Abstract
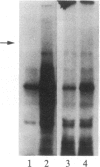
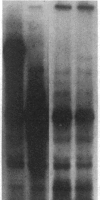
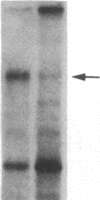
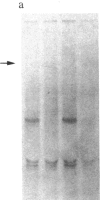
We have previously isolated three independent variants of Swiss 3T3 cells that are unable to generate a mitogenic response to epidermal growth factor (EGF). Each of the variants is unable to bind 125I-labeled EGF; each lacks a functional EGF receptor. We used an antiserum to murine EGF receptor to look for an EGF-receptor gene product in wild-type 3T3 cells and in the three EGF-nonresponsive variants. No cross-reactive material could be detected in any of the three variants, either in 125I-labeled cell extracts or in [35S]methionine metabolically labeled cells. 3T3 cells contained mRNA molecules homologous to a cDNA probe for the human EGF-receptor coding region. In contrast, no homologous RNA could be detected in any of the three variants. Analysis of genomic Southern blots of the DNA from 3T3 cells and the three EGF-nonresponsive variants indicated sequences from the EGF-receptor gene are present in the DNA of all four cell lines. These EGF-nonresponsive lines, which demonstrate proliferative responses to a variety of mitogens, will be ideal recipients for structure-function studies of the EGF receptor by transfection of the cloned gene.
Full text
PDF

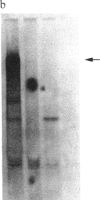
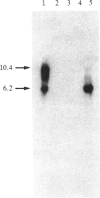
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonov A., Pruss R. M., Herschman H. R. Epidermal growth factor. Relationship between receptor regulation and mitogenesis in 3T3 cells. J Biol Chem. 1978 Jun 10;253(11):3970–3977. [PubMed] [Google Scholar]
- Bishayee S., Feinman J., Pittenger M., Michael H., Das M. Cell surface insertion of exogenous epidermal growth factor receptors into receptor- mutant cells: demonstration of insertion in the absence of added fusogenic agents. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1893–1897. doi: 10.1073/pnas.79.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler-Gralla E., Herschman H. R. Variants of 3T3 cells lacking mitogenic response to the tumor promoter tetradecanoyl-phorbol-acetate. J Cell Physiol. 1981 Apr;107(1):59–67. doi: 10.1002/jcp.1041070108. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen S., Fava R. A., Sawyer S. T. Purification and characterization of epidermal growth factor receptor/protein kinase from normal mouse liver. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6237–6241. doi: 10.1073/pnas.79.20.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Bowen-Pope D. F., Raines E., Ross R., Hunter T. Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell. 1982 Nov;31(1):263–273. doi: 10.1016/0092-8674(82)90426-3. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M., Miyakawa T., Fox C. F., Pruss R. M., Aharonov A., Herschman H. R. Specific radiolabeling of a cell surface receptor for epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2790–2794. doi: 10.1073/pnas.74.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Larco J. E., Todaro G. J. Sarcoma growth factor (SGF): specific binding to epidermal growth factor (EGF) membrane receptors. J Cell Physiol. 1980 Feb;102(2):267–277. doi: 10.1002/jcp.1041020218. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Firestone G. L. The role of protein glycosylation in the compartmentalization and processing of mouse mammary tumor virus glycoproteins in mouse mammary tumor virus-infected rat hepatoma cells. J Biol Chem. 1983 May 25;258(10):6155–6161. [PubMed] [Google Scholar]
- Fung Y. K., Lewis W. G., Crittenden L. B., Kung H. J. Activation of the cellular oncogene c-erbB by LTR insertion: molecular basis for induction of erythroblastosis by avian leukosis virus. Cell. 1983 Jun;33(2):357–368. doi: 10.1016/0092-8674(83)90417-8. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lin C. R., Chen W. S., Kruiger W., Stolarsky L. S., Weber W., Evans R. M., Verma I. M., Gill G. N., Rosenfeld M. G. Expression cloning of human EGF receptor complementary DNA: gene amplification and three related messenger RNA products in A431 cells. Science. 1984 May 25;224(4651):843–848. doi: 10.1126/science.6326261. [DOI] [PubMed] [Google Scholar]
- Porzig E. F., Stockdale F. E. Mammary serum factor initiates cell growth in 3T3 cell variants which are non-responsive to EGF. Exp Cell Res. 1982 Sep;141(1):47–52. doi: 10.1016/0014-4827(82)90066-0. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Herschman H. R. Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3918–3921. doi: 10.1073/pnas.74.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozek C. E., Davidson N. Drosophila has one myosin heavy-chain gene with three developmentally regulated transcripts. Cell. 1983 Jan;32(1):23–34. doi: 10.1016/0092-8674(83)90493-2. [DOI] [PubMed] [Google Scholar]
- Rubin R. A., Earp H. S. Dimethyl sulfoxide stimulates tyrosine residue phosphorylation of rat liver epidermal growth factor receptor. Science. 1983 Jan 7;219(4580):60–63. doi: 10.1126/science.6294827. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Stoscheck C. M., Carpenter G. Characteristics of antibodies to the epidermal growth factor receptor-kinase. Arch Biochem Biophys. 1983 Dec;227(2):457–468. doi: 10.1016/0003-9861(83)90476-9. [DOI] [PubMed] [Google Scholar]
- Stoscheck C. M., Carpenter G. Down regulation of epidermal growth factor receptors: direct demonstration of receptor degradation in human fibroblasts. J Cell Biol. 1984 Mar;98(3):1048–1053. doi: 10.1083/jcb.98.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger E., Herschman H. R. 3T3 variants unable to bind epidermal growth factor cannot complement in co-culture. Biochem Biophys Res Commun. 1984 Jan 13;118(1):60–64. doi: 10.1016/0006-291x(84)91067-2. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski G. P., Knight L., Piperno J. R., Walsh P. N. A rapid method for removal of [125I]iodide following iodination of protein solutions. Anal Biochem. 1980 Jul 15;106(1):118–122. doi: 10.1016/0003-2697(80)90126-8. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. H., Ishii S., Clark A. J., Sullivan M., Wilson R. K., Ma D. P., Roe B. A., Merlino G. T., Pastan I. Human epidermal growth factor receptor cDNA is homologous to a variety of RNAs overproduced in A431 carcinoma cells. 1984 Jun 28-Jul 4Nature. 309(5971):806–810. doi: 10.1038/309806a0. [DOI] [PubMed] [Google Scholar]