Summary
The ATM kinase plays a critical role in the maintenance of genetic stability. ATM is activated in response to DNA damage and is essential for cell cycle checkpoints. Here, we report that ATM is activated in mitosis in the absence of DNA damage. We demonstrate that mitotic ATM activation is dependent on the Aurora-B kinase and that Aurora-B phosphorylates ATM on serine 1403. This phosphorylation event is required for mitotic ATM activation. Further, we show that loss of ATM function results in shortened mitotic timing and a defective spindle checkpoint, and that abrogation of ATM Ser1403 phosphorylation leads to this spindle checkpoint defect. We also demonstrate that mitotically-activated ATM phosphorylates Bub1, a critical kinetochore protein, on Ser314. ATM-mediated Bub1 Ser314 phosphorylation is required for Bub1 activity and is essential for the activation of the spindle checkpoint. Collectively, our data highlight mechanisms of a critical function of ATM in mitosis.
Introduction
The ATM kinase is essential for cellular responses to ionizing radiation (IR) (Shiloh, 2003). Loss of ATM in humans causes the autosomal recessive disease Ataxia-Telangiectasia (A-T). Significant clinical phenotypes of A-T include progressive neuro-degeneration, immunodeficiency, cancer predisposition and hyper-radiosensitivity. At the cellular level, ATM-deficiency displays chromosomal instability, impaired cell cycle checkpoints, increased telomeric shortening, as well as enhanced radiosensitivity. ATM is a 3056 amino acid protein which belongs to the family of PI-3K like kinases (Lavin, 2008). Large-scale proteomic analyses of proteins phosphorylated on the ATM consensus sites in response to IR have identified more than 900 phosphorylation sites encompassing over 700 proteins (Matsuoka et al., 2007). ATM activation in response to DNA damage involves intermolecular autophosphorylation on several serine residues (Ser1981, Ser367 and Ser1893) which leads to dissociation of the inactive dimer to active monomers (Bakkenist and Kastan, 2003; Kozlov et al., 2006). ATM activation also requires NBS1 and MRE11, parts of the MRN complex with RAD50, as interaction with MRN stimulates ATM autophosphorylation and kinase activity (Cariveau et al., 2007; Difilippantonio et al., 2005; Dupre et al., 2006; Falck et al., 2005; Lee and Paull, 2004, 2005, 2007).
One of the significant but less studied phenotypes of ATM deficiency is abnormalities in mitosis. Early investigations reported that A-T lymphoblastoid cells have a defective spindle checkpoint after IR (Shigeta et al., 1999; Takagi et al., 1998). A recent study using a mouse knock-out model showed that ATM-deficient cells were defective in metaphase-anaphase transition which led to abnormal karyokinesis and aneuploidy (Shen et al., 2005). A-T patients have dramatically increased aneuploidy affecting different chromosomes in the cerebellum (Iourov et al., 2009). In addition, inhibition of ATM by an ATM inhibitor (KU55933) leads to increased accumulation of chromosome aberrations (White et al., 2008, 2010). These observations suggest a potential role of ATM in mitosis.
To date, studies have mainly been focused on elucidating molecular mechanisms of ATM in the DNA damage response (DDR). These findings have helped gain insight into many of the A-T phenotypes. However, due to the complexity of A-T phenotypes, many of them cannot simply be explained by lack of DDR when the gene is mutated. Therefore, roles of ATM outside DDR need to be investigated further. In this report, we show that ATM is activated in mitosis in an Aurora-B dependent manner. We demonstrate that Aurora-B phosphorylates ATM on Ser1403 both in vitro and in vivo. We also show that phosphorylation of ATM Ser1403 is required for mitotic ATM activation. Using isogenic cell lines, we demonstrate that ATM is essential for the spindle checkpoint. Mutation of ATM Ser1403 to alanine leads to a spindle checkpoint defect. Further, we find that ATM phosphorylates Bub1, a component of the kinetochore protein complex, on Ser314 to activate the spindle checkpoint. Thus, we elucidate the mechanism of mitotic ATM activation and its role in the spindle checkpoint.
Results
Aurora-B dependent ATM activation in mitosis in the absence of DNA damage
In an attempt to study the regulatory elements of ATM Ser1981 phosphorylation and ATM activation in the absence of DNA damage, we tested the hypothesis that ATM activation might be cell cycle dependent. We synchronized HeLa cells using thymidine double-block and harvested the cells at different time points to look at possible Ser1981 phosphorylation (S1981p). We could not detect S1981p in unsynchronized cells. In synchronized cells, there were no detectable signals in cells either at G0/G1 or S phases of the cell cycle. However, there was a weak signal of S1981p in cells accumulated in G2/M phases (Figure 1A). When cells were treated with nocodazole, noticeable S1981p was observed (Figure 1B). The pattern of S1981p is similar to that of Histone H3 Ser10 phosphorylation. Since nocodazole has been reported to cause DNA damage (Giunta et al., 2010), we utilized the comet assay (to detect DNA damage induced strand breaks) and immunofluorescence microscopy staining for γ-H2AX focus formation (an index of DNA DSBs), and found that nocodazole did not induce DNA damage (Supplemental Figure S1). Therefore, ATM Ser1981 is phosphorylated in an M-phase dependent manner in the absence of DNA damage. This is consistent with a report showing that ATM can be activated by mitotic stress (Shen et al., 2006). To further test that the signal of ATM Ser1981p is indeed a measurement of ATM activity, we immunoprecipitated ATM from unperturbed or mitotic (by mitotic shake-off) HeLa cells and measured the in vitro ATM kinase activity. Using Glutathione-S-Transferase (GST)-p53 peptides (a.a. 1-101 wild-type or S15A mutant) as substrates of ATM, we observed that ATM kinase activity increased about two-fold in mitosis (Figure 1C), indicating ATM is mitotically activated in the absence of DNA damage.
Figure 1. ATM is activated in an Aurora-B dependent manner in mitosis.

HeLa cells were synchronized by thymidine double block (A) or treated with nocodazole (B) for 16h before they were harvested. Total cell lysates were immunoblotted with indicated antibodies. (C) Mitotic HeLa cells were collected by mitotic shake-off. Endogenous ATM was immunoprecipitated and subjected to the in vitro kinase assay. (D) HeLa cells transiently transfected with control or Aurora-B siRNA (with or without siRNA-resistant Aurora-B) were exposed to mock or nocodazole. Total cell lysates were immunoblotted with indicated antibodies. Molecular weights shown are in kiloDalton (kD). Also see Figure S1.
