Abstract
The NO and cGMP signaling pathways are of broad physiological and pathological significance. We compared the NO/soluble guanylyl cyclase (sGC)/cGMP pathway in human glioma tissues and cell lines with that of healthy control samples and demonstrated that sGC expression is significantly lower in glioma preparations. Our analysis of GEO databases (National Cancer Institute) further revealed a statistically significant reduction of sGC transcript levels in human glioma specimens. On the other hand, the expression levels of particulate (membrane) guanylyl cyclases (pGC) and cGMP-specific phosphodiesterase (PDE) were intact in the glioma cells that we have tested. Pharmacologically manipulating endogenous cGMP generation in glioma cells through either stimulating pGC by ANP/BNP, or blocking PDE by 3-isobutyl-1-methylxanthine/zaprinast caused significant inhibition of proliferation and colony formation of glioma cells. Genetically restoring sGC expression also correlated inversely with glioma cells growth. Orthotopic implantation of glioma cells transfected with an active mutant form of sGC (sGCα1β1Cys105) in athymic mice increased the survival time by 4-fold over the control. Histological analysis of xenografts overexpressing α1β1Cys105 sGC revealed changes in cellular architecture that resemble the morphology of normal cells. In addition, a decrease in angiogenesis contributed to glioma inhibition by sGC/cGMP therapy. Our study proposes the new concept that suppressed expression of sGC, a key enzyme in the NO/cGMP pathway, may be associated with an aggressive course of glioma. The sGC/cGMP signaling-targeted therapy may be a favorable alternative to chemotherapy and radiotherapy for glioma and perhaps other tumors.
Introduction
Gliomas account for almost 75% of primary malignant brain tumors. Of 10,000 Americans diagnosed each year with malignant gliomas, only half will live beyond 1 year after diagnosis, and those will die within 2 years. Despite compelling advances in diagnostic imaging, surgery, radiation, and/or antineoplastic agents, the prognosis for people with glioma has remained largely unchanged (Jemal et al., 2008). Thus, new concepts in glioma etiology, therapy, and clinical management are needed.
The nitric oxide (NO) and cyclic 3′,5′-GMP (cGMP) pathway is one of the best characterized signaling cascades and plays a central role in several physiological processes, such as induction of vasodilation. Soluble guanylyl cyclase (sGC) is the only known receptor for NO. The α1β1 heterodimer is the predominant isoform of sGC that is obligatory for catalytic activity. Nitric oxide binds to the ferrous heme at histidine 105 of the β1 subunit and leads to an increase in sGC activity and cGMP production of at least 200-fold (Bian and Murad, 2003). On the other hand, the effects of NO can be attributed to the cGMP-independent pathway, which is mediated mainly by reactive oxygen/nitrogen species such as highly reactive peroxynitrite (ONOO−) (Bian et al., 2003, 2008). The role of NO and cGMP signaling in tumor biology has been extensively studied during the past 3 decades. Simple applications of NO or cGMP-regulating reagents to various cancer cell lines or animal models has generated controversial results, and whether the pathway is beneficial or detrimental in cancer is still open to question (Kimura and Murad, 1975; Criss and Murad, 1976; Rao, 2004; Mujoo et al., 2010). We suggest several reasons for this ambiguity: first, although the NO participates in normal signaling (e.g., vasodilation and neurotransmission), NO is also a cytotoxic or apoptotic molecule when produced at high concentrations by inducible nitric-oxide synthase (iNOS or NOS-2). In addition, the cGMP-dependent (NO/sGC/cGMP pathway) and cGMP-independent (NO/oxidative pathway) components may vary among different tissues and cell types. Furthermore, solid tumors contain two compartments: the parenchyma (neoplastic cells) and the stroma (nonmalignant supporting tissues including connective tissue, blood vessels, and, inflammatory cells) with different NO biology. Thus, the NO/sGC/cGMP signaling molecules in tumors as well as the surrounding tissue must be further characterized before targeting this signaling pathway for tumor therapy.
In this study, we demonstrate that perturbation of sGC/cGMP signal in glioma cells may play a significant role in tumor malignancy. Our experiments with genetic restoration of sGC gene expression or pharmacologic manipulation of endogenous cGMP generation in glioma cells resulted in inhibition of the growth of glioma cells and blockage of aggressive course of glioma. We suggest that manipulation of sGC/cGMP signaling may serve as a favorable alternative to current glioma treatment strategies. These concepts may also have applications to other tumors.
Materials and Methods
Cell lines, Cell Culture, and Biochemical Characterization of sGC/cGMP Signaling Molecules.
Glioma cell lines U87, U251, U373, A172, LN18, LN229, and D54 were obtained from American Type Culture Collection (Manassas, VA) and maintained at 37°C, 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (HyClone, Logan, UT) plus 1% penicillin/streptomycin mixture. The confluent cells were harvested, lysed, and processed by Western blot analysis, regular reverse transcription-PCR analysis, and quantitative real-time reverse transcription-PCR analysis as described in Supplemental Materials and Methods.
Generation of U87 Stable Transfectant Lines.
The protocol for generation of U87 stable transfected clones is described in Supplemental Materials and Methods.
Assay of cGMP/cAMP in Intact Cells.
For the activity assay, accumulation of cGMP/cAMP in tumor cells was determined in Dulbecco's phosphate-buffered saline containing 1 mM 3-isobutyl-1-methylxanthine (IBMX) (preincubation for 10 min with an sGC activator, 1 μM BAY 41-2272 or vehicle control). Then, an NO donor, spermine NONOate (0.1 mM) or forskolin (10 μM) was added and incubation continued for another 10 min. Medium was aspirated and cGMP/cAMP was extracted by rapid freezing of the plates at −80°C in the presence of 50 mM sodium acetate, pH 4.0, (0.3 ml per well), then measured by enzyme-linked immunosorbent assay as described previously (Kots et al., 2008). For the assay of the basal level, tumor cells were harvested without IBMX preincubation and further treatments.
Cell Viability and Colony Formation Assays.
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) assay was used to quantify surviving/proliferating cells as described in Supplemental Materials and Methods. The modified soft agar assay protocol for colony formation is described in Supplemental Materials and Methods.
Establishment of Xenograft Tumors, Efficacy Evaluation, and Characterization of Intracranial Xenografts.
Human glioma cell lines with or without stable transfection (at a concentration of 106 cells/5 μl) were resuspended in PBS and injected into the right frontal lobe of nude mice using a guide-screw system implanted within the skull as described previously (McCutcheon et al., 2000). Detailed protocols are described in Supplemental Materials and Methods. When the animals became moribund due to tumor progression, they were euthanized, and the brains were removed for histological and molecular analysis. The vasculature (CD31 staining) and cellular proliferation (Ki67 staining) in the tumors were evaluated by immunohistochemistry as described in Supplemental Materials and Methods.
