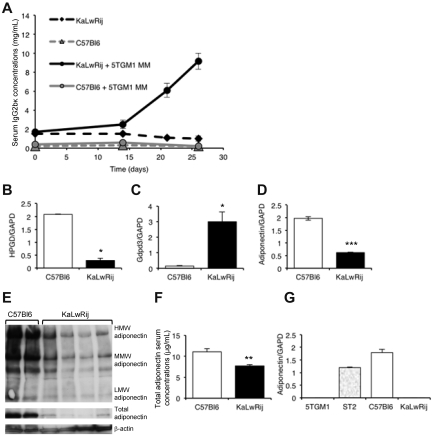
Figure 1.
Comparison of permissive KaLwRij and non-permissive C57Bl6 bone marrow microenvironments. (A) Tumor burden in non-permissive C57Bl6 and myeloma-permissive KaLwRij mice inoculated with 5TGM1 myeloma cells, determined by measuring myeloma-specific IgG2bκ concentrations. P < .01. n = 8. (B-D) Quantitative RT-PCR measurements of hydroxyprostaglandin dehydrogenase 15 (NAD HPGD), glycerophosphodiesterase domain containing 3 (Gdpd3), and adiponectin/GAPDH ratio in C57Bl6 and KaLwRij bone marrow. (E) Expression of adiponectin in bone marrow from C57Bl6 and KaLwRij mice was determined by Western blot. Each lane represents bone marrow from an individual mouse. (F) Serum concentrations of adiponectin in C57Bl6 and KaLwRij mice. (G) Quantitative RT-PCR measurements of adiponectin expression in 5TGM1 myeloma cells, and ST2, C57Bl6, and KaLwRij BM stromal cells (see also supplemental Figure 1). Data are mean ± SEM. *P < .05, **P < .01, and ***P < .001, compared with C57Bl6.
To investigate factors that were differentially expressed in the permissive KaLwRij microenvironment compared with non-permissive C57Bl6, microarray analysis was performed on pooled bone marrow samples from both strains. The microarray identified genes that were differentially expressed between the marrow of the KaLwRij and C57Bl6 mice (Table 1) and were verified by quantitative real-time PCR analysis. These included a significant increase in glycerophosphodiesterase domain-containing 3 and a significant reduction in hydroxyprostaglandin dehydrogenase 15 and adiponectin expression (Figure 1B-D). Myeloma is accompanied by a destructive bone disease; therefore, adiponectin drew particular interest based on its association with both cancer and bone biology. Expression of the adipokine adiponectin was significantly decreased in the bone marrow of the myeloma-permissive KaLwRij mice at both mRNA (Figure 1D) and protein (Figure 1E) levels. Because adiponectin is a secreted factor, we also measured serum concentrations and found that myeloma-permissive KaLwRij mice had significantly lower serum concentrations of total adiponectin compared with non-permissive C57Bl6 (Figure 1F). There was also a decrease in the HMW form of adiponectin in KaLwRij sera (supplemental Figure 1).
Table 1.
Differentially expressed genes in KaLwRij bone marrow
| Gene | Gene description | Fold change* |
|---|---|---|
| Abhd6 | Abhydrolase domain containing 6 | 2.01 increase |
| Abca13 | ATP-binding cassette, sub-family A (ABC1), member 13 | 2.15 increase |
| C5ar1 | Complement component 5a receptor 1 | 2.06 increase |
| Gdpd3 | glycerophosphodiester phosphodiesterase domain containing 3 | 32.7 increase |
| Mgl1 | Macrophage galactose N-acetyl-galactosamine specific lectin 1 | 2.19 decrease |
| Mrgpra2 | MAS-related GPR, member A2 | 2.08 increase |
| Mc2r | Melanocortin 2 receptor | 2.06 increase |
| Olfm4 | Olfactomedin 4 | 2.21 increase |
| Sirpb1 | Signal-regulatory protein-β1 | 2.17 increase |
| Stfa1 | Stefin A1 | 2.28 increase |
| Stfa2 | Stefin A2 | 2.55 increase |
| Stfa3 | Stefin A3 | 2.49 increase |
| Trdn | Triadin | 2.29 increase |
| Adipoq | Adiponectin, C1Q and collagen domain containing | 2.08 decrease |
| Cysltr2 | Cysteinyl leukotriene receptor 2 | 2.98 decrease |
| Gnal | guanine nucleotide binding protein, α-stimulating, olfactory type | 2.05 decrease |
| Grap2 | GRB2-related adaptor protein 2 | 2.38 decrease |
| Hist1h2bc | Histone cluster 1, H2bc | 2.27 decrease |
| Hist1h4c | Histone cluster 1, H4c | 3.18 decrease |
| Hpgd | Hydroxyprostaglandin dehydrogenase 15 (NAD) | 5.05 decrease |
| Tcte3 | t-complex–associated testis expressed 3 | 2.21 decrease |
| Tnfrsf26 | Tumor necrosis factor receptor superfamily, member 26, mRNA | 5.08 decrease |
Comparative microarray analysis of genes expressed in the BM of KaLwRij mice compared with C57Bl6 mice (pooled whole BM from 3 mice per strain).
Change in expression in KaLwRij bone marrow compared with C57Bl6 bone marrow.
Although adiponectin was originally identified as an adipose-specific factor, it has since been recognized that adiponectin is also secreted by a range of cell types, including BMSCs. To determine a cellular source of the differential expression of adiponectin in myeloma-permissive KaLwRij mice, we performed real-time PCR on a panel of BMSCs. Adiponectin was strongly expressed in both the normal ST2 BMSC line and in primary BMSCs from non-permissive C57Bl6 mice; however, primary BMSCs from myeloma-permissive KaLwRij mice did not express adiponectin (Figure 1G), providing further evidence for decreased adiponectin in the myeloma-permissive KaLwRij host microenvironment. We also determined that adiponectin is not expressed by 5TGM1 myeloma cells themselves (Figure 1G). These data demonstrate that the myeloma-permissive KaLwRij mice possess decreased circulating and bone marrow-derived adiponectin levels; however, the clinical relevance of these observations remained unknown.
