Abstract
Cocaine toxicity is a widespread problem in the United States, responsible for more than 500,000 emergency department visits a year. There is currently no U.S. Food and Drug Administration-approved pharmacotherapy to directly treat cocaine toxicity. To this end, we have developed a mutant bacterial cocaine esterase (DM-CocE), which has been previously shown to rapidly hydrolyze cocaine into inert metabolites, preventing and reversing toxicity with limited immunogenic potential. Herein we describe the ability of DM-CocE to hydrolyze the active cocaine metabolites norcocaine and cocaethylene and its inability to hydrolyze benzoylecgonine. DM-CocE hydrolyzes norcocaine and cocaethylene with 58 and 45% of its catalytic efficiency for cocaine in vitro as measured by a spectrophotometric assay. We have developed a mass spectrometry method to simultaneously detect cocaine, benzoylecgonine, norcocaine, and ecgonine methyl ester to quantify the effect of DM-CocE on normal cocaine metabolism in vivo. DM-CocE administered to rats 10 min after a convulsant dose of cocaine alters the normal metabolism of cocaine, rapidly decreasing circulating levels of cocaine and norcocaine while increasing ecgonine methyl ester formation. Benzoylecgonine was not hydrolyzed in vivo, but circulating concentrations were reduced, suggesting that DM-CocE may bind and sequester this metabolite. These findings suggest that DM-CocE may reduce cocaine toxicity by eliminating active and toxic metabolites along with the parent cocaine molecule.
Introduction
Cocaine is an addictive alkaloid derived from leaves of the South American shrub Erythroxylon coca. In the United States, more than 1.5 million people use cocaine (Substance Abuse and Mental Health Services Administration, Office of Applied Studies, 2010), resulting in more than 500,000 emergency room visits each year (Substance Abuse and Mental Health Services Administration, Office of Applied Studies, 2004). Although the addictive effects of cocaine are caused by cocaine's blockade of dopamine transporters in the brain, cocaine's toxicity is influenced by the blockade of norepinephrine transporters, effectively increasing both central and peripheral norepinephrine levels. The blockade of peripheral sodium channels amplifies the cardiovascular effects of cocaine including coronary vasospasm, increased heart rate, hypertension, and ventricular arrhythmia (Schrank, 1992; Freye and Levy, 2009). In addition to cardiac symptoms, the hallmarks of cocaine toxicity also include hyperthermia, convulsions, seizures, and psychosis (Schrank, 1992).
Because cocaine acts on several targets to cause a wide range of symptoms, it has been difficult to develop a pharmacotherapy to treat cocaine toxicity, and there is currently no U.S. Food and Drug Administration-approved therapy for this indication. Emergency personnel are limited to treating the symptoms of cocaine toxicity only.
Although cocaine causes many of the observed toxicities, it is quickly metabolized, with a half-life of approximately 1 to 2 h in humans, into compounds that also have strong physiological effects. The most abundant metabolite (40–50%), benzoylecgonine, is produced by hydrolysis of the methyl ester of cocaine by human liver carboxylesterase-1 (Dean et al., 1991). Benzoylecgonine is inactive at monoamine transporters; however, it has some vasoconstrictive effects possibly mediated through interactions with Ca2+ channels (Madden et al., 1995). Benzoylecgonine (t1/2 = 7 h) is the urinary metabolite measured in drug screens and may be the cause of latent chest pain associated with cocaine use.
When alcohol is coabused with cocaine, cocaethylene is also formed by human liver carboxylesterase-1 through a transesterification reaction with alcohol. This reaction decreases the hydrolysis of cocaine to benzoylecgonine. Cocaethylene has properties similar to those of the parent cocaine molecule. It is known to increase striatal dopamine concentrations in vivo (Iyer et al., 1995) and inhibit dopamine uptake in ex vivo assays (Jatlow et al., 1991). Behavioral studies have demonstrated that cocaethylene is more potent than cocaine for producing lethality but less potent for increasing locomotor activity and equipotent for producing convulsions (Katz et al., 1992). In addition, cocaethylene blocks cardiac sodium channels (Xu et al., 1994) and causes larger changes in blood pressure, oxygen saturation, cardiac output, and QT interval than cocaine (Wilson et al., 2001). The increased potency of cocaethylene at cardiac sodium channels may explain the behavioral observation of increased potency in lethality assays.
Norcocaine is formed by demethylation of cocaine by CYP3A4 in liver microsomes (Kloss et al., 1983; Pellinen et al., 1994) and is only a small fraction of cocaine's metabolites. However, norcocaine and its downstream products n-hydroxynorcocaine and norcocaine nitroxide are hepatotoxic (Thompson et al., 1979; Ndikum-Moffor et al., 1998). Furthermore, norcocaine blocks cardiac sodium channels in vitro (Crumb and Clarkson, 1992) and causes the same hemodynamic changes in vivo as caused by cocaine (Mahlakaarto et al., 1998).
The inactive metabolites ecgonine methyl ester and benzoic acid are formed by the serum enzyme butyrylcholinesterase, which accounts for approximately 40% of cocaine's initial metabolism. The bacterial enzyme cocaine esterase (CocE) catalyzes this same reaction but with a rate nearly 1000 times faster than the endogenous enzyme (Vmax = 2300 min−1) (Bresler et al., 2000; Larsen et al., 2002; Turner et al., 2002; Cooper et al., 2006; Gao et al., 2009). CocE is a 65-kDa (130-kDa dimer) (Narasimhan et al., 2010) α-β Serine hydrolase that was originally isolated from the Rhodococcus spp. MB1 soil bacterium (Bresler et al., 2000; Turner et al., 2002). We have added thermostabilizing mutations to CocE [T172R and G173Q (DM-CocE)] while preserving the hydrolytic function of the enzyme (Gao et al., 2009; Narasimhan et al., 2010). CocE is well classified as a cocaine-hydrolyzing molecule in buffer (Gao et al., 2009) and human plasma (Cooper et al., 2006; Brim et al., 2010); however, these studies only examined cocaine levels after CocE addition. Because the cocaine metabolites described above contain the same hydrolyzable ester linkage as cocaine, we investigated the ability of CocE to hydrolyze these metabolites. We have used both in vitro and in vivo studies and also developed a high-performance liquid chromatography-tandem mass spectrometry method (LC-MS/MS) to enable simultaneous quantification of cocaine, ecgonine methyl ester, benzoylecgonine, and norcocaine. By investigating these aspects, we will gain insight into the specificity of the stable DM-CocE mutant as a potential clinical therapy for cocaine toxicity.
