Abstract
Ribonucleotide reductase (RNR) catalyzes the rate-limiting step in the production of deoxyribonucleoside triphosphates (dNTPs) required for replicative and repair DNA synthesis. Mammalian RNR is a heteromeric enzyme consisting primarily of R1 and R2 subunits during the S phase of the cell cycle. We have shown previously that the presence of excess R2 subunits protects p53-deficient human colon cancer cells from cisplatin-induced DNA damage and replication stress. However, the mode of DNA repair influenced by changes in the level of the R2 subunit remained to be defined. In the present study, we demonstrated that depletion of BRCA1, an important factor of homologous recombination repair (HRR), preferentially sensitized stable R2-knockdown p53(−/−) HCT116 cells to the cytotoxicity of cisplatin and γ-H2AX induction. In accord with this finding, these R2-knockdown cells exhibited increased dependence on HRR, as evidenced by elevated levels of cisplatin-induced Rad51 foci and sister chromatid exchange frequency. Furthermore, stable knockdown of the R2 subunit also led to decreased cisplatin-induced gap-filling synthesis in nucleotide excision repair (NER) and a reduced dATP level in the G2/M phase of the cell cycle. These results suggest that an increased level of the R2 subunit extends the availability of dATP in the G2/M phase to promote the repair of NER-mediated single-strand gaps that are otherwise converted into double-strand breaks in the subsequent S phase. We propose that HRR becomes important for recovery from cisplatin-DNA lesions when the postexcision process of NER is restrained by reduced levels of the R2 subunit and dATP in p53-deficient cancer cells.
Introduction
Ribonucleotide reductase (RNR) catalyzes the rate-limiting step in the synthesis of the dNTPs that are required for both DNA replication and repair. The enzymatically active RNR complex is typically described as a heterotetramer consisting of two R1 and two R2 subunits (Nordlund and Reichard, 2006). In mammalian cells, the expression of the R2 subunit at the highest level occurs during the S phase, whereas the level of the R1 subunit remains relatively constant throughout the cell cycle (Engström et al., 1985). Therefore, the S phase-specific elevation of the R2 subunit leads to a marked increase in RNR activity required for replicative DNA synthesis (Eriksson and Martin, 1981). p53R2, a p53-inducible homolog of the R2 subunit, has been shown to play an important role in the synthesis of dNTPs designated for DNA damage repair (Tanaka et al., 2000). However, it is unclear how the majority of cancer cells with compromised p53 function provides dNTPs for DNA repair. Studies in our laboratory and others have provided evidence that the R2 subunit is also able to provide dNTPs for DNA repair when the p53-dependent induction of p53R2 is impaired (Zhou et al., 2003; Lin et al., 2004, 2007).
Cisplatin and other platinum-containing derivatives (i.e., carboplatin and oxaliplatin) are effective and widely used chemotherapeutic agents in the treatment of a broad range of malignancies, including ovarian, cervical, testicular, and non–small-cell lung cancer (Wang and Lippard, 2005). Cisplatin binds to the N7 positions of two adjacent purine bases in one strand of the DNA and forms 1,2-d(GpG) or 1,2-d(ApG) intrastrand cross-links comprising approximately 90% of the total lesions (Fichtinger-Schepman et al., 1985). It is believed that these major adducts are largely responsible for the cytotoxic and antiproliferative activities of cisplatin (Siddik, 2003). Thus, an increase in the ability of cancer cells to repair and/or tolerate cisplatin-induced intrastrand cross-linking lesions contributes to the phenotypes of intrinsic and acquired resistance to this agent. Nucleotide excision repair (NER) has long been considered a primary mechanism for the repair of cisplatin-induced DNA cross-links (Sibghatullah et al., 1989). The process involves dual incision on both sides of the damaged strand, filling of the resulting single-strand gap, and ligation (Mu et al., 1996). Emerging evidence has demonstrated that homologous recombination repair (HRR) also plays a role in the repair of DNA lesions caused by cisplatin and cells defective in components of HRR pathways are sensitive to cisplatin (Bhattacharyya et al., 2000; Zdraveski et al., 2000). HRR is necessary to repair double-strand breaks (DSBs) associated with stalled or collapsed replication forks (Arnaudeau et al., 2001). However, it is still unsettled whether cisplatin-intrastrand cross-links directly cause replication fork stalling and collapse. Contradictory evidence has shown that cisplatin-intrastrand cross-links can be replicatively bypassed through the mechanism of translesion synthesis (TLS). TLS is mediated by specialized low-fidelity polymerases that are able to incorporate nucleotides opposite lesions in an error-prone and mutagenic manner (Friedberg et al., 2002).
The tumor suppressor p53 is altered genetically and functionally at high frequency in a variety of human malignancies (Vogelstein et al., 2000). Wild-type p53 functions to maintain genomic integrity by triggering cell cycle arrest, apoptosis, and DNA repair in response to genotoxic and other stresses (Vogelstein et al., 2000). For cell cycle control, activated p53 induces the expression of p21 and 14-3-3 σ to stably block G1/S and G2/M transitions, respectively, until DNA damage is repaired (Vogelstein et al., 2000; Taylor and Stark, 2001). Furthermore, mounting evidence indicates that wild-type p53 prevents replication-associated DSBs and suppresses spontaneous homologous recombination (Mekeel et al., 1997; Janz and Wiesmüller, 2002; Kumari et al., 2004). Therefore, p53-compromised cancer cells are likely to implement HRR to counteract an increase in the burden of DNA damage during the S phase. Whether HRR activity in these cancer cells constitutes the determinant of chemotherapeutic or other DNA-damaging sensitivity requires further investigation.
In the present study, we determined the effects of a reduced level of the R2 subunit on DNA repair processes, based upon our previous findings that the presence of excess R2 subunits in p53-deficient HCT116 human colon cancer cells protects against the genotoxic and cytotoxic effects of cisplatin. We now present evidence that excess R2 subunits function to promote NER of cisplatin-induced primary DNA lesions in the G2/M phase of the cell cycle. Thus, a reduced level of the R2 subunit predisposes p53-deficient cancer cells to increase dependence on HRR of secondary lesions incurred in the subsequent S phase transition. These findings have clinical implications in treating human malignancies using RNR inhibitors in combination with platinum-based chemotherapy.
Materials and Methods
Cell Cultures and Reagents.
p53(+/+) and p53(−/−) HCT-116 human colon cancer cells were gifts from Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, MD). Vector and R2-knockdown p53(−/−) HCT-116 cell lines were established by stable transfection with the vector (pZZeomU6) and R2-short hairpin/siRNA expressing (pZZeomU6-R2-siRNA) constructs, respectively; they were maintained in the selective marker phleomycin (Zeocin; Invitrogen, Carlsbad, CA) as described previously (Lin et al., 2004). All cell lines were cultured in McCoy's 5A medium supplemented with 10% fetal bovine serum and penicillin-streptomycin antibiotics. Triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone) was developed and synthesized in our laboratory.
siRNA Transfection.
Cells were plated in 6-well or 96-well plates, grown for 24 h, and transfected with 50 nM siRNA using the Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's protocol. Transfected cells were incubated for appropriate times before assays. The control siRNA sequence used was 5′-GCGCGCUUUGUAGGAUUCGdTdT-3′ (Lin et al., 2007). The BRCA1 siRNA sequence used was 5′-UCACAGUGUCCUUUAUGUAdTdT-3′ (Kim et al., 2007). The ERCC1 siRNA sequence used was 5′-GGGAAUUUGGCGACGUAAUdTdT-3′. The checkpoint kinase 1 (Chk1)-siRNA sequence used was 5′-GCAACAGUAUUUCGGUAUAdTdT-3′. siRNAs were synthesized by Dharmacon RNA Technologies (Lafayette, CO). Endoribonuclease-prepared siRNA (esiRNA) that targets XPA or XPG mRNA was purchased from Sigma-Aldrich (Saint Louis, MO).
Western Blotting.
The procedure was performed as described previously (Lin et al., 2004, 2007). Gel images were quantified by densitometry with ImageQuaNT software (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). Anti-R2 (E-16), anti-p53R2 (N-16), anti-ERCC1 (FL297), anti-BRCA1 (D-9), and anti-Chk1 (G-4) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-actin (20-33) was from Sigma-Aldrich. Anti-γ-H2AX (Ser-139; JBW301) antibody was obtained from Millipore (Billerica, MA). Anti-phospho-Chk1 (Ser-345) antibody was from Cell Signaling Technology (Danvers, MA).
Cytotoxicity/Cell Viability Assay.