To identify regulatory elements of this mitotic ATM activation, we screened for proteins that interact with ATM specifically during mitosis. We identified several proteins, and one of them is the chromosome-passenger kinase Aurora-B. Since Aurora-B is activated in mitosis, we hypothesized that Aurora-B might be required for mitotic ATM activation. To test this hypothesis, we knocked-down Aurora-B by siRNA in HeLa cells. We found that in the absence of Aurora-B, Ser1981p was diminished in response to nocodazole treatment (Figure 1D). Reintroduction of an siRNA resistant Aurora-B into Aurora-B knock-down cells rescued Ser1981p (Figure 1D). It is noted that siRNA Aurora-B knock-down cells have a lower mitotic index (18%) than control cells (40%) in response to nocodazole (Supplemental Figure S1C). To exclude the possibility of defective ATM Ser1981p being a result of the lower mitotic index, we conducted a time-course experiment in control cells treated with nocodazole. At each time point, the mitotic index was recorded and Ser1981p was measured. We found that when control cells reached a mitotic index of 10%, Ser1981p was detectable. When the mitotic index reached 20%, similar to Aurora-B knock-down cells after nocodazole treatment, we observed a strong Ser1981p signal (Supplemental Figure S1D), indicating that lack of ATM Ser1981 phosphorylation in Aurora-B knock-down cells was not due to the reduced number of mitotic cells. Together, these observations indicate that Aurora-B is required for mitotic ATM activation.
Mitotic ATM activation is mechanistically distinct from IR-induced ATM activation
We then investigated whether mitotic ATM activation used a mechanism similar to that induced by IR. In response to DNA damage, ATM activation requires functional NBS1. However, we observed intact mitotic ATM activation in NBS1-deficient cells (Supplemental Figure S1E). We also observed that signaling pathways involving ATM and Aurora-B were different in response to mitotic stress versus DNA damage. For example, Aurora-B is inhibited in an ATM-dependent manner after DNA damage (Monaco et al., 2005; Tang et al., 2008), suggesting that Aurora-B is a downstream target of ATM in DDR. However, Aurora-B is activated during mitosis and functions as an upstream component of mitosis-dependent ATM activation (Figure 1D). Taken together, these results suggest that the mechanism governing mitotic ATM activation is different from mechanisms induced by IR.
Aurora-B phosphorylates ATM at Ser1403 in vitro
Aurora-B phosphorylates a series of targets to initiate cellular processes (Richie and Golden, 2005). Therefore, it is possible that Aurora-B might serve to activate ATM through phosphorylation. To test this possibility and to identify potential phosphorylation site(s), we conducted an in vitro kinase assay by immunoprecipitation of Aurora-B (endogenous in Figure 2A, exogenous in Figure 2B) from 293T cells. We studied the ATM protein sequence and found that several domains might be potential target sites for Aurora-B based on a consensus motif of the yeast Aurora-B homolog, Ipl1 (Guse et al., 2005; King et al., 2007). For initial screening, we included seven sequences (Supplemental Figure S2) which were individually linked to the GST tag. We found one of the peptides, which contained Ser1403 of ATM, could be detected with a strong phosphorylation signal, while the other peptides showed a weak or basal level of phosphorylation (Figure 2A). Since immunoprecipitation of endogenous Aurora-B could potentially bring down other associated kinases that could cause false-positive signals, we transfected flag-tagged wild-type Aurora-B (used as the bona fide kinase) or a kinase-dead Aurora-B (used as a negative control) into 293T cells. In order to test whether Ser1403 is the true in vitro phosphorylation site, we generated larger peptides containing the wild-type sequence or the serine to alanine mutation. We found a strong phosphorylation signal while using the wild-type ATM fragment as substrate (Figure 2B). However, mutation of serine to alanine (S1403A) completely abrogated this in vitro phosphorylation. Thus, Ser1403 of ATM appears to be the phosphorylation site for Aurora-B. To further confirm the in vitro phosphorylation, we used recombinant GST-His-Aurora-B as the pure kinase and applied the anti-phospho-Ser1403 antibody we generated for in vivo studies. As shown in Figure 2C, incubation of non-phosphorylated ATM Ser1403 peptides with the recombinant Aurora-B leads to enhanced phosphorylation, indicating that the phosphorylation event indeed happens in vitro. Ser1403 of ATM is well conserved among different organisms (Figure 2D), suggesting the functional importance of phosphorylation. It is noted that the Ser1403 sequence includes [K]×[K][S]-hydrophobic, which is slightly different from the [R/K]×[S/T]-hydrophobic or [R/K]-[R/K]-X(0-2)-[S/T] consensus motifs (Cheeseman et al., 2002; Kim et al., 2010; Meraldi et al., 2004; Tanno et al., 2010). Since this was a partial list of sequences used to screen for phosphorylation, we do not rule out the possibility of other potential Aurora-B phosphorylation sites existing on ATM.
Figure 2. Aurora-B phosphorylates ATM on Ser1403 in vitro.
(A) Immunoprecipitated Aurora-B was incubated with recombinant proteins consisting of GST fused peptides derived from the various regions of human ATM in the presence of 32P-labeled ATP. (B) Immunoprecipitated Flag-tagged wild-type (WT) or kinase-dead (KD) forms of Aurora-B were incubated with GST-fused peptides containing a.a. 1350-1450 fragments of ATM (either WT or serine 1403 mutated to alanine). (C) Phospho-(ATM-S1403p) or non-phospho-(ATM-S1403) peptides were incubated with the recombinant GST-His-Aurora-B protein in the presence of ATP. (D) Sequence homology of ATM around Ser1403 in different species. Also see Figure S2.
Mitotic-dependent ATM Ser1403 phosphorylation in vivo
The initial specificity test of the anti-phospho ATM Ser1403 antibody (ATM Ser1403p) was conducted using a non-phosphorylated blocking peptide (Supplemental Figure S3A). To further test the antibody specificity in vivo, we generated the flag-tagged Ser1403 to alanine mutant (S1403A) construct. We found that ATM S1403A was not detected by the antibody, while wild-type ATM displayed a strong signal when treated with nocodazole (Supplemental Figure S3B).
We then used this antibody to probe ATM for phosphorylation of Ser1403 in vivo. We found no reactivity was seen in cells treated with IR (Figure 3A). However, in extracts from nocodazole treated cells, significant reactivity was observed, demonstrating a mitotic-dependent ATM Ser1403 phosphorylation.
Figure 3. Aurora-B mediates ATM Ser1403 phosphorylation during mitosis.

(A) ATM Ser1403 is phosphorylated in response to nocodazole treatment. HeLa cells were treated with mock, IR (6Gy), or nocodazole and subjected to Western blot using the indicated antibodies. (B) EBV-transformed lymphoblast cell lines, possessing proficient (GM0536) or deficient (GM1526) ATM, were treated with nocodazole. Total cell lysates were treated with λ-phosphatase followed by Western blot. (C) HeLa cells were transfected with control or ATM shRNA followed by mock, nocodazole, or IR (6Gy) treatment. (D, E, F) Exponentially growing HeLa cells were stained with β-tubulin, CREST, ATM S1403p and DAPI. (G) HeLa cells were stained with ATM S1403p (red), Aurora-B (green) and DAPI (blue). Two representative images are shown (Image 1 and Image 2). Also see Figure S3.