Materials and Methods
Production and Purification of DM-CocE.
A pET24b plasmid containing DM-CocE (65 kDa) was prepared as described previously (Gao et al., 2009; Narasimhan et al., 2010) and transformed into BL21 cells. Inoculum for a BioFlo 3000 bioreactor (New Brunswick Scientific, Edison, NJ) was produced by growing BL21 cells in a 250-ml culture of Terrific broth (BD Biosciences, Franklin Lakes, NJ) containing kanamycin (50 μg/ml) at 37°C until reaching log-phase growth (approximately 8 h). The inoculum was added to 10 liters of Terrific Broth and kanamycin (50 μg/ml) in the bioreactor and grown at 37°C until the culture reached an OD600 of 5. Once the target turbidity was reached, the bioreactor was cooled to 18°C, and then the culture was induced by adding isopropyl β-d-thiogalactoside for a final concentration of 1 mM. After 16 h of induction, the culture was harvested by spinning down the broth at 4500g for 20 min. The resulting pellet was stored at −80°C.
Cell paste from 5 liters of a fermenter run was resuspended in phosphate-buffered saline, pH 7.4. The resuspended paste was passed through a French press twice or three times to lyse the cells. The maximum pressure for lysis is approximately 1100 psi. The lysate was clarified by spinning at 100,000g in an ultracentrifuge (XL-100K ultracentrifuge; Beckman Coulter, Fullerton, CA) using the rotor type Ti45. Clarified lysate was diluted by adding an equal volume of Q-buffer A (20 mM HEPES, pH 8.0) to a final volume of 1000 ml.
The clarified lysate (500 ml) was passed onto a 450-ml Q Sepharose HP column (Q Sepharose Fast Flow 17-0510-04, packed in-house; GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) at room temperature. The column was washed with 1000 ml of Q-buffer A followed by a gradient set to reach 50% Q-buffer B (20 mM HEPES, pH 8.0 + 1 M NaCl) within 1000 ml. Flow through and washes were saved for analysis. DM-CocE was eluted from the column using a gradient from 50 to 100% Q-buffer B over five column volumes (2250 ml). Fractions (25 ml) were collected at a flow rate of 10 ml/min. DM-CocE was monitored by running 5 μl of each fraction on an 8% SDS-polyacrylamide gel and staining with Coomassie Brilliant Blue stain according to established protocols.
Fractions from the first Q Sepharose HP separation were pooled (protein approximately 75% pure) and adjusted to 1 M ammonium sulfate (final volume 525 ml). Samples were applied to a 450-ml Phenyl Sepharose column (Phenyl Sepharose 6 Fast Flow 17-0973-03, packed in-house; GE Healthcare) preequilibrated with PS-buffer A (buffer A: 50 mM sodium phosphate, pH 7 + 1 M ammonium sulfate). The column was washed with 1000 ml of PS-buffer A followed by an additional wash in the form of a linear gradient to reach 30% PS-buffer B (buffer B: 50 mM sodium phosphate, pH 7) over two column volumes (∼1000 ml). DM-CocE was eluted with 30 to 100% PS-buffer B over five column volumes (2250 ml). Fractions (25 ml) were collected over the elution gradient. The presence and purity of DM-CocE were checked by running 5 μl of each fraction on an 8% SDS-polyacrylamide gel electrophoresis gel. Approximately 95% of the estimated DM-CocE protein was recovered and was 99% pure after phenyl Sepharose.
Fractions from the Phenyl Sepharose column were pooled and extracted twice with Triton X-114 to remove endotoxin. The twice-extracted protein was then diluted with Q-buffer A, and this was loaded on to a second Q Sepharose HP column (450 ml) to remove Triton X-114. Chromatography was performed as described above, and approximately 95% of the protein was recovered.
Spectrophotometric Cocaine and Cocaine Metabolite Assay.
DM-CocE (100 μl; 25 ng/ml final concentration) was added to a 96-well UV-permeable plate (Costar; Corning Life Sciences, Lowell, MA) containing either cocaine (Mallinckrodt, Hazelwood, MO), or a single cocaine metabolite (norcocaine, cocaethylene, and benzoylecgonine, 100 μl; 0.25, 0.5, 2.5, 5, 12.5, 25, 50, and 100 μM final concentrations; RTI International, Research Triangle Park, NC) in phosphate-buffered saline, pH 7.4, to give a final volume of 200 μl. The absorbance of cocaine at 240 nm was monitored over 15 min with readings every 10 s by a SpectraMax Plus 384 UV plate reader (Molecular Devices, Sunnyvale, CA) using SOFTmax Pro software (version 3.1.2). The specific extinction coefficient was used to convert the change in absorbance over time to the change in concentration over time. Extinction coefficients (ε) for the metabolites were determined using the absorbance of these metabolites at 240 nm and Beer's law (A = εbc). Our laboratory has determined these to be as follows: norcocaine = 8.7 l · mmol−1 · cm−1, benzoylecgonine = 7.5 l · mmol−1 · cm−1, and cocaethylene = 11.6 l · mmol−1 · cm−1. The rate of cocaine hydrolysis per mole of enzyme (kcat) and the Km were determined using Prism software (GraphPad Software Inc., San Diego, CA) to fit the data to the following equation: Rate = (kcat × [cocaine])/(Km + [cocaine]).
Metabolite Formation and Elimination.
Male Sprague-Dawley rats (300–350 g; Harlan Sprague-Dawley, Indianapolis, IN) were maintained in a temperature-controlled environment with a 12-h light/dark cycle, beginning at 7:00 AM. Rats were given ad libitum access to food and water and were allowed to acclimate to the room for at least 5 days before surgery. Rats were implanted with two indwelling femoral catheters. In brief, the rats were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg). Catheters were inserted into the femoral vein of both legs after the vein was manually separated from the femoral artery and nerve. Catheters were threaded under the skin and attached to stainless steel tubing that runs through a metal tether plate sutured to the back muscle. Animals were allowed to recover from the procedure for 5 days before use.