Adiponectin is decreased in the serum of patients with MGUS that subsequently progressed to myeloma
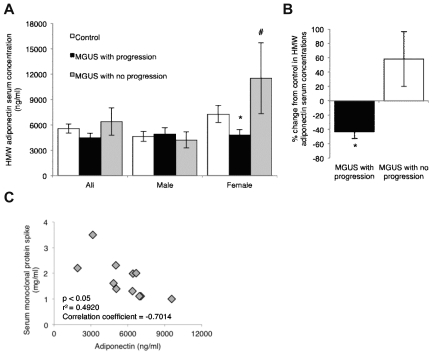
In almost all cases, myeloma is preceded by an unexplained non-malignant plasma cell disorder known as MGUS.17 Given the well-characterized similarities between the 5T Radl model of myeloma in KaLwRij mice and human myeloma, we next determined whether our observations of decreased adiponectin in myeloma-permissive KaLwRij mice translated to the clinical setting. In collaboration with Mayo Clinic colleagues, we obtained serum samples from patients with MGUS that subsequently progressed to myeloma and patients with MGUS that had not progressed to myeloma over an average period of 19 years. Patient samples were age-, sex-, and BMI-matched to normal controls (supplemental Table 1). Previous studies in the field of metabolism and diabetes suggest that HMW adiponectin is more biologically active and a better predictor of metabolic parameters.18,19 Measurement of HMW adiponectin in all patient samples revealed a trend toward a decrease in HMW adiponectin in those MGUS patients who progressed to myeloma, compared with controls or those patients who did not progress to myeloma (MGUS with progression, 4.5 ± 0.5 μg/mL; controls, 5.6 ± 0.5 μg/mL; MGUS with no progression, 6.4 ± 1.6 μg/mL; Figure 2A). Interestingly, when samples were stratified into male or female, no significant difference was observed in males between controls and MGUS patients with or without progression. However, in females, a significant reduction in serum HMW adiponectin was observed in those patients with MGUS who subsequently progressed to myeloma, compared with either control or MGUS patients who did not progress (MGUS with progression, 4.8 ± 0.7 μg/mL; controls, 7.3 ± 1.0 μg/mL; MGUS with no progression, 11.5 ± 4.2 μg/mL; Figure 2A-B). Furthermore, analysis of serum monoclonal protein concentrations in those patients with MGUS that subsequently progressed to myeloma revealed a significant negative correlation between concentrations of serum paraprotein and adiponectin (Figure 2C).
Figure 2.
Decreased serum adiponectin concentrations in female MGUS patients are associated with progression to myeloma. (A) Concentration of HMW adiponectin in serum from matched controls and patients with MGUS that subsequently progressed or did not progress to myeloma. Data are subdivided to demonstrate differences between male and female (control, n = 40; MGUS with progression, n = 20; and MGUS no progression, n = 20). (B) Percentage decrease from matched control in HMW adiponectin serum concentrations in MGUS patients who either progress or do not progress to myeloma. (C) Adiponectin expression relative to serum monoclonal protein expression in patients with MGUS who progress to myeloma. (A-B) Data are mean ± SEM. *P < .05, compared with control. #P < .05, compared with MGUS with progression.
Adiponectin is influenced by a number of different factors, including testosterone, obesity, diabetes, and metabolic syndrome. To exclude such factors from our analyses, patients and controls were matched for BMI, and no significant difference was detected in BMI measurements. In addition, no significant difference was detected in blood glucose levels or testosterone (supplemental Table 1). No patients had any evidence of metabolic syndrome, as defined by the International Diabetes Federation.20 These human data reinforce our observations from murine systems that decreased adiponectin is associated with a myeloma-permissive microenvironment and thus support the further use of the 5T Radl model to investigate the role of adiponectin in myeloma pathogenesis in vivo. Furthermore, the significant difference between adiponectin concentrations in MGUS patients who do or do not progress to myeloma suggests that adiponectin levels may function to suppress myeloma tumor development and represent a valid therapeutic target.
Lack of host-derived adiponectin exacerbates myeloma pathogenesis
To specifically address the contribution of host-derived adiponectin to myeloma development in vivo, we investigated myeloma development in adiponectin-deficient mice. To study 5T myeloma growth in genetically modified mice, it is necessary to use mice deficient in RAG-2, which are permissive to 5TGM1 myeloma cell growth and provide a tool for examining contributions of host-derived factors.5 RAG-2−/− mice were bred with adiponectin−/− mice to obtain littermate WT (RAG-2−/−:Adipo+/+) and homozygous deficient (RAG-2−/−:Adipo−/−) genotypes. Western blot analysis of serum confirmed that the double-knockout (RAG-2−/−:Adipo−/−) mice do not express total adiponectin or other circulating isotypes (supplemental Figure 2).
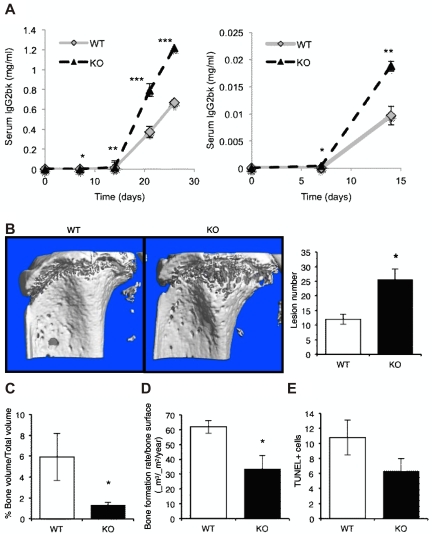
After inoculation with 5TGM1 myeloma cells, both WT and adiponectin-deficient mice developed myeloma over a period of ∼ 4 weeks. However, RAG-2−/−:Adipo−/− (Adipo−/−) mice had a significant increase in tumor burden, as measured by myeloma-specific IgG2bκ serum concentrations compared with WT mice (Figure 3A). This increase in tumor burden was seen as early as 1-week after tumor cell inoculation and persisted through the experimental end point. Accompanying this, myeloma-bearing Adipo−/− mice developed a more severe osteolytic bone disease with increased lesions penetrating the cortical bone (Figure 3B), decreased trabecular bone volume (Figure 3C), and decreased osteoblast activity (Figure 3D). Adipo−/− mice also showed a decrease in both osteoblasts and osteoclasts (data not shown). To determine whether adiponectin−/− mice had a significant bone phenotype at the time of tumor cell inoculation that might impact myeloma establishment, we evaluated their skeletal properties. Micro-CT analysis and histomorphometry found no significant differences between Adipo−/− and WT mice in any bone parameter measured (supplemental Table 2).