The specificity of the antibody was further demonstrated in lymphoblastic cell lines (GM0536 or GM1526) and HeLa cells transfected with control siRNA or ATM siRNA. The S1403p antibody does not recognize any signal in GM1526 (ATM-/-) cells, and λ-phosphatase abrogates the phosphorylation signal in GM0536 (ATM+/+) cells (Figure 3B). Concordantly, ATM siRNA knock-down in HeLa cells largely abrogates the phosphorylation signal observed in control cells (Supplemental Figure S3C).
Interestingly, we found that SMC1 Ser966, an ATM target site in response to IR (Kim et al., 2002; Yazdi et al., 2002), was not phosphorylated after nocodazole treatment despite mitotic ATM activation (Figure 3A), suggesting that M-phase activated ATM might have a different set of substrates than DNA damage activated ATM. Further experiments were conducted to compare ATM substrate phosphorylation in mitosis or in response to DNA damage in control or ATM knock-down cell lines (for the description of the cell lines, see Figure 5C). We found that p53 Ser15 phosphorylation was not induced in response to nocodazole treatment. However, Chk2 Thr68 phosphorylation is induced both in response to nocodazole and IR (Figure 3C), indicating that the substrate lists of ATM in response to mitosis and IR differ but are overlapping.
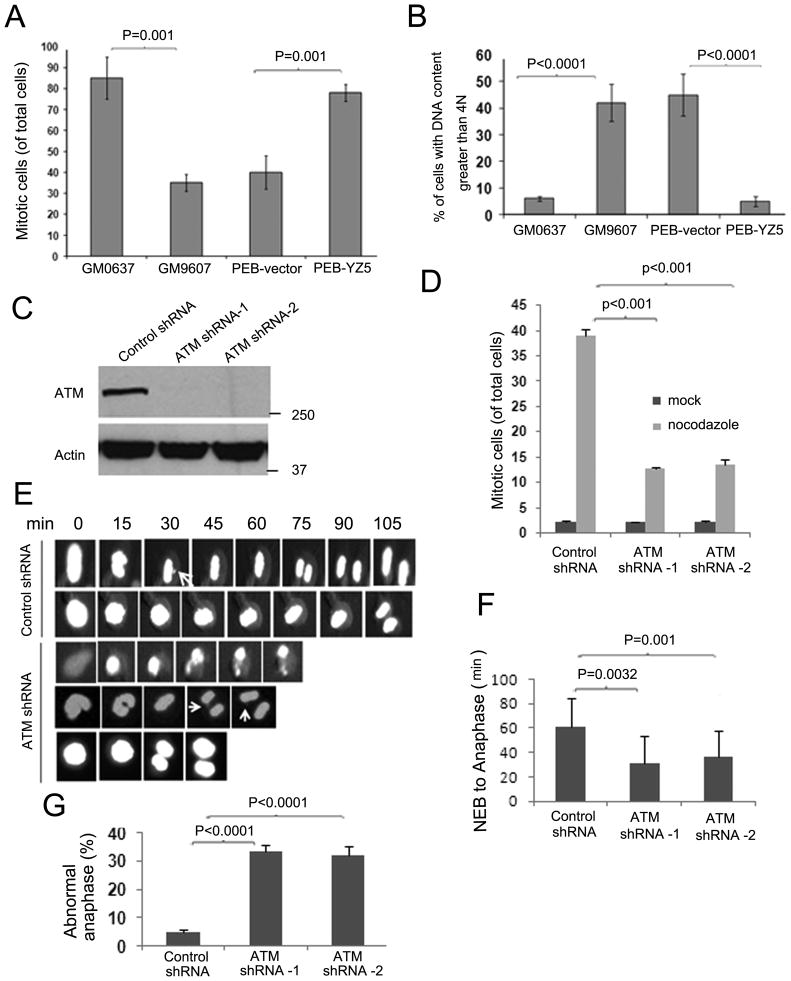
Figure 5. ATM deficiency leads to an impaired spindle checkpoint.
(A) Cells were treated with nocodazole and the mitotic index was measured by flow cytometric based Histone H3 S10p staining. The percentage of mitotic cells shown represents the averages of at least triplicate samples. Error bars are standard deviations. (B) Cells were treated with nocodazole for 48h and stained with propidium iodide to measure DNA content. Percentages of cells with greater than 4N DNA content are shown. Error bars are standard deviations of at least triplicate samples. (C) Generation of isogenic stable cell lines with control or ATM knock-down. HeLa cells were stably transfected with either control or ATM shRNA. ATM expression levels for control and ATM shRNA clones #1 and #2 are shown. (D) The isogenic cell lines were treated with nocodazole and the mitotic index was obtained using flow cytometric based Histone H3 S10p staining. The average percentages of mitotic cells are shown. Error bars are standard deviations of at least triplicate samples. (E) Control and ATM knock-down cells expressing H2B-GFP were imaged at the onset of mitosis to monitor chromosome dynamics. Representative fluorescence video microscopy series are shown. Arrows indicate maloriented and unaligned chromosomes. (F) The average time from nuclear envelope breakdown (NEB) to anaphase onset in stable cell lines expressing control or ATM shRNA was measured by time-lapse microscopy. (G) Percentage of aberrant anaphases in ATM-depleted and control cells as shown in Figure 5E. Error bars are standard deviations of at least triplicate samples. Also see Figure S4.
To further test ATM Ser1403 phosphorylation, we performed immunofluorescence microscopy, and found that no signal was detectable in interphase cells (Figure 3D, E, G). We found that ATM-Ser1403p has a diffused nuclear localization from prophase to anaphase (Figure 3D). More specifically, S1403p can be observed at chromosomes in prophase, the centromere in metaphase and the central mitotic spindle in anaphase (Figure 3D, E, F, Supplemental Figure S3E). S1403p signals also showed in the midbody of telophase (Figure 3D and G). Co-staining with Aurora-B showed that ATM S1403p and Aurora B co-localized in prophase (Figure 3G, Image 2, yellow arrow), in anaphase (Figure 3G, Image 1, yellow arrow) and in telophase (Figure 3G Image 2, yellow arrow). However, while ATM S1403p also localized to the spindle pole in prometaphase and metaphase, it did not co-localize with Aurora-B (Figure 3G, Image 1, green arrow). It is noted that the ATM S1403p antibody reveals no signal in ATM siRNA knock-down cells (Supplemental Figure S3D), further demonstrating the specificity of the antibody.
Aurora-B mediated ATM Ser1403 phosphorylation is required for mitotic ATM activation
We then performed Western blot analysis using the antibody in cells transfected with control or Aurora-B siRNA. We found that knocking-down Aurora-B abrogated ATM Ser1403 phosphorylation (Figure 4A), indicating that Aurora-B is required for the event. We also conducted experiments using Hesperadin as an Aurora-B inhibitor and found that Hesperadin abrogated ATM S1403p in a dose-dependent manner (Figure 4B). Combined with the Aurora-B in vitro kinase assay data, we conclude that Aurora-B phosphorylates ATM at Ser1403 during mitosis in vivo.
