Abstract
We conducted a meta-analysis focusing on studies with high potential for trichloroethylene (TCE) exposure to provide quantitative evaluations of the evidence for associations between TCE exposure and kidney, liver, and non-Hodgkin lymphoma (NHL) cancers. A systematic review documenting essential design features, exposure assessment approaches, statistical analyses, and potential sources of confounding and bias identified twenty-four cohort and case-control studies on TCE and the three cancers of interest with high potential for exposure, including five recently published case-control studies of kidney cancer or NHL. Fixed- and random-effects models were fitted to the data on overall exposure and on the highest exposure group. Sensitivity analyses examined the influence of individual studies and of alternative risk estimate selections. For overall TCE exposure and kidney cancer, the summary relative risk (RRm) estimate from the random effects model was 1.27 (95% CI: 1.13, 1.43), with a higher RRm for the highest exposure groups (1.58, 95% CI: 1.28, 1.96). The RRm estimates were not overly sensitive to alternative risk estimate selections or to removal of an individual study. There was no apparent heterogeneity or publication bias. For NHL, RRm estimates for overall exposure and for the highest exposure group, respectively, were 1.23 (95% CI: 1.07, 1.42) and 1.43 (95% CI: 1.13, 1.82) and, for liver cancer, 1.29 (95% CI: 1.07, 1.56) and 1.28 (95% CI: 0.93, 1.77). Our findings provide strong support for a causal association between TCE exposure and kidney cancer. The support is strong but less robust for NHL, where issues of study heterogeneity, potential publication bias, and weaker exposure-response results contribute uncertainty, and more limited for liver cancer, where only cohort studies with small numbers of cases were available.
Keywords: trichloroethylene, meta-analysis, kidney cancer, liver cancer, NHL, occupational exposure
1. Introduction
The interpretation of the epidemiologic studies on cancer and trichloroethylene (TCE) continues to be an area of considerable interest despite numerous reviews, including those of multidisciplinary expert panels whose conclusions have ranged widely due, in part, to differences in the qualitative evaluation of the epidemiologic data as well as in the studies available at the time [1–5]. Two advisory panels reviewing the epidemiologic evidence on cancer and TCE recommended meta-analysis as an approach to synthesize the data, noting individual studies had limited statistical power for relatively uncommon cancers such as kidney, liver, and non-Hodgkin lymphoma (NHL) [6,7].
In this paper, we present findings from a systematic review and a comprehensive meta-analysis of studies of occupational TCE exposure and cancer, focusing on studies with high potential for TCE exposure and following guidance for epidemiologic study reporting and meta-analysis practice [8–10]. We focus on three specific cancers of a priori interest from rodent bioassays of TCE exposure [11–13] and a broader qualitative review of the epidemiologic data—kidney cancer, liver cancer, and NHL. We consider current disease classifications for NHL and carry out a systematic evaluation of the literature. Our meta-analysis updates the literature covered by previous meta-analyses of TCE exposure and cancer [14–19], adding four case-control studies on NHL [20–23], one case-control study on renal cell carcinoma [24], two studies in a cohort of aerospace workers [25,26], and an updated mortality follow-up of a cohort of aircraft maintenance workers [27]. The incorporation of clear a priori guidelines for identifying studies with moderate-to-high probability of TCE exposure, inclusion of both cohort and case-control studies, supplemental examination of the highest exposure group in each study to reduce the impact of exposure misclassification, and assessments of heterogeneity and sensitivity provide insight for the evaluation of a causal link between TCE and these specific cancers.
2. Methods
A thorough search of the literature was carried out without restriction on year of publication or language to identify all studies that assessed the relationship between cancer and TCE following these approaches: a search of the bibliographic databases PubMed (http://www.ncbi.nlm.nih.gov/pubmed/), TOXNET (http://toxnet.nlm.nih.gov/) and EMBASE (http://www.embase.com/) using the terms “trichloroethylene cancer epidemiology” and ancillary terms, “degreasers,” “aircraft, aerospace or aircraft maintenance workers,” “metal workers,” and “electronic workers,” “trichloroethylene and cohort,” or, “trichloroethylene and case-control;” examination of bibliographies of reviews of the TCE epidemiologic literature such as those of the Institute of Medicine [28], National Research Council [5,6] and Scott and Chiu [4]; and review of bibliographies of individual studies and previous meta-analyses for relevant studies. Only studies in press or published in scientific journals, as of December 2010, or their additional analyses provided through personnel communication with the authors were considered. Studies with multiple published analyses based on updates to the same cohort are identified by the most recent publication.
2.1. Study Selection and Data Extraction
Studies selected for inclusion in the meta-analysis met the following criteria: (1) cohort or case-control design; (2) exposed and control groups in cohort studies and cases and controls in case-control studies are comparable and drawn from the same base population; (3) TCE exposure potential and some estimate of TCE exposure assessed for each subject by reference to industrial hygiene records, individual biomarkers, job-exposure matrices, expert assessment, water distribution models, or questionnaire responses (case-control studies); and (4) relative risk (RR) estimates for kidney cancer, liver cancer, or NHL.
The general approach for selecting RR estimates and associated confidence intervals (CIs) was to pick a single RR estimate for overall TCE exposure versus no TCE exposure. When multiple estimates were available for the same study based on different subcohorts with different inclusion criteria, the preference for overall exposure was to select the RR estimate that represented the largest population in the study, while trying to minimize the likelihood of TCE exposure misclassification. A subcohort with more restrictive inclusion criteria was selected if the goal for the definition of the subcohort was to reduce exposure misclassification (e.g., including only subjects with more probable TCE exposure) but not if the goal was to reflect subjects with greater exposure (e.g., routine versus any exposure). When available, RR estimates from internal analyses were chosen over standardized incidence or mortality ratios (SIRs, SMRs), and adjusted RR estimates were selected over crude estimates. Odds ratios in case-control studies were considered to approximate the RR, or more specifically the rate ratio, as the cancers of interest are rare diseases in both the exposed and unexposed groups, with lifetime risks considerably less than 10% [29]. No correction was made to cause-specific mortality estimates based on an overall standardized mortality ratio to adjust for the healthy worker bias. In separate analyses, a RR estimate for the highest exposure group was selected, with a preference for cumulative exposure if available; however, often duration was the sole exposure metric presented.
2.2. Statistical Methods
Random-effects models were used for the primary analyses, employing the methodology of DerSimonian and Laird [30], with fixed-effect analyses conducted for comparison. Both approaches combine effect measures (in this case, RR estimates) weighted by the inverse variance; however, the random-effects model takes estimates of between-study, as well as within-study, variation into account. The meta-analysis calculations are based on natural-logarithm-transformed relative risk (log RR) values. For the weights, either an estimate of the standard error (SE) of the log RR, from which to estimate the variance, was obtained from the CIs of the RR estimate [31] or, for SMRs and SIRs, an estimate of the variance of the log RR was calculated directly as the inverse of the observed number of deaths (or cases) [32]. In some case-control studies, no overall odds ratio (OR) was reported and a crude OR estimate was used, with the variance of the log OR estimated in accordance with the method proposed by Woolf [33], as described by Breslow and Day [34]. All analyses were performed using Microsoft Excel® spreadsheets and the software package Comprehensive Meta-Analysis, Version 2 (© 2006, Biostat, Inc.); forest plots were created using SAS, Version 9.2 (© 2002–2008, SAS Institute, Inc.). We calculated summary meta-relative-risk (RRm) estimates for overall TCE exposure and for the highest exposure groups in studies that provided data by exposure group. Examination of the highest exposure group was undertaken not to provide an estimate of risk associated with a specific exposure level, as exposure assessments differed greatly in quality and in the exposure metrics used, but, rather, to identify subjects with the greatest potential for TCE exposure, reducing potential bias from exposure misclassification. Additionally, we conducted analyses of the sensitivity of the summary estimates to alternative study inclusion selections or to alternative selections of a study’s RR estimate and influence analyses to examine the impact of individual studies on the summary estimates. Heterogeneity was assessed using the Q-statistic [30] and the I2-value [35]. Publication bias was assessed a number of ways, including funnel plots, the “trim and fill” procedure of Duval and Tweedie [36], forest plots of studies sorted by the SE, and cumulative meta-analyses after sorting studies by the SE.
3. Results
Twenty-four studies met the inclusion criteria and were eligible for meta-analysis. Table 1 lists the literature reference, study design, number of subjects, follow-up period, exposure assessment approach and whether RRs were based on incidence or on mortality. Eleven cohort studies were identified, four European studies of cancer incidence in degreasers from predominantly iron and metal industries [37–40] and seven studies in the United States of mortality in electrical, aerospace or aircraft maintenance workers [26,27,41–43] or of both incidence and mortality in aircraft maintenance and aerospace workers [25,44]. The study periods and base populations in Zhao et al. [25] and Boice et al. [26] overlap, although the studies differ in their exposure assessment approach. Zhao et al. [25] is preferred for the primary analysis given its larger number of TCE-exposed subjects, larger number of kidney cancer and NHL deaths, semi-quantitative exposure assessment, and internal referent group; Boice et al. [26] was used in the sensitivity analyses. Radican et al. [27] updated mortality in the National Cancer Institute (NCI) cohort of Blair et al. [44] and is preferred in the main analysis, given its additional 10 years of follow-up; the incidence findings reported in the earlier publication by Blair et al. [44] were used in the sensitivity analyses. The analysis treats Greenland et al. [42], a case-control study of multiple cancer sites nested within an occupational cohort, as a cohort study because the OR was estimated from incidence density sampling [45]. Of the thirteen case-control studies, seven were of NHL [20–23,46–48], five of renal cell carcinoma [24,49–52] and one of multiple cancer sites that included NHL and renal cell carcinoma [53]. No case-control studies of primary liver cancer or liver and biliary cancers were identified which fulfilled the inclusion criteria.
Table 1.