On the day of the experiment, rats were placed in Plexiglas cages (49 cm long × 23 cm wide × 21 cm high) containing cob bedding. A long piece of catheter tubing was extended from the stainless steel tubing of the metal tether plate out of the cage so that rats could be infused, and blood could be drawn without direct handling. At time t = 0, rats were given a 10-s bolus of 5.6 mg/kg cocaine (5.6 mg/ml) followed by a bolus of either DM-CocE (0.32 mg/kg, 0.32 mg/ml) or PBS at t = 10 min. Blood was sampled (200 μl) at t = 2, 5, 9, 10.75, 12, 15, 20, 40, and 60 min and immediately added to 5 μl of 500 mM EDTA plus 20 μl of 1 M NaF to prevent coagulation and further cocaine hydrolysis, respectively. Plasma was separated from blood cells by centrifugation at 1500g (rcf) in a bench top microcentrifuge, flash-frozen in liquid nitrogen, and stored at −80°C until preparation for mass spectrometry.
Experiments conformed to the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (Institute of Laboratory Animal Resources, 1996). In addition, all experiments were approved by the University of Michigan Committee on the Use and Care of Animals.
Preparation of Plasma Samples for Mass Spectrometry.
The plasma fraction from each 200-μl blood sample was added to 570 μl of acetonitrile (ACN), 20 μl of 1 M NaF, and 2 μl of internal standard solution containing 750 nM deuterium-labeled norcocaine, cocaine, benzoylecgonine, and ecgonine methyl ester (Cerilliant Corporation, Round Rock, TX). Samples were vortexed for 30 s and centrifuged at 25,000g (rcf) at 20°C for 30 min. The supernatant was removed and added to a microcentrifuge tube. Samples were centrifuged a second time using the same conditions, and the supernatants were transferred to clean tubes. After evaporation to dryness in a vacuum centrifuge, samples were stored at −80°C and analyzed within 1 week of preparation.
For mass spectral analysis, the dried samples were reconstituted with 30 μl of 10 mM ammonium formate (pH 4.6)-ACN [97:3 (v/v)] to yield a 50 nM final concentration of each internal standard. To achieve concentrations of cocaine, norcocaine, benzoylecgonine, and ecgonine methyl ester within the limits of quantification, further dilutions (varying along the time course) were prepared with 10 mM ammonium formate (pH 4.6)-ACN [97:3 (v/v)] containing 50 nM concentrations of internal standards. Samples were vortexed for 30 s and then were centrifuged at 13,600g (rcf) for 20 min. Aliquots of the supernatants were transferred to polypropylene autosampler vials for analysis within 12 h.
Preparation of Calibration Standards for Mass Spectrometry.
Calibration standards of cocaine, benzoylecgonine, ecgonine methyl ester (4.0–0.03 μM), and norcocaine (0.4–0.003 μM) were prepared by addition of different concentrations of authentic standards into plasma from untreated Sprague-Dawley rats (Valley Biomedical, Winchester VA). All standards were stored at −80°C until sample preparation. A full series of calibration standards was prepared with every set of experimental samples. Calibration stocks (20 μl) were extracted with 68 μl of ACN, 4 μl of 1 M NaF, and 2 μl of a 2.5 μM concentration of each deuterium-labeled norcocaine, cocaine, benzoylecgonine, and ecgonine methyl ester as described above. Calibration standards were reconstituted to 100 μl, resulting in final internal standard concentrations of 50 nM.
Quantification of Cocaine Metabolites by LC-MS/MS.
Analysis was performed on a Prominence HPLC system (Shimadzu, Kyoto, Japan) interfaced directly to the TurboIonSpray source of an API 3000 triple quadrupole mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA). Separation was achieved with a Thermo Hypersil Gold column (50 × 2.1 mm i.d., 1.9-μm packing) maintained at 45°C using a binary gradient and a flow rate of 0.45 ml/min. The flow was split approximately 1 to 3.5 so that 0.13 ml/min was directed into the ionization source. Solvent A was 10 mM ammonium formate, pH 4.6, and solvent B was ACN. The gradient program was as follows: 2% B at 0 min, hold 2% B for 1 min, 18% B at 2 min, 40% B at 10 min, 100% B at 11 min, 2% B at 12 min, and reequilibrate at 2% B for 3 min. Each analysis was completed in 15 min. Aliquots (4 μl) were injected onto the column, and the sample tray was cooled to 10°C to prevent sample degradation.
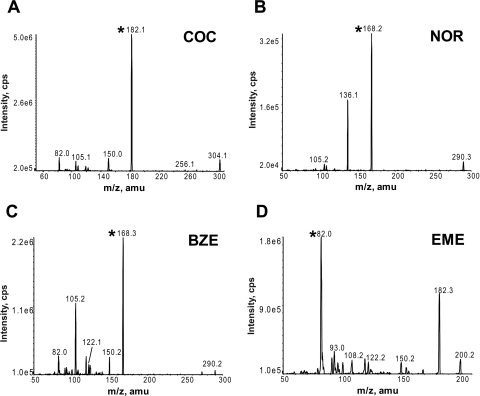
Positive ions were generated in the source using purified air for the source gases under the following conditions and settings: turbo electrospray ionization gas, 7.0 l/min; nebulizer setting, 15; ion spray voltage, 3000 V; and source gas temperature, 400°C. Compound-specific ionization parameters were optimized for cocaine, each metabolite, and the internal standards as shown in Table 1. Nitrogen was used as the curtain gas at a setting of 12. Mass analysis was performed by single reaction monitoring (SRM) with 100-ms dwell times using the precursor-to-product ion pairs determined from the MS/MS spectra collected by direct infusion of authentic standards (Fig. 1). Nitrogen served as the collision gas (collisionally activated dissociation = 12). The chromatogram was divided into three time segments so that individual periods contained a subset of one or two target compounds. The corresponding SRM transitions were scanned only during the time segments in which they eluted. Period 1 spanned the first 2 min, period 2 covered the next 1.7 min, and period 3 extended for the remainder of the chromatogram. Precursor/product ion pairs for the SRM transitions and their respective scan periods are listed in Table 2. Analyst software (version 1.4.2; Applied Biosystems/MDS Sciex, Toronto, ON, Canada) was used for instrument control, data acquisition, and quantitative analysis. Calibration curves were constructed from the standard samples. The ratios of the peak areas of the target compounds to the corresponding deuterium-labeled internal standards were plotted as a function of the analyte concentration normalized to the internal standard concentration. Calibration curves were generated using a least-squares linear regression analysis with 1/x weighting, not including the origin.
TABLE 1.