Statistical Analysis.
Results are expressed as mean ± S.E.M. A one-way analysis of variance was performed for multiple comparisons, and if there was a significant variation between the treatment groups, the mean values for a treated group were compared with those of the control by Student's t test; p values of less than 0.05 were considered statistically significant. The n values indicate the numbers of animals used in the experiment.
Results
Absence of sGC Signaling in Human Glioma Tissues and Cells.
The key intracellular receptor for NO is sGC, which produces the second messenger cGMP. Pathologically high concentrations of NO are produced by iNOS, which is overexpressed in various inflammation-related conditions, including glioma (Cuny et al., 2002). To explore the functional significance of increased NO on sGC, we examined cGMP accumulation in primary cultured brain tumor cells, including glioma specimens. As demonstrated in Supplemental Fig. S1 (also refer to Fig. 4B), the basal levels of cGMP in malignant brain tumors were markedly low, and unresponsive to stimulation by either NO donor spermine NONOate or 3-(4-amino-5-cyclopropylpyrimidine-2-yl)-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine (Bay41-2272), an allosteric sGC activator. Thus, the sGC-catalyzed cGMP production seems to be impaired or abolished in glioma and other malignant brain tumors.
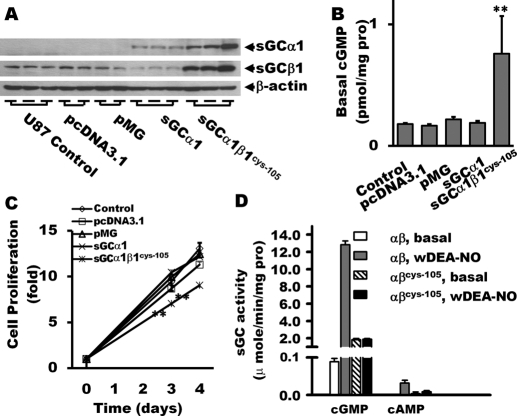
Fig. 4.
Proliferation analysis of sGCα1 and sGC α1β1Cys105-expressing stable clones. Overexpression of sGCα1 and sGCα1β1Cys105 mutant in U87 glioma cells was confirmed by Western blot (A). Lanes 1 to 3, U87 control; lanes 4 to 7, vector alone; lanes 8 and 9, sGCα1 overexpression; and lanes 11 to 13, sGCα1β1Cys105 overexpression. The lysates were also probed for the basal cGMP levels in U87 glioma cells (B). Codelivery of sGCα1 and sGCβ1Cys105 in U87 glioma cells suppressed cellular proliferation (C). Basal and NO-stimulated activity (1 μM DEA-NO) of the wild-type and mutant αβCys105 sGC in generation of cGMP and cAMP were evaluated (D). n = 6 per group for cGMP and cAMP assays; n = 18 per group for proliferation assay (MTT). **, P < 0.01 (versus control groups).
sGC is a heterodimer composed of α and β subunits (Kamisaki et al., 1986). To date, four isoforms of sGC subunit have been identified (α1, α2, β1, and β2) in mammals (Zabel et al., 1998), and the majority of functional sGC heterodimer is α1β1, which is expressed ubiquitously. To further explore the status of sGC expression in gliomas, we collected glioma cell lines of U87, U251, U373, A172, LN18, LN229, and D54, and investigated protein expression levels of the sGC α1 and β1 (Fig. 1A). The sGCα1 subunit was undetectable in most glioma cell lines. The sGCβ1 subunit was significantly decreased in all glioma cells. Furthermore, the protein levels of the sGC subunits were either undetectable or very low in human glioma tissues compared with that in normal adjacent tissues (Supplemental Fig. S2).
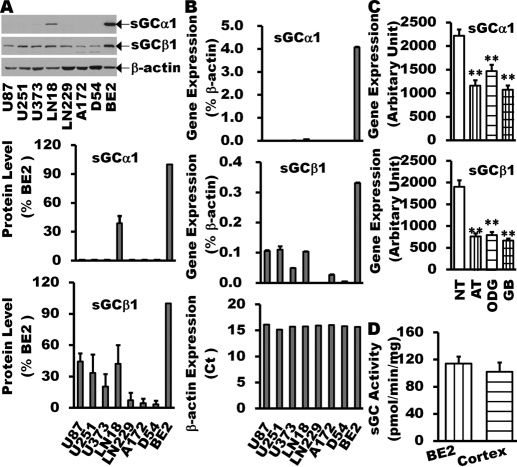
Fig. 1.
Reduction of sGC expression in human glioma tissues and cell lines. sGC expression in glioma cell lines (U87, U251, U373, A172, LN18, LN229, and D54) was examined by Western blot (A) and real time-Q-PCR (B) and compared with that in BE2 human neuroblastoma cell line, which normally expresses both sGC α1 and β1 subunits at levels similar to those in normal human cortex (D) (Bonkale et al., 1995; Corbalán et al., 2002; Sharina et al., 2008). The GEO database analysis of sGC gene expression in human glioma tissues of different grade (C) showed that the expression of sGC α1 and β1 is markedly decreased in astrocytoma (n = 26), oligodendrocytoma (n = 50), and glioblastoma multiforme (n = 81) compared with normal brain tissues (n = 23). Data are mean ± S.E.M. n = 3 to 6 per group for glioma cell lines. **, P < 0.01 (versus normal brain tissues).
Real-time-Q-PCR of sGCα1 and β1 transcript levels showed a pattern similar to protein expression (Fig. 1B). sGCα1 mRNA was almost undetectable in all glioma cell line samples. sGCβ1 mRNA was detectable, but at very low levels in glioma tumor cells.
A significant difference in gene expression has been demonstrated in human cancer cell lines compared with the corresponding human tumor tissues (Ertel et al., 2006). To obtain gene expression profiles from human glioma tissues, we accessed the Gene Expression Omnibus (GEO) at the National Center for Biotechnology Information (NCBI) and analyzed the expression of sGC genes in human glioma of different grades. As shown in Fig. 1C, the expression levels of sGCα1 and β1are markedly decreased in astrocytoma (n = 26), oligodendrocytoma (n = 50), and glioblastoma multiforme (n = 81) compared with normal brain tissues (n = 23).
It is worth noting that sGC expression is not only diminished in malignant glioma, but it also inversely correlates with the malignancy of the brain tumors. Additional data illustrating those findings are presented in supplementary materials (Supplemental Figs. S2 and S3).