Key characteristics of epidemiologic cohort and case-control studies of TCE exposure included in the meta-analysis.
| Reference | Study Design | Study population and size | Outcome and Sites Examined | Exposure assessment and exposure surrogate | |
|---|---|---|---|---|---|
| Anttila et al. [38] | C | Finnish workers (n = 3,974) biologically monitored using U-TCA (n = 3,089), 1965–1982, FU 1965–1991 (M), 1967–1992 (I). | I, M | K, L, NHL | Subjects from several industries, primarily metal. Using the Ikeda et al. [54] relationship for TCE exposure to U-TCA, TCE exposures were roughly 4 ppm (median) and 6 ppm (mean). Overall TCE exposure, mean U-TCA, years since 1st U-TCA measurement. |
| Axelson et al. [37] | C | Swedish workers biologically monitored using U-TCA (n = 1,670), 1955–1975, FU 1958–1987 (I). | I | K, L, NHL | Roughly ¾ of cohort had U-TCA concentrations equivalent to <20 ppm TCE. Overall TCE exposure mean U-TCA, years since 1st biological monitoring measurement. |
| Boice et al. [41] | C | Aircraft-manufacturing workers with ≥1 year at Lockheed Martin (Burbank, CA) (n = 77,965; 2,267 with routine TCE; 3,176 with intermittent TCE), FU 1960–1996. | M | K, L, NHL | TCE subcohort. JEM for potential TCE exposure for (1) routine or (2) intermittent or routine basis without semi-quantitative intensity estimate. Overall TCE exposure, exposure duration. |
| Boice et al. [26] | C | Aerospace workers with ≥6 months employment at Rockwell/ Rocketdyne (Santa Susana Field Laboratory and nearby facilities) (n = 41,351; 1,111 with TCE exposure), FU 1948–1999. Overlaps cohort of Zhao et al. [25]. | M | K, L, NHL | TCE subcohort. Potential TCE exposure assigned to test-stand workers whose tasks included the cleaning or flushing of rocket engines (engine flush) (n = 639 subjects) or for general utility cleaning (n = 472). JEM for TCE without semi-quantitative intensity estimates. Vapor degreasing with TCE before 1966 and PCE afterwards. Overall TCE exposure, exposure duration. |
| Brüning et al. [49] | CC | Histologically confirmed RCC (n = 134), from hospitals (Arnsberg, Germany), 1992−2000; hospital controls (n = 401). | I | K (RCC) | Self-reported exposure and JEM of Pannett et al. [55] to assign cumulative exposure to TCE and PCE. Cumulative exposure, exposure duration. |
| Charbotel et al. [50] | CC | RCC (n = 87), from urologists’ files and area teaching hospitals (Arve Valley region, France), 1993–2003; urologist or general practitioner patient controls (n = 316). | I | K (RCC) | Semi-quantitative cumulative TCE exposure and presence/absence of peak TCE exposure assigned to subjects using a JTEM designed using information obtained from questionnaires and routine atmospheric monitoring of workshops or biological monitoring (U-TCA) of workers carried out since the 1960s. Cumulative exposure (low, 62.4 ppm-year; medium, 253.2 ppm-year; high, 925.0 ppm-year), cumulative exposure + peaks. |
| Cocco et al. [21] | CC | Histologically confirmed NHL from 7 European countries (Czech Republic, Finland, France, Germany, Ireland, Italy, and Spain) (n = 2,348), 1998−2004; hospital (4 participating countries) or population controls (all others) (n = 2,462). | I | NHL | IH assessment of 43 agents, including TCE, by confidence, exposure intensity, and exposure frequency, in each participating center. Overall TCE exposure, cumulative TCE exposure for subjects assessed with high degree of confidence. |
| Dosemeci et al. [51] | CC | Histologically confirmed RCC (n = 438), 1988−1990, Minnesota Cancer Registry; controls identified using RDD or, if ≥65 years, from HCFA records (n = 687). | I | K (RCC) | Occupational history of TCE exposure using job title and JEM of Gomez et al. [56]. Overall TCE exposure. |
| Greenland et al. [42] | Nested CC | Cancer deaths among pensioned workers, GE transformer plant (Pittsfield, MA) (n = 12 kidney, 9 liver and biliary, 15 NHL), 1969–1984; controls were non-cancer deaths among pensioned workers (n = 1,202). | M | K, L, NHL | IH assessment from interviews and position descriptions. TCE (no/any exposure) assigned to individual subjects using JEM. Overall TCE exposure. |
| Hansen et al. [39] | C | Workers biologically monitored using U-TCA and air-TCE (n = 803), 1947–1989, FU 1968–1998. | I | K, L, NHL | U-TCA from 1947−1989; air TCE measurements from 1974. Overall, TCE exposure to cohort as extrapolated from air TCE and U-TCA measurements, using Ikeda et al. [54], was 4 ppm (median) and 12 ppm (mean). Overall TCE exposure, year 1st employed, employment duration, mean exposure, cumulative exposure. |
| Hardell et al. [46] | CC | Histologically confirmed cases of NHL in males from Swedish (Umea) hospital (n = 105), 1974−1978; population controls or, if case deceased, from causes-of-death registry (n = 335). | I | NHL | Self-reported overall TCE exposure. |
| Miligi et al. [20] | CC | NHL, including CLL, cases (n = 1,428) identified through surveys of hospital and pathology departments or specialized hematology centers in 8 areas in Italy, 1991−1993; population controls (n = 1,530). | I | NHL+ CLL | TCE exposure assigned using JEM and assessed using exposure probability, intensity and duration. TCE exposure intensity, exposure duration. |
| Moore et al. [24] | CC | Histologically confirmed RCC identified in hospitals in four European countries (Czech Republic, Poland, Romania, Russia) (n = 1,097), 1999−2003; hospital controls with diagnoses unrelated to smoking or genitourinary disorders (n = 1,476). | I | K (RCC) | Specialized job-specific questionnaire for specific jobs or industries of interest focused on solvent exposures, includingTCE, with exposure assignment by frequency, intensity and confidence of TCE exposure. Overall TCE exposure, duration (total hours, years), cumulative exposure (cases: 0, 0.83, 1.95, 7.25 ppm-years for 25th percentile, median, and 75th percentile) and average intensity (cases: 0, 0.08, 0.08, and 0.44 ppm for 25th percentile, median, and 75th percentile). |
| Morgan et al. [43] | C | Aerospace workers with >6 months during 1950–1985 at Hughes (Tucson, AZ) (n = 20,503; 4,733 with TCE exposure), FU 1950–1993. | M | K, L, NHL | TCE subcohort. TCE exposure intensity assigned using JEM. “High TCE exposure” job classification defined as >50 ppm. Overall TCE exposure, cumulative exposure, peak exposure. |
| Nordstrom et al. [47] | CC | Histologically confirmed cases of hairy-cell leukemia in males (n = 111), Swedish Cancer Registry, 1987−1992;a population controls (n = 400). | I | NHL (HCL) | Self-reported overall TCE exposure. |
| Persson and Fredrikson [48] | CC | Histologically confirmed B-cell NHL from two hospitals in Sweden: Oreboro, 1964−1986, or Linkoping, 1975−1984 (n = 199); controls from previous studies, randomly selected from population registers (n = 479). | I | NHL | Self-reported overall TCE exposure. |
| Pesch et al. [52] | CC | Histologically confirmed RCC from German hospitals (5 regions) (n = 935), 1991−1995; controls randomly selected from residency registries (n = 4,298). | I | K (RCC) | TCE and other exposures assigned by questionnaire assessing occupational history using job title (JEM approach), job task (JTEM approach), or self-reported exposure. Cumulative exposure. |
| Purdue et al. [22] | CC | Histologically confirmed NHL identified from four SEER areas (Los Angeles County, Detroit metropolitan area, Seattle-Puget Sound and Iowa) (n = 1,321), 1998–2000; population controls from RDD, or Medicare file, if ≥65 years (n = 1,057). | I | NHL | Specialized job-specific modules asked for detailed information on individual jobs and focused on solvent exposures, including TCE; assessment by expert industrial hygienist blinded to case and control status by levels of probability, frequency, and intensity. Overall exposure, average weekly exposure, years exposed, average exposure intensity, and cumulative exposure. Cumulative exposure categories of 0, 1–46,800 ppm-hour, 46,801–112,320 ppm-hour, 112,321–234,000 ppmhour and >234,000 ppm-hour. |
| Radican et al. [27] (mortality follow-up of Blair et al. [44]) | C | Civilian aircraft-maintenance workers with at least 1 year in 1952−1956 at Hill Air Force Base (UT) (n = 14,455; 7,204 ever exposed to TCE), FU 1952–2000 (M), 1973–1990 (I). | I, M | K, L, NHL | TCE assigned to individual subjects using JEM. TCE replaced in 1968 with 1,1,1-trichloroethane in bench-top degreasing activities and was discontinued in 1978 in vapor degreasing activities. Median TCE exposures were ~10 ppm for rag and bucket; 100−200 ppm for vapor degreasing. Overall TCE exposure, cumulative exposure, continuous or intermittent exposures, and peak exposure. Cumulative exposure categories of 0–5 unit-hour, 5–25 unit-hour, and >25 unit-hour. |
| Raaschou-Nielsen et al. [40] | C | Blue-collar workers employed >1,968 at 347 Danish TCE-using companies (n = 40,049; 14,360 with presumably higher-level exposure to TCE). FU to 1997. | I | K, L, NHL | Employers had documented TCE usage but no information on individual subjects, with job type and company size as variables identified as increasing the likelihood for TCE exposure. Subjects from iron and metal, electronics, painting, printing, chemical, and dry-cleaning industries. Median exposures to TCE were 40−60 ppm for the years before 1970, 10−20 ppm for 1970 to 1979, and approximately 4 ppm for 1980 to 1989. Overall TCE exposure, employment duration, year 1st employed, and company size. |
| Siemiatycki [53] | CC | Histologically confirmed NHL or kidney cancer in males, diagnosed in 16 large Montreal-area hospitals (215 = NHL, 177 K), 1979−1985; population-based controls identified from electoral lists and RDD (n = 533). | I | K, NHL | TCE and other exposure assigned on semi-quantitative scale from work histories by team of chemists and industrial hygienists. Overall TCE exposure, substantial exposure. |
| Wang et al. [23] | CC | Histologically confirmed NHL cases among females (n = 601), Connecticut Cancer Registry, 1996−2000; population-based female controls from RDD or, if ≥65 years of age, random selection from Medicare and Medicaid Service files (n = 717). | I | NHL | TCE intensity and probability of exposure assigned on semi-quantitative scale using JEM (Gomez et al. [56]). Overall TCE exposure, intensity, exposure probability. |
| Zhao et al. [25] | C | Aerospace workers with >2 years of employment at Rockwell/ Rocketdyne’s Santa Susana Field Laboratory, 1950–1993, FU 1950–2001 (M, n = 6,044), 1988–2000 (I, n = 5,049). Overlaps cohort of Boice et al.[26]. | I, M | K, NHL+ other LHP cancers | TCE and other exposures assigned on semi-quantitative scale from work history using JEM. Cumulative TCE score. |
Upon a review of the case series, Nordstrom et al. [47] found one case was diagnosed in 1993.