Optimized compound-specific source parameters for electrospray ionization
| Compound | Declustering Potential | Focusing Potential | Entrance Potential | Collision Energy | Collision Cell Exit Potential |
|---|---|---|---|---|---|
| V | V | V | eV | V | |
| Cocaine | 34 | 140 | 10 | 31 | 12 |
| d3-Cocaine | 44 | 148 | 10 | 33 | 11 |
| Norcocaine | 34 | 140 | 10 | 27 | 10.5 |
| d3-Norcocaine | 34 | 158 | 10 | 27 | 11 |
| Benzoylecgonine | 42 | 192 | 10 | 32 | 10 |
| d3-Benzoylecgonine | 36 | 161 | 10 | 32 | 11 |
| Ecgonine methyl ester | 34 | 134 | 10 | 35 | 10.5 |
| d3-Ecgonine methyl ester | 42 | 145 | 10 | 35 | 8 |
Fig. 1.
MS/MS spectra of cocaine and cocaine metabolites. Product ion MS/MS spectra were collected by direct infusion of a 5 to 10 μM solution of the authentic standards and deuterium-labeled internal standards using the optimized electrospray ionization source parameters listed in Table 1. The product ions selected for SRM transitions are indicated by an asterisk on the spectra. A, cocaine (COC) MS/MS spectrum; the SRM product ion is m/z 182.1. B, norcocaine (NOR) MS/MS spectrum; the SRM product ion is m/z 168.2. C, benzoylecgonine (BZE) MS/MS spectrum; the SRM product ion is m/z 168.3. D, ecgonine methyl ester (EME) MS/MS spectrum; the SRM product ion is m/z 82.0. Product ions selected for the deuterium-labeled internal standards are identical to the unlabeled standards except that the m/z values are shifted by 3 atomic mass units (amu).
TABLE 2.
Precursor-to-product ion pairs for SRM transitions
| Compound | Precursor Ion | Product Ion | Scan Period |
|---|---|---|---|
| m/z | m/z | ||
| Cocaine | 304.2 | 182.2 | 3 |
| d3-Cocaine | 307.2 | 185.2 | 3 |
| Norcocaine | 290.2 | 168.2 | 3 |
| d3-Norcocaine | 293.3 | 171.3 | 3 |
| Benzoylecgonine | 290.2 | 168.2 | 2 |
| d3-Benzoylecgonine | 293.3 | 171.2 | 2 |
| Ecgonine methyl ester | 200.1 | 82.0 | 1 |
| d3-Ecgonine methyl ester | 203.2 | 85.0 | 1 |
Results
Effect of DM-CocE on Cocaine Metabolites In Vitro.
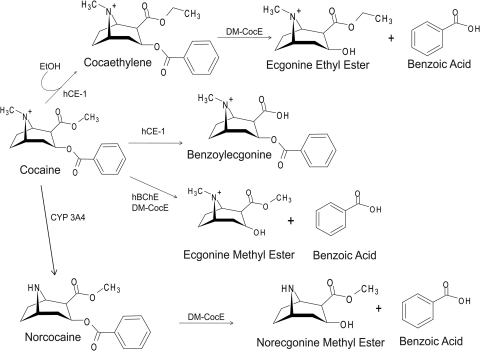
Cocaine esterase hydrolyzes cocaine at the ester linkage between the tropane and benzoyl rings, producing the inactive metabolites ecgonine methyl ester and benzoic acid, the same reaction catalyzed by human serum butyrylcholinesterase (Fig. 2). Norcocaine (formed by CYP3A4) and cocaethylene and benzoylecgonine (formed by human liver carboxyesterase-1), all retain the ester linkage found in the parent molecule (Fig. 2). The ability of DM-CocE to hydrolyze these metabolites was assessed using the spectrophotometric assay. The extinction coefficient for each metabolite and cocaine was determined experimentally (data not shown) and used to convert the loss of absorbance at 240 nm over time to the loss of metabolite. Cocaethylene, the cocaine metabolite formed by human liver carboxyesterase-1 in the presence of ethanol, was hydrolyzed by CocE with a statistically significant lower kcat and a statistically significant higher Km than cocaine (18.9 μM for cocaethylene compared with 14.2 μM for cocaine), resulting in a 44% reduction in catalytic efficiency (Fig. 3). DM-CocE hydrolysis of norcocaine did not display a statistically significantly different kcat but did have a significantly lower kcat/Km than cocaine (Fig. 3). This was due to the statistically significant higher Km (29.0 for norcocaine compared with 14.19 for cocaine), which resulted in the approximately 50% reduction in catalytic efficiency. The loss of the benzoyl group of cocaethylene and norcocaine results in the products shown in Fig. 2. Of interest, benzoylecgonine was not hydrolyzed by DM-CocE. Even when higher concentrations of DM-CocE were assessed (100 ng/ml compared with 25 ng/ml), benzoylecgonine was not hydrolyzed according to Michaelis-Menten kinetics to any measurable level.
Fig. 2.
Major cocaine metabolites. Cocaine is rapidly converted into several main metabolites. Human liver carboxylesterase-1 (hCE-1) demethylates 40 to 45% of cocaine to benzoylecgonine. hCE-1 also catalyzes the conversion of cocaine into the more potent metabolite cocaethylene when alcohol [ethanol (EtOH)]is present. Liver P450 CYP3A4 N-demethylates approximately 5 to 10% of cocaine to norcocaine. Serum butyrylcholinesterase (hBChE) (Km = 14 μM, kcat = 3.9 min−1) (Xie et al., 1999) hydrolyzes 40 to 45% of cocaine into ecgonine methyl ester and benzoic acid. DM-CocE also catalyzes this reaction with a faster rate (Km = 14 μM, kcat = 1432 min−1). DM-CocE hydrolyzes the hydrolysis of norcocaine to norecgonine methyl ester and benzoic acid (Km = 29 μM, kcat = 1307 min−1) as well as that of cocaethylene to ecgonine ethyl ester and benzoic acid (Km = 19 μM, kcat = 1099 min−1).
Fig. 3.
Hydrolysis of cocaine metabolites by DM-CocE. DM-CocE can hydrolyze the active cocaine metabolites cocaethylene and norcocaine but cannot hydrolyze benzoylecgonine, as determined by the spectrophotometric cocaine hydrolysis assay. Benzoylecgonine was not hydrolyzed; therefore, no data (n.d.) are reported in B to D. A, Michaelis-Menten saturation curves of DM-CocE hydrolysis of cocaine compared with those for the three major metabolites. B, the catalytic efficiency of DM-CocE for cocaethylene and norcocaine is 58 and 45%, respectively, that of cocaine (p < 0.001, Student's t test). C, the kcat of DM-CocE for norcocaine was unchanged from the kcat for cocaine. Cocaethylene had a significantly reduced kcat as determined by Student's t test (p < 0.01). D, the Km values of DM-CocE for both cocaethylene and norcocaine were significantly elevated above that for cocaine (p < 0.05 and p < 0.001, respectively).