Figure 4. Aurora-B phosphorylates ATM Ser1403 to activate ATM in mitosis.
(A) Aurora-B is required for ATM Ser1403 phosphorylation in mitosis. HeLa cells, transfected with control or Aurora-B siRNA, were treated with mock or nocodazole and subjected to Western blot using the indicated antibodies. (B) HeLa cells were treated with the Aurora-B inhibitor, Hesperadin, in the absence or presence of nocodazole. (C) ATM Ser1403 phosphorylation is required for mitotic ATM activation. Vector, WT, S1403A or S1981A mutants of ATM were transiently transfected into 293T cells and treated with nocodazole. Exogenous proteins were then immunoprecipitated with an anti-Flag antibody followed by Western blot using the indicated antibodies.
To test whether Ser1403 phosphorylation is required for mitotic ATM activation, we expressed vector-only, wild-type, S1403A or S1981A mutants of ATM into 293T cells and immunoprecipitated the flag-tagged ATMs. Using anti-S1403p and anti-S1981p antibodies, we found that S1403A abrogated mitotic-dependent Ser1981 phosphorylation (Figure 4C). However, the S1981A mutation did not affect Ser1403 phosphorylation. Therefore, ATM Ser1403 phosphorylation is required for mitotic ATM activation. This conclusion is further supported by evidence that ATM-mediated Bub1 Ser314 phosphorylation is abrogated by S1403A expression (Figure 7C).
Figure 7. ATM phosphorylates Bub1 on Ser314 to activate the spindle checkpoint.
Isogenic HeLa cell lines with proficient (control shRNA) or deficient (ATM shRNA) ATM were treated with mock or nocodazole and subjected to immunoblotting with the indicated antibodies. (C) HeLa cells transfected with vector, WT or S1403A ATM were treated with nocodazole and immunoblotted with the indicated antibodies. (D) HeLa cells were transfected with vector, WT or S314A Bub1. 36 h after transfection, cells were treated with nocodazole and subjected to flow cytometry analysis. The averages of the mitotic index of at least three independent samples are shown. Error bars are standard deviations.
ATM is required for the spindle checkpoint
Aneuploidy has been documented in ATM-deficient cell lines, animal models and clinical samples (Iourov et al., 2009; Shen et al., 2005; Shigeta et al., 1999; Takagi et al., 1998). However, no report exists on whether ATM is required for the spindle checkpoint in the absence of DNA damage. To test this possibility, we first utilized SV-40 transformed fibroblast cell lines with proficient (GM0637) or deficient (GM9607) ATM to examine the spindle checkpoint in the presence of nocodazole. As shown in Figure 5A, GM9607 cells displayed a significantly impaired mitotic response compared to GM0637 cells. Using isogenic fibroblast cell lines with deficient ATM (PEB-vector) or wild-type ATM reconstituted (PEB-YZ5) (Ziv et al., 1997), we found that the spindle checkpoint defect in PEB-vector cells was rescued by wild-type ATM complementation. We also incubated cells with nocodazole for a prolonged period (48 h) and found that while GM0637 and PEB-YZ5 cells had 6% of cells possessing greater than 4N DNA content, at least 40% of GM9607 and PEB-vector cells underwent another round of DNA replication (with larger than 4N DNA content) in the absence of cell division (Figure 5B).
In order to fully investigate the role of ATM in mitosis, we generated isogenic HeLa cell lines stably expressing either control shRNA or ATM shRNA (Figure 5C). Compared with control cells, ATM knock-down cells failed to arrest in mitosis after nocodazole treatment (Figure 5D and Supplemental Figure S4A). We then monitored the chromosome dynamics and timing of mitosis by time-lapse microscopy. Analysis of the results revealed that ATM knock-down cells proceeded into anaphase despite the presence of maloriented and unaligned chromosomes (Figure 5E, bottom; Supplemental Video S1); whereas control cells did not progress into anaphase until the completion of chromosome alignment on the metaphase plate (Figure 5E, top; Supplemental Video S2). In addition, we found that ATM knock-down cells exited mitosis much earlier than control cells. The time between nuclear envelope breakdown (NEB) and anaphase onset was 61.4±22.7 min (n=21) in control cells. In ATM knock-down cells, the time was only 31.5±21.7 min (n=20) for ATM shRNA-1 and 36.8±20.5 min (n=21) for ATM shRNA-2 (Figure 5F). Moreover, the number of lagging chromosomes increased in ATM knockdown cells in comparison with control cells (Figure 5G). Since shortened mitotic timing is often associated with decreased Mad2 expression levels, we assessed a potential off-target effect on Mad2. As shown in Supplemental Figure S4B, Mad 2 expression is not altered in ATM knock-down cells. Taken together, these investigations strongly demonstrate an essential role of ATM in the spindle checkpoint.
Aurora-B mediated ATM Ser1403 phosphorylation is required for the spindle checkpoint
To investigate the functional significance of Aurora-B phosphorylation of ATM, we transfected vector-only, wild-type ATM (ATM-WT), ATM-S1403A or ATM-S1403D plasmids into HeLa cells and assessed spindle checkpoint activation. We found that following nocodazole treatment, cells expressing S1403A showed a significantly lower mitotic index than vector or ATM-WT transfected cells (Figure 6A and 6B). However, S1403D, which mimics phosphorylation, did not affect the checkpoint activation. Further, time-lapse microscopy experiments indicated that cells expressing S1403A showed shortened mitotic timing (Figure 6C, 6D and Supplemental Videos S3-5), in which the time from NEB to anaphase onset was 40.9±8.1 min (n=66), compared with cells expressing vector (59.5 ±19.4 min, n=81) and ATM-WT (58.4+19.9 min, n=66).
Figure 6. ATM Ser1403 phosphorylation is critical for activation of the spindle checkpoint.
(A)HeLa cells transfected with vector, WT, S1403A, or S1403D ATM were exposed to nocodazole for 16h and stained for Histone H3p to obtain the mitotic index. Average percentages of mitotic cells are shown. Error bars are standard deviations of at least triplicate samples. (B) Expressions of flag-tagged ATM proteins were assessed by Western blot. (C) Live cell time-lapse imaging. (D) Vector, ATM-WT or ATM-S1403A was co-transfected with H2B-GFP into HeLa cells. The time from NEB to anaphase onset was measured by time-lapse microscopy. Each diamond in the scatter plot represents a single cell. Cell numbers were 81 (vector), 66 (ATM-WT) and 66 (ATM-S1403A). (E) The EBV-transformed lymphoblast cell line, GM1526, and (F) the SV-40 transformed fibroblast cell line, GM9607, were transfected with vector, WT, or S1403A ATM by electroporation. 36h after transfection, cells were treated with mock or nocodazole followed by flow-cytometry based assay to assess the mitotic index. Expression of transfected proteins is also shown. Error bars are standard deviations of at least triplicate samples. (G) Vector, WT or S1403A ATM was co-transfected with H2B-GFP into GM9607 cells. The time from NEB to anaphase onset was measured by time-lapse microscopy. Cell numbers were 49 (vector), 51 (ATM-WT) and 52 (ATM-S1403A). (H) GM9607 cells were transfected with vector, WT, S1981A or S1403A ATM. 36h after transfection, cells were treated with mock or IR (6Gy), and 2h later cells were subjected to H3 Ser10p staining by flow cytometry. Average percentages of mitotic cells are shown. Error bars are standard deviations of at least triplicate samples.