C = cohort, CA = California, CC = case-control, CLL = chronic lymphocytic leukemia, FU = follow-up, HCFA = Health Care Financing Administration, HCL = hairy cell leukemia, I = incidence, IH = industrial hygiene, JEM = job-exposure matrix, JTEM = job-task-exposure matrix, K = kidney cancer, L = liver and biliary tract cancer, LHP = lymphohematopoietic, M = mortality, MA = Massachusettes, NHL = non-Hodgkin lymphoma, PCE = perchloroethylene, ppm = parts per million, RCC = renal cell carcinoma, RDD = random digit dialing, TCE = trichloroethylene, US = United States, U-TCA = urinary trichloroacetic acid, UT = Utah.
A listing of studies not meeting the selection criteria and the reasons for the exclusions can be found in the Supplemental Material.
3.1. Kidney Cancer
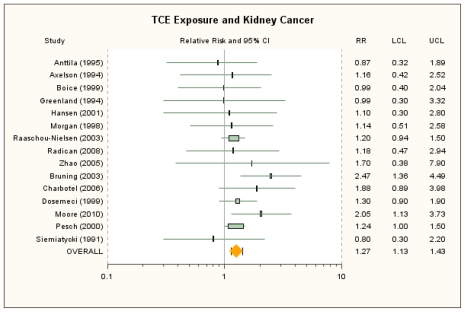
Primary and alternate RR estimates for kidney cancer from the individual cohort and case-control studies are presented in Table 2, and a forest plot of the primary RR estimates and the summary RRm estimate for overall exposure is displayed in Figure 1. The RRm from the primary random-effects meta-analysis of the 15 studies was 1.27 (95% CI: 1.13, 1.43) and was identical to the RRm from the fixed-effect model. The studies of Pesch et al. [52], Raaschou-Nielsen et al. [40], and Dosemeci et al. [51] contributed about 75% of the weight, although no single study was overly influential and removal of individual studies resulted in RRm estimates that were all statistically significant and that ranged from 1.24 (removing Brüning et al. [49]) to 1.30 (removing Raaschou-Nielsen et al. [40]). Similarly, the RRm estimate was not highly sensitive to alternate RR estimate selections. Use of the 13 alternate RR selections, individually, resulted in RRm estimates that ranged from 1.21 (95% CI: 1.09, 1.34) to 1.32 (95% CI: 1.17, 1.49) (see Supplemental Material).
Table 2.
RR estimates for kidney cancer associated with TCE exposure (overall and highest exposure group) from cohort and case-control studies.
| Overall Exposure | Highest TCE Exposure Group | |||||
|---|---|---|---|---|---|---|
| Study | RR (95% CI) | Alternate RR estimates | Exposure Category | RR (95% CI) | Alternate RR estimates | Comments |
| Cohort Studies | ||||||
| Anttila et al. [38] | 0.87 (0.32, 1.89) | None | 100+ μmol/L U-TCA a | 1.0 assumed b | ICD-7 180. SIR. Reported high exposure group results for some cancer sites but not kidney. | |
| Axelson et al. [37] | 1.16 (0.42, 2.52) | 1.07 (0.39, 2.33) with estimated female contribution to SIR addedc | ≥2 year exposure and 100+ mg/L U-TCA | 1.0 assumed b | ICD-7 180. SIR reported for males only, but there was a small female component to the cohort. Reported high exposure group results for some cancer sites but not kidney. | |
| Boice et al. [41] | 0.99 (0.4, 2.04) | None | ≥5 years exposure | 0.69 (0.22, 2.12) | None | ICD-9 189.0−189.2. Overall exposure SMR for potential routine exposure; results for any potential exposure not reported. Mortality RR for highest exposure group for potential routine or intermittent exposure, adjusted for date of birth, dates 1st and last employed, race, and sex; referent group is workers not exposed to any solvent. |
| Greenland et al. [42] | 0.99 (0.30, 3.32) | None | NA | b | ICD-8 codes not specified, presumably all of 189. Mortality OR from nested case-control study. | |
| Hansen et al. [39] | 1.1 (0.3, 2.8) c | None | ≥1,080 months × mg/m3 | 1.0 assumed b | ICD-7 180. SIR. Reported high exposure group results for some cancer sites but not kidney. | |
| Morgan et al. [43] | 1.14 (0.51, 2.58) (Morgan et al. [57]) | 1.32 (0.57, 2.6) Published SMR | High cumulative exposure score | 1.59 (0.68, 3.71) | 1.89 (0.85, 4.23) for medium/high peak | ICD-7 180, ICD-8, -9 189.0−189.2. Overall mortality RR from Morgan et al. [57]. RRs adjusted for age and sex. |
| Raaschou-Nielsen et al. [40] | 1.20 (0.94, 1.50) | 1.20 (0.98, 1.46) for ICD-7 180; c 1.4 (1.0, 1.8) for subcohort with expected higher exposures |
≥5 years in subcohort with expected higher exposure levels | 1.7 (1.1, 2.4) | 1.6 (1.1, 2.2) for ≥5 years in total cohort; c 1.4 (0.99, 1.9) ICD-7 180 ≥5 years in total cohort c |
ICD-7 180.0 (RCC). |
| Radican et al. [27] | 1.18 (0.47, 2.94) | None | >25 unit-years | 1.11 (0.35, 3.49) d | Incidence RR: 0.9 (0.3, 3.2) (Blair et al. [44]) d | ICD-8, -9 189.0, ICD-10 C64. Mortality RR adjusted for age, sex and race, with workers with no chemical exposures as referent group. |
| Zhao et al. [25] | 1.7 (0.38, 7.9)e | Incidence RR: 2.0 (0.47, 8.2); e Mortality RR no lag: 0.89 (0.22, 3.6); e Incidence RR no lag : 2.1 (0.56, 8.1);e SMR: 2.22 (0.89, 4.57) (Boice et al. [26]) |
High exposure score | 7.40 (0.47, 116) | Mortality RR: 1.82 (0.09, 38.6); Incidence RR no lag: 7.71 (0.65, 91.4); Mortality RR no lag: 0.96 (0.09, 9.91); Mortality RR: 2.12 (0.63, 7.11) for ≥5 years as test stand mechanic (Boice et al. [26]); 3.13 (0.74, 13.2) for ≥4 test-year engine flush (Boice et al. [26]) |
ICD-9 189. Mortality RR for males only for overall exposure with 20-year lag; adjusted for age, SES, time since first employment, exposure to other carcinogens. Overall mortality results reflect same number exposed cases (10 with no lag) as do incidence results. Overall RRs estimated by combining across exposure groups. Incidence RR for highest TCE exposure group reflects more exposed cases than does the mortality results and is used in primary analysis. Boice et al. [26] cohort, with seven exposed deaths, overlaps Zhao et al. [25] cohort. |
| Case-Control Studies: f | ||||||
| Brüning et al. [58] | 2.47 (1.36, 4.49) | 1.80 (1.01, 3.20) for longest job held in industry with TCE exposure | ≥20 years self-assessed exposure | 2.69 (0.84, 8.66) | None | RCC. OR for self-assessed TCE exposure adjusted for age, sex, and smoking. |
| Charbotel et al. [50] | 1.88 (0.89, 3.98) | 1.64 (0.95, 2.84) for full study; 1.68 (0.97, 2.91) for full study with 10-year lag | High cumulative dose | 3.34 (1.27, 8.74) | 3.80 (1.27, 11.40) for high + peaks; Full study, high: 2.16 (1.02, 4.60) + peaks: 2.73 (1.06, 7.07); Full study with 10-year lag, high: 2.16 (1.01, 4.65) + peaks: 3.15 (1.19, 8.38); Full study, additional adjustment, high: 1.96 (0.71, 5.37) + peaks: 2.63 (0.79, 8.83) |
RCC. ORs for subgroups with good confidence about exposure assessment. Matched on sex and age, and adjusted for smoking and BMI. Highest exposure group alternate estimates with additional adjustment were also adjusted for exposure to cutting fluids and other petroleum oils. |
| Dosemeci et al. [51] | 1.30 (0.9, 1.9) | None | NA | b | RCC. OR adjusted for age, sex, smoking, hypertension and/or use of diuretics and/or anti-hypertension drugs, BMI. | |
| Moore et al. [24] | 2.05 (1.13, 3.73) | 1.63 (1.04, 2.54) for all subjects | ≥1.58 ppm × years | 2.23 (1.07, 4.64) | 2.02 (1.14, 3.59) for all subjects | RCC. Subgroup with high-confidence assessments. OR adjusted for age, sex, and center. |
| Pesch et al. [52] | 1.24 (1.03, 1.49) | 1.13 (0.98, 1.30) with German JEM | Substantial | 1.4 (0.9, 2.1) d | 1.2 (0.9, 1.7) for JEM d | RCC. JTEM approach. Crude ORs and CIs for overall TCE exposure calculated from data provided by Pesch [59], as described in methods section. ORs for highest exposure group adjusted for age, study center, and smoking. |
| Siemiatycki [53] | 0.8 (0.3, 2.2) | None | Substantial | 0.8 (0.2, 3.4) | None | “Kidney cancer.” SE and 95% CI calculated from reported 90% CI. OR for males only, adjusted for age, income, and cigarette smoking index. |
Mean personal trichloroacetic acid in urine. 1 μmol/L = 0.1634 mg/L.