Cells were seeded into 96-well plates at 1500 cells/well in complete McCoy's medium and incubated for 24 h. Cells were then transfected with siRNA as described above. After 24 h, cells were treated with various concentrations of cisplatin (Calbiochem/EMD Biosciences, San Diego, CA) in triplicate continuously for 72 h, or 1 h followed by a 72-h drug-free period. For other cytotoxic agents, cells were treated continuously for 72 h. At the end of treatment, the MTS tetrazolium reagent (CellTiter 96 One solution; Promega, Madison, WI) was added to each well and the incubation was continued for an additional 2 h at 37°C. The absorbance of soluble MTS tetrazolium formazan produced by viable cells was measured at 490 nm using a microplate reader.
Cell Synchronization.
Cells were grown for 24 h and then treated with 3 mM thymidine (Sigma-Aldrich) for 16 h. Cells were then washed and gown in thymidine-free growth medium for 8 h. Cells were exposed to 1.5 mM l-mimosine (Calbiochem, San Diego, CA), 3 mM thymidine, or 100 ng/ml nocodazole (Enzo Life Sciences, Plymouth Meeting, PA) for 16 h to obtain synchronized cell populations of G1, S, and G2/M phases, respectively. Cells were then collected for Western blotting, flow cytometry analyses, and dNTP measurements. Because double-thymidine blocks for obtaining S phase-enriched cell populations interfered with the dynamics of dNTP pools, the second thymidine treatment was replaced with 2 μg/ml aphidicolin (Calbiochem) only for dNTP measurements.
Flow Cytometry.
To determine DNA content, cells were collected by trypsinization and fixed/permeabilized with 70% ethanol for 1 h, and fixed cells were incubated with 1 mg/ml RNase I at 37°C for 30 min, stained with 50 μg/ml propidium iodide (Sigma-Aldrich), and analyzed for DNA content by flow cytometry using a FACScalibur cell sorter (BD Biosciences, San Jose, CA). To determine the level of γ-H2AX for each of the cell cycle phases, cells were labeled with 10 μM BrdU (5-bromo-2′-deoxyuridine; BD Biosciences) during the final hour of cisplatin treatment. Cells were then collected, permeabilized, fixed, and treated with DNase using a BrdU Flow kit (BD Biosciences). Thereafter, fixed cells were stained with Alexa Fluor 647 conjugated anti-γ-H2AX (Cell Signaling Technology) and fluorescein isothiocyanate-conjugated anti-BrdU antibodies (BD Biosciences), followed by counterstaining of DNA with 7-aminoactinomycin D (BD Biosciences). Bivariate analysis of BrdU incorporation and DNA content was performed by flow cytometry using a FACScalibur cell sorter (BD Biosciences) and FlowJo software (Tree Star, Inc., Ashland, OR). Cell populations of G1, S, and G2/M phases were gated and further analyzed for the level of γ-H2AX staining in each population.
Immunofluorescent Microscopy.
Cells grown in chamber slides (BD Biosciences) were treated with 10 μM cisplatin for 1 h and incubated in drug-free medium for 6 h. Cells were then fixed with 2% formaldehyde and permeabilized in cooled 100% methanol. The slides were blocked with 1 or 3% bovine serum albumin in phosphate-buffered saline and stained with a rabbit anti-Rad51 (H-92) antibody (Santa Cruz Biotechnology) followed by incubation with an Alexa Fluor 488 conjugated anti-rabbit IgG antibody (Invitrogen). Nuclei were counterstained with 1 μM TO-PRO-3 (Invitrogen), and the slides were then mounted with coverslips using the Anti-Fade reagent (Invitrogen). For analyses of γ-H2AX and BRCA1 foci, slides were stained with Alexa Fluor 488-conjugated anti-γ-H2AX (Cell Signaling Technology) and mouse anti-BRCA1 (D-9; Santa Cruz) antibodies, followed by incubation with an Alexa Fluor 546 conjugated anti-mouse IgG antibody (Invitrogen). Immunofluorescence of target proteins and nuclei were viewed with a Zeiss Laser Scanning System (LSM510; Zeiss, Thornwood, NY) and confocal images were analyzed with a Zeiss LSM Image Browser.
Quantification of dNTP Levels.
Cellular deoxyribonucleoside triphosphates were quantified using an enzymatic assay based upon the DNA polymerase-catalyzed incorporation of deoxyribonucleotides into DNA as described previously (Lin et al., 2004, 2007). Approximately 106 cells were collected for the assay.
Host Cell Reactivation Assay.
The pGL3-control plasmid was treated with cisplatin at concentrations of 0, 0.5, 1, and 2.5 μM in a reaction buffer (3 mM NaCl and 1 mM sodium phosphate, pH 7.4) and incubated at 37°C for 18 h. The plasmid was then purified by ethanol precipitation and resuspended in Tris-EDTA buffer. Cells were grown in 12-well plates for 24 h and then transfected with 0.01 μg of pRL-TK plasmid together with 0.1 μg of cisplatin-damaged pGL3 plasmid per well using the Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's protocol. For cotransfection with siRNA, 50 nM siRNA was used along with the plasmids. Transfected cells were incubated for 48 h and then assayed for both firefly (pGL3; Promega) and Renilla reniformis (pRL-TK; Promega) luciferase activities using the Dual-Luciferase Reporter Assay system (Promega) and a luminometer (Turner Designs, Sunnyvale, CA). Firefly luciferase activity was normalized against R. reniformis luciferase activity to correct for the transfection efficiency in each well. Relative luciferase activity was expressed as a percentage of the activity in each cell line transfected with non–cisplatin-treated pGL3 plasmid. Transfection of cells with cisplatin-damaged plasmid had no effect on cell viability.
Sister Chromatid Exchange Assay.
Cells were pulsed with 10 μM cisplatin for 1 h and then exposed to 10 μM BrdU for 40 h. During the last 2 h of BrdU treatment, colcemid (Invitrogen) was added to cells at a final concentration of 0.1 μg/ml. Cells were then harvested, incubated in a hypotonic buffer (0.075 M KCl), fixed in methanol/glacial acetic acid [3:1 (v/v)], and dropped onto slides. Slides were dried, stained with 0.5 μg/ml Hoechst 33258 (Invitrogen), and exposed to UVA light. Subsequently, slides were incubated in 2× standard saline citrate at 62°C, stained with 4% Giemsa (Invitrogen), and then air-dried. Metaphase spreads were viewed by light microscopy, and images were taken for analyses. Forty-two metaphase spreads were scored for sister chromatid exchanges (SCEs) per data point.
DNA Repair Incorporation Assay.
Measurement of DNA repair incorporation in the G2/M phase of the cell cycle was performed by flow cytometry as described by Selden et al. (1993) with some modifications. Cells were treated with increasing concentrations of cisplatin for 4 h in the presence of 10 μM 5-ethynyl 2′-deoxyuridine (EdU). Thereafter, cells were collected, fixed, and permeabilized. EdU incorporation into DNA was detected by Alexa Fluor 488 azide using the Click-it EdU flow cytometry kit (Invitrogen). Total DNA was counterstained with 7-aminoactinomycin D. Bivariate analyses of EdU incorporation and DNA content were performed by flow cytometry using a FACScalibur or LSRII cell sorter (BD Biosciences) and FlowJo software. A total of 20,000 cells per sample were sorted and the G2/M cell population was gated for analysis of increases in EdU incorporation. S phase cells with intensive EdU incorporation were excluded by gating analysis. The cisplatin-induced increase in EdU incorporation in the G2/M phase was the difference between cisplatin-treated cells and an untreated control (in percentage of total cells).
Statistical Analysis.
Data are shown as the mean ± S.E. Data were compared with the respective control in each experiment using the paired Student's t test. A p value <0.05 was considered statistically significant.
Results
Loss of p53 Leads to Enhancement of Cisplatin- Induced γ-H2AX and Chk1 Activation in HCT116 Cells.
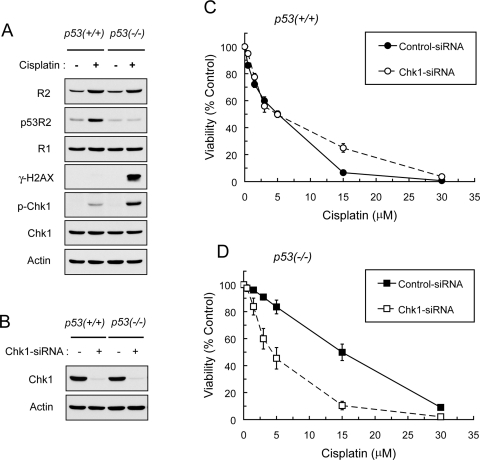
Given that wild-type p53 is required to induce cell cycle arrest at G1/S and G2/M transitions (Vogelstein et al., 2000; Taylor and Stark, 2001), the effects of p53 ablation on cisplatin-induced DNA damage response, including phosphorylation of histone H2AX and Chk1, were determined. Radiation- and DNA damaging agents-induced DSBs trigger phosphorylation of H2AX at Ser-139 (γ-H2AX) (Rogakou et al., 1998; Furuta et al., 2003). Concurrently, Chk1 is phosphorylated at Ser317 and Ser345 to mediate the activation of S and G2/M checkpoints (Zhao et al., 2002). Our results show that treatment with cisplatin induced p53R2, a p53-inducible analog of the R2 subunit, in p53(+/+) but not in p53(−/−) HCT116 cells (Fig. 1A). Conversely, cisplatin-induced γ-H2AX and Chk1 phosphorylation occurred at a greater level in p53(−/−) than in p53(+/+) cells. Cell cycle analyses showed that p53(−/−) exhibited a loss of G1 arrest associated with continued S phase entry in response to cisplatin-induced DNA damage (Supplemental Fig. 1)
Fig. 1.