Expression of NPR, PDE, and PKG in Glioma Cells.
cGMP is also produced when natriuretic peptide receptors (NPRs = membrane-bound [particulate] guanylyl cyclase, pGC) are stimulated with natriuretic peptides including atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) (Murad et al., 1987). Using a real time-Q-PCR approach, we quantified the amount of mRNA transcripts of the receptors, and detected the expression of NPR1 (GC-A), NPR2 (GC-B), and guanylyl cyclase type C (GC-C) in all tested samples (Fig. 2A). It has been known that NPR2 represents the main natriuretic peptide receptor in the brain (Wilcox et al., 1991), and it shares structural similarities with NPR1.
Fig. 2.
pGCs, PDEs, and PKG expression in glioma cell lines. Real time-Q-PCR analysis of glioma cell lines (U87, U251, U373, A172, LN18, LN229, D54) determined expression profiles of NPR1, -2, -3 (A), PDEs (B), and PKG (D and E). The profile of PDE isoform in U87 cells was also assayed (C). Data are mean ± S.E.M. n = 3 to 6 per group for glioma cell lines.
Inhibition of specific phosphodiesterases (PDEs) results in cellular responses by elevating cGMP signals derived from the activation of sGC and/or pGC (Bian et al., 2008). We examined the profile of PDE expression in U87 cells (Fig. 2B) and detected PDE2, PDE5, PDE8, PDE10, and PDE11 expression. PDE5 is a highly cGMP-specific isoform. PDE5 inhibitors have been the subject of many recent clinical and basic-science studies beside erectile dysfunction (Bhuiyan et al., 2006). We performed Q-PCR analysis with various glioma cell lines (Fig. 2C) and found that U87, A172, and LN18 have higher levels of PDE5 expression.
One of the major receptors for cGMP is the cGMP-dependent protein kinase (PKG), which phosphorylates serine and threonine residues in many cellular proteins. Two PKG families (PKG 1 and PKG 2) are derived from separate genes (prkg1 and prkg2). Our Q-PCR analysis (Fig. 2, D and E) with glioma cell lines revealed at least three patterns of expression: PKG1-dominant (U87), PKG2-dominant (A172 or Ln18), equal expression of PKG1 and PKG2 (D54), and PKG-negative (U373).
Inhibition of Glioma Cell Proliferation by Pharmacologically Restoring cGMP Signaling.
To verify whether restoring sGC function through cGMP elevation may influence proliferation of glioma cells, we investigated the effect of 8-bromo-cGMP, a membrane-permeable cGMP analog resistant to PDE hydrolysis. As demonstrated in Fig. 3A, 8-bromo-cGMP significantly reduced proliferation of glioma cells in a concentration-dependent manner.
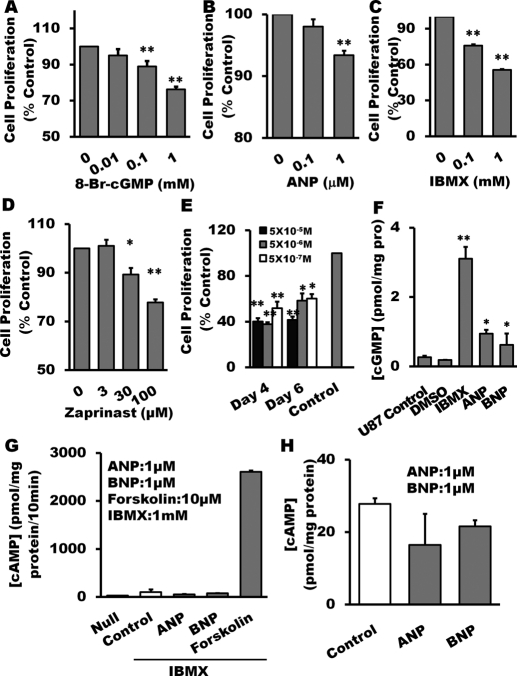
Fig. 3.
Inhibition of glioma cell proliferation by pharmacological restoration of cGMP. Treatment with 8-bromo-cGMP (A), particulate GC agonist ANP (B), nonselective PDE inhibitor IBMX (C), and PDE5-specific inhibitor zaprinast (D) for 24 h, respectively, suppressed U87 cell proliferation measured by MTT assay. Combined treatment of ANP with 0.1 mM IBMX for 4 or 6 days inhibited U87 cell proliferation (E). After 24-h treatment with ANP (1 μM), IBMX (1 mM), and 8-Br-cAMP (1 mM), cGMP accumulation in U87 glioma cells was increased significantly (F). Adenylyl cyclase activity (G) and cAMP accumulation (H) in U87 glioma cells was measured after stimulation with cGMP-promoting reagents. 10 μM forskolin was used as positive control. Data are mean ± S.E.M. n = 6 per group, *, P < 0.05; **, P < 0.01 (versus control groups).
On the basis of NPR expression profile in glioma cell lines (Fig. 2A), we tested the hypothesis that elevating endogenous cGMP through NPRs stimulation should have similar effects as 8-bromo-cGMP on tumor proliferation. As demonstrated in Fig. 3B, ANP did inhibit proliferation of U87 glioma cells in a concentration-dependent manner.
Inhibiting PDE activity is another approach to elevate intracellular cGMP. Considering the fact that multiple PDE isoforms are expressed in U87 cells (Fig. 2B), we used a nonselective PDE inhibitor IBMX and showed (Fig. 3C) that IBMX concentration-dependently suppressed glioma cell proliferation. As shown in Fig. 2C, U87 cells express higher levels of PDE5. We then treated the cells with a PDE5-specific inhibitor zaprinast and also demonstrated a significant attenuation of cell proliferation (Fig. 3D). To further confirm our observations, we treated U87 cells with an NPR agonist in combination with a PDE inhibitor. The combined treatment concentration-dependently inhibited tumor cell growth for up to 6 days (Fig. 3E). We did not observe marked morphological changes of the glioma cells treated with 8-bromo-cAMP, ANP, and IBMX.
To verify the effects of these pharmacological agents on the levels of intracellular cGMP, the assay for cGMP accumulation was performed. As demonstrated in Fig. 3F, 24-h treatment with ANP (1 μM) or IBMX (1 mM) markedly increased cGMP accumulation in U87 glioma cells. In summary, glioma cell proliferation was significantly inhibited upon pharmacological restoration of sGC function through increasing intracellular cGMP levels. To further rule out possible cAMP involvement, we measured cAMP accumulation (Fig. 3, G and H) in U87 glioma cells under stimulation of the cGMP-promoting agents. In contrast to the magnitude of forskolin-stimulated cAMP, IBMX, ANP, and BNP failed to significantly influence the levels of cAMP.
Inhibition of Glioma Cell Proliferation by Genetically Restoring sGC .