Figure 3.
Lack of host-derived adiponectin exacerbates myeloma pathogenesis. (A) Female age-matched adiponectin-deficient (KO) mice or WT littermate controls were inoculated with 5TGM1 myeloma cells. Tumor burden was measured by serum IgG2bκ ELISA. Data demonstrate tumor burden with respect to time from tumor inoculation. Days 0 to 15 are expanded in the right panel graph to demonstrate differences in tumor burden at early time points. (B) Representative images of tibiae of myeloma-bearing WT and adiponectin knockout mice. Osteolytic lesions through cortical bone were quantitated after micro-CT analysis. (C) Trabecular bone volume was quantitated by micro-CT analysis. (D) Bone formation rates were quantitated by dynamic histomorphometry. (E) Using immunohistochemistry, apoptotic myeloma cells in bone marrow were quantitated based on TUNEL positivity (see also supplemental Figure 2 and supplemental Table 2). Data are mean ± SEM. *P < .05, **P < .01, and ***P < .001, compared with WT (WT, n = 3; KO, n = 6).
Histologic assessment of the bone marrow demonstrated a decrease in myeloma cells undergoing apoptosis in Adipo−/− mice compared with WT mice (Figure 3E). These data provide evidence in support of a myeloma-suppressive effect of host-derived adiponectin and suggest a proapoptotic mechanism of action. There was no difference in myeloma cell proliferation in the Adipo−/− mice compared with WT mice (data not shown). The significant increase in both tumor burden and the associated osteolytic bone disease observed in the Adipo−/− mice provide strong evidence that decreased adiponectin plays a role in myeloma pathogenesis.
Adiponectin induces myeloma cell apoptosis
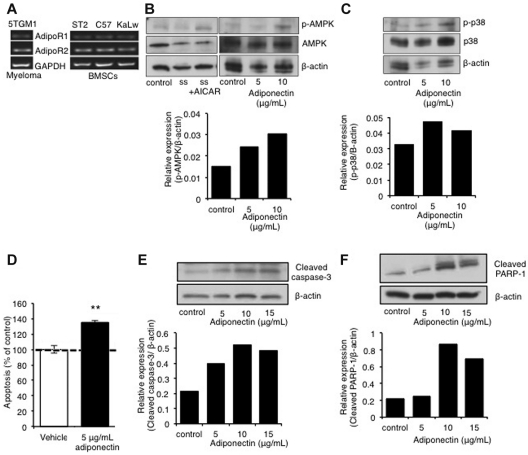
The function of adiponectin in myeloma biology is completely unknown. Because our studies demonstrated a significant decrease in host-derived adiponectin in myeloma-permissive KaLwRij mice, we next investigated adiponectin receptor expression in cells of the bone marrow microenvironment. Primary BMSCs from both C57Bl6 and KaLwRij mice expressed both adiponectin receptors 1 (AdipoR1) and 2 (AdipoR2; Figure 4A), as did 5TGM1 myeloma cells. The detection of adiponectin receptor expression on 5TGM1 myeloma cells suggested that bone marrow–derived adiponectin might have direct effects on myeloma cells. Adiponectin signaling has been previously reported to require phosphorylation-dependent activation of AMP kinase and MAP kinase.21,22 Treatment of 5TGM1 myeloma cells with recombinant adiponectin for 48 hours resulted in activation of the downstream signaling kinase, AMPK (Figure 4B). The level of AMPK activation was comparable with the activation seen after treatment with the known AMPK activator, aminoimidazole carboxamide ribonucleotide (Figure 4B). In addition, adiponectin treatment also resulted in the activation of p38 (Figure 4C).
Figure 4.
Adiponectin induces myeloma cell apoptosis. (A) Adiponectin receptor expression measured by RT-PCR in 5TGM1 myeloma cells, ST2, C57Bl6, and KaLwRij BMSCs. (B) Western blot of AMPK activation after treatment of 5TGM1 myeloma cells with 5 to 15 μg/mL adiponectin for 48 hours. (C) Western blot of p38 kinase activation after treatment of 5TGM1 myeloma cells with 5 to 10 μg/mL adiponectin for 48 hours. (D) Proportion of apoptotic 5TGM1 myeloma cells, as measured by annexin V/propidium iodide staining and flow cytometric analysis, after treatment with 5 μg/mL adiponectin for 48 hours. **P < .01, compared with control (data represent 3 independent experiments). (E) Western blot of cleavage of caspase 3 after treatment of 5TGM1 myeloma cells with 5 to 15 μg/mL adiponectin for 48 hours. (F) Western blot of cleavage of PARP-1 after treatment of 5TGM1 myeloma cells with 5 to 15 μg/mL adiponectin for 48 hours.
Adiponectin is known to induce apoptosis in a number of solid tumors, including breast cancer. Its role in myeloma is unknown; however, our in vivo studies suggest a tumor-suppressive effect. Furthermore, the activation of AMPK has been shown to inhibit growth of myeloma cells and other types of cancer cells.23–25 Given this inhibition, we determined the downstream effects of adiponectin on myeloma cells. 5TGM1 myeloma cells treated with adiponectin showed a significant decrease in viability and a significant increase in the percentage of apoptotic cells compared with vehicle-treated control cells (Figure 4D). Moreover, there was an increase in expression of cleaved caspase-3 (Figure 4E) and PARP-1 cleavage (Figure 4F) present in 5TGM1 myeloma cells, indicative of apoptotic signaling activation. Although these studies indicate a mechanism through which adiponectin exerts a tumor-suppressive effect, the potential for targeting adiponectin clinically remained to be demonstrated.