A, loss of p53 augmented cisplatin-induced γ-H2Ax and Chk1 activation in HCT-116 cells. p53(+/+) and p53(−/−) cells were treated with 20 μM cisplatin for 24 h. Total protein was examined for the levels of R2, p53R2, R1, γ-H2AX (Ser-139), phospho-Chk1 (Ser-345), Chk1, and actin by Western blot analyses. B, cells were transiently transfected with 50 nM control- or Chk1-siRNA. At 48 h after transfection, cells were harvested, and Western blotting was used to assess the protein levels of Chk1 and actin. The levels of actin protein were used to demonstrate approximately equal loading of protein in each lane. C and D, Chk1 was necessary to protect against the cytotoxicity of cisplatin in p53(−/−)HCT-116 cells. Cells were transfected with 50 nM control- or Chk1-siRNA for 24 h and then treated with various concentrations of cisplatin for 72 h before measurement of viability by the MTS colorimetric assay. Points are means ± S.E. from three independent experiments, each performed in at least triplicate wells.
To elucidate the importance of Chk1 activation in p53(−/−) cells, we tested the impact of Chk1 abrogation on the sensitivity of p53(+/+) and p53(−/−) cells to cisplatin. Transient transfection of these cells with Chk1-siRNA produced substantial depletion of Chk1 protein as determined by Western blot analysis (Fig. 1B). Depletion of Chk1 resulted in a pronounced sensitization of p53(−/−) cells to the cytotoxicity of cisplatin but failed to do so in p53(+/+) cells (Fig. 1, C and D). These results suggest that a lack of p53 function incurs replication-associated γ-H2AX/DSBs, and Chk1 becomes necessary to halt cell cycle progression in S and G2/M phases for the enhancement of cell survival after DNA damage. Thus, we chose to focus on p53(−/−) HCT116 cells for the following studies.
Cisplatin Produces a Time- and Concentration- Dependent Induction of γ-H2AX Predominantly in S- and G2/M-Phase Populations of p53(−/−) HCT116 cells.
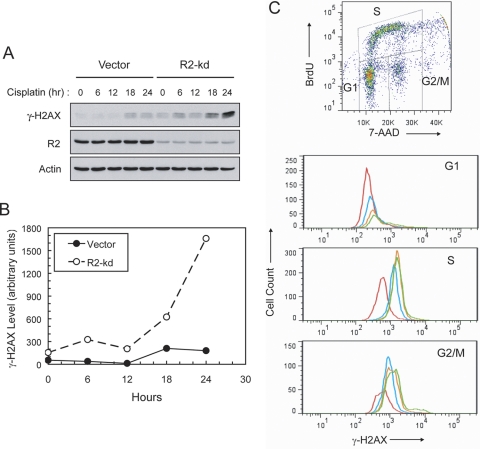
We have demonstrated previously that an increased level of the R2 subunit of RNR alleviates cisplatin-induced DNA damage and Chk1 activation in p53(−/−) HCT116 cells (Lin et al., 2007). To further determine the role of the R2 subunit in the kinetics of DSB formation, a time course study of cisplatin-induced γ-H2AX was performed using Western blot analyses. The R2 subunit in p53(−/−) HCT116 cells was stably down-regulated by a short hairpin/siRNA vector and remained permanently at a 15 to 20% level compared with that in vector control cells (Lin et al., 2004; Lin et al., 2007). In response to cisplatin treatment, these R2-knockdown cells initially exhibited a minor elevation in γ-H2AX at 6 h followed by a decline at 12 h (Fig. 2, A and B). A second pronounced increase in γ-H2AX occurred after 12 h and continued to rise through 18 h to the maximum level detected at 24 h. In contrast, vector cells displayed only a slight increase in γ-H2AX at 18 h and remained relatively low up to 24 h. Unlike radiation, which induces γ-H2AX within minutes (Rogakou et al., 1998), cisplatin-induced γ-H2AX was gradual, and the occurrence of maximal induction was evident after 18 h. We have confirmed that the accentuated induction of γ-H2AX at 24 h is independent of apoptosis in R2-knockdown cells (Lin et al., 2007).
Fig. 2.
A, time course of γ-H2AX induction in vector and stable R2-knockdown p53(−/−) HCT-116 cells (vector and R2-kd, respectively, labeled in all figures and tables). Cells were pulsed with 30 μM cisplatin for 1 h and incubated until harvested at the indicated times. Total protein was analyzed by Western blotting to assess the levels of γ-H2AX, R2, and actin. B, the intensity of the γ-H2AX bands was quantified by densitometry and normalized against that of the actin bands. The normalized γ-H2AX induction plotted against time is shown. C, cisplatin-induced γ-H2AX occurred primarily in S and G2/M phases of the cell cycle. R2-knockdown cells were exposed to 0 (red), 2.5 (blue), 7.5 (orange), and 15 (green) μM cisplatin for 24 h and 10 μM BrdU during the final hour. Cells were fixed, stained, and analyzed for bivariate distribution of BrdU incorporation and DNA content (top) by flow cytometry. G1, S, and G2/M cell populations were gated and further analyzed for the level of γ-H2AX in response to cisplatin treatment.
To ascertain whether cisplatin-induced γ-H2AX occurred in a cell cycle phase-specific manner, the increase in γ-H2AX by cisplatin in each cell cycle phase was determined by flow cytometry. Cells pulsed with BrdU were stained and gated for each population of the cell-cycle phases. Treatment of R2-knockdown cells with cisplatin for 24 h led to a concentration-dependent reduction in the G1 cell population. These G1 cells exhibited a minimal increase in the level of γ-H2AX (Fig. 2C). However, the treatment caused a pronounced increase in the accumulation of S and G2/M populations with considerably elevated levels of γ-H2AX. These results collectively suggest that cisplatin-induced γ-H2AX/DSBs occur as cells progressed through S and G2/M phases and that the level of DSBs is augmented by stable depletion of the R2 subunit of RNR in p53(−/−) HCT116 cells.
Depletion of BRCA1 Causes Enhancement of Cisplatin-Induced γ-H2AX and Cytotoxicity in R2-Knockdown p53(−/−) HCT116 Cells.
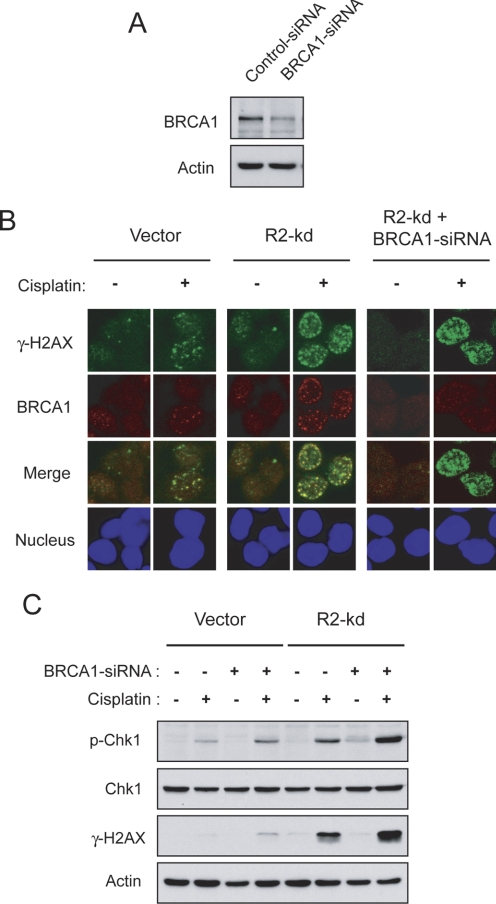
Because stable R2 knockdown caused an increase in the level of cisplatin-induced γ-H2AX/DSBs (Fig. 2), we determined whether disruption of HRR by depletion of BRCA1 had differential effects on the sensitivity of R2-knockdown cells to cisplatin compared with vector cells. BRCA1, an important factor of HRR, interacts with Rad51 to promote the repair of DSBs (Bhattacharyya et al., 2000). Transient transfection of p53(−/−) HCT116 cells with BRCA1-siRNA caused a considerable reduction in BRCA1 protein as confirmed by Western blott analysis (Fig. 3A). We also performed confocal microscopy to visualize the subcellular localization of γ-H2AX and BRCA1 and their physical interaction in response to cisplatin. After cisplatin treatment for 6 h, BRCA1 foci predominantly colocalized with γ-H2AX foci in the nucleus (Fig. 3B). Depletion of BRCA1 abolished cisplatin-induced BRCA1 foci but had no effect on the formation of γ-H2AX foci, indicating that BRCA1 is subsequently recruited to the sites of cisplatin-induced DSBs for the repair process. To evaluate the overall intensity of incurred DNA damage and corresponding checkpoint activation, Western blot analyses of the levels of γ-H2AX and Chk1 phosphorylation were conducted after 24-h cisplatin treatment. In vector cells, depletion of BRCA1 slightly increased cisplatin-induced γ-H2AX and Chk1 phosphorylation (Fig. 3C). In contrast, BRCA1 depletion resulted in pronounced enhancement of cisplatin-induced γ-H2AX and Chk1 phosphorylation in R2-knockdown cells, suggesting that abrogation of BRCA1 function deters the repair of DSBs and exacerbates S and G2/M checkpoint activation.