Development of High-Performance Liquid Chromatography-Tandem Mass Spectrometry Assay.
To expand on the spectrophotometric assay and to validate these findings in a physiological setting, we sought to monitor the elimination of cocaine and its metabolites in vivo. This required an assay that could simultaneously detect cocaine, ecgonine methyl ester, benzoylecgonine, and norcocaine in a plasma matrix. A Thermo Hypersil Gold column with 1.9-μm particles and dimensions of 50 mm × 2.1 mm i.d. provided the best combination of retention and resolution of cocaine and the three metabolites of interest (Fig. 4). Ecgonine methyl ester was slightly retained on this column, and cocaine and norcocaine were nearly baseline-resolved. The LINAC technology (Loboda et al., 2000) incorporated into the collision cell prevents any cross-talk from the small amount of overlap between the cocaine and norcocaine peaks, thus allowing accurate quantification of these two compounds. Chromatographic peaks were narrow and symmetrical with the exception of that for ecgonine methyl ester, which displayed a slight amount of tailing. The mass spectroscopy scan dwell time was shortened from the instrument default of 300 ms to 100 ms to characterize the peaks more exactly. Furthermore, time segmentation was used to divide the chromatogram into three periods to reduce the number of SRM transitions that were monitored during each scan cycle. Instead of continuously collecting data for all eight SRM transitions throughout the entire chromatogram, only those transitions corresponding to the compounds eluting during that time period were scanned. As a result of all these improvements, the final method was accurate and precise, with good linearity for all the target compounds (Fig. 5).
Fig. 4.
LC-MS/MS analysis of cocaine and cocaine metabolites. Total ion chromatogram of a 4.00 μM calibration standard showing separation of ecgonine methyl ester (EME), benzoylecgonine (BZE), cocaine (COC), and norcocaine (NOR) on a Hypersil Gold ultra-performance liquid chromatography column (50 mm × 2.1 mm i.d., 1.9-μm particles) using 10 mM ammonium formate (pH 4.6) as solvent A and acetonitrile as solvent B at a flow rate of 0.45 ml/min. Then 4-μl aliquots were injected onto the column, and compounds were eluted over an 11-min gradient that began with an initial hold at 2% B for 1 min, increased to 18% B in the next minute, gradually ramped to 40% B over 8 min, and finally increased to 100% B in 1 min. Selected reaction monitoring was used for compound detection and quantification. The chromatogram was divided into three time segments during which only the relevant SRM transitions were scanned. This allowed for more accurate characterization of chromatographic peaks and, thus, more precise quantification.
Fig. 5.
LC-MS/MS calibration curves for cocaine and cocaine metabolites. Calibration curves were constructed for each compound by calculating the ratios of the LC-MS/MS peak areas of the target compounds to the corresponding deuterium-labeled internal standards and plotting the ratios as a function of analyte concentration normalized to the concentration of internal standard (IS). Data were fitted to a curve using least-squares linear regression analysis with 1/x weighting. The linear range for cocaine (COC), benzoylecgonine (BZE), and ecgonine methyl ester (EME) was 0.0313 to 4.00 μM. Because of the extremely low concentrations of norcocaine (NOR) observed in the in vivo studies, the calibration curve was adjusted correspondingly and ranged from 0.00313 to 0.400 μM. Regression coefficients (R2) were all ≥0.995 and accuracies typically ranged from 67 to 130%.
Effect of DM-CocE on Cocaine Metabolites In Vivo.
Male Sprague-Dawley rats were given a dose of 5.6 mg/kg cocaine at time t = 0 and a dose of 0.32 mg/kg DM-CocE at time t = 10 min. Blood samples were taken over 60 min for analysis by LC-MS/MS. Cocaine, benzoylecgonine, ecgonine methyl ester, and norcocaine were quantified simultaneously in each serum sample using the method developed for this study.
Cocaine produced an initial concentration of 7.1 to 27.1 μM in rats. In the animals receiving only PBS vehicle at the 10-min time point, the concentration of cocaine slowly fell over the 60-min period because of slow natural hydrolysis. The majority of cocaine was converted into benzoylecgonine and ecgonine methyl ester, with norcocaine produced as a minor metabolite in the rats, as expected (Fig. 6). The natural metabolism of cocaine was significantly and predictably altered by the administration of DM-CocE 10 min after cocaine dosing. Cocaine was rapidly metabolized by DM-CocE to levels below the limit of quantification within 2 min after administration. The cocaine was immediately converted into ecgonine methyl ester, and peak concentrations ranged from 9.1 to 11.4 μM at t = 12 min. Consistent with the in vitro results, DM-CocE also eliminated the norcocaine that was formed before esterase administration. The concentration of norcocaine decreased from 10 nM at t = 9 min to levels below the quantification limit of 3 nM at t = 10:45, only seconds after the administration of DM-CocE. Although DM-CocE did not hydrolyze benzoylecgonine in vitro, a rapid reduction and then stabilization of benzoylecgonine concentration was observed in rats after DM-CocE administration.
Fig. 6.
Hydrolysis of cocaine metabolites by DM-CocE in Sprague-Dawley rats. At time 0, 5.6 mg/kg cocaine is injected intravenously, followed 10 min later by either PBS or 0.32 mg/kg DM-CocE. Blood samples were taken throughout the time course and analyzed by mass spectrometry for cocaine metabolites simultaneously. A, cocaine is rapidly eliminated by DM-CocE. The concentration of cocaine rises again at t = 40 min because of the elimination of DM-CocE and redistribution of cocaine. B, benzoylecgonine concentrations are affected by DM-CocE, most likely because of binding and sequestration by DM-CocE. In addition, the absence of high concentrations of cocaine after 10 min and 45 s reduces the substrate for hCE-1 production of benzoylecgonine. C, ecgonine methyl ester concentrations are higher in DM-CocE-treated animals (equal to that of the original cocaine concentrations) demonstrating the production of EME by DM-CocE. D, norcocaine is rapidly eliminated by DM-CocE.