We also conducted complementation experiments expressing vector only, wild-type or S1403A ATM into ATM-deficient cell lines, GM1526 and GM9607. To ensure transfection efficiency, we utilized electroporation transfection and reached at least 60% efficiency in both cell lines. GM1526 cells expressing wild-type ATM showed a normal response to nocodazole (Figure 6E), however, introduction of S1403A failed to rescue the A-T phenotype, indicating a critical role of ATM Ser1403 phosphorylation in the spindle checkpoint. We confirmed this result in GM9607 cells (Figure 6F). Additionally, time-lapse experiments showed that GM9607 cells had significantly shortened mitotic timing (Figure 6G), and reintroducing wild-type ATM increased mitotic timing. However, S1403A mutant ATM was not capable of rescuing the phenotype. Together, these results strongly demonstrate that ATM Ser1403 phosphorylation is critical for activation of the spindle checkpoint.
To investigate whether S1403A can rescue A-T phenotypes in response to IR, we conducted complementation experiments in GM9607 cells. We transfected vector, wild-type, S1981A or S1403A into the cells and assessed the IR-induced G2/M checkpoint. We found that while ATM S1981A failed to rescue the defect in A-T cells, either wild-type or S1403A was capable of restoring the checkpoint (Figure 6H). This indicates that ATM Ser1403 phosphorylation is not essential for the IR-induced response. Combined with our data showing that Ser1403 phosphorylation is not induced by IR (Figure 3A), these observations provide further evidence that ATM is activated by different mechanisms in mitosis than in response to IR.
ATM phosphorylates Bub1 Ser314 to activate the spindle checkpoint
To fully study how ATM regulates the spindle checkpoint, we investigated the protein list that was identified previously by proteomic analysis (Matsuoka et al., 2007). Among the proteins involved in mitosis, Bub1 is one of the critical kinases for spindle checkpoint activation. Bub1 phosphorylates H2A at Thr121 to regulate chromosome segregation (Kawashima et al., 2010). We found that ATM knock-down cells have impaired H2A Thr121 phosphorylation in response to nocodazole treatment (Figure 7A), indicating that Bub1 activity in mitosis is regulated in an ATM-dependent manner. Since Bub1 Ser314 is indicated to be a phosphorylation site of ATM in DDR (Matsuoka et al., 2007), we hypothesized that ATM might phosphorylate Bub1 on Ser314 in mitosis. To test this notion, we generated an anti-phospho-Bub1 Ser314 antibody (Ser314p) and tested potential in vivo phosphorylation in the isogenic ATM cells lines. We found that Ser314p is induced in cells treated with nocodazole only when ATM is present (Figure 7B), and ATM shRNA knock-down cells showed defective Ser314p. These data demonstrate that ATM is required for Bub1 Ser314 phosphorylation in mitosis. Further experiments revealed that over-expression of the ATM S1403A mutant abrogated Ser314p (Figure 7C), indicating that Bub1 Ser314 phosphorylation is dependent on mitotic ATM activation.
To study the functional significance of ATM-mediated Bub1 phosphorylation, we generated flag-tagged constructs expressing wild-type or S314A mutant of Bub1. Over-expression of the S314A mutant Bub1 in HeLa cells led to an impaired spindle checkpoint (Figure 7D). These data demonstrate that mitotic-dependent ATM phosphorylation of Bub1 on Ser314 is required for activation of the spindle checkpoint.
Discussion
Mitosis is the critical cell cycle stage when sister chromatids are segregated. The spindle checkpoint, along with other mitotic control systems, is required to guard against errors in chromosome segregation which may lead to aneuploidy (Wells, 1996). One of the significant cellular phenotypes of ATM deficiency is chromosomal instability (Lavin, 2008). Spindle checkpoint defects as well as aneuploidy have been documented in human cell lines, animal models and clinical samples (Iourov et al., 2009; Shen et al., 2005; Shigeta et al., 1999; Takagi et al., 1998). However, mechanisms behind how loss of ATM leads to these phenotypes was less clear. Here, we provide strong evidence that ATM plays a critical role in mitotic progression and is essential for spindle checkpoint activation. ATM is activated in mitosis using a mechanism distinct from that used in response to IR. The mitotic-dependent kinase Aurora-B is the primary enzyme required for mitotic ATM activation. Aurora-B phosphorylates ATM on Ser1403 to activate the kinase, and ATM phosphorylates Bub1 on Ser314 to initiate the spindle checkpoint. These findings highlight a previously undescribed mechanism of ATM in mitotic cell cycle control.
Unlike most of the spindle checkpoint components, including Mad1, Mad2, BubR1 and Bub1, the ATM gene is not essential for embryonic survival. This creates a discrepancy as genes involved in the mitotic spindle checkpoint are thought to be essential for survival (Baker et al., 2005; Yamada and Gorbsky, 2006). However, Mad1, Mad2, BubR1 and Bub1 all have multiple functions in addition to the spindle checkpoint which may be responsible for embryonic lethality when knocked out. Indeed, Mad2 and Bub1 heterozygous cells possess a defective spindle checkpoint but are still viable (Dobles et al., 2000; Jeganathan et al., 2007; Michel et al., 2001). Concordantly, deficiency of the spindle checkpoint does not necessarily lead to embryonic lethality (Burds et al., 2005). Therefore, it is reasonable that ATM is required for the spindle checkpoint but is not an essential gene. Further supporting this notion is the evidence that the Chk2 kinase also participates in spindle checkpoint activation (Stolz et al., 2010) and is non-essential (Chen and Poon, 2008). One possible explanation for both ATM and Chk2 being components of the spindle checkpoint while not being essential for viability might be that they indirectly participate in activation of the spindle checkpoint. For example, ATM controls the activity of a kinetochore kinase Bub1, which regulates the components of the anaphase promoting complex/cyclosome (APC/C). Chk2, on the other hand, sends signals to Brca1 by phosphorylation to activate the spindle checkpoint.