To provide additional evidence supporting the inhibitory effects of cGMP on tumor growth, we established three stable clones of U87 cells overexpressing the sGCα1 subunit alone (Fig. 4A, Western blot, lanes 8–10) or in combination with the sGCβ1Cys105 mutant (Fig. 4A, lanes 11–13). The sGCβ1Cys105 mutant was created and characterized previously by our group (Martin et al., 2003). It has a constitutively elevated activity that allows observing the effect of sGC function with less inference from endogenous NO (Martin et al., 2003). It is worth noting that overexpression of the α1β1Cys105 sGC heterodimer resulted in higher basal level of cGMP (Fig. 4B).
To verify the effect of genetically restored sGC activity on glioma cell proliferation, the MTT assay was performed, and the proliferation curves were plotted in Fig. 4C. The proliferation of U87 cells was markedly inhibited by overexpression of α1β1Cys105 sGC. Delivery of only sGCα1 failed to suppress cellular proliferation, probably because the insufficient expression of sGCβ1 in the cells could not generate threshold basal level of cGMP (Fig. 4B). To further clarify the action of sGC enzyme, we measured basal and NO-stimulated activity of the wild-type and mutant α1β1Cys105 sGC in generation of cGMP and cAMP (Fig. 4D). NO donor DEA-NO (1 μM) significantly increased cGMP generation of wild-type sGC, but had no influence on cGMP synthesis by the α1β1Cys105 sGC mutant. Neither basal nor NO-stimulated wild-type and mutant αβCys105 sGC catalyzed significant synthesis of cAMP.
Inhibition of Glioma Cell Colony Formation by Pharmacologically Increasing cGMP or Genetically Restoring sGC.
The cellular microenvironment is important for modulation of cancer cell growth. Growing cells within a three-dimensional (3D) support system may simulate a natural microenvironment affecting tumor cell proliferation, morphology, signaling, and responses to therapeutic agents (Fischbach et al., 2007). Thus, we used the colony formation assay to further evaluate the influence of sGC signaling on glioma cell growth. We treated the cells with 1 μM ANP and assessed the inhibitory effect of the natriuretic peptide on colony number and size. There were fewer colonies (the size ≥1 mm2) formed in the ANP-treated group than in the control group (Fig. 5, A and B). In addition, the average size of the colonies was reduced by 40% in the ANP-treated group (Fig. 5, A and C).
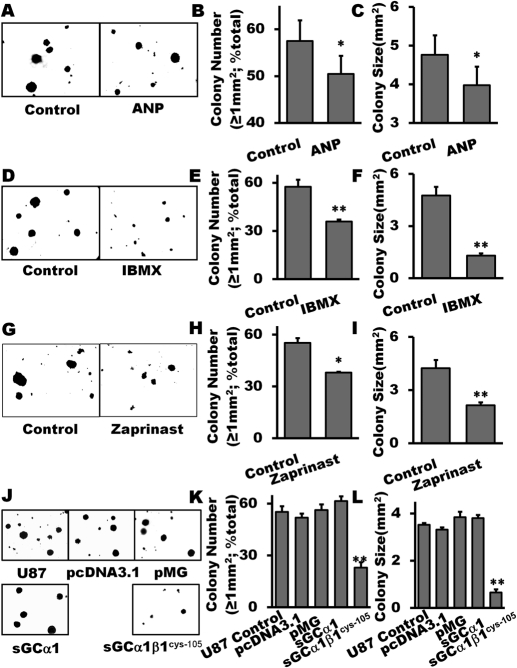
Fig. 5.
Inhibition of glioma colony formation by pharmacological restoration of cGMP or genetical restoration of sGC. Treatment with 1 μM ANP (A–C), IBMX (0.1 μM; D–F) and zaprinast (30 μM, G–I), respectively, markedly inhibited the number of larger colonies with the size ≥1 mm2 and reduced average size of colonies of U87 glioma cells. The clones of the U87 glioma cell with stable overexpression of α1β1Cys105 sGC formed fewer and smaller colonies (J–L). Reagents were freshly added every 3 days for a period of 3 weeks. Colonies were counted at day 21 using an inverted microscope with a digital camera photo system. Data are mean ± S.E.M. n = 6 per group, *, P < 0.05; **, P < 0.01 (versus control groups).
The effects of elevating intracellular cGMP by IBMX on U87 glioma colony formation are shown in Fig. 5, D to F. IBMX significantly decreased colony (with size ≥1 mm2) number and size by 33 and 67%, respectively. To further confirm the efficacy of PDE inhibition, we used the PDE5-specific blocker zaprinast and observed significant suppression of tumorigenesis (Fig. 5, G–I).
Genetically restoring sGC by overexpression of α1β1Cys105 sGC in U87 glioma cells also significantly attenuated colony formation. The average size of colonies was reduced from 3.5 mm2 for the controls to 0.7 mm2 for the α1β1Cys105 sGC-overexpressing clones (Fig. 5, J and K). The number of larger colonies (size ≥1 mm2) was also markedly less in the α1β1Cys105sGC-transfected colonies (Fig. 5, J and L). Neither the U87 control nor U87 with an empty vector (pcDNA or pMG) showed any changes in the size or number of colonies. Similar results were obtained in the stable clones overexpressing the sGC α1 subunit.
Intracerebral Glioma Xenograft Assay for sGC Transfectants.
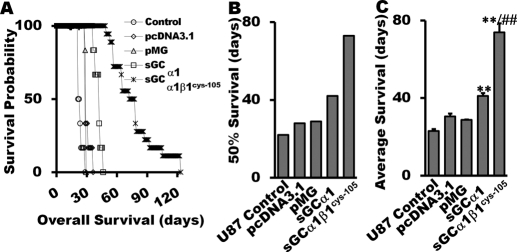
In vivo antitumor activity of sGC / cGMP was evaluated in athymic nude mice with intracerebral xenotransplantation of U87 cells with or without prior transfection. The data of Fig. 6A indicate that animals inoculated with α1β1Cys105 sGC-transfected cells had significantly longer survival time. The 50% survival times of the mice inoculated with the α1β1Cys105 sGC-expressing cells (n = 18) were extended to 73 days (332% of control group; Fig. 6, A and B). The longest average survival time of animals inoculated with the α1β1Cys105 sGC-transfected cells was more than 124 days (4.6-fold over control). The average survival times and 50% survival times of different groups of tumor-bearing mice are shown in Fig. 6, B and C. Unlike cell proliferation and colony formation assays, intracranial xenograft with the sGCαl transfectant prolonged average survival time in a statistically significant manner (Fig. 6B).
Fig. 6.
Intracerebral human glioma xenograft assay for sGC transfectants. In vivo antitumor activity of sGC / cGMP was evaluated in athymic mice with intracerebral xenotransplantation of U87 cells with or without transfection. The survival probability (A), average survival time (B), and 50% survival time (C) were plotted. Data are mean ± S.E.M. n = 6 for U-87 control, U87 with empty vectors, and U87-overexpressing sGCα1; n = 18 for U87 glioma cells transfected with α1β1Cys105 sGC subunits. **, P < 0.01(versus control groups). ##, P < 0.01 (versus sGCα1-overexpressing group).