Anttila et al. [38], Axelson et al. [37] and Hansen et al. [39] report a RR estimate for highest TCE exposure groups and other cancers, but not kidney. A risk estimate of 1.0 is assigned for highest exposure in these studies to account for potential publication bias. For the SE (of the log RR) estimates for these null values, SE estimates from cancer types in the highest exposure group that were expected to have similar numbers of cases were generally used (See Appendix C of U.S. EPA [60] for further details). For Greenland et al. [42] and Dosemeci et al. [51], a risk of 1.0 is not assumed for highest exposure since only overall results are presented in those studies.
Male and female results combined assuming Poisson distribution. Details of the approach used to estimate the female contribution for Axelson et al. [37] are presented in U.S. EPA [60].
Male and female results combined using inverse-variance weighting, as in a fixed-effect meta-analysis.
To derive an overall RR estimate, results were combined across exposure groups using inverse-variance weighting, under assumptions of group independence, although the exposure groups share a referent group and, hence, are not actually independent.
The RR estimates are all ORs for incident cases.
BMI = body mass index, CI = confidence interval, cum = cumulative, ICD = International Classification of Diseases, JTEM = job-task-exposure matrix, NA = not available, OR = odds ratio, RCC = renal cell carcinoma, RR = relative risk, SE = standard error, SES = socioeconomic status, SIR = standardized incidence ratio, SMR = standardized mortality ratio, TCE = trichloroethylene, U-TCA = urinary trichloroacetic acid.
Figure 1.
Forest plot of overall TCE exposure and kidney cancer from random-effects meta-analysis. Individual study results are plotted with 95% confidence intervals. Symbol sizes reflect relative weights of the 15 cohort and case-control studies.
There was no observable heterogeneity across the studies of overall exposure (I2 = 0%). Nevertheless, subgroup analyses examining cohort and case-control studies separately were carried out as sensitivity analyses. The random-effects model yielded RRm estimates of 1.16 (95% CI: 0.96, 1.40) for the cohort studies and 1.48 (1.15, 1.91) for the case-control studies, with RRm estimates not statistically significant different (p = 0.12) between cohort or case-control studies. No heterogeneity was observed in the cohort subgroup (I2 = 0%), and low-to-moderate heterogeneity in the case-control subgroup was suggested by the I2-value of 41% (p = 0.14 for heterogeneity). No evidence of publication bias was observed in the data set for kidney cancer and overall TCE exposure. The trim-and-fill procedure determined that there was no imbalance in the funnel plot indicative of publication bias (see Supplemental Material).
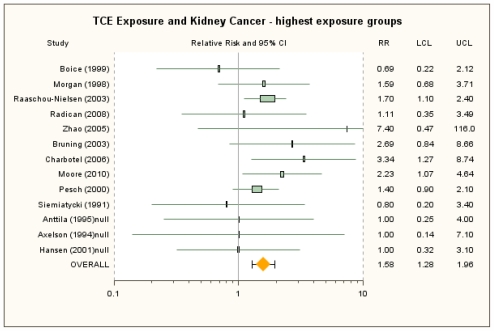
For the highest exposure groups, the primary random-effects meta-analysis, in which null estimates (RR = 1.0) were included for three studies which reported exposure-group results for select other cancers but not for kidney cancer, to address ostensible reporting bias, yielded an RRm estimate of 1.58 (95% CI: 1.28, 1.96) (see Figure 2) [37–39]. In an analysis of only the ten studies reporting results by exposure level, the RRm estimate was 1.64 (95% CI: 1.31, 2.04), similar to the RRm from the primary analysis. No single study was overly influential, as removal of individual studies resulted in RRm estimates that were all statistically significant and that ranged from 1.52 (removing Raaschou-Nielsen et al. [40]) to 1.64 (removing Pesch et al. [52]). Similarly, the RRm estimate was not highly sensitive to alternate RR estimate selections. Use of the 18 alternate selections, individually, resulted in RRm estimates that ranged from 1.47 (95% CI: 1.20, 1.79) to 1.60 (95% CI: 1.29, 1.98), with most alternate selections yielding RRm estimates in the narrow range 1.54–1.60 (see Supplemental Material). There was no observable heterogeneity across the studies for any of the analyses conducted with the highest exposure groups other than a negligible amount of heterogeneity observed in the sensitivity analysis with the Pesch et al. [52] alternate RR estimate (I2 = 0.64%).
Figure 2.
Forest plot of highest TCE exposure group and kidney cancer from random-effects meta-analysis. Individual study results are plotted with 95% confidence intervals. A risk estimate of 1.0 is assigned for highest exposure in Anttila et al. [38], Axelson et al. [37] and Hansen et al. [39] to account for presumed reporting bias. Symbol sizes reflect relative weights of the 13 cohort and case-control studies.
3.2. Liver Cancer
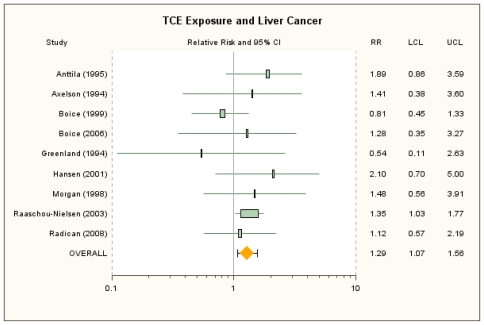
The RRm estimate from the random-effects meta-analysis of the nine independent cohort studies of overall TCE exposure and liver and gall bladder/biliary passages was 1.29 (95% CI: 1.07, 1.56) (Figure 3), identical to that from the fixed-effect model. Individual study RR estimates and alternatives are in Table 3. Relative risk estimates in these studies were generally based on fewer than 10 events. The number of events in Raaschou-Nielsen et al. [40] was over 2-fold higher than the numbers in other studies and contributed about 53% of the total weight. The RRm estimate decreases somewhat without this large study and, as expected, has less precision (RRm = 1.22; 95% CI: 0.93, 1.61). The influence analysis showed no other single study was overly influential; removal of any of the other individual studies resulted in RRm estimates that were all statistically significant and that ranged from 1.24 (removing Anttila et al. [38]) to 1.39 (removing Boice et al. [41]). The RRm, furthermore, was not highly sensitive to the seven alternate RR estimate selections, nor to the simultaneous substitution of results for liver cancer alone for the three studies for which these were available, which yielded a RRm of 1.25 (95% CI: 0.99, 1.57) (see Supplemental Material).
Figure 3.
Forest plot of overall TCE exposure and liver cancer from random-effects meta-analysis. Individual study results are plotted with 95% confidence intervals. Symbol sizes reflect relative weights of the 9 cohort studies.
Table 3.
Selected RR estimates for liver cancer associated with TCE exposure (overall and highest exposure group) from cohort and case-control studies.
| Overall Exposure | Highest TCE Exposure Group | |||||
|---|---|---|---|---|---|---|
| Study | RR (95% CI) | Alternate RR estimates | Exposure Category | RR (95% CI) | Alternate RR estimates | Comments |
| Cohort Studies | ||||||
| Anttila et al. [38] | 1.89 (0.86, 3.59) a | 2.27 (0.74, 5.29) for 155.0 alone | 100+ μmol/L U-TCA b | 2.74 (0.33, 9.88) | None | ICD-7 155.0 + 155.1. SIR. ICD-7 155.0 for highest exposure group. |
| Axelson et al. [37] | 1.41 (0.38, 3.60) | 1.34 (0.36, 3.42) with estimated female contribution to SIR added c | 100+ mg/L U-TCA | 3.7 (0.09, 21) c | Exclude study | ICD-7 155. SIR reported for males only, but there was a small female component to the cohort. No cases were observed in highest exposure group (i.e., >2 years and 100+ U-TCA), so combined with <2 years and 100+ subgroup and estimated female results. |
| Boice et al. [41] | 0.81 (0.45, 1.33) | 0.54 (0.15, 1.38) for potential routine exposure | ≥5 year exposure | 0.94 (0.36, 2.46) | None | ICD-9 155 + 156. Overall SMR for any potential exposure. Highest exposure mortality RR for any potential exposure, adjusted for date of birth, dates 1st and last employed, race, and sex; referent group is workers not exposed to any solvent. |
| Greenland et al. [42] | 0.54 (0.11, 2.63) | None | NA | d | ICD-8 155 + 156. Mortality OR from nested case-control study. | |
| Hansen et al. [39] | 2.1 (0.7, 5.0) c | None | ≥1,080 months × mg/m3 | 1.0 assumed d | ICD-7 155. SIR. Reported high exposure group results for some cancer sites but not liver. | |
| Morgan et al. [43] | 1.48 (0.56, 3.91) | 0.98 (0.36, 2.13) Published SMR | High cumulative exposure score | 1.19 (0.34, 4.16) | 0.98 (0.29, 3.35) for medium/high | ICD-7 155, ICD-8, −9 155 + 156. Overall mortality RR as reported in Morgan et al. [57] RRs adjusted for age and sex. |
| Raaschou-Nielsen et al. [40] | 1.35 (1.03, 1.77) a,c | 1.28 (0.89, 1.80) for ICD-7 155.0 c | ≥5 years | 1.2 (0.7, 1.9) a,c | 1.1 (0.5, 2.1) ICD-7 155.0 (liver only) c | ICD-7 155.0 + 155.1. SIR. |
| Radican et al. [27] | 1.12 (0.57, 2.19) | 1.25 (0.31, 4.97) for ICD-8, −9 155.0 | >25 unit-year | 1.49 (0.67, 3.34) e | None | ICD-8, −9 155 + 156, ICD-10 C22–C24. Mortality HR adjusted for age, sex and race, with workers with no chemical exposures as referent group. |
| Zhao et al. [25]/Boice et al. [26] | 1.28 (0.35, 3.27) | 1.0 assumed for Zhao et al. [25] d | High exposure score | 1.0 assumed for Zhao et al. [25] d | ICD-9 155 + 156. Overall SMR for males from Boice et al. [26] used in lieu of Zhao et al. [25], who do not report liver cancer results. Highest exposure group RR for liver cancer not reported by Zhao et al. [25] or Boice et al. [26]. | |
Observed and expected numbers of cases combined assuming Poisson distribution for ICD codes identified in comments column.