L-4F increases adiponectin production in vitro and in vivo
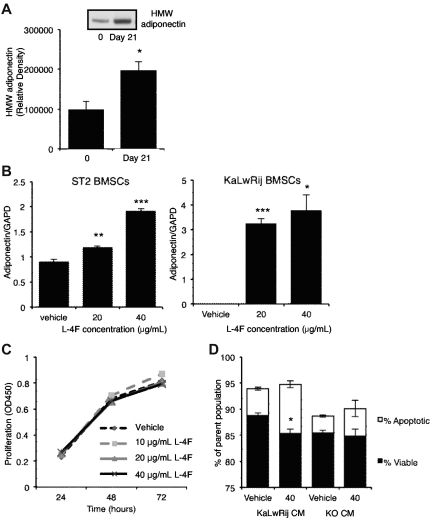
Because our data strongly suggest that a decrease in host-derived adiponectin promotes myeloma, we hypothesized that pharmacologic enhancement of adiponectin levels may represent a novel therapeutic approach. To increase adiponectin production, we used an apolipoprotein mimetic peptide (L-4F) reported to increases serum concentrations of HMW adiponectin in obese mice.26 First, the low basal levels of adiponectin in KaLwRij mice were increased to a level comparable with normal C57Bl6 mice (Figure 5A). To determine whether L-4F could induce adiponectin expression in BMSCs, particularly from KaLwRij mice with decreased adiponectin expression, normal ST2 BMSCs and primary BMSCs isolated from myeloma-permissive KaLwRij mice were treated with L-4F. Both ST2 and KaLwRij BMSCs showed a significant induction of adiponectin expression within 48 hours. This increase in expression was demonstrated at both 20 and 40 μg/mL (Figure 5B). Interestingly, the KaLwRij BMSCs showed adiponectin expression after L-4F treatment, despite basal expression being undetectable. In contrast, 5TGM1 myeloma cells treated with L-4F failed to induce adiponectin expression, which remained undetectable (data not shown).
Figure 5.
L-4F treatment increases circulating adiponectin in vivo and increases adiponectin expression by BMSCs. (A) Expression of HMW adiponectin, as measured by Western blot, in mice treated with L-4F for 21 days, compared with baseline. A representative Western blot image is shown. Data are mean ± SEM of 5 mice. *P < .05. (B) Quantitative real-time PCR measurement of adiponectin/GAPDH ratios in ST2 and KaLwRij BMSCs after treatment with L-4F for 48 hours. **P < .01 and ***P < .005, compared with control. (C) Myeloma cell proliferation, as measured by MTS assay, after treatment with 10 to 40 μg/mL L-4F for 24 to 72 hours. (D) Myeloma cells were treated with 20% conditioned media from KaLwRij or adiponectin knockout BMSCs that had been treated with 40 μg/mL L-4F or vehicle control. Apoptotic and viable cells were quantitated by annexin V/propidium iodide staining and flow cytometric analysis. *P < .05, compared with KaLwRij vehicle control. Data are mean ± SEM.
Increasing concentrations of L-4F did not inhibit 5TGM1 myeloma cell viability (Figure 5C). However, treatment of 5TGM1 myeloma cells with conditioned media from KaLwRij BMSCs treated with L-4F showed a significant increase (82%) in the percentage of apoptotic cells (Figure 5D). In contrast, treatment of 5TGM1 myeloma cells with conditioned media from L-4F–treated Adipo−/− BMSCs had no significant effect on apoptosis, indicating that L-4F can indirectly induce myeloma cell apoptosis via induction of adiponectin in BMSCs. These results suggest that BMSCs of the myeloma-permissive KaLwRij mice are not irreversibly altered and support the therapeutic potential for increasing adiponectin levels using L-4F in vivo.
L-4F reduces myeloma tumor burden via adiponectin in vivo
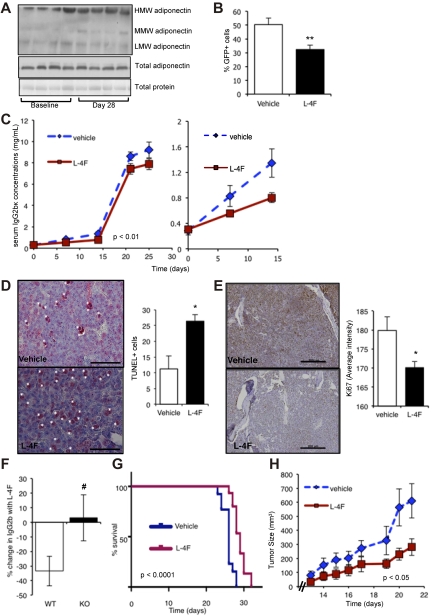
Given that L-4F increases adiponectin expression and production in vitro, we next addressed whether L-4F treatment of myeloma-bearing KaLwRij mice had an antitumor effect. Our data from human subjects who develop myeloma demonstrate that decreased adiponectin levels are present before myeloma cell presence. Because our human data suggest a role for adiponectin in the early stages of myeloma development, we first investigated the effect of L-4F administered as pretreatment, to increase host adiponectin levels, before myeloma cell inoculation. After 28 days of daily L-4F treatment, KaLwRij mice showed an increase in both HMW and total adiponectin levels present in serum (Figure 6A), similar to that seen in normal C57Bl6 animals. At the 28-day time point, mice were inoculated with 5TGM1 myeloma cells. Treatment with L-4F or vehicle was continued throughout the experiment to maintain the change in the host microenvironment. Mice treated with L-4F had a significant reduction in tumor burden throughout disease progression (Figure 6B-C). Interestingly, the decrease in tumor burden was seen early in myeloma development, suggesting that changes in adiponectin are important for tumor establishment. Immunohistochemical analysis of the bone marrow demonstrated a significant increase in the number of apoptotic myeloma cells in mice treated with L-4F compared with vehicle-treated mice (Figure 6D). There was also a significant decrease in Ki67 staining and a 55% decrease in phospho-histone H3-positive myeloma cells in the bone marrow of mice treated with L-4F, indicative of a decrease in overall myeloma cell proliferation (Figure 6E). In contrast, L-4F treatment of myeloma-bearing mice from time of tumor inoculation had no effect on tumor burden (data not shown), suggesting that modification of the host microenvironment, before myeloma cells appear, is critical for the anti-myeloma effect of L-4F and adiponectin. Importantly, the anti-myeloma effects of L-4F were not observed in adiponectin-deficient mice. There was a 33.4% ± 10.2% decrease in serum IgG2bk in WT mice treated with L-4F (P < .05), but no significant decrease in tumor burden in L-4F–treated adiponectin-deficient littermates (Figure 6F). These in vivo results confirm that the effects of L-4F to reduce myeloma tumor burden are mediated through adiponectin.