Discussion
This work describes not only the ability of cocaine esterase to eliminate cocaine metabolites but also demonstrates the utility of simultaneous detection of the major cocaine metabolites. Our data show that although DM-CocE is an enzyme-specific for cocaine, it can also hydrolyze the active cocaine metabolites norcocaine and cocaethylene but not benzoylecgonine. These data suggest several structure-function relationships among DM-CocE and its substrates. Compared with the parent molecule, norcocaine lacks the N-methyl group of the tropane ring. This leads to a loss of positive charge on the nitrogen, but does not affect the maximum potential of DM-CocE to mediate catalysis, which is demonstrated by the equivalent kcat of cocaine and norcocaine. This loss of charge, however, does lead to the decreased ability of norcocaine to bind DM-CocE, as reflected by the higher Km of this reaction. It is possible that norcocaine, with a slightly more negative charge than cocaine, is not properly oriented in the enzyme active site pocket containing the negatively changed hydroxyl group of serine 117 that initiates the nucleophilic attack on the benzoyl ester linkage of cocaine. Cocaethylene retains the same charge as the parent molecule and contains an additional methyl group. The larger molecular size of cocaethylene may decrease its capacity to bind to the active site of DM-CocE, most likely because of simple steric hindrance, thus decreasing its hydrolysis by DM-CocE.
No activity against benzoylecgonine was detected in our in vitro assay. This result was surprising, considering that, compared with cocaine, benzoylecgonine is only slightly smaller and retains most of the charge properties. Loss of the methyl group exposes a hydroxyl group, which could create a small, negative charge that reduces binding, similar to norcocaine. However, the in vivo data suggest that DM-CocE may be binding to benzoylecgonine, because there is a significant and rapid decrease in the concentration of benzoylecgonine after the addition of DM-CocE that persists for approximately 2 min, at which point the concentration stabilizes. This observation suggests that the lack of enzyme hydrolysis observed with DM-CocE and benzoylecgonine is most likely due to an inability to hydrolyze the molecule, not an inability to bind.
To further validate our in vitro results, we developed a LC-MS/MS method to simultaneously detect and quantify cocaine and its major metabolites. Most drug screening assays include cocaine and at least one major metabolite, usually benzoylecgonine, and an increasing number of these screens are now performed using LC-MS/MS technology (Dams et al., 2007; Badawi et al., 2009; Shakleya et al., 2010; Wang et al., 2010). Whereas the methods have been well documented (Dams et al., 2007; Badawi et al., 2009; Shakleya et al., 2010; Wang et al., 2010), the goal of a screen is to incorporate as many compounds as possible into a single assay. Comprehensive screens may even require the use of expensive ultra-performance liquid chromatography instrumentation (Badawi et al., 2009) to achieve sufficient resolution of all components. The study described in this report centers on the metabolism of a single drug (cocaine), so a simple, targeted method for the analysis of cocaine, norcocaine, benzoylecgonine, and ecgonine methyl ester was developed.
Because of the structural similarities of these compounds, the product ion spectra generated in MS/MS are also very similar. Many overlapping fragment ions were observed, although the relative abundance varied from compound to compound. As a result, good chromatographic separation is required for accurate quantitative analysis because these compounds could not be separated entirely by the mass spectrometer. Traditional microbore HPLC columns (3 μm, 150 mm × 2.0 mm i.d.) containing stationary phases such as C18 and phenyl hexyl in combination with H2O-ACN-formic acid solvent gradients did not resolve cocaine and norcocaine. Furthermore, ecgonine methyl ester, the most polar metabolite, eluted in or very near to the void volume of these columns and was often obscured by nonretained salts and low-molecular-weight contaminants. Ecgonine methyl ester was also not strongly retained on columns that combine polar end capping with hydrophobic alkyl chains to provide mixed-mode retention mechanisms (e.g., Synergi Hydro RP; Phenomenex, Torrance, CA). Peak shapes were significantly improved by the substitution of 10 mM ammonium formate, pH 4.6, for 0.1% formic acid in water as the aqueous solvent, but cocaine and norcocaine were still not resolvable, despite the ion-pairing capabilities of ammonium formate.
Because cocaine and norcocaine could not be separated on the basis of stationary phase selectivity, it was necessary to use a column with a smaller particle size and, thus, higher separation efficiency and improved resolution. There have been reports in the literature of the use of ultra-performance liquid chromatography columns in conventional HPLC systems (Loboda et al., 2000; Woodruff and Pereira, 2005; Cho et al., 2009). When used at flow rates lower than the optimum, the back pressure generated in the system stays within the limits of the HPLC plumbing, yet large improvements in chromatographic resolution can be achieved. Our final conditions using a Thermo Hypersil Gold column allowed cocaine and its three metabolites analyzed in this study to be retained and resolved well (Fig. 4) and yielded linear calibration curves (Fig. 5).
In vivo results obtained by analyzing plasma samples with this method supported the in vitro data, both demonstrating that DM-CocE was effective in hydrolyzing cocaine and norcocaine, but not benzoylecgonine. The in vivo concentrations of cocaine and norcocaine well illustrate the loss of DM-CocE activity over time. After rapid elimination of cocaine to levels below the quantification limit, the plasma concentrations of both these molecules begin to increase 20 to 40 min after cocaine injection. We have previously shown that the concentration of CocE in the serum of mice and rats decreases rapidly over time with a half-life of approximately 1 to 2 h (Brim et al., 2010, 2011). Taken together, these data suggest that this may also be occurring with DM-CocE, and loss of DM-CocE from the circulation probably leads to the reduction in the rate of cocaine elimination. At the reduced removal rate, more cocaine can be found in this compartment, as the return to equilibrium of cocaine is established by redistribution throughout the body. A full kinetic profile of the DM-CocE enzyme will be reported elsewhere (Brim et al., 2011).
A CocE with a longer circulating half-life is needed if all cocaine is to be eliminated at the DM-CocE doses tested here. However, the levels of cocaine that redistribute into the plasma 50 min after DM-CocE administration only range from 13.7 to 92.4 nM, a level that is not toxic and far lower than that observed in recreational cocaine users (Couper and Logan, 2004). Attempts to prolong the circulating half-time of CocE through modification by polyethylene glycol polymers are currently being pursued (Narasimhan et al., 2011).
We have demonstrated that DM-CocE is able to hydrolyze not only cocaine but also the active and dangerous cocaine metabolites norcocaine and cocaethylene using an LC-MS/MS method to simultaneously quantify cocaine and three of its major metabolites in rat plasma. Reduction in physiologically active levels of norcocaine and cocaethylene along with the parent molecule gives CocE a property not seen with engineered human butyrylcholinesterases (Gao and Brimijoin, 2004; Pan et al., 2005; Brimijoin et al., 2008; Gao et al., 2008) and further strengthens the preclinical evidence that CocE may be an effective treatment for cocaine toxicity in humans.