Mean personal trichloroacetic acid in urine. 1 μmol/L = 0.1634 mg/L.
Male and female results combined assuming Poisson distribution. Details of the approach used to estimate the female contribution for Axelson et al. [37] are presented in U.S. EPA [60].
Hansen et al. [39] and Zhao et al. [25] report a RR estimate for highest TCE exposure groups and other cancers, but not liver. A risk estimate of 1.0 is assigned for highest exposure in these studies, and as an alternate overall RR estimate for the Zhao et al. [25] study, which does not report any liver results, to account for potential publication bias. For the SE (of the log RR) estimates for these null values, SE estimates from cancer types that were expected to have similar numbers of cases were generally used (See Appendix C of U.S. EPA [60] for further details). For Greenland et al. [42], a risk of 1.0 is not assumed for highest exposure since only overall results are presented in that study.
Male and female results combined using inverse-variance weighting, as in a fixed-effect meta-analysis.
HR = hazard ratio, ICD = International Classification of Diseases, NA = not available, OR = odds ratio, RR = relative risk, SIR = standardized incidence ratio, SMR = standardized mortality ratio, TCE = trichloroethylene, U-TCA = urinary trichloroacetic acid.
Analysis of the nine studies of overall exposure revealed no apparent heterogeneity (I2-values were 0%). Since all studies on liver cancer were of cohort design, no analyses were conducted examining cohort and case-control studies separately. Funnel plots and other tests performed to examine potential publication bias in the TCE liver cancer data set did not find any evidence of missing studies or of a relationship between RR estimate and study size (see Supplemental Material).
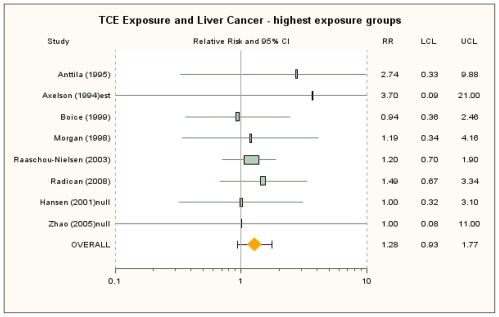
The RRm estimate from the random-effects meta-analysis of the six studies presenting results for highest exposure groups was 1.32 (95% CI: 0.93, 1.86). The RRm estimate from the primary random-effects meta-analysis with null RR estimates (i.e., 1.0) included for Hansen et al. [39] and Zhao et al. [25] to address presumed reporting bias was 1.28 (95% CI: 0.93, 1.77) (Figure 4). The RRm estimate for liver cancer in the highest exposure groups was lower than that for overall exposure and primarily reflects the lower observed RR estimate for the highest exposure group in Raaschou-Nielsen et al. [40], the study carrying the greatest weight. No other single study was overly influential, and the RRm estimate was not sensitive to alternate RR selections (see Supplemental Material). There was no observable heterogeneity across the eight studies for any of the analyses conducted with the highest exposure groups (I2-values were 0%).
Figure 4.
Forest plot of highest TCE exposure group and liver cancer from random-effects meta-analysis. Individual study results are plotted with 95% confidence intervals. A risk estimate of 1.0 is assigned for highest exposure in Hansen et al. [39] and Zhao et al. [25] to account for presumed reporting bias. Symbol sizes reflect relative weights of the 8 cohort studies.
3.3. Non-Hodgkin Lymphoma
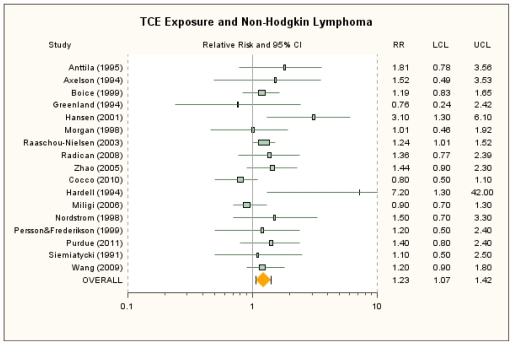
Primary and alternate RR estimates for NHL in the nine cohort and eight case-control studies are presented in Table 4, and a forest plot for overall exposure is displayed in Figure 5. The RRm estimate from the primary random-effects meta-analysis of overall exposure was 1.23 (95% CI: 1.07, 1.42). This RRm estimate was not overly sensitive to removal of individual studies, with resulting RRm estimates that were all statistically significant and that ranged from 1.18 (removing Hansen et al. [39]) to 1.27 (removing Miligi et al. [20] or Cocco et al. [21]). Removal of Hardell et al. [46], whose RR estimate of 7.2 is a relative outlier, only decreased the RRm estimate to 1.21 (95% CI: 1.07, 1.38), since this study contributes little weight to the meta-analysis. Similarly, the primary RRm estimate was not highly sensitive to the alternate RR estimate selections, with which RRm estimates ranged from 1.20 (95% CI: 1.03, 1.39) to 1.28 (95% CI: 1.09, 1.49); nor was it sensitive to restriction of the analysis to the 13 studies for which RR estimates for the traditional definition of NHL were available (RRm = 1.27, 95% CI: 1.05, 1.55) (see Supplemental Material). Low-to-moderate heterogeneity was observed in the primary analysis of overall exposure (I2-value was 26%; p = 0.16) and in each of the meta-analyses with alternative RR selections (I2 = 25% to 34%; p = 0.09 to 0.17). Subgroup analyses examined cohort and case-control studies and overall exposure separately to investigate the heterogeneity. In cohort studies, the RRm was 1.33 (95% CI: 1.13, 1.58) and, in case-control studies, 1.11 (95% CI: 0.89, 1.38). The subgroup RRm estimates were not statistically significantly different (p = 0.19, under the random-effects model). There was evidence of potential publication bias, as the funnel plot appeared asymmetrical and suggested some relationship between RR estimate and study size (see Supplemental Material). Using Duval and Tweedie’s trim-and-fill procedure to counter-balance the apparent asymmetry of the more extreme values in the funnel plot yielded a RRm estimate of 1.15 (95% CI: 0.97, 1.36). Thus, if there is publication bias in this data set, it does not appear to account completely for the finding of an increased NHL risk.
Table 4.
Selected RR estimates for NHL associated with TCE exposure (overall and highest exposure group) from cohort and case-control studies.