Figure 6.
L-4F has anti-myeloma effects in vivo. (A) Western blot of the different molecular weight isoforms of adiponectin in serum of KaLwRij mice treated with L-4F for 28 days. Each lane represents serum from one mouse. (B) KalwRij mice were treated with L-4F for 28 days before inoculation of 5TGM1 myeloma cells. Treatment was continued for a further 25 days, at which point mice were killed. The proportion of GFP-positive myeloma cells in BM was quantitated by flow cytometry. **P < .01, compared with vehicle control. (C) KaLwRij mice were treated as described in panel B and tumor burden monitored by serum IgG2bκ ELISA. Two-way ANOVA demonstrated a significant difference in the rate of tumor development in L-4F-treated mice compared with control (P < .01). Days 0 to 15 are expanded in the second panel to demonstrate differences in tumor burden detectable at early time points. (D) Apoptotic myeloma cells in the BM were quantitated by TUNEL staining. A representative image is shown. White asterisks represent TUNEL-positive cells. Bar represents 50 μm. *P < .05, compared with vehicle control. (E) Proliferation was quantitated in the BM by immunostaining for Ki-67. Bars represent 200 μm. *P < .05, compared with vehicle control (Vehicle, n = 9; L-4F, n = 10). (F) Adiponectin-deficient mice (KO) or WT controls were treated with L-4F for 28 days before inoculation of 5TGM1 myeloma cells. Treatment was continued for a further 25 days, at which point mice were killed. Tumor burden was monitored by serum IgG2bκ ELISA. Data are expressed as percentage change in serum IgG2bk after treatment with L-4F. #P < .05 compared with WT. (G) KaLwRij mice were treated with L-4F for 28 days before inoculation of 5TGM1 myeloma cells. Treatment was continued until time of paraplegia, as a surrogate for survival. Data are displayed as a Kaplan-Meier plot, and a log-rank (Mantel-Cox) test demonstrated a significant increase in survival with L-4F treatment (P < .0001, n = 15). (H) KaLwRij mice were inoculated subcutaneously with 5TGM1 myeloma cells and treated with L-4F. Tumor volume was measured daily (Vehicle, n = 7; L-4F, n = 8). Two-way ANOVA demonstrated a significant difference in the rate of tumor development in L-4F-treated mice compared with control (P < .05). Data are mean ± SEM.
To further investigate the anti-myeloma effects of L-4F in vivo, we examined whether L-4F treatment could increase survival or impair myeloma cell growth independent of the bone marrow microenvironment. Myeloma-bearing mice treated with L-4F showed a significant increase in survival compared with vehicle-treated animals (P < .0001, Figure 6G). Finally, because adiponectin is a secreted factor detected in the serum, we used L-4F in a plasmacytoma model to test the anti-myeloma effects independent from the bone marrow microenvironment. KaLwRij mice were treated daily with L-4F after subcutaneous injection of 5TGM1 myeloma cells. L-4F treatment resulted in a significant decrease in tumor growth, compared with vehicle-treated mice (P < .05, Figure 6H), that was associated with a significant 8-fold increase in myeloma cell apoptosis.
L-4F decreases myeloma bone disease
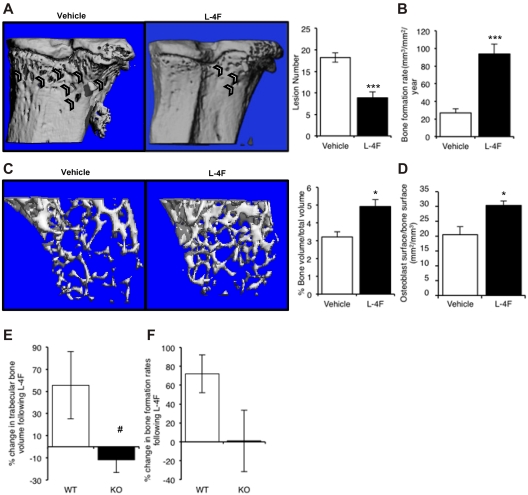
Myeloma is associated with a destructive osteolytic disease; therefore, when investigating new potential therapeutic options, it is important to consider potential benefits for myeloma bone disease in addition to antitumor effects. As previously described and commensurate with human myeloma bone disease, myeloma-bearing KaLwRij mice have reduced trabecular bone volume, increased numbers of both osteolytic lesions and osteoclasts, and decreased osteoblast numbers and bone formation rates. Micro-CT analysis showed that treatment with L-4F resulted in significantly fewer osteolytic lesions within cortical bone, relative to vehicle-treated mice (Figure 7A). Histomorphometric analysis demonstrated a 26% increase in trabecular bone volume, no significant differences in osteoclast numbers, and a significant increase in bone formation rates in L-4F–treated mice compared with vehicle (Figure 7B).
Figure 7.