Characteristic Glioma Xenograft Changes in sGC Transfectants.
Histological analysis showed that glioma xenograft was clearly distinct from the surrounding host brain tissue and had pathological features of glioblastoma (Fig. 7A). The tumor tissue obtained from mice inoculated with the α1β1Cys105 sGC-transfected cells had significantly fewer microvessels as assessed by CD31 staining (Fig. 7, B and C) and quantified using a method previously developed by our group (Zhu et al., 2008). Immunostaining for Ki-67, a marker associated with cell proliferation, was used to evaluate the fraction of growing cells in malignant neoplasia. A marked reduction in Ki-67 positive cells was observed in double-sGC transfectant-derived tumors (Fig. 7, D and E). It is notable that xenografts in mice inoculated with the α1β1Cys105 sGC-transfected cells exhibited a heterogeneous population of tumor cells (Fig. 7A) with different nuclear density. We have observed decreased angiogenesis in both tumor cell populations. However, increased Ki-67 labeling occurred predominantly in the areas with higher nuclear density. It will be interesting to have more information about the heterogeneous population of tumor cells in the α1β1Cys105 sGC xenograft model.
Fig. 7.
Characterization of glioma xenograft with sGC transfectants. H.E. staining showed tumor mass derived from sGC α1β1Cys105-overexpressing cells with well defined pathological features of glioblastoma (A; 100× and 400× magnification). The xenografts with α1β1Cys105 sGC are composed of heterogeneous populations of tumor cells (A, 400× magnification) with different nuclear density. The tumor tissue derived from α1β1Cys105 sGC transfected clone had significantly fewer microvessels according to CD31 staining (B and C; 200× magnification). Significant reduction in Ki-67 staining, a marker associated with cell proliferation, was observed in sGC transfectant-derived tumors (D and E; 200× magnification). Data are mean ± S.E.M. n = 3 to 4 for each group. **, P < 0.01(versus control groups).
Discussion
The role of cyclic nucleotides in the regulation of cell proliferation and tumor growth was noted as early as in the 1960s (Ryan and Heidrick, 1968). cAMP and cGMP were thought to act as biological antagonists in the regulation of cell growth at the beginning (Goldberg et al., 1975), and elevated cGMP levels have been observed in certain human tumor tissues (Hadden et al., 1972; DeRubertis et al., 1976; Takemoto et al., 1982). Increased urinary cGMP in rats with hepatoma or renal tumor implants was also detected by Murad et al. (1975) and Criss and Murad (1976). Inhibition of proliferation by cGMP was initially observed in vascular smooth muscle cells (Garg and Hassid, 1989) and then extended to endothelial cells (Yang et al., 1994) and the cells from nervous system [e.g., cerebellar, glial (Garg et al., 1992), and neuroblastoma cells (Murillo-Carretero et al., 2002)]. Frattola et al. (1976) reported a lower activity of guanylyl cyclase in human neurinomas and glioblastomas. Our current study demonstrates an altered expression of sGC in malignant brain tumors and suggests a therapeutic approach by manipulating sGC/cGMP signaling either pharmacologically or genetically. Stable clones with the overexpressed heterodimer α1β1Cys105 sGC resulted in elevated cGMP levels and inhibited colony formation in vitro and extended survival of tumor-bearing mice in vivo.
The Cancer Genome Atlas (TCGA) pilot project selected glioma as the first cancer for which molecular genetic changes would be characterized (McLendon et al., 2008; Parsons et al., 2008). Analysis of nearly 21,000 genes in some 20 patients with glioblastoma by Vogelstein's team and 600 genes in 91 glioblastoma patients by the TCGA team (McLendon et al., 2008; Parsons et al., 2008) confirmed that cancer cells are diverse with numerous genetic aberrations and epigenetic modifications. However, no apparent genetic mutations of sGC were reported in these studies. We propose that an event affecting the transcription and translation of sGC, rather than a functional mutation of the protein, could be important during the development of some cancers.
The common sGC α1β1 heterodimer is expressed ubiquitously, and the β1 subunit has the binding site for NO. Our group (Martin et al., 2003) created a constitutively active mutant with the β1subunit His-105 residue substituted with Cys105. This heme-deficient sGCα1β1Cys105 heterodimer has markedly higher basal activity compared with the heme-containing wild-type enzyme. Our current study with the stable clones expressing α1β1Cys105 sGC suggests a potential therapeutic role for the mutant enzyme. Several possible mechanisms have been reported for the effects of cyclic nucleotides on cancer growth, including suppression of proliferation by cell cycle arrest in G0-G1 in human hepatocellular carcinoma cells (Wang et al., 2005), induction of apoptosis in human colon adenocarcinoma cells (Shailubhai et al., 2000), and induction of cell differentiation in human Caco-2 intestinal epithelial cells (Basson and Hong, 1996). Our study revealed an interesting finding that inhibition by overexpression of the α1β1Cys105 sGC was more potent in the colony formation assay than that in the MTT assay. MTT quantifies cell survival, proliferation, and mitochondrial function. cGMP inhibits proliferation (decreased MTT) but increases mitochondrial function (increased MTT) (Ruiz-Stewart et al., 2004). Thus, smaller effect of sGC/cGMP in the MTT assay versus the colony assay may imply an inhibition of cell-cell communication. Significant reduction of angiogenesis in xenografts of mice inoculated with α1β1Cys105 sGC-transfected cells further supports this hypothesis (Fig. 7, B and C).
As illustrated in Fig. 7A, glioma xenografts of U-87 control or U-87 with the empty vector (pcDNA or pMG) exhibited morphology of cancer cells with hypertrophic nucleolus, decreased cytoplasmic/nuclear ratio, and shrunken cytoplasm. It is notable that xenografts expressing α1β1Cys105 sGC had heterogeneous populations of tumor cells (Fig. 7, 400×), a large population of cells resembling the morphology of the normal cells. We propose that sGC/cGMP signaling normalizes glioma cellular architecture through a prodifferentiation mechanism. It is generally accepted that tumor malignancy correlates with undifferentiated (anaplastic) status. Our group has previously reported low levels of sGCα1 and β1 expression in undifferentiated embryonic stem cells from both human and mouse, and the embryonic stem cells regain sGC expression while entering differentiation (Krumenacker et al., 2006; Mujoo et al., 2006). Tumorigenesis and organogenesis are similar in many respects, and many types of cancer (including brain tumor) contain cancer stem-like cells (Singh et al., 2004; Yuan et al., 2004). Thus, we isolated a population of CD133-positive cancer stem-like cells from human glioma and did not detect sGCα1 expression when the expression of sGCβ1 mRNA was very low (data not shown). Together with our findings that restoring sGC function inhibits glioma growth and normalizes cellular architecture, we suggest that involvement of a prodifferentiation mechanism in sGC-targeted therapy may be an alternative or complementary approach to toxic treatments such as chemotherapy and radiation.