| Overall Exposure | Highest TCE Exposure Group | |||||
|---|---|---|---|---|---|---|
| Study | RR (95% CI) | Alternate RR estimates | Exposure Category | RR (95% CI) | Alternate RR estimates | Comments |
| Cohort Studies | ||||||
| Anttila et al. [38] | 1.81 (0.78, 3.56) | None | 100+ μmol/L U-TCA a | 1.4 (0.17, 5.04) | None | ICD-7 200 + 202. SIR. |
| Axelson et al. [37] | 1.52 (0.49, 3.53) | 1.36 (0.44, 3.18) with estimated female contribution to SIR added b | ≥2 year exposure and 100+ mg/L U-TCA | 6.25 (0.16, 34.83) | 5.62 (0.14, 31.3) with estimated female contribution added b | ICD-7 200 + 202. SIR reported for males only, but there was a small female component to the cohort. |
| Boice et al. [41] | 1.19 (0.83, 1.65) | 1.19 (0.65, 1.99) for potential routine exposure | ≥5 years exposure | 1.62 (0.82, 3.22) | None | ICD-9 200 + 202. Overall exposure SMR for any potential exposure. Mortality RR for highest exposure group for any potential exposure adjusted for date of birth, dates 1st and last employed, race, and sex; referent group is workers not exposed to any solvent. |
| Greenland et al. [42] | 0.76 (0.24, 2.42) | None | NA | ICD-8 200–202. Mortality OR from nested case-control study. Overall exposure only. | ||
| Hansen et al. [39] | 3.1 (1.3, 6.1) b | None | ≥1,080 months × mg/m3 | 2.7 (0.56, 8.0) b | 3.7 (1.0, 9.5) for >75 months exposure duration; b 2.9 (0.79, 7.5) for >19 mg/m3 mean exposure b |
ICD-7 200 + 202. SIR for highest exposure group presented only for males; female results estimated and combined with male results. |
| Morgan et al. [43] | 1.01 (0.46, 1.92) | 1.36 (0.35, 5.21) RR for ICD 200 |
High cumulative exposure score | 0.81 (0.1, 6.49) | 1.31 (0.28, 6.08) for medium/high peak | ICD 200 + 202, ICD Revision 7, 8, or 9, depending on year of death. Overall SMR reported by Mandel et al. [18] Alternative overall mortality RR for ICD 200 as reported in Morgan et al. [57] and adjusted for age and sex. Mortality RR for highest exposure group is for ICD 200 only and adjusted for age and sex. |
| Raaschou-Nielsen et al. [40] | 1.24 (1.01, 1.52) | 1.5 (1.2, 2.0) for subcohort with expected higher exposures | ≥5 years in subcohort with expected higher exposure levels | 1.6 (1.1, 2.2) | 1.45 (0.99, 2.05) for ≥5 years in full cohort b | ICD-7 200 + 202. SIR. |
| Radican et al. [27] | 1.36 (0.77, 2.39) | None | >25 unit-years | 1.41 (0.71, 2.81)c | 0.97 (0.42, 2.2) for incidence (Blair et al. [44])c | ICD-8,-9 200 + 202; ICD-10 C82–C85. Mortality RR adjusted for age, sex and race, with workers with no chemical exposures as referent group. |
| Zhao et al. [25] | 1.44 (0.90, 2.30) d | Incidence RR: 0.77 (0.42, 1.39); d SMR for ICD-9 200 + 202: 0.21 (0.01, 1.18) (Boice et al. [26]) | High exposure score | 1.30 (0.52, 3.23) | Incidence RR: 0.20 (0.03, 1.46) | Most lymphohematopoietic cancers, ICD-9 200–208, ICD-10, C81–C95, ICD-O 2, morphology code 9590–9716, 9723, 9800–9980. Mortality RRs used in primary analyses since reflect more exposed cases than do the incidence results. Males only; adjusted for age, SES, time since first employment. |
| Case-Control Studies:e | ||||||
| Cocco et al. [21] | 0.8 (0.5, 1.1) | None | High cumulative exposure | 0.7 (0. 4, 1.3) | None | NHL. Grouping consistent with traditional definition provided by author. Incidence OR. High-confidence subgroup. Adjusted for age, sex, center, and education. |
| Hardell et al. [46] | 7.2 (1.3, 42) | None | NA | NHL. Rappaport classification system. Incidence OR. Males only; controls matched for age, place of residence, vital status. Overall exposure only. | ||
| Miligi et al. [20] | 0.93 (0.67, 1.29) | None | Medium/high exposure intensity | 1.2 (0.7, 2.0) | 1.0 (0.5, 2.6) for med/high intensity and >15 years | NHL + CLL. NCI Working Formulation. Adjusted OR for overall exposure not presented; overall crude incidence OR calculated as described in methods section. OR for highest exposure group adjusted for age, sex, education, and area. |
| Nordstrom et al. [47] | 1.5 (0.7, 3.3) | None | NA | HCL. Incidence OR. Males only; controls matched for age and county; analysis controlled for age. Overall exposure only. | ||
| Perrson and Frederikson [48] | 1.2 (0.5, 2.4) | None | NA | NHL. Classification system not specified. Incidence OR. Controls selected from same geographic areas; OR stratified on age and sex. Overall exposure only. | ||
| Purdue et al. [22] | 1.4 (0.8, 2.4) | None | Cumulative exposure > 234,000 ppm-hours | 3.3 (1.1, 10.1) | 2.3 (1.0, 5.0) for highest exposure tertile (>112,320 ppm-hours) | ICD-O-3 codes 967–972. Incidence OR. Probable-exposure subgroup. Adjusted for age, sex, SEER center, race, and education. |
| Siemiatycki [53] | 1.1 (0.5, 2.5) | None | Substantial | 0.8 (0.2, 3.3) | None | ICD-9 200 + 202. Incidence OR. SE and 95% CI calculated from reported 90% CIs; males only; adjusted for age, income, and cigarette smoking index. |
| Wang et al. [23] | 1.2 (0.9, 1.8) | None | Medium-high intensity | 2.2 (0.9, 5.4) | None | ICD-O M-9590–9595, 9670–9688, 9690–9698, 9700–9723. Incidence OR. Females only; adjusted for age, family history of lymphohematopoietic cancers, alcohol consumption, and race. |
Mean personal trichloroacetic acid in urine. 1 μmol/L = 0.1634 mg/L.
Male and female results combined assuming Poisson distribution. Details of the approach used to estimate the female contribution for Axelson et al. [37] are presented in U.S. EPA [60].
Male and female results combined using inverse-variance weighting, as in a fixed-effect meta-analysis.
To derive an overall RR estimate, results were combined across exposure groups using inverse-variance weighting, under assumptions of group independence, although the exposure groups share a referent group and, hence, are not actually independent.
The RR estimates are all ORs for incident cases.
CI = confidence interval, CLL = chronic lymphocytic leukemia, HCL = hairy cell leukemia, ICD = International Classification of Diseases, NCI = National Cancer Institute, NHL = non-Hodgkin lymphoma, NA = not available, OR = odds ratio, RR = relative risk, SES = socioeconomic status, SIR = standardized incidence ratio, SMR = standardized mortality ratio, TCE = Trichloroethylene, U-TCA = urinary trichloroacetic acid.
Figure 5.
Forest plot of overall TCE exposure and non-Hodgkin lymphoma from random-effects meta-analysis. Individual study results are plotted with 95% confidence intervals. Symbol sizes reflect relative weights of the 17 cohort and case-control studies.
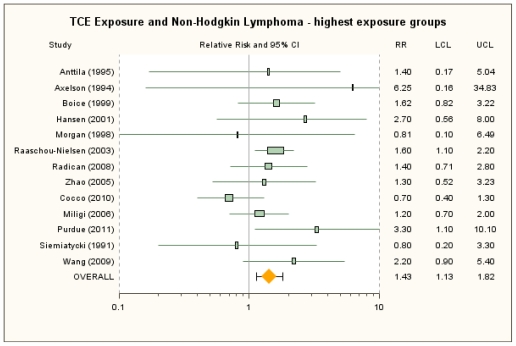
The RRm estimate from the primary random-effects meta-analysis of the highest exposure groups from the 13 studies with results presented by exposure level was 1.43 (95% CI: 1.13, 1.82) (see Figure 6). No single study was overly influential; removal of individual studies resulted in RRm estimates that ranged from 1.38 (removing Purdue et al. [22]) to 1.57 (removing Cocco et al. [21]). Similarly, use of the nine alternative RR selections produced RRm estimates in a narrow range from 1.40 (95% CI: 1.09, 1.80) to 1.49 (95% CI: 1.14, 1.93) (see Supplemental Material). Low heterogeneity was observed across the 13 studies of NHL in the highest exposure groups (I2 = 14%; p = 0.30), and low to low-to-moderate heterogeneity was apparent in each of the meta-analyses with alternative RR selections (I2 = 9% to 33%; p = 0.12 to 0.37). Subgroup analyses examined the highest exposure groups in cohort and case-control studies, separately, to investigate the heterogeneity. In cohort studies, the RRm was 1.60 (95% CI: 1.24, 2.08) and, for case-control studies, 1.29 (95% CI: 0.76, 2.20). The subgroup RRm estimates were not statistically significantly different (p = 0.47, under the random-effects model).
Figure 6.
Forest plot of highest TCE exposure group and non-Hodgkin lymphoma from random-effects meta-analysis. Individual study results are plotted with 95% confidence intervals. Symbol sizes reflect relative weights of the 13 cohort and case-control studies.
4. Discussion
Individual studies in our analysis had low power to evaluate TCE exposure and cancer risk, but meta-analysis provided a tool to combine underpowered studies and to systematically assess the associations of TCE exposure and various cancers. In addition to the analyses of overall exposure, results were combined across the highest exposure groups, although individual studies used different exposure definitions, as an attempt to identify subjects with the greatest exposure potential and reduce potential exposure misclassification problems. We were unable to explore the shape of exposure-response relationships, as was recently done for benzene [61], given the few studies with quantitative TCE exposure data.
For kidney cancer, the elevated RRm estimates for overall TCE exposure and the highest exposure groups in the primary and alternative analyses provide robust support for a small, statistically significant increased risk, without evidence of heterogeneity or publication bias. The lack of observed heterogeneity provides evidence of consistency in kidney cancer risk estimates from independent epidemiologic studies of different industries with high potential for TCE exposure, regardless of study design. We did observe a slightly larger RRm estimate for case-control than for cohort studies; however, the difference across study designs was not statistically significant. Two case-control studies were carried out in geographic areas with a high frequency and a high degree of TCE exposure and were designed with a priori hypotheses to test for the effects of TCE exposure on renal cell cancer risk [49,50], and a third study was carried out in four central and eastern European countries with high renal cell cancer rates unexplained by established risk factors [24]. The higher exposures in these case-control studies compared to cohort studies may, in part, contribute to our finding of a larger RRm estimate for case-control studies.
We also found support for a small increased risk of NHL from TCE exposure, but less support than that for kidney cancer. Some potential publication bias in the TCE NHL data set was suggested by some of the tests used. In addition, low-to-moderate heterogeneity was observed for the NHL studies, although it was not statistically significant. Subgroup analysis of the cohort and case-control studies separately explained some of the differences in NHL risk across studies, although study design, itself, is unlikely to be an underlying cause of heterogeneity and is probably a surrogate for some other differences across these studies that may be associated with study design. Instead, temporal NHL classification changes and study differences in NHL classification, as well as differences in exposure definitions, levels, and prevalence, are possible alternative explanations. If TCE exposure increases the risk of NHL, the effects should be more apparent in the highest exposure groups and, indeed, our analyses did observe this finding. Our observations, furthermore, suggest an increased risk of liver cancer. The liver cancer results, however, are relatively underpowered with respect to numbers of studies and cases, and the study that provides the greatest weight used the weak exposure surrogate of duration of employment for categorizing exposure level.