L-4F prevents myeloma bone disease and increases bone volume and bone formation in non–tumor-bearing mice. (A) KaLwRij mice were treated with L-4F for 28 days before inoculation of 5TGM1 myeloma cells. Treatment was continued for a further 25 days, at which point mice were killed. Representative images of tibiae of vehicle and L-4F treated are shown. Osteolytic lesions through cortical bone were quantitated after micro-CT analysis. Vehicle, n = 9; L-4F, n = 10. (B) Bone formation rates in myeloma-bearing control and L-4F–treated mice were quantitated by dynamic histomorphometry. (C) KaLwRij mice were treated with L-4F for 28 days. Micro-CT analysis demonstrated a significant increase in trabecular bone volume. N = 5 per group. (D) Osteoblast number in control and L-4F–treated mice was quantitated by histomorphometry. (E) Adiponectin-deficient mice (KO) or WT controls were treated with L-4F for 28 days before inoculation of 5TGM1 myeloma cells. Treatment was continued for a further 25 days, at which point mice were killed. Trabecular bone volume was quantitated by micro-CT analysis, and rates of bone formation were quantitated by dynamic histomorphometry (F). (E-F) Data are presented as percentage change in response to treatment with L-4F. Data are mean ± SEM. *P < .05 and ***P < .001, compared with vehicle. #P < .05, compared with WT.
To determine whether L-4F had effects on bone independent of tumor cell presence, we assessed the bone parameters in nontumor mice treated with vehicle or L-4F. After L-4F treatment, mice showed a significant increase in trabecular bone volume, measured by micro-CT, compared with vehicle-treated animals (Figure 7C). Histomorphometry showed that L-4F treatment induced a significant increase in both osteoblasts (Figure 7D) and bone formation rates (data not shown). In contrast to the increase in osteoblast number and activity seen in response to L-4F, there was no significant change in the number of osteoclasts (data not shown). As such, these data provide strong evidence that L-4F has positive effects on both myeloma bone disease and normal bone, probably mediated through increasing osteoblastic bone formation. Importantly, the effects of L-4F on myeloma bone disease were not observed in adiponectin-deficient mice; myeloma-bearing adiponectin-knockout mice treated with L-4F had no increase in trabecular bone volume (Figure 7E) or rates of bone formation (Figure 7F), compared with WT mice. This confirms that the effects of L-4F to reduce myeloma bone disease are mediated through adiponectin.
Discussion
The present study identifies adiponectin as a novel mediator in the pathogenesis of myeloma and provides direct in vivo evidence for an association between decreased adiponectin levels and myeloma establishment and progression. In the preclinical murine model of myeloma, decreased circulating adiponectin was observed in the myeloma-permissive KaLwRij mice, compared with nonpermissive C57Bl6 mice. These observations were supported by clinical evidence demonstrating that MGUS patients who progressed to myeloma had decreased serum adiponectin concentrations. Adiponectin deficiency exacerbated myeloma pathogenesis, whereas increasing circulating adiponectin in myeloma-bearing mice resulted in reduced tumor burden, prevention of myeloma bone disease, and increased overall survival. These data demonstrate that adiponectin is tumor-suppressive and decreased levels contribute to myeloma pathogenesis. Furthermore, we have established the potential therapeutic benefit of increasing adiponectin levels in the treatment of myeloma.
In almost all cases, myeloma is preceded by the non-malignant plasma cell disorder MGUS. However, not all patients with MGUS will develop myeloma, with studies demonstrating a rate of progression of 1% per year, with a rate of disease progression of 10% at 10 years and 21% at 20 years.27 The underlying mechanisms behind the progression of MGUS to myeloma are not well understood, and it is currently not possible to definitively identify the patient with MGUS who will develop myeloma. In the present study, we identified a significant reduction in adiponectin concentrations in a subset of MGUS patients who subsequently progressed to myeloma, compared with those MGUS patients who did not. Furthermore, we observed a negative correlation between adiponectin concentrations and monoclonal protein levels, which have previously been shown to predict progression to myeloma. No association was observed between adiponectin concentrations and evidence of bone disease. Strikingly, these results were only observed in female patients. Adiponectin concentrations are increased in females, and our data are in support of this.28,29 This gender difference may be a result of testosterone-mediated inhibition of adiponectin secretion.29,30 However, we found no significant difference in testosterone levels in patients with MGUS, suggesting that the reduction in adiponectin in female patients with MGUS who progress to myeloma is not dependent on testosterone. There is no evidence to suggest that gender is associated with risk of progression from MGUS to myeloma, although the incidence of myeloma is greater in males than in females. Although only a subset of our overall cohort, our results raise the intriguing possibility that serum adiponectin concentrations may be a beneficial biomarker in predicting the likelihood of myeloma progression from MGUS in women.
To date, there are very few studies measuring circulating adiponectin in plasma cell disorders, with only 2 studies indicating a potential association between abnormal adipokine production and myeloma.31,32 However, adiponectin is inversely correlated with obesity, and there is increasing evidence to suggest an association between obesity, MGUS, and myeloma.33,34 Interestingly, in the present study, all samples were age-, sex-, and BMI-matched; therefore, it is unlikely that the differences observed in adiponectin concentrations are simply a reflection of changes in obesity. Furthermore, we have confirmed that differences in glucose, testosterone, or the presence of a metabolic syndrome were not associated with progression from MGUS to myeloma. Our studies suggest that differences in host-derived adiponectin may be a major contributing factor in myeloma pathogenesis, which is a unique and unexplored concept.
In this study, we demonstrate that reduced or absent adiponectin promotes myeloma progression and we provide strong evidence for a tumor-suppressive role for adiponectin in myeloma. Adiponectin can directly induce apoptosis of myeloma cells, myeloma cell apoptosis is reduced in myeloma-bearing adiponectin-deficient mice, and increasing adiponectin via L-4F increased apoptosis of myeloma cells in vivo. Adiponectin-induced apoptosis was associated with an activation of AMPK that has been shown to inhibit myeloma cell growth in vitro, supporting AMPK activation as a potential mechanism by which adiponectin can reduce myeloma tumor burden in vivo.23 Adiponectin haploinsufficiency was shown to promote mammary tumor development, but recently, 2 independent studies have identified a proangiogenic effect of adiponectin in mammary tumor development where later stages of the disease were associated with either an increase in tumor growth or metastasis.11–13 In light of these studies, we specifically analyzed myeloma growth at different stages of disease development and identified no evidence of enhanced tumor growth in response to adiponectin. Myeloma-bearing adiponectin-deficient mice had a higher tumor burden than their WT littermate controls. Intriguingly, the increased tumor burden was apparent not only at death but also very early in disease development. Furthermore, the potent anti-myeloma effect of L-4F, mediated via adiponectin, was only observed when mice were treated with L-4F before tumor inoculation, and was again apparent as early as 7 days after tumor inoculation. These results, combined with our observation of decreased adiponectin in MGUS patients who progress to myeloma, suggest that host-derived adiponectin has a protective effect against myeloma establishment and progression, which manifests early in disease development.