Fig. 3.
Effects of BRCA1 depletion on cisplatin-induced γ-H2AX formation and Chk1 phosphorylation in p53(−/−) HCT-116 cells. A, vector cells were transiently transfected with 50 nM control- or BRCA1-siRNA. At 48 h after transfection, cells were harvested and protein levels of BRCA1 and actin were assessed by Western blotting. B, colocalization of cisplatin-induced γ-H2AX and BRCA1 foci in nuclei. Vector and R2-knockdown cells were transiently transfected with 50 nM control-siRNA or BRCA1-siRNA. At 24 h after transfection, cells were treated with 10 μM cisplatin for 1 h and incubated in drug-free medium for 6 h before staining and imaging for γ-H2AX (green) and BRCA1 (red) foci and nuclei (blue) by immunofluorescent microscopy. C, BRCA1 depletion augmented cisplatin-induced γ-H2AX and Chk1 phosphorylation in R2-knockdown cells. Vector and R2-knockdown cells were transiently transfected with 50 nM control-siRNA or BRCA1-siRNA. At 24 h after transfection, cells were treated with 10 μM cisplatin for 24 h, and total protein was examined for the levels of phospho-Chk1, Chk1, γ-H2AX, and actin by Western blot analyses.
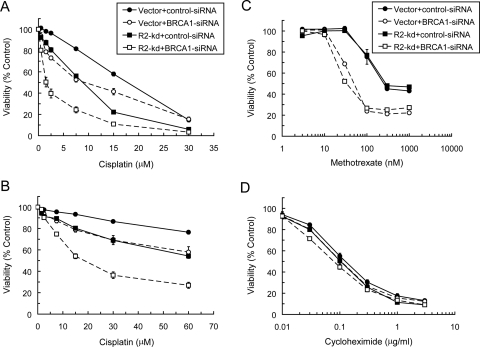
Furthermore, we examined the effects of BRCA1 depletion on the sensitivity of R2-knockdown cells to cisplatin. Transient depletion of BRCA1 caused a moderate increase in the sensitivity of vector cells but resulted in a marked sensitization of R2-knockdown cells to cisplatin (Fig. 4A). BRCA1 depletion-mediated sensitization of vector and R2-knockdown cells to cisplatin was 1.8- and 5.3-fold, respectively, at the IC50 concentration (Table 1). In contrast to the effects of BRCA1 depletion, depletion of ERCC1, a key component of NER, equally sensitized vector and R2-silencing cells to cisplatin (Table 1); the fold sensitization at the IC50 concentrations in vector and R2-silencing cells was 2.9- and 3.0-fold, respectively. Pulse treatment with cisplatin for 1 h, which allowed cells to recover from DNA damage, also yielded similar profiles of sensitization by BRCA1 (Fig. 4B). To substantiate the finding that the effects of impaired HRR and/or reduced RNR activity were unique to cisplatin-induced DNA damage, we determined the sensitivity of cells to methotrexate, a thymidylate synthase inhibitor that abrogates thymidylate and replicative DNA syntheses (Bertino, 2009). Because the inhibitory effects of methotrexate on DNA synthesis are independent of RNR, both vector and R2-knockdown cells manifested similar sensitivity to methotrexate and became equally sensitized by BRCA1 depletion (Fig. 4C). The large sensitization by BRCA1 depletion may be due to the fact that methotrexate-induced thymidylate stress causes DSBs and increases HR activity (Mishina et al., 1991). Finally, we also demonstrated that neither BRCA1 depletion nor R2 knockdown produced discernible effects on the sensitivity of these cells to cycloheximide, a protein synthesis inhibitor (Fig. 4D). These findings suggest that R2-knockdown p53(−/−) cells are subject to greater sensitization to cisplatin by BRCA1 depletion or HRR impairment.
Fig. 4.
Depletion of BRCA1 sensitized R2-knockdown p53(−/−) HCT116 cells to the cytotoxicity of cisplatin. Cells were transfected with 50 nM control- or BRCA1-siRNA for 24 h and then treated with various concentrations of cisplatin for 72 h (A) or 1 h followed by a 72 h drug-free period (B) before the measurement of viability by the MTS colorimetric assay. Cells transfected as described in A and B were treated with various concentrations of methotrexate (C) or cycloheximide (D) for 72 h before the measurement of viability. Points are the means ± S.E. from three independent experiments, each performed in triplicate wells.
TABLE 1.
Sensitization of vector and R2-knockdown p53(−/−) HCT-116 cells to cisplatin by BRCA1- and ERCC1-siRNA
The 50% inhibitory concentration (IC50) of cisplatin was determined using the MTS assay. Values shown are the means ± S.E. from three independent experiments, each performed in at least triplicate wells. p values (paired t test) were obtained using the IC50 of cisplatin and fold sensitization (F.S.) was calculated as the ratio of the IC50, in both cases for BRCA1- or ERCC1-transfected cells compared with that for control-siRNA transfected cells.
| p53(−/−) HCT-116 cells | BRCA1-siRNA |
ERCC1-siRNA |
||||
|---|---|---|---|---|---|---|
| IC50 | p | F.S. | IC50 | p | F.S. | |
| μM | μM | |||||
| Vector | ||||||
| − siRNA | 17.7 ± 0.6 | 18.5 ± 0.7 | ||||
| + siRNA | 9.7 ± 2.0 | 0.031 | 1.8 | 6.3 ± 0.4 | 0.0012 | 2.9 |
| R2-kd | ||||||
| − siRNA | 8.8 ± 0.4 | 8.3 ± 0.4 | ||||
| + siRNA | 1.7 ± 0.3 | 0.00015 | 5.3 | 2.7 ± 0.3 | 0.00043 | 3.0 |
R2-Knockdown p53(−/−) HCT116 Cells Exhibit an Increase in HRR of Cisplatin-Induced DNA Damage.
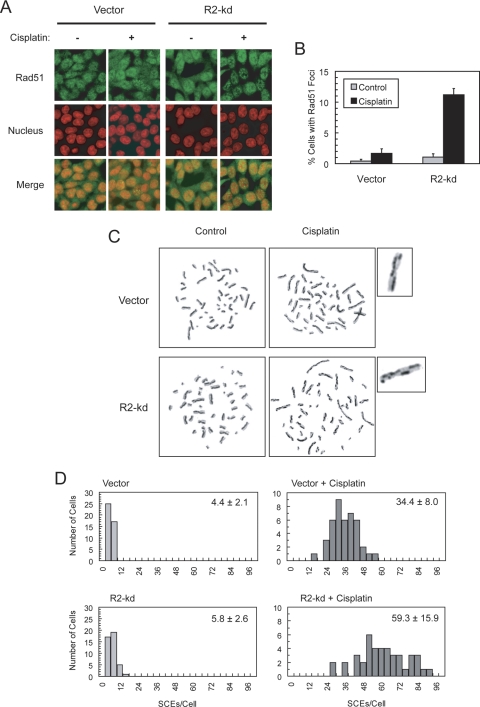
To further determine whether stable R2 knockdown had effects on the use of HRR to contend with cisplatin-induced DNA damage, we performed confocal microscopy to assess the cisplatin-induced formation of nuclear foci of HRR complexes consisting of Rad51. Rad51 is a nuclear recombinase required for the process of homologous pairing and strand exchange during the repair of DSBs (Baumann and West, 1998). Compared with vector cells, R2-knockdown cells exhibited a slight elevation in the basal level of Rad51 foci and a pronounced increase in cisplatin-induced Rad51 foci in the nuclei (Fig. 5, A and B).
Fig. 5.