Our analysis approach has several advantages to previous meta-analyses of TCE exposure and cancer. The selection criteria adopted for this meta-analysis were intended to identify informative studies for the evaluation of TCE exposure and cancer, i.e., studies with reduced systematic errors. Neither Henschler et al. [62] nor Vamvakas et al. [63], two studies with incomplete cohort identification or potential selection bias of study controls, met our inclusion criteria. Their inclusion in another recent meta-analysis may have contributed to the observed heterogeneity in kidney cancer RR estimates in that analysis [16]. Despite these and other differences in study selection, such as the inclusion of studies lacking documented TCE exposure, that meta-analysis reported RRm estimates of 1.24 (95% CI: 1.06, 1.45) to 1.42 (95% CI: 1.13, 1.77) for overall exposure and kidney cancer [16], similar to our finding. Studies with low potential for TCE exposure also did not meet our selection criteria, as our analysis focused on studies in which TCE exposure potential was inferred to each subject by reference to industrial hygiene records, individual biomarkers, job-exposure matrices, or questionnaire responses, in order to reduce exposure misclassification bias, although this bias would not have been completely eliminated. Inclusion of studies of lower exposure potential in meta-analyses can have important implications for identifying a cancer hazard [64–66]. Additionally, we allowed use of a broader definition of NHL, more consistent with the updated WHO classification [67]. Use of this broader definition of NHL may have introduced some downward bias, since NHL subtypes unrelated to TCE may have been included. Last, our analysis updates other meta-analyses by the inclusion of recently published studies on NHL and kidney cancer, and we also included two studies of overlapping cohorts in alternate analyses. Two previous meta-analyses of TCE and NHL [18] or kidney cancer [16] chose to include Boice et al. [26] rather than Zhao et al. [25]. Despite the fact that Zhao et al. [25] did not report NHL results and we had to use results for a broader category of lymphohematopoietic cancers that included NHL, we preferred Zhao et al. [25] for our primary analysis because of their use of a semi-quantitative cumulative exposure assessment approach, use of an internal referent population, and much larger number of lymphohematopoietic cancer cases, which include NHLs, than Boice et al. [26] Our sensitivity analysis showed this study choice did not greatly affect the summary RRm for NHL and overall TCE exposure.
Interpretation of our findings on kidney cancer, liver cancer and NHL within a causal framework can be challenging in light of the modest RRm estimates and, for NHL, the low-to-moderate heterogeneity and potential publication bias. In general, the observed RRm estimates for overall TCE exposure suggest increased risks of 20%–30% for liver cancer, kidney cancer, and NHL. Increased risks suggested by the RRm estimates for the highest exposure groups were further elevated for kidney cancer (58%) and NHL (43%). Large RR estimates are considered strong evidence of causality; however, modest-sized risks may reflect a lower level of exposure or an agent of lower potency.
Consideration of potential confounding as an alternative explanation for our observations is important. Obesity, high body mass index (BMI), and smoking are known risk factors for kidney cancer [68]. Any confounding in cohort studies related to obesity is likely small given the generally healthy nature of an employed population. All kidney cancer case-control studies controlled for BMI, except Pesch et al. [52] and Moore et al. [24], and for smoking, except Moore et al. [24]. Moore et al. [24] reported that neither smoking nor BMI significantly changed the overall association with TCE exposure. Information on smoking for individual subjects is commonly lacking in cohort studies. Use of internal controls, which occurred in five of the cohort studies [25–27,41,43], generally minimizes effects of potential confounding due to smoking or socioeconomic status, since exposed and referent subjects are drawn from the same target population. Unlike for kidney cancer, a pattern of increased lung cancer risk was not apparent in the cohort studies; the RRm for lung cancer from the nine cohort studies in our meta-analysis was 0.96 (95% CI: 0.76, 1.21) for overall TCE exposure and 0.96 (95% CI: 0.72, 1.27) for the highest exposure groups. Although smoking was more prevalent in the Raaschou-Nielsen et al. [40] cohort than in the background population, if smoking fully explains the observed 40% excess lung cancer risk in this study, the expected contribution to renal cell carcinoma risk from smoking based on RRs for lung cancer and kidney cancer observed in five smoking cohorts [69] is estimated as 1%–6%, far smaller than the 20% and 40% excess in renal cell carcinoma risk observed in the cohort and subcohort, respectively. These observations suggest that confounding by smoking is not an issue for the kidney cancer results.
Job titles such as a degreaser often have potential for several exposures, including mineral oils, hydrazine, and other solvents, besides TCE [25,27,38,41]. Mineral oils such as cutting fluids, common to some job titles with potential TCE exposures, were included as covariates in the statistical analyses of Zhao et al. [25], who also examined hydrazine and several other co-exposures, and of Charbotel et al. [50,70] or were evaluated as a single exposure for cases and controls in some other studies [49,71]. Although Brüning et al. [49] reported an association with cutting oil exposure and kidney cancer, cutting oil exposure did not appear highly correlated with TCE exposure, as only 5 cases reported exposure to cutting oils compared to 25 cases reporting TCE exposure. Karami et al. [71], who examined mineral oil or cutting fluid exposure among cases and controls in Moore et al. [24], found no association for cutting oil mists or other mineral oil mists and kidney cancer. Cutting oils and mineral oils have not been associated with kidney cancer in other cohort or case-control studies [72,73]. Potential co-exposure to other solvents and other chemicals is unlikely to provide an alternative explanation for our robust findings, as the studies included in our analysis varied in the pattern, level, and specific types of co-exposures.
Risk factors for liver cancer include Hepatitis C viruses and heavy alcohol consumption in the United States and Northern Europe, where Hepatitis B prevalence is low [74]. In addition, nonalcoholic steatohepatitis, reflecting obesity and metabolic syndrome, was recently identified as contributing to liver cancer risk [68,75]. Heavy alcohol consumption is unlikely a confounder for liver cancer, as four of the nine independent cohort studies also reported on cirrhosis mortality, with no observed positive association with TCE exposure [26,27,41,43]. The generally healthy nature of an employed population reduces concern about confounding related to obesity and Hepatitis C.
Few risk factors have been identified for NHL, with the exception of viruses and suspected factors such as family history of NHL and lymphoproliferative diseases or immunosuppression [76]. Smoking is weakly associated with the follicular type of NHL [68,77]; however, any potential confounding by smoking in the case-control studies is reduced by the inclusion of several NHL types in our definition. Altered immunity may be a possible mode of action for TCE and NHL, as Lan et al. [78] recently reported decreased lymphocyte subsets among TCE-exposed workers.
In conclusion, our analysis updates the literature review since past meta-analyses on TCE exposure and NHL, liver cancer and kidney cancer, adopting criteria to identify studies that minimize biases associated with exposure misclassification and subject selection. The consistency of increased kidney cancer RR estimates across a large number of independent studies of different designs and populations from different countries and industries and the robust summary RRm estimates across various influence and sensitivity analyses provide strong support for a causal association. Although the RRm for kidney cancer was modest, neither chance nor confounding related to BMI, smoking, or exposure to cutting oils could provide alternative explanations for the observed increase in risk for this site. The support is strong but less robust for NHL, where issues of (non-statistically significant) study heterogeneity, potential publication bias, and weaker exposure-response results contribute greater uncertainty, and more limited for liver cancer, where only cohort studies with small numbers of cases are available. Although we did not examine exposure-response relationships using statistical models, biological gradients are supported for kidney cancer and NHL based on meta-analyses of only the highest exposure groups, which yielded higher summary RRm estimates than for overall TCE exposure. Other human, animal and pharmacokinetic data linking TCE and these cancers provide further support and biological plausibility to our findings [60].
Supplementary Material
Acknowledgements
We thank the TCE team for their insightfulness and constructive input, particularly Chiu, W. for his technical guidance and leadership, Cooper, G. and Christensen, K. for their critical review of the manuscript, and Clark, R., Bussard, D. and White, P. for providing management support for this work.