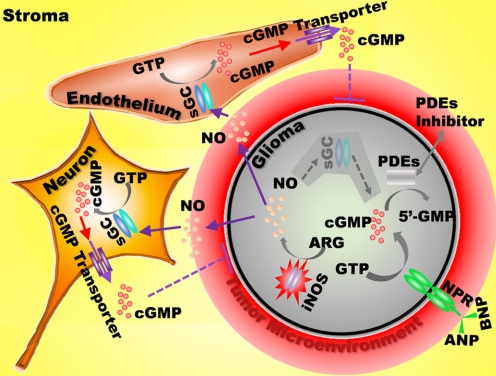
Our current study suggests two possible roles of NO/cGMP signaling in malignant tumors (also see Scheme 1). First, iNOS expression and NO overproduction may contribute to the formation of inflammatory cancer microenvironment. Second, sGC/cGMP signaling may influence proliferation and/or differentiation of the tumor cells. Our current observations suggest that although sGC-deficient glioma cells lack the cGMP-mediated response to NO, this highly permeant gas can act upon adjacent stroma and increase cGMP levels in nonmalignant supporting tissues, including connective tissue and blood vessels. Although the elevated cGMP in surrounding tissues cannot cross the cell membrane to enter the cancer cells, it can be released and detected in extracellular fluids of patients or animals with tumors as discussed above (Murad et al., 1975; Criss and Murad, 1976).
Scheme 1.
NO/cGMP signaling in malignant tumor. NO and cGMP participate in regulation of inflammation in the cancer microenvironment and contribute to proliferation and/or differentiation of tumor cells and surrounding supporting cells. The iNOS/NO/cGMP signaling pathway is not functional in glioma because of sGC-deficiency. However, higher concentration of NO generated by malignant cells and/or its microenvironment can penetrate cell membrane and increase cGMP levels in nonmalignant stroma cells. cGMP can be released into the intercellular space through cGMP transporters; however, extracellular cGMP cannot cross the cell membrane to re-enter the cells.
The cross-talk between the cGMP and cAMP pathways has been noticed for years. To clarify the involvement of cAMP in our experimental settings, we measured cAMP levels (Fig. 3, G and H) in glioma cells treated with cGMP-manipulating pharmacological agents. As showed in Fig. 3G, the 10 μM forskolin (in the presence of 1 mM IBMX) excited a 23-fold increase in cAMP compared with that stimulated by 1 mM IBMX alone. It is worth to note that 100 μM forskolin is commonly used for inhibition of cancer cell proliferation and growth both in vitro and in vivo (Yamanaka et al., 2010, 2011). In contrast, for human U138 glioma cells an enhanced proliferation by cAMP signaling was reported (Farias et al., 2008). To further verify the possible influence of sGC on the cAMP pathway, we examined the cAMP-forming capacity of both wild-type and Cys105-mutant sGC (Fig. 4D). We found only an insignificant formation of cAMP by NO-induced wild-type sGC and detected only traces of cAMP in samples with α1β1Cys105 sGC enzyme (Fig. 4D). These data strongly suggest that the observed effect of sGC-dependent inhibition of glioma cell proliferation through a cAMP/protein kinase A-dependent pathway is unlikely.
cGMP-dependent protein kinase (cGK), also called protein kinase G (PKG), is a serine/threonine-specific protein kinase which exists in two isoforms, type-I and type II. PGK1 has been shown to have tumor suppressor properties in colon carcinoma (Deguchi et al., 2004). However, PKG1α has been recently reported to promote DNA synthesis and cell proliferation in human ovarian cancer cells (Leung et al., 2010). PKG2 is widely expressed in normal brain tissue. We thus screened human glioma cell lines for expression of PKG isoforms (Fig. 2D) and found four different phenotypes. Although some cell lines express PKG1 (e.g., U87), PKG2 (e.g., A172 or Ln18), or both (e.g., D54), other cells are PGK-negative (e.g., U373). The U87 glioma cells are characterized as highly tumorigenic, low-invasive, and PKG2 deficient. Westermark's group (Swartling et al., 2009) reported that overexpression of PKG2 in U87 cells inhibited cell proliferation and xenograft growth. Together with our current findings, the experimental evidence supports our hypothesis that normal function of the sGC-cGMP signaling axis may be important for the prevention and/or treatment of glioma. Further studies are warranted to elucidate the detailed involvement of PKG, as well as protein kinase A, in sGC/cGMP signaling-targeted glioma therapy.
In summary, we found that sGC expression is lower or diminished in human glioma tissues and cell lines and propose that sGC is a novel tumor susceptibility gene in human glioma. Restoring sGC/cGMP signaling genetically or pharmacologically significantly inhibited glioma growth. Orthotopic xenograftment of glioma cells with the α1β1Cys105 sGC stable clone in athymic mice increased the survival time by 4-fold over the control. This increase in survival time exceeds the results recently reported for therapy of glioma with the combination of bevacizumab [Avastin; Genentech (South San Francisco, CA)VEGF-A antibody] and temozolomide (an alkylating agent) (Mathieu et al., 2008). New approaches in cancer therapy that facilitate selective targeting of cancers have been emerging in recent years. For instance, the estrogen and progesterone receptor assays have had important effects on treatment of breast cancer. Our current research provides genetic, proteomic, and functional evidence from in vitro and in vivo experiments supporting a role for sGC/cGMP in the diagnosis and therapy of human malignant intracranial tumors. The NO pathways may facilitate development of new protocols involving activation or inhibition of iNOS, sGC, pGC, PKG, and PDE to treat human malignant tumors by influencing NO and cGMP signaling.
Supplementary Material
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM076695-02]; the John S. Dunn [Grants 74Y-1-9135, 000447] and Robert A. Welch Foundations [Grants AU-1437, L-AU-0002]; The Department of Defense [Grant T5-0004271project]; and The George Washington University. The work was also supported in part by the NHLBI Grant 5R01HL088128 [E.M.]. The authors sincerely thank the supports of Dr. Raymond Sawaya of the Department of Neurosurgery and Brain Tumor Center of The University of Texas M. D. Anderson Cancer Center.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/mol.111.073585.