A and B, R2-knockdown p53(−/−) HCT-116 cells exhibited an elevated level of cisplatin-induced Rad51 foci formation. Cells were pulsed with 10 μM cisplatin for 1 h and incubated with cisplatin-free medium for 6 h before fixation. Slides were subsequently stained and imaged for Rad51 foci (green) and nuclei (red) by confocal microscopy. The percentage of cells with ≥ 10 Rad51 foci for at least 500 cells counted in each treatment group is presented as a bar graph; error bars represent S.E. C and D, R2-knockdown cells exhibited an increase in cisplatin-induced SCE. Cells were pulsed with 10 μM cisplatin for 1 h and then exposed to 10 μM BrdU for two cell cycles of DNA replication. Metaphase spreads were prepared, and at least 40 metaphase cells were scored for the number of SCEs. A representative metaphase exhibiting an approximately average SCE level in each treatment group is shown. One chromosome from each cisplatin-treated cell is enlarged to indicate the sites of SCE. Histograms show the frequency of cells with the numbers of SCE. The mean of SCE ± S.E. per cell is indicated for each histogram.
To substantiate the findings of an increase in Rad51 foci formation, we ascertained whether the frequency of SCE, which is indicative of HRR of cisplatin-induced DNA lesions, was also elevated in R2-knockdown cells. SCE occurs as a result of repair by HR for collapsed replication forks during normal replication or DNA strand damage (Sonoda et al., 1999). It also reflects the outcome of a successful HRR process, because only surviving cells that undergo mitosis are examined and scored for SCE. Vector and R2-knockdown cells had a comparable level of basal SCE in the absence of exogenous DNA damage (Fig. 5, C and D). In both vector and R2-knockdown cells, treatment with cisplatin caused a marked increase in SCE, a noticeably greater level being observed in R2-knockdown cells. Given that Chk1 is required for HRR (Sørensen et al., 2005), the results of Rad51 foci formation and SCE assays are consistent with enhanced Chk1 phosphorylation in R2-knockdown cells (Fig. 3C) (Lin et al., 2007), suggesting that stable knockdown of the R2 subunits leads to an increase in the use of HR to repair cisplatin-induced DNA lesions in p53-deficient cells.
R2-Knockdown p53(−/−) HCT116 Cells Exhibit Reduced NER of Cisplatin-Induced DNA Damage.
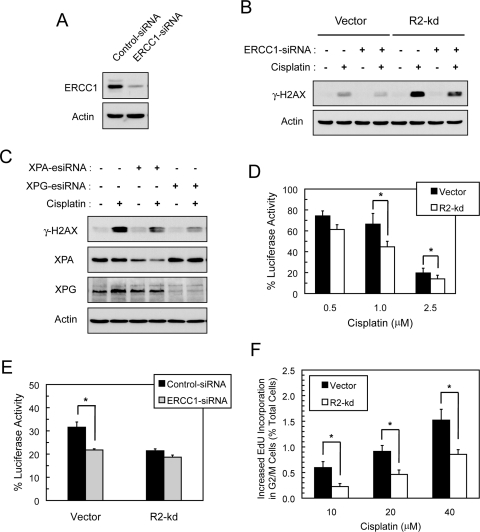
Because cisplatin-DNA intrastrand cross-links can be effectively repaired by NER, we determined the effects of depletion of ERCC1, a key factor of NER, on the accumulation of cisplatin-induced γ-H2AX. ERCC1 complexes with XPF to mediate the 5′-incision at cisplatin-intrastrand cross-links in the excision step of NER (Mu et al., 1996). Transfection of p53(−/−) HCT116 cells with ERCC1-siRNA caused a substantial reduction in ERCC1 protein as shown by Western blotting (Fig. 6A). In contrast to BRCA1, depletion of ERCC1 attenuated the levels of cisplatin-induced γ-H2AX in both vector and R2-knockdown cells (Fig. 6B). We ascertained whether abrogation of other key components of NER required for the incision step produced similar effects. XPA functions as a principal DNA damaging recognition/binding protein to recruit the components of the excision complex, and XPG serves to mediate 3′-incision at DNA lesions (Cleaver and States, 1997). Similar to ERCC1, abrogation of XPA or XPG also attenuated cisplatin-induced γ-H2AX in R2-knockdown cells (Fig. 6C). These findings suggest that damage-recognition and excision steps of NER are required to elicit the accumulation of γ-H2AX caused by cisplatin.
Fig. 6.
R2-knockdown p53(−/−) HCT116 cells exhibited a decrease in NER of cisplatin-induced DNA damage. A, vector cells were transiently transfected with 50 nM control- or ERCC1-siRNA. At 48 h after transfection, cells were harvested and the protein levels of ERCC1 and actin were assessed by Western blotting. B, depletion of ERCC1 attenuated cisplatin-induced γ-H2AX. Vector and R2-knockdown cells were transiently transfected with 50 nM control-siRNA or ERCC1-siRNA. At 24 h after transfection, cells were treated with 10 μM cisplatin for 24 h, and total protein was examined for the levels of γ-H2AX and actin by Western blotting. C, XPA and XPG depletion attenuated cisplatin-induced γ-H2AX in R2-knockdown cells. R2-knockdown cells were transiently transfected with 50 nM control-siRNA, XPA-esiRNA, or XPG-esiRNA. At 24 h after transfection, cells were treated with 10 μM cisplatin for 24 h and total protein was examined for the levels of γ-H2AX and actin by Western blotting. The XPA protein levels after knockdown were 49 and 36% of control for untreated and cisplatin-treated cells, respectively. D, host cell reactivation of cisplatin-damaged luciferase reporter plasmid in p53(−/−) HCT-116 cells. The pGL3 plasmid was treated with the indicated cisplatin concentrations and then transfected into cells. At 48 h after transfection, cells were harvested and assayed for luciferase activities to determine the percentage recovery from cisplatin-induced damage to the plasmid DNA. Values are the means ± S.E. from three independent experiments. *, p < 0.05, paired t test. E, effects of ERCC1 depletion on host cell reactivation of cisplatin-damaged luciferase reporter plasmid in p53(−/−) HCT-116 cells. Cells were cotransfected with control- or ERCC1-siRNA and pGL3 plasmid treated with 1 μM cisplatin, and then harvested at 48 h after transfection. Luciferase activities were measured and calculated as described in D. Values are means ± S.E. from a representative experiment of two, each performed in triplicate. *, p < 0.05, paired t test. F, R2-knockdown cells displayed a decrease in cisplatin-induced EdU incorporation at the G2/M phase of the cell cycle. Cells were treated with 0, 10, 20, and 40 μM cisplatin together with 10 μM EdU for 4 h. Subsequently cells were fixed, stained, and analyzed by flow cytometry. Bivariate distribution of EdU incorporation and DNA content was used to determine increases in cisplatin-induced EdU incorporation in the G2/M cell population above the values of untreated controls. Values are percentages of total cells sorted and expressed as the means ± S.E. from three independent experiments. *, p < 0.05, paired t test.
To assess whether stable R2 knockdown affected the efficiency of NER of cisplatin-induced DNA lesions, we performed host cell reactivation assays for the recovered transcriptional activity of cisplatin-damaged luciferase reporter plasmid transfected into intact cells. Both vector and R2-knockdown cells exhibited a decrease in reactivated luciferase activity as the cisplatin concentration increased (Fig. 6D). R2-knockdown cells exhibited relatively lower levels of reactivated luciferase activity than those of vector cells. To corroborate the finding that R2-knockdown cells had a reduced NER activity, we cotransfected cells with ERCC1-siRNA and cisplatin-damaged reporter plasmid to determine the recovery of luciferase activity. Depletion of ERCC1 resulted in a decrease in reactivated luciferase activity in vector cells but had no effect on R2-knockdown cells (Fig. 6E). In this assay, the effects of R2 knockdown are presumably epistatic with those of ERCC1 depletion in disrupting the NER pathway. Thus, the results suggest that stable knockdown of the R2 subunit causes a decrease in the capacity of intact cells to perform NER of cisplatin-induced DNA damage to the reporter plasmid.
Because cells in the G1 phase are devoid of RNR activity to prevent premature onset of DNA replication (Chabes et al., 2003), we postulated that stable knockdown of the R2 subunit decreased RNR activity in the G2/M phase and thus impeded the gap-filling synthesis step of NER. To test this hypothesis, we performed flow cytometry analyses to determine the incorporation of EdU into the G2/M cell population indicative of DNA repair synthesis. Cisplatin treatment produced a concentration-dependent increase in EdU incorporation in the G2/M populations of both vector and R2-knockdown cells (Fig. 6F). R2-knockdown cells displayed considerably lower levels of cisplatin-induced EdU incorporation than did vector cells. In contrast, there was no difference between vector and R2-knockdown cells in cisplatin-induced EdU incorporation in the G1 phase (data not shown). These results suggest that although the excision step of NER continues functioning, stable knockdown of the R2 subunit hinders gap-filling synthesis after the excision of cisplatin-intrastrand cross-links in the G2/M phase of the cell cycle.