A more detailed mechanism by which ATM is activated in mitosis requires further investigation. During mitosis, chromosomes condense and attach to spindles that pull the sister chromatids to opposite sides of the cell. The chromosome condensation process, regulated by histone modifications (such as H3 Ser10 phosphorylation), might stimulate ATM activation through a different mechanism than that induced by DNA DSBs. It is noted that, unlike in response to IR, NBS1 is not involved in mitotic ATM activation, nor do NBS1 deficient cells have mitotic checkpoint defects (data not shown). This is consistent with findings that ATM activation in response to oxidative stress is different from that induced by DSBs (Guo et al., 2010). During oxidative stress, the active form of ATM is a disulfide crosslinked dimer. In the present case, however, mitotic ATM activation still involves autophosphorylation (at least on Ser1981), and therefore might also have a dimer to monomer conversion. Since this is a mitosis-specific process, it requires a signal cascade to be activated at the onset of prophase and turned off at the end of telophase or cytokinesis. This is supported by our data that Aurora-B dependent ATM Ser1403 phosphorylation is required for the activation process. Although it is not clear how Ser1403 phosphorylation activates the enzyme, it is likely that phosphorylation on this well conserved site produces a structure change leading to autophosphorylation and activation.
ATM functions by phosphorylating a list of downstream targets to facilitate cell cycle checkpoints in response to DNA damage (Kastan and Bartek, 2004). For example, ATM phosphorylates SMC1 to initiate the S phase checkpoint and radiosensitivity in response to radiation treatment (Kim et al., 2002). It is interesting that this DNA damage induced event does not happen in mitosis despite ATM activation (Figure 3A). Further, we also found that p53 Ser15 was not phosphorylated in the mitotic response. On the contrary, Chk2 Thr68 phosphorylation can be induced by both mitotic stress and IR-induced DNA damage. These data indicate that ATM phosphorylates a different but overlapping list of substrates to regulate mitotic progression. Indeed, several mitotic spindle checkpoint proteins, such as Bub1, Mad1, Sgo1 and p31comet are among the substrates in DDR (Matsuoka et al., 2007). Our data showing that ATM phosphorylates Bub1 on Ser314 to activate the spindle checkpoint provide critical evidence on the mechanisms of ATM activation in mitosis. All these evidence support the notion that mitotic regulatory machinery and DNA damage pathways crosstalk (Yu, 2007).
ATM is on a growing list of DDR proteins involved in the mitotic spindle checkpoint. Other proteins, such as Chk1, Chk2, Brca1-Bard1 and Brca2, have been reported to participate in the spindle checkpoint (Ayoub et al., 2009; Joukov et al., 2006; Marmorstein et al., 2001; Stolz et al., 2010; Zachos et al., 2007). Depletion of Chk2 phosphorylation of Brca1 in mitosis leads to spindle checkpoint defects and chromosomal instability. Chk1-deficient cells show a spindle checkpoint failure which correlates with decreased Aurora-B kinase activity and impaired phosphorylation and kinetochore localization of BubR1. Furthermore, Chk1 phosphorylates Aurora-B to enhance its catalytic activity in vitro (Bourke et al., 2007; Zachos et al., 2007). Meanwhile, Chk1 is a downstream target of ATM in DDR (Zhou et al., 2002); therefore, it is likely that there is a feedback loop of the signaling pathway linking Chk1, Aurora-B and ATM in mitosis. It is also interesting to note that several other DNA damage responsive proteins (such as H2AX and 53BP1) are hyper-phosphorylated in mitosis in the absence of DNA damage (Jullien et al., 2002; McManus and Hendzel, 2005). These reports provide further evidence for the concept of mitotic ATM activation. Taken together, our data combined with the literature support a notion that mitotic checkpoints share common molecular components with the DDR machinery.
In summary, we reveal that ATM is activated during mitosis in the absence of DNA damage. Mitotic ATM activation is governed by Aurora-B phosphorylation on Ser1403. Mitotically-activated ATM phosphorylates Bub1 on Ser314 to activate the spindle checkpoint.
Experimental Procedures
Cell lines and culture
HeLa, HEK 293T (from ATCC), PEB-vector and PEB-YZ5 cells (provided by Dr. Yosef Shiloh) were maintained in DMEM containing 10% FBS and 50μg/ml of penicillin/streptomycin (HyClone). GM0637, GM9607 and GM1526 (from NIGMS) were maintained in RPMI1640. Stable cell lines were generated by transfecting control or ATM shRNA (Santa Cruz Biotechnology) into HeLa cells using FuGENE HD transfection Reagent (Roche). Puromycin resistant colonies were individually selected and detected by Western blot. Also see supplemental experimental procedures.
Cell Synchronization and Flow Cytometry
Double thymidine block was used to synchronize cells at the G1/S boundary. For mitotic arrest, 200nM nocodazole (Sigma) was added to the medium and incubated for 16h. For flow cytometry analysis, cells were permeabilized with 0.1% triton X-100 after fixation in 70% ethanol and stained with FITC conjugated Histone H3p antibody (Millipore). For DNA content, cells were stained with propidium iodide (Sigma). Data acquisition and analysis were performed by using a FACS Calibur flow cytometer (Becton Dickinson) with CellQuest software.
siRNAs
Control and Aurora B siRNA were synthesized by Dharmacon Research Inc. (Lafayette, CO) and transfected using Oligofectamine Reagent (Invitrogen). 36h after transfection, cells were incubated for 16h in media containing nocodazole and then analyzed.
Plasmids, Antibodies and Reagents
The ATM peptides were synthesized by Integrated DNA Technologies and subcloned into the vector pGEX-2T to make GST-fusion peptides. GST-p53 and GST-p53-S15A, Flag tagged ATM and S1981A plasmids were provided by Dr. Michael B. Kastan. The ATM-S1403A and S1403D mutants were generated using the QuikChange II XL Mutagenesis Kit (Stratagene) according to the manufacturer's protocol. The Aurora B siRNA resistant construct was created by Cellomics Technology (Baltimore, MD). Antibodies against ATM-S1403p and Bub1-S314p were generated by EzBioLab (Westfield, IN). The centromere antibody was kindly provided by Dr. William R. Brinkley to Dr. Kitagawa. For commercial antibodies, see Supplemental Experimental Procedures.
Live cell time-lapse imaging
To monitor chromosome dynamics, the GFP-tagged H2B plasmid (AddGene, MA) was transfected into cells. 48h later, cells were imaged every 15min for 24h in the IncuCyte system. All images were analyzed with the IncuCyte software and Image J. For details see Supplemental Experimental Procedures.
Immunofluorescence
Cells were fixed in 4% paraformaldehyde for 15min and blocked with 1% BSA for 30min. Antibodies against β-tubulin, ATM S1403p and Aurora B, and CENPE were incubated for 2h. Cells were then probed with fluorescent-conjugated secondary antibodies for 1h and observed through a Leica DM IRE2 motorized fluorescence microscope equipped with an HCX PL APO 63× or 100× oil immersion lens and an ORCA-ER high resolution digital charge coupled device camera (Hamamatsu, NJ).