Our studies suggest that adiponectin represents a novel potential therapeutic target in myeloma and raise the possibility of L-4F as a treatment for this fatal malignancy. Indeed, an oral version of L-4F is currently in clinical trials for the treatment of cardiovascular disease.35 L-4F is an apolipoprotein peptide mimetic developed for the treatment of atherosclerosis, which we and others found to significantly increase serum adiponectin concentrations in vivo.26 L-4F had no effect in adiponectin-deficient mice, suggesting that the anti-myeloma effects are mediated through adiponectin. However, we cannot exclude the possibility that the effects of L-4F may be the result of changes in the microenvironment of adiponectin-deficient mice, which have previously been shown to influence lung and melanoma growth.36 The striking effect of L-4F to reduce myeloma development when administered preventatively, combined with our observations of decreased adiponectin in MGUS patients who progress to myeloma, raise the intriguing potential for L-4F and related compounds as a prophylactic approach for those MGUS patients identified at high risk for myeloma development.
Destructive osteolytic bone disease is a major clinical feature of myeloma. We show that L-4F has positive effects on bone, in the presence or absence of tumor cells, suggesting that L-4F may be a viable treatment option for other conditions in which bone health is compromised. Our data suggest that adiponectin improves bone mass mainly through stimulation of osteoblasts. Conflicting studies in the literature indicate both a positive and negative effect of adiponectin on the skeleton.37–39 The additional benefits of L-4F on bone suggest that increasing adiponectin may be beneficial for MGUS patients who experience bone loss, as recently demonstrated by Drake et al, who found that MGUS patients experience a generalized bone loss despite the absence of lytic lesions.40
In conclusion, our studies using human and murine systems have collectively identified a novel mechanism whereby decreased adiponectin promotes tumor growth and myeloma bone disease. Moreover, our model has highlighted a clinical association between adiponectin and myeloma progression from the clinically identified, yet poorly understood, non-malignant plasma cell disorder MGUS. We have clearly demonstrated the potential for targeting adiponectin in the treatment of myeloma and the associated bone disease, identifying not only adiponectin as a therapeutic target but also apolipoprotein peptide mimetics, such as L-4F, as novel therapies for the treatment of myeloma. The inevitable fatality associated with a diagnosis of myeloma and the unpredictable nature of MGUS progression make it imperative to identify mechanisms that will ultimately enhance our understanding of disease progression and lead to new therapeutic approaches, such as targeting adiponectin, to both prevent myeloma development, and to treat patients with established myeloma and their associated bone disease.
Supplementary Material
Acknowledgments
The authors thank the Vanderbilt University Institute of Imaging Science, the Vanderbilt Medical Center Flow Cytometry Shared Resource, and the Vanderbilt Functional Genomics Shared Resource for their help with the initial analysis of microarray data, as well as F. Elefteriou, S. Hayward, and B. Fingleton for their critiques of this work.
This work was supported by the National Institutes of Health/National Cancer Institute (R01 CA-137116, C.M.E.; and P01 CA-40035, G.R.M.), the American Society of Bone and Mineral Research (C.M.E.), the Elsa U. Pardee Foundation (C.M.E.), and Leukaemia and Lymphoma Research (C.M.E.). Collection of samples from patients with MGUS and normal controls was supported in part by the National Institutes of Health/National Cancer Institute (P01 CA62242) and National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR027065). The Vanderbilt Medical Center Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.A.F. and C.M.E. conceived and designed the experiments and wrote the paper; M.T.D., J.R.E., and G.R.M. contributed ideas and discussed experiments; J.A.F. and S.T.L. performed the experiments; M.T.D. and R.A.K. provided human clinical samples and discussed analysis; and J.A.F. analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claire M. Edwards, Botnar Research Centre, University of Oxford Institute of Musculoskeletal Sciences, Old Road, Oxford OX3 7LD, United Kingdom; e-mail: claire.edwards@ndorms.ox.ac.uk.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Fowler JA, Edwards CM, Croucher PI. Tumor-host cell interactions in the bone disease of myeloma. Bone. 2010;48(1):121–128. doi: 10.1016/j.bone.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radl J, Croese JW, Zurcher C, Van Den Enden-Vieveen MHM, Margreet de Leeuw A. Animal model of human disease: multiple myeloma. Am J Pathol. 1988;132(3):593–597. [PMC free article] [PubMed] [Google Scholar]
- 4.Radl J, de Glopper E, Schuit HER, Zurcher C. Idiopathic paraprotienemia: II. Transplantation of the paraprotein-producing clone from old to young C587Bl/KaLwRij mice. J Immunol. 1979;122(2):609–613. [PubMed] [Google Scholar]
- 5.Fowler JA, Mundy GR, Lwin ST, Lynch CC, Edwards CM. A murine model of myeloma that allows genetic manipulation of the host microenvironment. Dis Model Mech. 2009;2(11):604–611. doi: 10.1242/dmm.003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrett IR, Dallas S, Radl J, Mundy GR. A murine model of human myeloma bone disease. Bone. 1997;20(6):515–520. doi: 10.1016/s8756-3282(97)00056-2. [DOI] [PubMed] [Google Scholar]
- 7.Berner HS, Lyngstadaas SP, Spahr A, et al. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35(4):842–849. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Miyoshi Y, Funahashi T, Kihara S, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9(15):5699–5704. [PubMed] [Google Scholar]
- 9.Schaffler A, Scholmerich J, Buechler C. Mechanisms of disease: adipokines and breast cancer: endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nat Clin Pract Endocrinol Metab. 2007;3(4):345–354. doi: 10.1038/ncpendmet0456. [DOI] [PubMed] [Google Scholar]