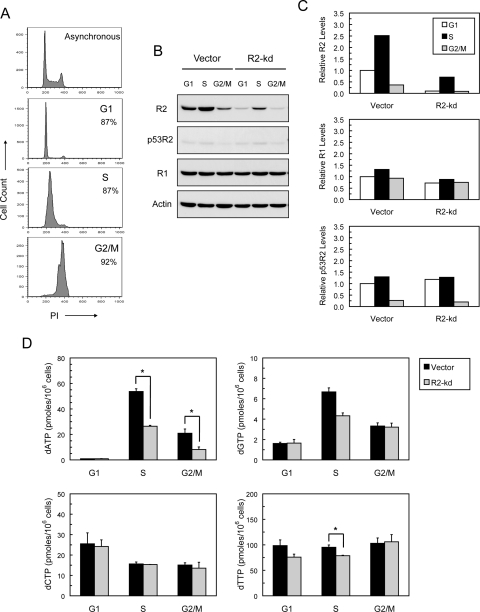
Stable Knockdown of the R2 Subunit Reduces the dATP Level in the G2/M Phase of the Cell Cycle.
To determine the effects of stable knockdown of the R2 subunit on dNTP levels in each of the cell cycle stages, we measured dNTP levels in cell populations enriched for the G1, S, and G2/M phases (Fig. 7A). Cells enriched for the S phase displayed a prominent increase in the R2 subunit, with the level in R2-knockdown cells being considerably attenuated (Fig. 7, B and C). In vector cells, the levels of the R2 subunit in G1 and G2/M cells were also higher than those in R2-knockdown cells. In contrast, the levels of the R1 subunit were comparable between vector and R2-knockdown cells and remained relatively constant throughout the cell cycle. p53R2 was basally expressed in the G1 and S phases, the levels being lowest in the G2/M phase.
Fig. 7.
Effects of stable knockdown of the R2 subunit on the levels of dNTPs related to cell cycle stages. A, enrichment of cells in the G1, S, and G2/M phases of the cell cycle. Cells were synchronized at G1, S, and G2/M, and subsequently collected, fixed, stained, and analyzed for DNA content by flow cytometry. Cells synchronized at each of the cell cycle phases are shown, and the percentage of cells is indicated to confirm the degree of synchronicity. B, the levels of the R2, R1, and p53R2 protein in each cell cycle phase. Cells synchronized at each cell cycle phase were collected and analyzed for the protein levels of R2, R1, p53R2, and actin by Western blotting. C, the intensity of R2, R1, and p53R2 bands shown in B was quantified by densitometry and normalized against that of the actin bands. The normalized protein levels at each phase relative to that at the G1 phase in vector cells (set as 1) are shown. D, the levels of dNTPs in the G1, S, and G2/M phases of the cell cycle. Cells synchronized at G1, S, and G2/M phases were collected and assayed for the levels of dNTPs. Values are the means ± S.E. from three independent experiments. *, p < 0.05, paired t test.
In line with the levels of the R2 subunit, there were 59- and 30-fold increases in dATP levels, and 4.1- and 2.6-fold increases in dGTP levels in the S phase compared with the G1 phase in vector and R2-knockdown cells, respectively (Table 2). In contrast, dCTP and dTTP levels showed minimal changes (0.6- and 1.0-fold, respectively, in both vector and R2-knockdown cells). The levels of dATP and dGTP in G1 cells were markedly low (Fig. 7D), indicating a depressed RNR activity to prevent replicative DNA synthesis. In the G2/M phase, the level of dATP was 23- and 9-fold greater than that of G1 cells in vector and R2-knockdown cells, respectively. The changes in levels of dGTP, dCTP, and dTTP in the G2/M phase were 2.0-, 0.6-, and 1.0/1.4-fold, respectively, in both vector and R2-knockdown cells. Among these changes in dNTP levels, R2-knockdown cells displayed a significant reduction in the dATP level compared with vector cells in the G2/M phase. Given that the large elevation in the dATP level corresponds to replicative DNA synthesis occurring in the S phase, these results suggest that stable knockdown of the R2 subunit leads to a suppressed dATP level in the G2/M phase and negatively affects repair DNA synthesis in p53-deficient cells.
TABLE 2.
Fold increases in dNTP levels in the S and G2/M phases compared with that in the G1 phase of the cell cycle in Vector and R2-knockdown p53(−/−) HCT-116 cells
The fold increases were calculated as the ratio of the dNTP levels in the S and G2/M phases compared with that in the G1 phase.
| S Phase |
G2/M Phase |
|||
|---|---|---|---|---|
| Vector | R2-kd | Vector | R2-kd | |
| dATP | 59 | 30 | 23 | 9.0 |
| dGTP | 4.1 | 2.6 | 2.1 | 2.0 |
| dCTP | 0.6 | 0.6 | 0.6 | 0.6 |
| dTTP | 1.0 | 1.0 | 1.0 | 1.4 |
Discussion
Cisplatin produces mostly intrastrand cross-links in DNA and therefore imparts unique genotoxic and cytotoxic activities against cancer cells. Based upon our findings, we favor the concept that the major intrastrand cross-links produce an excessive level of single-strand gaps that subsequently cause lethality to G1 checkpoint-compromised, p53-deficient cancer cells. Therefore, the efficiency of gap-filling synthesis in NER plays a crucial role in determining the sensitivity of these cells to cisplatin. Stable knockdown of the R2 subunit of RNR hinders this process and sensitizes p53(−/−) HCT116 cells to cisplatin. However, we cannot completely rule out the possibility that the minor adducts of interstrand cross-links are also lethal lesions contributing to the therapeutic efficacy of cisplatin. Interstrand cross-links produce direct obstruction of DNA replication, which requires extensive and coordinated repair by NER, HRR, and TLS (Muniandy et al., 2010). Each of these mechanisms is a prerequisite in sequential steps to complete the repair process. It is thus conceivable that a few interstrand cross-links are sufficient to produce lethality in cells. Although it is difficult to separate the lethal events produced by a mixture of cisplatin intra- and interstrand cross-links, we have examined the sensitivity of p53(−/−) HCT116 cells to mitomycin C, an interstrand cross-linking agent. The results show that R2-knockdown cells exhibit a very minor enhancement of the sensitivity to mitomycin C compared with vector cells (data not shown). These findings support our assertion that cisplatin-induced lethality is largely attributable to the major DNA adducts of intrastrand cross-links.
Whether cisplatin-intrastrand cross-links constitute physical barriers to replication polymerases and directly cause stalled/collapsed replication forks remains debatable. Increasing evidence indicates that TLS plays an important role in the bypass of these DNA lesions for enhancing cell survival at the cost of an increased mutation frequency (Friedberg, 2005). The cell line used in our studies, HCT116 cells, harbors an MLH1/mismatch repair deficiency that enhances replication bypass of cisplatin-induced lesions and contributes to increased resistance to cisplatin (Vaisman et al., 1998; Lin and Howell, 2006). In addition, a loss of p53 function is reportedly to promote TLS and increase the mutation frequency (Avkin et al., 2006; Lin and Howell, 2006). We have found that cisplatin-induced impediment of S phase transition in synchronized cells does not occur until the second round of S phase transition (Supplemental Fig. 2). This phenomenon coincides with a delay in the accumulation of cisplatin-induced DSBs in R2-knockdown cells (Fig. 2, A and B). These observations affirm that TLS-mediated replication bypass of primary cisplatin-intrastrand cross-links is likely to occur during the first round of S-phase transition in p53(−/−) HCT116 cells.
In mammalian cells, it is well known that S-phase-specific elevation of the R2 subunit confers a marked increase in RNR activity to supply dNTPs for replicative DNA synthesis (Eriksson and Martin, 1981). However, the role of the R2 subunit in postreplication repair synthesis remains poorly understood. We propose that excess R2 subunits impart RNR activity or surplus dNTPs available for DNA repair after completion of DNA replication. We have observed that the levels of purine dNTPs (dATP and dGTP) show the most prominent changes, corresponding to the demand of replicative DNA synthesis (Fig. 7D; Table 2). In vector cells, the levels of the R2 subunit and dATP remain elevated in the G2/M phase and are considerably greater than those in R2-knockdown cells. In contrast, pyrimidine dNTP pools are relatively stable regardless of changes in the level of the R2 subunit. We speculate that the salvage pathway (Sandrini and Piskur, 2005) in p53(−/−) HCT116 cells is highly operative to supplement pyrimidine nucleotide pools independent of the cell cycle phases. Given that a larger overall dNTP pool is present in the S phase (Table 2), there should be no difference between vector and R2-knockdown cells in the NER of damaged DNA during this period. However, as cells enter the G2/M phase and the following G1 phase, purine dNTP pools become critically limited (Fig. 7D). We propose that a significant reduction in the dATP level in the G2/M phase contributes to a decrease in the cisplatin-induced gap-filling synthesis of NER in R2-knockdown cells (Fig. 6F). This assumption is supported by several lines of evidence that hydroxyurea treatment results in decreased purine dNTP pools, increased UV-induced single strand breaks (Snyder, 1984; Hunting and Dresler, 1985), and retarded lagging strand gap-filling DNA synthesis (Martin et al., 1977). Our findings also demonstrate that treatment with triapine, which is 1000 times more potent than hydroxyurea, causes sustained and pronounced depletion of dATP and dGTP, whereas dCTP and dTTP are less affected and rapidly rebound to normal levels (Supplemental Fig. 3A). A reduction of replicative DNA synthesis is correlated with the suppression of the dATP level (Supplemental Fig. 3, B and C). Furthermore, the very low levels of dATP in the G1 phase (Fig. 7D) correspond to the absence of replicative DNA synthesis during this period. These observations further strengthen the concept that dATP/purine dNTP pools critically govern the ability of cells to carry out repair DNA synthesis in the G2/M phases of the cell cycle.