Immunoprecipitation and Western blot
Cells lysates were immunoprecipitated with antibodies and protein A/G Sepharose overnight, and eluted with Laemmli buffer after washing. For Western Blot, protein extracts were separated by SDS-PAGE and transferred to nitrocellulose membranes; blots were probed with antibodies and visualized by using the Pierce chemiluminescence detection system.
In vitro kinase assays
In vitro kinase assays for ATM were performed as described (Lim et al., 2000). For Aurora-B kinase assays, immunoprecipitated Aurora-B (endogenous or flag tagged) was used as a kinase with ATM peptides as substrates in the presence of 5μCi of P32-ATP. After 30min incubation at 30°C, proteins were analyzed by SDS/PAGE followed by autoradiography. In another set of experiments (Figure 2C), the in vitro Aurora B kinase assay was carried out using ATM-S1403 and S1403p peptides as substrates. The recombinant GST-His-Aurora B was incubated with peptides in kinase buffer (Cell Signaling) with 5mM ATP for 1h at 30°C. The samples were separated by SDS-PAGE and detected by the ATM-S1403p antibody.
Statistics
Data are presented as the mean ± standard deviation for at least three independent experiments. The results were tested for significance using the unpaired Student t test. Values of P≤0.05 were determined to be significant.
Supplementary Material
Highlights.
ATM is activated in mitosis in an Aurora-B dependent manner;
Aurora-B phosphorylates ATM on serine 1403;
ATM serine 1403 phosphorylation is required for mitotic activation;
ATM phosphorylates Bub1 on serine 314 to activate of the spindle checkpoint.
Acknowledgments
We thank Michael B. Kastan (St Jude Children's Research Hospital) and Yosef Shiloh (Tel Aviv University) for providing reagents. We thank Min Li (BCM) for helpful comments on the manuscript. We thank Rebecca Danforth and Kathryn Brinkman for proof-reading of the manuscript. Time-lapse imaging was performed at the TMHRI Advanced Cellular and Tissue Microscope Core Facility. Flow cytometry was conducted at UAB Comprehensive Center and the TMHRI Flow Cytometry Core. This work was supported by NIH grants R01CA133093, R01ES016354, R21NS061748, R01GM68418, R01CA121225, and U54CA149169.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayoub N, Rajendra E, Su X, Jeyasekharan AD, Mahen R, Venkitaraman AR. The carboxyl terminus of Brca2 links the disassembly of Rad51 complexes to mitotic entry. Curr Biol. 2009;19:1075–1085. doi: 10.1016/j.cub.2009.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Chen J, van Deursen JM. The mitotic checkpoint in cancer and aging: what have mice taught us? Curr Opin Cell Biol. 2005;17:583–589. doi: 10.1016/j.ceb.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- Bourke E, Dodson H, Merdes A, Cuffe L, Zachos G, Walker M, Gillespie D, Morrison CG. DNA damage induces Chk 1-dependent centrosome amplification. EMBO Rep. 2007;8:603–609. doi: 10.1038/sj.embor.7400962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariveau MJ, Tang X, Cui XL, Xu B. Characterization of an NBS1 C-terminal peptide that can inhibit ataxia telangiectasia mutated (ATM)-mediated DNA damage responses and enhance radiosensitivity. Mol Pharmacol. 2007;72:320–326. doi: 10.1124/mol.107.036681. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Anderson S, Jwa M, Green EM, Kang J, Yates JR, 3rd, Chan CS, Drubin DG, Barnes G. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111:163–172. doi: 10.1016/s0092-8674(02)00973-x. [DOI] [PubMed] [Google Scholar]
- Difilippantonio S, Celeste A, Fernandez-Capetillo O, Chen HT, Reina San Martin B, Van Laethem F, Yang YP, Petukhova GV, Eckhaus M, Feigenbaum L, et al. Role of Nbs1 in the activation of the Atm kinase revealed in humanized mouse models. Nat Cell Biol. 2005;7:675–685. doi: 10.1038/ncb1270. [DOI] [PubMed] [Google Scholar]
- Dobles M, Liberal V, Scott ML, Benezra R, Sorger PK. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell. 2000;101:635–645. doi: 10.1016/s0092-8674(00)80875-2. [DOI] [PubMed] [Google Scholar]
- Dupre A, Boyer-Chatenet L, Gautier J. Two-step activation of ATM by DNA and the Mre11-Rad50-Nbs1 complex. Nat Struct Mol Biol. 2006;13:451–457. doi: 10.1038/nsmb1090. [DOI] [PubMed] [Google Scholar]
- Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- Giunta S, Belotserkovskaya R, Jackson SP. DNA damage signaling in response to double-strand breaks during mitosis. J Cell Biol. 2010;190:197–207. doi: 10.1083/jcb.200911156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Deshpande R, Paull TT. ATM activation in the presence of oxidative stress. Cell Cycle. 2010;9:4805–4811. doi: 10.4161/cc.9.24.14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guse A, Mishima M, Glotzer M. Phosphorylation of ZEN-4/MKLP1 by aurora B regulates completion of cytokinesis. Curr Biol. 2005;15:778–786. doi: 10.1016/j.cub.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Iourov IY, Vorsanova SG, Liehr T, Kolotii AD, Yurov YB. Increased chromosome instability dramatically disrupts neural genome integrity and mediates cerebellar degeneration in the ataxia-telangiectasia brain. Hum Mol Genet. 2009;18:2656–2669. doi: 10.1093/hmg/ddp207. [DOI] [PubMed] [Google Scholar]
- Jeganathan K, Malureanu L, Baker DJ, Abraham SC, van Deursen JM. Bub1 mediates cell death in response to chromosome missegregation and acts to suppress spontaneous tumorigenesis. J Cell Biol. 2007;179:255–267. doi: 10.1083/jcb.200706015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V, Groen AC, Prokhorova T, Gerson R, White E, Rodriguez A, Walter JC, Livingston DM. The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell. 2006;127:539–552. doi: 10.1016/j.cell.2006.08.053. [DOI] [PubMed] [Google Scholar]
- Jullien D, Vagnarelli P, Earnshaw WC, Adachi Y. Kinetochore localisation of the DNA damage response component 53BP1 during mitosis. J Cell Sci. 2002;115:71–79. doi: 10.1242/jcs.115.1.71. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science. 2010;327:172–177. doi: 10.1126/science.1180189. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kwon HR, Bae CD, Park J, Hong KU. Specific primary sequence requirements for Aurora B kinase-mediated phosphorylation and subcellular localization of TMAP during mitosis. Cell Cycle. 2010;9:2027–2036. doi: 10.4161/cc.9.10.11753. [DOI] [PubMed] [Google Scholar]