- 10.Dieudonne MN, Bussiere M, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2006;345(1):271–279. doi: 10.1016/j.bbrc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 11.Lam JB, Chow KH, Xu A, et al. Adiponectin haploinsufficiency promotes mammary tumor development in MMTV-PyVT mice by modulation of phosphatase and tensin homolog activities. PLoS One. 2009;4(3):e4968. doi: 10.1371/journal.pone.0004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denzel MS, Hebbard LW, Shostak G, Shapiro L, Cardiff RD, Ranscht B. Adiponectin deficiency limits tumor vascularization in the MMTV-PyV-mT mouse model of mammary cancer. Clin Cancer Res. 2009;15(10):3256–3264. doi: 10.1158/1078-0432.CCR-08-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landskroner-Eiger S, Qian B, Muise ES, et al. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin Cancer Res. 2009;15(10):3265–3276. doi: 10.1158/1078-0432.CCR-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dallas SL, Garrett IR, Oyayobi BO, et al. Ibandronate reduces osteolytic lesions but not tumour burden in a murine model of myeloma bone disease. Blood. 1999;93(5):1697–1706. [PubMed] [Google Scholar]
- 15.Edwards CM, Edwards JR, Lwin ST, et al. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111(5):2833–2842. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeBlanc R, Catley LP, Hideshima T, et al. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res. 2002;62(17):4996–5000. [PubMed] [Google Scholar]
- 17.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113(22):5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bluher M, Brennan AM, Kelesidis T, et al. Total and high-molecular weight adiponectin in relation to metabolic variables at baseline and in response to an exercise treatment program: comparative evaluation of three assays. Diabetes Care. 2007;30(2):280–285. doi: 10.2337/dc06-1362. [DOI] [PubMed] [Google Scholar]
- 19.Salani B, Briatore L, Andraghetti G, Adami GF, Maggi D, Cordera R. High-molecular weight adiponectin isoforms increase after biliopancreatic diversion in obese subjects. Obesity (Silver Spring) 2006;14(9):1511–1514. doi: 10.1038/oby.2006.174. [DOI] [PubMed] [Google Scholar]
- 20.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423(6941):762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 22.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 23.Baumann P, Mandl-Weber S, Emmerich B, Straka C, Schmidmaier R. Activation of adenosine monophosphate activated protein kinase inhibits growth of multiple myeloma cells. Exp Cell Res. 2007;313(16):3592–3603. doi: 10.1016/j.yexcr.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Angulo AM, Meric-Bernstam F. Metformin: a therapeutic opportunity in breast cancer. Clin Cancer Res. 2010;16(6):1695–1700. doi: 10.1158/1078-0432.CCR-09-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodard J, Platanias LC. AMP-activated kinase (AMPK)-generated signals in malignant melanoma cell growth and survival. Biochem Biophys Res Commun. 2010;398(1):135–139. doi: 10.1016/j.bbrc.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson SJ, Drummond G, Kim DH, et al. L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J Lipid Res. 2008;49(8):1658–1669. doi: 10.1194/jlr.M800046-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 28.Pajvani UB, Du X, Combs TP, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin: implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278(11):9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 29.Bottner A, Kratzsch J, Muller G, et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89(8):4053–4061. doi: 10.1210/jc.2004-0303. [DOI] [PubMed] [Google Scholar]
- 30.Xu A, Chan KW, Hoo RL, et al. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280(18):18073–18080. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 31.Reseland JE, Reppe S, Olstad OK, et al. Abnormal adipokine levels and leptin-induced changes in gene expression profiles in multiple myeloma. Eur J Haematol. 2009;83(5):460–470. doi: 10.1111/j.1600-0609.2009.01311.x. [DOI] [PubMed] [Google Scholar]
- 32.Dalamaga M, Karmaniolas K, Panagiotou A, et al. Low circulating adiponectin and resistin, but not leptin, levels are associated with multiple myeloma risk: a case-control study. Cancer Causes Control. 2009;20(2):193–199. doi: 10.1007/s10552-008-9233-7. [DOI] [PubMed] [Google Scholar]
- 33.Birmann BM, Giovannucci E, Rosner B, Anderson KC, Colditz GA. Body mass index, physical activity, and risk of multiple myeloma. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1474–1478. doi: 10.1158/1055-9965.EPI-07-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landgren O, Rajkumar SV, Pfeiffer RM, et al. Obesity is associated with an increased risk of monoclonal gammopathy of undetermined significance (MGUS) among African-American and Caucasian women. Blood. 2010;116:1056–1059. doi: 10.1182/blood-2010-01-262394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bloedon LT, Dunbar R, Duffy D, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49(6):1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Y, Lodish HF. Adiponectin deficiency promotes tumor growth in mice by reducing macrophage infiltration. PLoS One. 2010;5(8):e11987. doi: 10.1371/journal.pone.0011987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oshima K, Nampei A, Matsuda M, et al. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun. 2005;331(2):520–526. doi: 10.1016/j.bbrc.2005.03.210. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi N, Kukita T, Li YJ, et al. Adiponectin inhibits osteoclast formation stimulated by lipopolysaccharide from Actinobacillus actinomycetemcomitans. FEMS Immunol Med Microbiol. 2007;49(1):28–34. doi: 10.1111/j.1574-695X.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 39.Williams GA, Wang Y, Callon KE, et al. In vitro and in vivo effects of adiponectin on bone. Endocrinology. 2009;150(8):3603–3610. doi: 10.1210/en.2008-1639. [DOI] [PubMed] [Google Scholar]
- 40.Drake M, Ng A, Kumar S, et al. Increases in serum levels of Dickkopf 1 are associated with alterations in skeletal microstructure in monoclonal gammopathy of undetermined significance [abstract]. J Bone Miner Res. 2009;24(suppl 1) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.