- cGMP
- cyclic 3′,5′-GMP
- sGC
- soluble guanylyl cyclase
- iNOS
- inducible nitric-oxide synthase
- PCR
- polymerase chain reaction
- IBMX
- 3-isobutyl-1-methylxanthine
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
- Bay41-2272
- 3-(4-amino-5-cyclopropylpyrimidine-2-yl)-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine
- Q-PCR
- quantitative polymerase chain reaction
- GC
- guanylyl cyclase
- pGC
- particulate guanylyl cyclase
- NPR
- natriuretic peptide receptor
- ANP
- atrial natriuretic peptide
- BNP
- brain natriuretic peptide
- PDE
- phosphodiesterase
- PKG
- cGMP-dependent protein kinase.
Authorship Contributions
Participated in research design: Murad and Bian.
Conducted experiments: Zhu, Li, Zheng, Martin, Kots, Krumenacker, Choi, Bögler, and Bian.
Contributed new reagents or analytic tools: Martin and McCutcheon.
Performed data analysis: Zhu, Choi, and Bian.
Wrote or contributed to the writing of the manuscript: Zhu, Weisbrodt, Bögler, Murad, and Bian.
References
- Basson MD, Hong F. (1996) Regulation of human Caco-2 intestinal epithelial brush border enzyme activity by cyclic nucleotides. Cancer Lett 99:155–160 [DOI] [PubMed] [Google Scholar]
- Bhuiyan MB, Murad F, Fant ME. (2006) The placental cholinergic system: localization to the cytotrophoblast and modulation of nitric oxide. Cell Commun Signal 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian K, Doursout MF, Murad F. (2008) Vascular system: role of nitric oxide in cardiovascular diseases. J Clin Hypertens (Greenwich) 10:304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian K, Gao Z, Weisbrodt N, Murad F. (2003) The nature of heme/iron-induced protein tyrosine nitration. Proc Natl Acad Sci USA 100:5712–5717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian K, Harari Y, Zhong M, Lai M, Castro G, Weisbrodt N, Murad F. (2001) Down-regulation of inducible nitric-oxide synthase (NOS-2) during parasite-induced gut inflammation: a path to identify a selective NOS-2 inhibitor. Mol Pharmacol 59:939–947 [DOI] [PubMed] [Google Scholar]
- Bian K, Murad F. (2003) Nitric oxide (NO)–biogeneration, regulation, and relevance to human diseases. Front Biosci 8:d264–278 [DOI] [PubMed] [Google Scholar]
- Bonkale WL, Winblad B, Ravid R, Cowburn RF. (1995) Reduced nitric oxide responsive soluble guanylyl cyclase activity in the superior temporal cortex of patients with Alzheimer's disease. Neurosci Lett 187:5–8 [DOI] [PubMed] [Google Scholar]
- Corbalán R, Chatauret N, Behrends S, Butterworth RF, Felipo V. (2002) Region selective alterations of soluble guanylate cyclase content and modulation in brain of cirrhotic patients. Hepatology 36:1155–1162 [DOI] [PubMed] [Google Scholar]
- Criss WE, Murad F. (1976) Urinary excretion of cyclic guanosine 3′:5′-monophosphate and cyclic adenosine 3′:5′-monophosphate in rats bearing transplantable liver and kidney tumors. Cancer Res 36:1714–1716 [PubMed] [Google Scholar]
- Cuny E, Loiseau H, Penchet G, Ellie E, Arsaut J, Vital A, Vincendeau P, Demotes-Mainard J. (2002) Association of elevated glial expression of interleukin-1beta with improved survival in patients with glioblastomas multiforme. J Neurosurg 96:294–301 [DOI] [PubMed] [Google Scholar]
- Deguchi A, Thompson WJ, Weinstein IB. (2004) Activation of protein kinase G is sufficient to induce apoptosis and inhibit cell migration in colon cancer cells. Cancer Res 64:3966–3973 [DOI] [PubMed] [Google Scholar]
- DeRubertis FR, Chayoth R, Field JB. (1976) The content and metabolism of cyclic adenosine 3′,5′-monophosphate and cyclic guanosine 3′,5′-monophosphate in adenocarcinoma of the human colon. J Clin Invest 57:641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel A, Verghese A, Byers SW, Ochs M, Tozeren A. (2006) Pathway-specific differences between tumor cell lines and normal and tumor tissue cells. Mol Cancer 5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias CB, Lima RC, Lima LO, Flores DG, Meurer L, Brunetto AL, Schwartsmann G, Roesler R. (2008) Stimulation of proliferation of U138-MG glioblastoma cells by gastrin-releasing peptide in combination with agents that enhance cAMP signaling. Oncology 75:27–31 [DOI] [PubMed] [Google Scholar]
- Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, Mooney DJ. (2007) Engineering tumors with 3D scaffolds. Nat Methods 4:855–860 [DOI] [PubMed] [Google Scholar]
- Frattola L, Carenzi A, Cerri C, Kumakura K, Trabucchi M. (1976) Regulation of the cyclic guanosine 3′-5′ monophosphate system in human brain tumors. Acta Neurol Scand 54:382–390 [DOI] [PubMed] [Google Scholar]
- Garg UC, Devi L, Turndorf H, Goldfrank LR, Bansinath M. (1992) Effect of nitric oxide on mitogenesis and proliferation of cerebellar glial cells. Brain research 592:208–212 [DOI] [PubMed] [Google Scholar]
- Garg UC, Hassid A. (1989) Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 83:1774–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg ND, Haddox MK, Nicol SE, Glass DB, Sanford CH, Kuehl FA, Jr, Estensen R. (1975) Biologic regulation through opposing influences of cyclic GMP and cyclic AMP: the Yin Yang hypothesis. Adv Cyclic Nucleotide Res 5:307–330 [PubMed] [Google Scholar]
- Hadden JW, Hadden EM, Haddox MK, Goldberg ND. (1972) Guanosine 3′:5′-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci USA 69:3024–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. (2008) Cancer statistics, 2008. CA Cancer J Clin 58:71–96 [DOI] [PubMed] [Google Scholar]
- Kamisaki Y, Saheki S, Nakane M, Palmieri JA, Kuno T, Chang BY, Waldman SA, Murad F. (1986) Soluble guanylate cyclase from rat lung exists as a heterodimer. J Biol Chem 261:7236–7241 [PubMed] [Google Scholar]
- Kimura H, Murad F. (1975) Increased particulate and decreased soluble guanylate cyclase activity in regenerating liver, fetal liver, and hepatoma. Proc Natl Acad Sci USA 72:1965–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kots AY, Choi BK, Estrella-Jimenez ME, Warren CA, Gilbertson SR, Guerrant RL, Murad F. (2008) Pyridopyrimidine derivatives as inhibitors of cyclic nucleotide synthesis: application for treatment of diarrhea. Proc Natl Acad Sci USA 105:8440–8445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumenacker JS, Katsuki S, Kots A, Murad F. (2006) Differential expression of genes involved in cGMP-dependent nitric oxide signaling in murine embryonic stem (ES) cells and ES cell-derived cardiomyocytes. Nitric Oxide 14:1–11 [DOI] [PubMed] [Google Scholar]