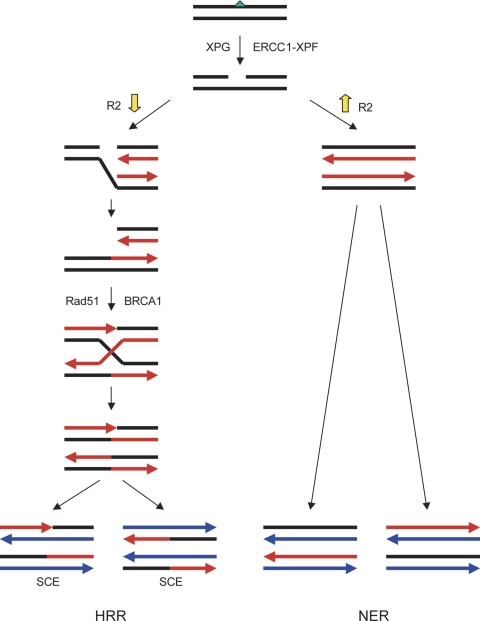
Herein we provide evidence that when the dATP/dNTP pool is limited by a reduced level of the R2 subunit, retarded postexcision process of NER (gap-filling synthesis) in the G2/M phase gives rise to an increased level of single-strand gaps in DNA. If the p53-mediated G1 checkpoint is compromised, these gaps are likely converted into DSBs when advancing replication forks collapse at these gapped lesions (Kuzminov, 2001). Replication-associated DSBs are potentially lethal to cells that undergo active DNA replication and therefore require HRR to resume S-phase progression (Arnaudeau et al., 2001; Lundin et al., 2003). Based upon our findings, we propose the model of DNA repair pathways for cisplatin-induced DNA damage illustrated in Fig. 8. HRR functions to complement NER when XPG and ERCC1-XPF mediated single-strand gaps are excessive or not completely repaired before S-phase entry. Therefore, stable knockdown of the R2 subunit renders p53-deficient cells increasingly dependent on HRR of cisplatin-induced DNA lesions.
Fig. 8.
HRR complements NER of cisplatin-induced DNA damage when gap-filling synthesis is hindered by a reduced level of the R2 subunit of RNR. Green triangle, cisplatin-DNA intrastrand cross-link; black lines, the original DNA strands; red lines, the DNA strands from round one of DNA replication; blue lines, the DNA strands from round two of DNA replication.
Chk1 is phosphorylated by ATR to activate S and G2/M checkpoints in response to DNA damage and stalled replication (Liu et al., 2000). Thus, Chk1 functions to prevent the replication of damaged DNA, thereby maintaining genome stability and enhancing cell survival (Bartek and Lukas, 2003). We have found that R2-knockdown p53(−/−) HCT116 cells exhibit enhanced Chk1 phosphorylation, corresponding to an increase in γ-H2AX in response to cisplatin-induced DNA damage (Fig. 3C). In addition, we have shown that the time course of induced Chk1 phosphorylation closely parallels that of γ-H2AX (Lin et al., 2007). Because of a reduced NER capacity, enhanced Chk1 activation indicates that these cells encounter an increased level of replication-associated DNA damage and the impediment of cell cycle progression in S and G2/M phases. It is noteworthy that Chk1 has also been shown to interact with Rad51 to promote HRR of replication-associated DSBs (Sørensen et al., 2005). These findings provide additional support to our conclusion that stable knockdown of the R2 subunit increases reliance on HRR of cisplatin-induced DNA lesions in p53-deficient cancer cells.
The clinical implications of our findings in the treatment of human malignancies are exemplified not only by our in vivo studies with the active combination of cisplatin and triapine (Finch et al., 2000) but also by a recently published phase I study. This clinical trial reported that 100% of patients with advanced cervical cancer exhibited a complete clinical response and remained disease-free for 18 months with the combination regimen of cisplatin, radiation, and triapine (Kunos et al., 2010). A follow-up phase II trial is currently under way. The success of this phase I trial may be attributed to the combination of the following factors. First, cervical cancer is known to harbor inactivated p53 pathways by the HPV protein E6 (Werness et al., 1990). These cancer cells are likely to exhibit an increased reliance on the HRR pathway because of the lack of a G1 checkpoint response. Second, triapine inhibits the RNR R2 subunit and therefore reduces the NER of cisplatin-induced DNA damage during the transition of the G2/M phase. Third, radiation produces extensive DSBs that compete with the capacity of HRR for cisplatin-induced DNA damage. The inclusion of radiation and triapine serves to incapacitate both the NER and HRR pathways for the repair of cisplatin-induced DNA damage, thereby leading to enhanced activity of cisplatin against p53-compromised cervical cancer cells. Thus, a better understanding of the mechanisms underlying the repair of cisplatin-induced DNA damage will assist in devising rational chemotherapeutic strategies toward the goal of curing human malignancies.
Supplementary Material
Acknowledgments
We thank Dr. Yilun Liu (Yale University) for advice on Rad51 foci studies, Rocco Carbone (Yale University) for assistance in flow cytometry, and members of Dr. Sartorelli's laboratory for helpful discussion.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported in part by the National Institutes of Health National Cancer Institute [Grant P01-CA129186]; and by the Wendy Will Case Cancer Fund, Inc.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/mol.111.074708.
- RNR
- ribonucleotide reductase
- dNTP
- deoxyribonucleoside triphosphate
- NER
- nucleotide excision repair
- HRR
- homologous recombination repair
- DSB
- double-strand break
- TLS
- translesion synthesis
- esiRNA
- endoribonuclease-prepared siRNA
- siRNA
- small intefering
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- BrdU
- 5-bromo-2′-deoxyuridine
- EdU
- 5-ethynyl 2′-deoxyuridine
- SCE
- sister chromatid exchange
- Chk1
- checkpoint kinase 1
- H2AX
- H2A histone family, member X
- γ-H2AX
- phosphorylated H2AX
- HR
- homologous recombination
- XP
- xeroderma pigmentosum.
Authorship Contributions
Participated in research design: Z. P. Lin, Lee, Cory, Glazer, and Sartorelli.
Conducted experiments: Z. P. Lin, Lee, F. Lin, and Belcourt.
Contributed new reagents or analytic tools: F. Lin and Li.
Performed data analysis: Z. P. Lin, Lee, and Li.
Wrote or contributed to the writing of the manuscript: Z. P. Lin, Glazer, and Sartorelli.