Acknowledgments
We thank Davina Barron and Alyssa Cunningham for their excellent technical assistance. Thank you to Dr. R. D. Davenport for providing us with human plasma samples.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA028086-01, DA025100, DA021416]; the National Institutes of Health National Institute of General Medical Sciences [Grant GM007767]; and Reckitt Benckiser.
J.H.W. and R.K.S. have received funding from and have been consultants to Reckitt Benckiser Pharmaceuticals. R.L.B., D.N., J.H.W., and R.K.S. are inventors on a patent for DM-CocE and other cocaine esterases: Landry D, MacDonald J, Stojanovic MN, Sunahara RK, Narasimhan D, Woods JH, Tesmer JJG, and Brim RL (2008), inventors; The Trustees of Columbia University in the City of New York, The Regents of the University of Michigan, et al., assignees. Thermostabilization of proteins. World patent WO2009009669. 2008 Oct 7.
This work was previously presented in part at the following conference: Brim RL, Sunahara RK, Woods JH (2010) Ability of bacterial cocaine esterase to hydrolyze active cocaine metabolites, and function in the presence of commonly coabused drugs. Annual Meeting of the American Society of Pharmacology and Experimental Therapeutics; 2010 Apr 24–28; Anaheim CA. American Society for Pharmacology and Experimental Therapeutics, Rockville, MD. This article is based on a thesis submitted in partial fulfillment of the requirements for a doctoral degree: Brim RL (2011) Investigations into the therapeutic potential of a bacterial cocaine esterase for the treatment of cocaine toxicity and cocaine abuse. Ph.D. thesis, University of Michigan: Ann Arbor.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/mol.111.074534.
- CocE
- cocaine esterase
- DM-CocE
- T172R/G173Q-CocE
- LC
- liquid chromatography
- MS/MS
- tandem mass spectroscopy
- PBS
- phosphate-buffered saline
- rcf
- relative centrifugal force
- ACN
- acetonitrile
- HPLC
- high-performance liquid chromatography
- SRM
- single reaction monitoring.
Authorship Contributions
Participated in research design: Brim, Noon, Collins, Sunahara, and Woods.
Conducted experiments: Brim, Noon, and Collins.
Contributed new reagents or analytic tools: Nichols and Narasimhan.
Performed data analysis: Brim and Noon.
Wrote or contributed to the writing of the manuscript: Brim, Noon, Sunahara, and Woods.
References
- Badawi N, Simonsen KW, Steentoft A, Bernhoft IM, Linnet K. (2009) Simultaneous screening and quantification of 29 drugs of abuse in oral fluid by solid-phase extraction and ultraperformance LC-MS/MS. Clin Chem 55:2004–2018 [DOI] [PubMed] [Google Scholar]
- Bresler MM, Rosser SJ, Basran A, Bruce NC. (2000) Gene cloning and nucleotide sequencing and properties of a cocaine esterase from Rhodococcus sp. strain MB1. Appl Environ Microbiol 66:904–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim RL, Nance MR, Youngstrom DW, Narasimhan D, Zhan CG, Tesmer JJ, Sunahara RK, Woods JH. (2010) A thermally stable form of bacterial cocaine esterase: a potential therapeutic agent for treatment of cocaine abuse. Mol Pharmacol 77:593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim RL, Noon KR, Stein A, Nichols J, Narasimhan D, Ko MC, Sunahara RK, Woods JH. (2011) The fate of bacterial cocaine esterase in Sprague-Dawley rats: an in vivo study of CocE distribution and elimination and mediated cocaine hydrolysis. doi:10.1124/jpet.111.186049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimijoin S, Gao Y, Anker JJ, Gliddon LA, Lafleur D, Shah R, Zhao Q, Singh M, Carroll ME. (2008) A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacology 33:2715–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Kim JD, Lee WY, Chung BC, Choi MH. (2009) Quantitative metabolic profiling of 21 endogenous corticosteroids in urine by liquid chromatography-triple quadrupole-mass spectrometry. Anal Chim Acta 632:101–108 [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Narasimhan D, Sunahara RK, Mierzejewski P, Jutkiewicz EM, Larsen NA, Wilson IA, Landry DW, Woods JH. (2006) Rapid and robust protection against cocaine-induced lethality in rats by the bacterial cocaine esterase. Mol Pharmacol 70:1885–1891 [DOI] [PubMed] [Google Scholar]
- Couper FJ, Logan BK. (2004) Drug and Human Performance Fact Sheets, National Highway Traffic Safety Administration, Washington DC [Google Scholar]
- Crumb WJ, Jr, Clarkson CW. (1992) Characterization of the sodium channel blocking properties of the major metabolites of cocaine in single cardiac myocytes. J Pharmacol Exp Ther 261:910–917 [PubMed] [Google Scholar]
- Dams R, Choo RE, Lambert WE, Jones H, Huestis MA. (2007) Oral fluid as an alternative matrix to monitor opiate and cocaine use in substance-abuse treatment patients. Drug Alcohol Depend 87:258–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean RA, Christian CD, Sample RH, Bosron WF. (1991) Human liver cocaine esterases: ethanol-mediated formation of ethylcocaine. FASEB J 5:2735–2739 [DOI] [PubMed] [Google Scholar]
- Freye E, Levy J. (2009) Pharmacology and Abuse of Cocaine, Amphetamines, Ecstasy and Related Designer Drugs: A Comprehensive Review on Their Mode of Action, Treatment of Abuse and Intoxication, Springer, Dordrecht, The Netherlands [Google Scholar]
- Gao D, Narasimhan DL, Macdonald J, Brim R, Ko MC, Landry DW, Woods JH, Sunahara RK, Zhan CG. (2009) Thermostable variants of cocaine esterase for long-time protection against cocaine toxicity. Mol Pharmacol 75:318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Brimijoin S. (2004) An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther 310:1046–1052 [DOI] [PubMed] [Google Scholar]