- Leung EL, Wong JC, Johlfs MG, Tsang BK, Fiscus RR. (2010) Protein kinase G type Ialpha activity in human ovarian cancer cells significantly contributes to enhanced Src activation and DNA synthesis/cell proliferation. Mol Cancer Res 8:578–591 [DOI] [PubMed] [Google Scholar]
- Martin E, Sharina I, Kots A, Murad F. (2003) A constitutively activated mutant of human soluble guanylyl cyclase (sGC): implication for the mechanism of sGC activation. Proc Natl Acad Sci USA 100:9208–9213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu V, De Nève N, Le Mercier M, Dewelle J, Gaussin JF, Dehoux M, Kiss R, Lefranc F. (2008) Combining bevacizumab with temozolomide increases the antitumor efficacy of temozolomide in a human glioblastoma orthotopic xenograft model. Neoplasia 10:1383–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon IE, Friend KE, Gerdes TM, Zhang BM, Wildrick DM, Fuller GN. (2000) Intracranial injection of human meningioma cells in athymic mice: an orthotopic model for meningioma growth. J Neurosurg 92:306–314 [DOI] [PubMed] [Google Scholar]
- McLendon R, Friedman A, Bigner D, Van Meir EG, Brat DJ, Mastrogianakis M, Olson JJ, Mikkelsen T, Lehman N, et al. , for Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujoo K, Krumenacker JS, Wada Y, Murad F. (2006) Differential expression of nitric oxide signaling components in undifferentiated and differentiated human embryonic stem cells. Stem Cells Dev 15:779–787 [DOI] [PubMed] [Google Scholar]
- Mujoo K, Sharin VG, Martin E, Choi BK, Sloan C, Nikonoff LE, Kots AY, Murad F. (2010) Role of soluble guanylyl cyclase-cyclic GMP signaling in tumor cell proliferation. Nitric Oxide 22:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad F, Kimura H, Hopkins HA, Looney WB, Kovacs CJ. (1975) Increased urinary excretion of cyclic guanosine monophosphate in rats bearing Morris hepatoma 3924A. Science 190:58–60 [DOI] [PubMed] [Google Scholar]
- Murad F, Leitman DC, Bennett BM, Molina C, Waldman SA. (1987) Regulation of guanylate cyclase by atrial natriuretic factor and the role of cyclic GMP in vasodilation. Am J Med Sci 294:139–143 [DOI] [PubMed] [Google Scholar]
- Murillo-Carretero M, Ruano MJ, Matarredona ER, Villalobo A, Estrada C. (2002) Antiproliferative effect of nitric oxide on epidermal growth factor-responsive human neuroblastoma cells. J Neurochem 83:119–131 [DOI] [PubMed] [Google Scholar]
- Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et al. (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao CV. (2004) Nitric oxide signaling in colon cancer chemoprevention. Mutat Res 555:107–119 [DOI] [PubMed] [Google Scholar]
- Ruiz-Stewart I, Tiyyagura SR, Lin JE, Kazerounian S, Pitari GM, Schulz S, Martin E, Murad F, Waldman SA. (2004) Guanylyl cyclase is an ATP sensor coupling nitric oxide signaling to cell metabolism. Proc Natl Acad Sci USA 101:37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan WL, Heidrick ML. (1968) Inhibition of cell growth in vitro by adenosine 3′,5′-monophosphate. Science 162:1484–1485 [DOI] [PubMed] [Google Scholar]
- Shailubhai K, Yu HH, Karunanandaa K, Wang JY, Eber SL, Wang Y, Joo NS, Kim HD, Miedema BW, Abbas SZ, et al. (2000) Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res 60:5151–5157 [PubMed] [Google Scholar]
- Sharina IG, Jelen F, Bogatenkova EP, Thomas A, Martin E, Murad F. (2008) Alpha1 soluble guanylyl cyclase (sGC) splice forms as potential regulators of human sGC activity. J Biol Chem 283:15104–15113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. (2004) Identification of human brain tumour initiating cells. Nature 432:396–401 [DOI] [PubMed] [Google Scholar]
- Swartling FJ, Ferletta M, Kastemar M, Weiss WA, Westermark B. (2009) Cyclic GMP-dependent protein kinase II inhibits cell proliferation, Sox9 expression and Akt phosphorylation in human glioma cell lines. Oncogene 28:3121–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto DJ, Dunford C, Vaughn D, Kramer KJ, Smith A, Powell RG. (1982) Guanylate cyclase activity in human leukemic and normal lymphocytes. Enzyme inhibition and cytotoxicity of plant extracts. Enzyme 27:179–188 [DOI] [PubMed] [Google Scholar]
- Wang SW, Pan SL, Guh JH, Chen HL, Huang DM, Chang YL, Kuo SC, Lee FY, Teng CM. (2005) YC-1 [3-(5′-Hydroxymethyl-2′-furyl)-1-benzyl Indazole] exhibits a novel antiproliferative effect and arrests the cell cycle in G0-G1 in human hepatocellular carcinoma cells. J Pharmacol Exp Ther 312:917–925 [DOI] [PubMed] [Google Scholar]
- Wilcox JN, Augustine A, Goeddel DV, Lowe DG. (1991) Differential regional expression of three natriuretic peptide receptor genes within primate tissues. Mol Cell Biol 11:3454–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H, Oue T, Uehara S, Fukuzawa M. (2010) Forskolin, a Hedgehog signal inhibitor, inhibits cell proliferation and induces apoptosis in pediatric tumor cell lines. Mol Med Report 3:133–139 [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Oue T, Uehara S, Fukuzawa M. (2011) Hedgehog signal inhibitor forskolin suppresses cell proliferation and tumor growth of human rhabdomyosarcoma xenograft. J Pediatr Surg 46:320–325 [DOI] [PubMed] [Google Scholar]
- Yang W, Ando J, Korenaga R, Toyo-oka T, Kamiya A. (1994) Exogenous nitric oxide inhibits proliferation of cultured vascular endothelial cells. Biochem Biophys Res Commun 203:1160–1167 [DOI] [PubMed] [Google Scholar]
- Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, Black KL, Yu JS. (2004) Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene 23:9392–9400 [DOI] [PubMed] [Google Scholar]
- Zabel U, Weeger M, La M, Schmidt HH. (1998) Human soluble guanylate cyclase: functional expression and revised isoenzyme family. Biochem J 335:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wei X, Bian K, Murad F. (2008) Effects of nitric oxide on skin burn wound healing. J Burn Care Res 29:804–814 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.