References
- Arnaudeau C, Lundin C, Helleday T. (2001) DNA double-strand breaks associated with replication forks are predominantly repaired by homologous recombination involving an exchange mechanism in mammalian cells. J Mol Biol 307:1235–1245 [DOI] [PubMed] [Google Scholar]
- Avkin S, Sevilya Z, Toube L, Geacintov N, Chaney SG, Oren M, Livneh Z. (2006) p53 and p21 regulate error-prone DNA repair to yield a lower mutation load. Mol Cell 22:407–413 [DOI] [PubMed] [Google Scholar]
- Bartek J, Lukas J. (2003) Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3:421–429 [DOI] [PubMed] [Google Scholar]
- Baumann P, West SC. (1998) Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem Sci 23:247–251 [DOI] [PubMed] [Google Scholar]
- Bertino JR. (2009) Cancer research: from folate antagonism to molecular targets. Best Pract Res Clin Haematol 22:577–582 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. (2000) The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem 275:23899–23903 [DOI] [PubMed] [Google Scholar]
- Chabes AL, Pfleger CM, Kirschner MW, Thelander L. (2003) Mouse ribonucleotide reductase R2 protein: a new target for anaphase-promoting complex-Cdh1-mediated proteolysis. Proc Natl Acad Sci USA 100:3925–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver JE, States JC. (1997) The DNA damage-recognition problem in human and other eukaryotic cells: the XPA damage binding protein. Biochem J 328:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström Y, Eriksson S, Jildevik I, Skog S, Thelander L, Tribukait B. (1985) Cell cycle-dependent expression of mammalian ribonucleotide reductase. Differential regulation of the two subunits. J Biol Chem 260:9114–9116 [PubMed] [Google Scholar]
- Eriksson S, Martin DW., Jr. (1981) Ribonucleotide reductase in cultured mouse lymphoma cells. Cell cycle-dependent variation in the activity of subunit protein M2. J Biol Chem 256:9436–9440 [PubMed] [Google Scholar]
- Fichtinger-Schepman AM, van der Veer JL, den Hartog JH, Lohman PH, Reedijk J. (1985) Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry 24:707–713 [DOI] [PubMed] [Google Scholar]
- Finch RA, Liu M, Grill SP, Rose WC, Loomis R, Vasquez KM, Cheng Y, Sartorelli AC. (2000) Triapine (3-aminopyridine-2-carboxaldehyde- thiosemicarbazone): A potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem Pharmacol 59:983–991 [DOI] [PubMed] [Google Scholar]
- Friedberg EC. (2005) Suffering in silence: the tolerance of DNA damage. Nat Rev Mol Cell Biol 6:943–953 [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Wagner R, Radman M. (2002) Specialized DNA polymerases, cellular survival, and the genesis of mutations. Science 296:1627–1630 [DOI] [PubMed] [Google Scholar]
- Furuta T, Takemura H, Liao ZY, Aune GJ, Redon C, Sedelnikova OA, Pilch DR, Rogakou EP, Celeste A, Chen HT, et al. (2003) Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J Biol Chem 278:20303–20312 [DOI] [PubMed] [Google Scholar]
- Hunting DJ, Dresler SL. (1985) Dependence of u.v.-induced DNA excision repair on deoxyribonucleoside triphosphate concentrations in permeable human fibroblasts: a model for the inhibition of repair by hydroxyurea. Carcinogenesis 6:1525–1528 [DOI] [PubMed] [Google Scholar]
- Janz C, Wiesmüller L. (2002) Wild-type p53 inhibits replication-associated homologous recombination. Oncogene 21:5929–5933 [DOI] [PubMed] [Google Scholar]
- Kim H, Chen J, Yu X. (2007) Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response. Science 316:1202–1205 [DOI] [PubMed] [Google Scholar]
- Kumari A, Schultz N, Helleday T. (2004) p53 protects from replication-associated DNA double-strand breaks in mammalian cells. Oncogene 23:2324–2329 [DOI] [PubMed] [Google Scholar]
- Kunos CA, Waggoner S, von Gruenigen V, Eldermire E, Pink J, Dowlati A, Kinsella TJ. (2010) Phase I trial of pelvic radiation, weekly cisplatin, and 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) for locally advanced cervical cancer. Clin Cancer Res 16:1298–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzminov A. (2001) Single-strand interruptions in replicating chromosomes cause double-strand breaks. Proc Natl Acad Sci USA 98:8241–8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Howell SB. (2006) DNA mismatch repair and p53 function are major determinants of the rate of development of cisplatin resistance. Mol Cancer Ther 5:1239–1247 [DOI] [PubMed] [Google Scholar]
- Lin ZP, Belcourt MF, Carbone R, Eaton JS, Penketh PG, Shadel GS, Cory JG, Sartorelli AC. (2007) Excess ribonucleotide reductase R2 subunits coordinate the S phase checkpoint to facilitate DNA damage repair and recovery from replication stress. Biochem Pharmacol 73:760–772 [DOI] [PubMed] [Google Scholar]
- Lin ZP, Belcourt MF, Cory JG, Sartorelli AC. (2004) Stable suppression of the R2 subunit of ribonucleotide reductase by R2-targeted short interference RNA sensitizes p53(−/−) HCT-116 colon cancer cells to DNA-damaging agents and ribonucleotide reductase inhibitors. J Biol Chem 279:27030–27038 [DOI] [PubMed] [Google Scholar]
- Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, et al. (2000) Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev 14:1448–1459 [PMC free article] [PubMed] [Google Scholar]
- Lundin C, Schultz N, Arnaudeau C, Mohindra A, Hansen LT, Helleday T. (2003) RAD51 is involved in repair of damage associated with DNA replication in mammalian cells. J Mol Biol 328:521–535 [DOI] [PubMed] [Google Scholar]
- Martin RF, Radford I, Pardee M. (1977) Accumulation of short DNA fragments in hydroxyurea treated mouse L-cells. Biochem Biophys Res Commun 74:9–15 [DOI] [PubMed] [Google Scholar]
- Mekeel KL, Tang W, Kachnic LA, Luo CM, DeFrank JS, Powell SN. (1997) Inactivation of p53 results in high rates of homologous recombination. Oncogene 14:1847–1857 [DOI] [PubMed] [Google Scholar]
- Mishina Y, Ayusawa D, Seno T, Koyama H. (1991) Thymidylate stress induces homologous recombination activity in mammalian cells. Mutat Res 246:215–220 [DOI] [PubMed] [Google Scholar]
- Mu D, Hsu DS, Sancar A. (1996) Reaction mechanism of human DNA repair excision nuclease. J Biol Chem 271:8285–8294 [DOI] [PubMed] [Google Scholar]
- Muniandy PA, Liu J, Majumdar A, Liu ST, Seidman MM. (2010) DNA interstrand crosslink repair in mammalian cells: step by step. Crit Rev Biochem Mol Biol 45:23–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund P, Reichard P. (2006) Ribonucleotide reductases. Annu Rev Biochem 75:681–706 [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273:5858–5868 [DOI] [PubMed] [Google Scholar]
- Sandrini MP, Piskur J. (2005) Deoxyribonucleoside kinases: two enzyme families catalyze the same reaction. Trends Biochem Sci 30:225–228 [DOI] [PubMed] [Google Scholar]
- Selden JR, Dolbeare F, Clair JH, Nichols WW, Miller JE, Kleemeyer KM, Hyland RJ, DeLuca JG. (1993) Statistical confirmation that immunofluorescent detection of DNA repair in human fibroblasts by measurement of bromodeoxyuridine incorporation is stoichiometric and sensitive. Cytometry 14:154–167 [DOI] [PubMed] [Google Scholar]
- Sibghatullah, Husain I, Carlton W, Sancar A. (1989) Human nucleotide excision repair in vitro: repair of pyrimidine dimers, psoralen and cisplatin adducts by HeLa cell-free extract. Nucleic Acids Res 17:4471–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddik ZH. (2003) Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22:7265–7279 [DOI] [PubMed] [Google Scholar]
- Snyder RD. (1984) The role of deoxynucleoside triphosphate pools in the inhibition of DNA-excision repair and replication in human cells by hydroxyurea. Mutat Res 131:163–172 [DOI] [PubMed] [Google Scholar]
- Sonoda E, Sasaki MS, Morrison C, Yamaguchi-Iwai Y, Takata M, Takeda S. (1999) Sister chromatid exchanges are mediated by homologous recombination in vertebrate cells. Mol Cell Biol 19:5166–5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen CS, Hansen LT, Dziegielewski J, Syljuåsen RG, Lundin C, Bartek J, Helleday T. (2005) The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol 7:195–201 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Arakawa H, Yamaguchi T, Shiraishi K, Fukuda S, Matsui K, Takei Y, Nakamura Y. (2000) A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature 404:42–49 [DOI] [PubMed] [Google Scholar]
- Taylor WR, Stark GR. (2001) Regulation of the G2/M transition by p53. Oncogene 20:1803–1815 [DOI] [PubMed] [Google Scholar]
- Vaisman A, Varchenko M, Umar A, Kunkel TA, Risinger JI, Barrett JC, Hamilton TC, Chaney SG. (1998) The role of hMLH1, hMSH3, and hMSH6 defects in cisplatin and oxaliplatin resistance: correlation with replicative bypass of platinum-DNA adducts. Cancer Res 58:3579–3585 [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. (2000) Surfing the p53 network. Nature 408:307–310 [DOI] [PubMed] [Google Scholar]
- Wang D, Lippard SJ. (2005) Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4:307–320 [DOI] [PubMed] [Google Scholar]
- Werness BA, Levine AJ, Howley PM. (1990) Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248:76–79 [DOI] [PubMed] [Google Scholar]
- Zdraveski ZZ, Mello JA, Marinus MG, Essigmann JM. (2000) Multiple pathways of recombination define cellular responses to cisplatin. Chem Biol 7:39–50 [DOI] [PubMed] [Google Scholar]
- Zhao H, Watkins JL, Piwnica-Worms H. (2002) Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc Natl Acad Sci USA 99:14795–14800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Liu X, Mo X, Xue L, Darwish D, Qiu W, Shih J, Hwu EB, Luh F, Yen Y. (2003) The human ribonucleotide reductase subunit hRRM2 complements p53R2 in response to UV-induced DNA repair in cells with mutant p53. Cancer Res 63:6583–6594 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.