- Gao Y, LaFleur D, Shah R, Zhao Q, Singh M, Brimijoin S. (2008) An albumin-butyrylcholinesterase for cocaine toxicity and addiction: catalytic and pharmacokinetic properties. Chem Biol Interact 175:83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Iyer RN, Nobiletti JB, Jatlow PI, Bradberry CW. (1995) Cocaine and cocaethylene: effects on extracellular dopamine in the primate. Psychopharmacology (Berl) 120:150–155 [DOI] [PubMed] [Google Scholar]
- Jatlow P, Elsworth JD, Bradberry CW, Winger G, Taylor JR, Russell R, Roth RH. (1991) Cocaethylene: a neuropharmacologically active metabolite associated with concurrent cocaine-ethanol ingestion. Life Sci 48:1787–1794 [DOI] [PubMed] [Google Scholar]
- Katz JL, Terry P, Witkin JM. (1992) Comparative behavioral pharmacology and toxicology of cocaine and its ethanol-derived metabolite, cocaine ethyl-ester (cocaethylene). Life Sci 50:1351–1361 [DOI] [PubMed] [Google Scholar]
- Kloss MW, Rosen GM, Rauckman EJ. (1983) N-Demethylation of cocaine to norcocaine. Evidence for participation by cytochrome P-450 and FAD-containing monooxygenase. Mol Pharmacol 23:482–485 [PubMed] [Google Scholar]
- Larsen NA, Turner JM, Stevens J, Rosser SJ, Basran A, Lerner RA, Bruce NC, Wilson IA. (2002) Crystal structure of a bacterial cocaine esterase. Nat Struct Biol 9:17–21 [DOI] [PubMed] [Google Scholar]
- Loboda A, Krutchinsky A, Loboda O, McNabb J, Spicer V, Ens W, Standing KG. (2000) Novel LINAC II electrode geometry to crease an axial field in a mulipole ion guide, in Proceedings of the 48th ASMS Conference on Mass Spectrometry and Allied Topics; 2000 June 11–15; Long Beach, CA American Society for Mass Spectrometry, Santa Fe, NM [Google Scholar]
- Madden JA, Konkol RJ, Keller PA, Alvarez TA. (1995) Cocaine and benzoylecgonine constrict cerebral arteries by different mechanisms. Life Sci 56:679–686 [DOI] [PubMed] [Google Scholar]
- Mahlakaarto J, Ruskoaho H, Huttunen P, MacDonald E, Pasanen M. (1998) Norcocaine is a potent modulator of haemodynamic responses, plasma catecholamines and cardiac hormone release in conscious rats. Toxicology 128:101–111 [DOI] [PubMed] [Google Scholar]
- Narasimhan D, Collins G, Nance MA, Nichols J, Chan J, Edwald E, Ko MC, Woods JH, Tesmer JJ, Sunahara RK. (2011) Subunit stabilization and polyethylene glycolation of cocaine esterase improves in vivo residence time. Mol Pharmacol 80:1056–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan D, Nance MR, Gao D, Ko MC, Macdonald J, Tamburi P, Yoon D, Landry DM, Woods JH, Zhan CG, et al. (2010) Structural analysis of thermostabilizing mutations of cocaine esterase. Protein Eng Des Sel 23:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndikum-Moffor FM, Schoeb TR, Roberts SM. (1998) Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther 284:413–419 [PubMed] [Google Scholar]
- Pan Y, Gao D, Yang W, Cho H, Yang G, Tai HH, Zhan CG. (2005) Computational redesign of human butyrylcholinesterase for anticocaine medication. Proc Natl Acad Sci USA 102:16656–16661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellinen P, Honkakoski P, Stenbäck F, Niemitz M, Alhava E, Pelkonen O, Lang MA, Pasanen M. (1994) Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol 270:35–43 [DOI] [PubMed] [Google Scholar]
- Schrank K. (1992) Cocaine-related emergency department presentations, in Acute Cocaine Intoxication: Current Methods of Treatment (Sorer H. ed), National Institutes of Health, Rockville, MD [Google Scholar]
- Shakleya DM, Dams R, Choo RE, Jones H, Huestis MA. (2010) Simultaneous liquid chromatography-mass spectrometry quantification of urinary opiates, cocaine, and metabolites in opiate-dependent pregnant women in methadone-maintenance treatment. J Anal Toxicol 34:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies (2004) Drug Abuse Warning Network, 2003: Interim National Estimates of Drug-Related Emergency Department Visits. DAWN Series D-26. DHHS publication no. SMA 04-3972 United States Department of Health and Human Services, Rockville, MD [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies (2010) Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. NSDUH Series H-38A, DHHS Publication no. SMA 10–4586 (Findings) United States Department of Health and Human Services, Rockville, MD [Google Scholar]
- Thompson ML, Shuster L, Shaw K. (1979) Cocaine-induced hepatic necrosis in mice—the role of cocaine metabolism. Biochem Pharmacol 28:2389–2395 [DOI] [PubMed] [Google Scholar]
- Turner JM, Larsen NA, Basran A, Barbas CF, 3rd, Bruce NC, Wilson IA, Lerner RA. (2002) Biochemical characterization and structural analysis of a highly proficient cocaine esterase. Biochemistry 41:12297–12307 [DOI] [PubMed] [Google Scholar]
- Wang IT, Feng YT, Chen CY. (2010) Determination of 17 illicit drugs in oral fluid using isotope dilution ultra-high performance liquid chromatography/tandem mass spectrometry with three atmospheric pressure ionizations. J Chromatogr B Analyt Technol Biomed Life Sci 878:3095–3105 [DOI] [PubMed] [Google Scholar]
- Wilson LD, Jeromin J, Garvey L, Dorbandt A. (2001) Cocaine, ethanol, and cocaethylene cardiotoxicity in an animal model of cocaine and ethanol abuse. Acad Emerg Med 8:211–222 [DOI] [PubMed] [Google Scholar]
- Woodruff MJ, Pereira L. (2005) The use of 1.9 μm particles in HPLC to increase speed and efficiency. LCGC N Am Appl Notebook September 2 [Google Scholar]
- Xie W, Altamirano CV, Bartels CF, Speirs RJ, Cashman JR, Lockridge O. (1999) An improved cocaine hydrolase: the A328Y mutant of human butyrylcholinesterase is 4-fold more efficient. Mol Pharmacol 55:83–91 [DOI] [PubMed] [Google Scholar]
- Xu YQ, Crumb WJ, Jr, Clarkson CW. (1994) Cocaethylene, a metabolite of cocaine and ethanol, is a potent blocker of cardiac sodium channels. J Pharmacol Exp Ther 271:319–325 [PubMed] [Google Scholar]