- Kim ST, Xu B, Kastan MB. Involvement of the cohesin protein, Smc1, in Atm-dependent and independent responses to DNA damage. Genes Dev. 2002;16:560–570. doi: 10.1101/gad.970602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King EM, Rachidi N, Morrice N, Hardwick KG, Stark MJ. Ipl1p-dependent phosphorylation of Mad3p is required for the spindle checkpoint response to lack of tension at kinetochores. Genes Dev. 2007;21:1163–1168. doi: 10.1101/gad.431507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov SV, Graham ME, Peng C, Chen P, Robinson PJ, Lavin MF. Involvement of novel autophosphorylation sites in ATM activation. EMBO J. 2006;25:3504–3514. doi: 10.1038/sj.emboj.7601231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- Lee JH, Paull TT. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science. 2004;304:93–96. doi: 10.1126/science.1091496. [DOI] [PubMed] [Google Scholar]
- Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- Lee JH, Paull TT. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26:7741–7748. doi: 10.1038/sj.onc.1210872. [DOI] [PubMed] [Google Scholar]
- Lim DS, Kim ST, Xu B, Maser RS, Lin J, Petrini JH, Kastan MB. ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway. Nature. 2000;404:613–617. doi: 10.1038/35007091. [DOI] [PubMed] [Google Scholar]
- Marmorstein LY, Kinev AV, Chan GK, Bochar DA, Beniya H, Epstein JA, Yen TJ, Shiekhattar R. A human BRCA2 complex containing a structural DNA binding component influences cell cycle progression. Cell. 2001;104:247–257. doi: 10.1016/s0092-8674(01)00209-4. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- McManus KJ, Hendzel MJ. ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Mol Biol Cell. 2005;16:5013–5025. doi: 10.1091/mbc.E05-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P, Honda R, Nigg EA. Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr Opin Genet Dev. 2004;14:29–36. doi: 10.1016/j.gde.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Michel LS, Liberal V, Chatterjee A, Kirchwegger R, Pasche B, Gerald W, Dobles M, Sorger PK, Murty VV, Benezra R. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature. 2001;409:355–359. doi: 10.1038/35053094. [DOI] [PubMed] [Google Scholar]
- Monaco L, Kolthur-Seetharam U, Loury R, Murcia JM, de Murcia G, Sassone-Corsi P. Inhibition of Aurora-B kinase activity by poly(ADP-ribosyl)ation in response to DNA damage. Proc Natl Acad Sci U S A. 2005;102:14244–14248. doi: 10.1073/pnas.0506252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richie CT, Golden A. Chromosome segregation: Aurora B gets Tousled. Curr Biol. 2005;15:R379–382. doi: 10.1016/j.cub.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Shen K, Wang Y, Brooks SC, Raz A, Wang YA. ATM is activated by mitotic stress and suppresses centrosome amplification in primary but not in tumor cells. J Cell Biochem. 2006;99:1267–1274. doi: 10.1002/jcb.20848. [DOI] [PubMed] [Google Scholar]
- Shen KC, Heng H, Wang Y, Lu S, Liu G, Deng CX, Brooks SC, Wang YA. ATM and p21 cooperate to suppress aneuploidy and subsequent tumor development. Cancer Res. 2005;65:8747–8753. doi: 10.1158/0008-5472.CAN-05-1471. [DOI] [PubMed] [Google Scholar]
- Shigeta T, Takagi M, Delia D, Chessa L, Iwata S, Kanke Y, Asada M, Eguchi M, Mizutani S. Defective control of apoptosis and mitotic spindle checkpoint in heterozygous carriers of ATM mutations. Cancer Res. 1999;59:2602–2607. [PubMed] [Google Scholar]
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- Stolz A, Ertych N, Kienitz A, Vogel C, Schneider V, Fritz B, Jacob R, Dittmar G, Weichert W, Petersen I, et al. The CHK2-BRCA1 tumour suppressor pathway ensures chromosomal stability in human somatic cells. Nat Cell Biol. 2010;12:492–499. doi: 10.1038/ncb2051. [DOI] [PubMed] [Google Scholar]
- Takagi M, Delia D, Chessa L, Iwata S, Shigeta T, Kanke Y, Goi K, Asada M, Eguchi M, Kodama C, et al. Defective control of apoptosis, radiosensitivity, and spindle checkpoint in ataxia telangiectasia. Cancer Res. 1998;58:4923–4929. [PubMed] [Google Scholar]
- Tang X, Hui ZG, Cui XL, Garg R, Kastan MB, Xu B. A novel ATM-dependent pathway regulates protein phosphatase 1 in response to DNA damage. Mol Cell Biol. 2008;28:2559–2566. doi: 10.1128/MCB.01711-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno Y, Kitajima TS, Honda T, Ando Y, Ishiguro K, Watanabe Y. Phosphorylation of mammalian Sgo2 by Aurora B recruits PP2A and MCAK to centromeres. Genes Dev. 2010;24:2169–2179. doi: 10.1101/gad.1945310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells WA. The spindle-assembly checkpoint: aiming for a perfect mitosis, every time. Trends Cell Biol. 1996;6:228–234. doi: 10.1016/0962-8924(96)10018-0. [DOI] [PubMed] [Google Scholar]
- White JS, Choi S, Bakkenist CJ. Irreversible chromosome damage accumulates rapidly in the absence of ATM kinase activity. Cell Cycle. 2008;7:1277–1284. doi: 10.4161/cc.7.9.5961. [DOI] [PubMed] [Google Scholar]
- White JS, Choi S, Bakkenist CJ. Transient ATM kinase inhibition disrupts DNA damage-induced sister chromatid exchange. Sci Signal. 2010;3:ra44. doi: 10.1126/scisignal.2000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada HY, Gorbsky GJ. Spindle checkpoint function and cellular sensitivity to antimitotic drugs. Mol Cancer Ther. 2006;5:2963–2969. doi: 10.1158/1535-7163.MCT-06-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi PT, Wang Y, Zhao S, Patel N, Lee EY, Qin J. SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint. Genes Dev. 2002;16:571–582. doi: 10.1101/gad.970702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. Chk1: a double agent in cell cycle checkpoints. Dev Cell. 2007;12:167–168. doi: 10.1016/j.devcel.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Zachos G, Black EJ, Walker M, Scott MT, Vagnarelli P, Earnshaw WC, Gillespie DA. Chk1 is required for spindle checkpoint function. Dev Cell. 2007;12:247–260. doi: 10.1016/j.devcel.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XY, Wang X, Hu B, Guan J, Iliakis G, Wang Y. An ATM-independent S-phase checkpoint response involves CHK1 pathway. Cancer Res. 2002;62:1598–1603. [PubMed] [Google Scholar]
- Ziv Y, Bar-Shira A, Pecker I, Russell P, Jorgensen TJ, Tsarfati I, Shiloh Y. Recombinant ATM protein complements the cellular A-T phenotype. Oncogene. 1997;15:159–167. doi: 10.1038/sj.onc.1201319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.