Footnotes
Sources of Financial Support
No external sources of funding were used for analysis or preparation of manuscript.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
References
- 1.International Agency for Research on Cancer (IARC) Monographs on the Evaluation of Carcinogenic Risks to Humans. Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals. Vol. 63. International Agency for Research on Cancer; Lyon, France: 1995. [PMC free article] [PubMed] [Google Scholar]
- 2.National Toxicology Program (NTP) 11th report on carcinogens. Rep. Carcinog. 2005:1-A32. [PubMed] [Google Scholar]
- 3.Ruden C. Principles and practices of health risk assessment under current EU regulations. Regul. Toxicol. Pharmacol. 2006;44:14–23. doi: 10.1016/j.yrtph.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Scott C.S., Chiu W.A. Trichloroethylene cancer epidemiology: A consideration of select issues. Environ. Health Perspect. 2006;114:1471–1478. doi: 10.1289/ehp.8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Research Council (NRC) Contaminated Water Supplies at Camp Lejeune—Assessing Potential Health Effects. The National Academies Press; Washington, DC, USA: 2009. [PubMed] [Google Scholar]
- 6.National Research Council (NRC) Assessing the Human Health Risks of Trichloroethylene: Key Scientific Issues. The National Academies Press; Washington, DC, USA: 2006. [Google Scholar]
- 7.Ward E.M., Schulte P.A., Straif K., Hopf N.B., Caldwell J.C., Carreon T., Demarini D.M., Fowler B.A., Goldstein B.D., Hemminki K., et al. Research recommendations for selected IARC-classified agents. Environ. Health Perspect. 2010;118:1355–1362. doi: 10.1289/ehp.0901828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blair A., Burg J., Foran J., Gibb H., Greenland S., Morris R., Raabe G., Savitz D., Teta J., Wartenberg D. Guidelines for application of meta-analysis in environmental epidemiology. Regul. Toxicol. Pharmacol. 1995;22:189–197. doi: 10.1006/rtph.1995.1084. [DOI] [PubMed] [Google Scholar]
- 9.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 10.Vandenbroucke J.P., von Elm E., Altman D.G., Gotzsche P.C., Mulrow C.D., Pocock S.J., Poole C., Schlesselman J.J., Egger M. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. Epidemiology. 2007;18:805–835. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute (NCI) Technical Report Series. NCI; Bethesda, MD, USA: 1976. Carcinogenesis Bioassay of Trichloroethylene, NCI-CG-TR-2. [PubMed] [Google Scholar]
- 12.NTP. Technical Report Series. NTP; Research Triangle Park, NC, USA: 1988. Toxicology and Carcinogenesis Studies of Trichloroethylene (CAS No. 79-01-6) in Four Strains of Rats (ACI, August, Marshall, Osborne-Mendel) (Gavage Studies) [PubMed] [Google Scholar]
- 13.NTP. Technical Report Series. NTP; Research Triangle Park, NC, USA: 1990. Carcinogenesis Studies of Trichloroethylene (without Epichlorohydrin) in F344/N Rats and B6C3F1 Mice (Gavage Studies) [PubMed] [Google Scholar]
- 14.Alexander D.D., Kelsh M.A., Mink P.J., Mandel J.H., Basu R., Weingart M. A meta-analysis of occupational trichloroethylene exposure and liver cancer. Int. Arch. Occup. Environ. Health. 2007;81:127–143. doi: 10.1007/s00420-007-0201-4. [DOI] [PubMed] [Google Scholar]
- 15.Alexander D.D., Mink P.J., Mandel J.H., Kelsh M.A. A meta-analysis of occupational trichloroethylene exposure and multiple myeloma or leukaemia. Occup. Med. (Lond.) 2006;56:485–493. doi: 10.1093/occmed/kql083. [DOI] [PubMed] [Google Scholar]
- 16.Kelsh M.A., Alexander D.D., Mink P.J., Mandel J.H. Occupational trichloroethylene exposure and kidney cancer: A meta-analysis. Epidemiology. 2010;21:95–102. doi: 10.1097/EDE.0b013e3181c30e92. [DOI] [PubMed] [Google Scholar]
- 17.Kelsh M.A., Weingart M., Mandel J.H., Mink P., Alexander D., Basu R., Kalmes R., Goodman M. A meta-analysis of epidemiology studies of occupational TCE exposure and selected cancers. Presented at the Third Meeting on Assessing Human Health Risks of Trichloroethylene; Irvine, CA, USA. 2005. [Google Scholar]
- 18.Mandel J.H., Kelsh M.A., Mink P.J., Alexander D.D., Kalmes R.M., Weingart M., Yost L., Goodman M. Occupational trichloroethylene exposure and non-Hodgkin’s lymphoma: A meta-analysis and review. Occup. Environ. Med. 2006;63:597–607. doi: 10.1136/oem.2005.022418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wartenberg D., Reyner D., Scott C.S. Trichloroethylene and cancer: Epidemiologic evidence. Environ. Health Perspect. 2000;108(Suppl 2):161–176. doi: 10.1289/ehp.00108s2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miligi L., Costantini A.S., Benvenuti A., Kriebel D., Bolejack V., Tumino R., Ramazzotti V., Rodella S., Stagnaro E., Crosignani P., et al. Occupational exposure to solvents and the risk of lymphomas. Epidemiology. 2006;17:552–561. doi: 10.1097/01.ede.0000231279.30988.4d. [DOI] [PubMed] [Google Scholar]
- 21.Cocco P., t’Mannetje A., Fadda D., Melis M., Becker N., de Sanjose S., Foretova L., Mareckova J., Staines A., Kleefeld S., et al. Occupational exposure to solvents and risk of lymphoma subtypes: Results from the Epilymph case-control study. Occup. Environ. Med. 2010;67:341–347. doi: 10.1136/oem.2009.046839. [DOI] [PubMed] [Google Scholar]
- 22.Purdue M.P., Bakke B., Stewart P., De Roos A.J., Schenk M., Lynch C.F., Bernstein L., Morton L.M., Cerhan J.R., Severson R.K., et al. A case-control study of occupational exposure to trichloroethylene and non-Hodgkin lymphoma. Environ. Health Perspect. 2011;119:232–238. doi: 10.1289/ehp.1002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang R., Zhang Y., Lan Q., Holford T.R., Leaderer B., Zahm S.H., Boyle P., Dosemeci M., Rothman N., Zhu Y., et al. Occupational exposure to solvents and risk of non-Hodgkin lymphoma in Connecticut women. Am. J. Epidemiol. 2009;169:176–185. doi: 10.1093/aje/kwn300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore L.E., Boffetta P., Karami S., Brennan P., Stewart P.S., Hung R., Zaridze D., Matveev V., Janout V., Kollarova H., et al. Occupational trichloroethylene exposure and renal carcinoma risk: Evidence of genetic susceptibility by reductive metabolism gene variants. Cancer Res. 2010;70:6527–6536. doi: 10.1158/0008-5472.CAN-09-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y., Krishnadasan A., Kennedy N., Morgenstern H., Ritz B. Estimated effects of solvents and mineral oils on cancer incidence and mortality in a cohort of aerospace workers. Am. J. Ind. Med. 2005;48:249–258. doi: 10.1002/ajim.20216. [DOI] [PubMed] [Google Scholar]
- 26.Boice J.D., Jr, Marano D.E., Cohen S.S., Mumma M.T., Blot W.J., Brill A.B., Fryzek J.P., Henderson B.E., McLaughlin J.K. Mortality among Rocketdyne workers who tested rocket engines, 1948–1999. J. Occup. Environ. Med. 2006;48:1070–1092. doi: 10.1097/01.jom.0000240661.33413.b5. [DOI] [PubMed] [Google Scholar]
- 27.Radican L., Blair A., Stewart P., Wartenberg D. Mortality of aircraft maintenance workers exposed to trichloroethylene and other hydrocarbons and chemicals: Extended follow-up. J. Occup. Environ. Med. 2008;50:1306–1319. doi: 10.1097/JOM.0b013e3181845f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Institute of Medicine (IOM) Gulf War and Health. Insecticides and Solvents. Vol. 2 The National Academies Press; Washington, DC, USA: 2003. [Google Scholar]
- 29.Altekruse S.F., Kosary C.L., Krapcho M., Neyman N., Aminou R., Waldron W., Ruhl J., Howlader N., Tatalovich Z., Cho H., et al. Surveillance, Epidemiology, End Results (SEER) Cancer Statistics Review, 1975–2007. National Cancer Institute; Bethesda, MD, USA: 2010. [accessed on 30 March 2011]. Available online: http://seer.cancer.gov/csr/1975_2007/ [Google Scholar]
- 30.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 31.Rothman K.J., Greenland S. Modern Epidemiology. 2nd Edition. Lippincott Williams & Wilkins; Philedelphia, PA, USA: 1998. [Google Scholar]
- 32.Breslow N.E., Day N.E. Statistical Methods in Cancer Research. Volume II—The Design and Analysis of Cohort Studies. International Agency for Research on Cancer; Lyon, France: 1987. [PubMed] [Google Scholar]
- 33.Woolf B. On estimating the relation between blood group and disease. Ann. Hum. Genet. 1955;19:251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 34.Breslow N.E., Day N.E. Statistical Methods in Cancer Research. Volume I—The Analysis of Case-Control Studies. International Agency for Research on Cancer; Lyon, France: 1980. [PubMed] [Google Scholar]
- 35.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duval S., Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 37.Axelson O., Selden A., Andersson K., Hogstedt C. Updated and expanded Swedish cohort study on trichloroethylene and cancer risk. J. Occup. Med. 1994;36:556–562. [PubMed] [Google Scholar]
- 38.Anttila A., Pukkala E., Sallmen M., Hernberg S., Hemminki K. Cancer incidence among Finnish workers exposed to halogenated hydrocarbons. J. Occup. Environ. Med. 1995;37:797–806. doi: 10.1097/00043764-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Hansen J., Raaschou-Nielsen O., Christensen J.M., Johansen I., McLaughlin J.K., Lipworth L., Blot W.J., Olsen J.H. Cancer incidence among Danish workers exposed to trichloroethylene. J. Occup. Environ. Med. 2001;43:133–139. doi: 10.1097/00043764-200102000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Raaschou-Nielsen O., Hansen J., McLaughlin J.K., Kolstad H., Christensen J.M., Tarone R.E., Olsen J.H. Cancer risk among workers at Danish companies using trichloroethylene: A cohort study. Am. J. Epidemiol. 2003;158:1182–1192. doi: 10.1093/aje/kwg282. [DOI] [PubMed] [Google Scholar]
- 41.Boice J.D., Jr, Marano D.E., Fryzek J.P., Sadler C.J., McLaughlin J.K. Mortality among aircraft manufacturing workers. Occup. Environ. Med. 1999;56:581–597. doi: 10.1136/oem.56.9.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenland S., Salvan A., Wegman D.H., Hallock M.F., Smith T.J. A case-control study of cancer mortality at a transformer-assembly facility. Int. Arch. Occup. Environ. Health. 1994;66:49–54. doi: 10.1007/BF00386579. [DOI] [PubMed] [Google Scholar]
- 43.Morgan R.W., Kelsh M.A., Zhao K., Heringer S. Mortality of aerospace workers exposed to trichloroethylene. Epidemiology. 1998;9:424–431. [PubMed] [Google Scholar]
- 44.Blair A., Hartge P., Stewart P.A., McAdams M., Lubin J. Mortality and cancer incidence of aircraft maintenance workers exposed to trichloroethylene and other organic solvents and chemicals: Extended follow up. Occup. Environ. Med. 1998;55:161–171. doi: 10.1136/oem.55.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothman K.J., Greenland S., Lash T.L. Modern Epidemiology. 3rd Edition. Wolters Kluwer, Lippincott Williams and Wilkins; Philadelphia, PA, USA: 2008. [Google Scholar]
- 46.Hardell L., Eriksson M., Degerman A. Exposure to phenoxyacetic acids, chlorophenols, or organic solvents in relation to histopathology, stage, and anatomical localization of non-Hodgkin’s lymphoma. Cancer Res. 1994;54:2386–2389. [PubMed] [Google Scholar]
- 47.Nordstrom M., Hardell L., Magnuson A., Hagberg H., Rask-Andersen A. Occupational exposures, animal exposure and smoking as risk factors for hairy cell leukaemia evaluated in a case-control study. Br. J. Cancer. 1998;77:2048–2052. doi: 10.1038/bjc.1998.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Persson B., Fredrikson M. Some risk factors for non-Hodgkin’s lymphoma. Int .J. Occup. Med. Environ. Health. 1999;12:135–142. [PubMed] [Google Scholar]
- 49.Bruning T., Pesch B., Wiesenhutter B., Rabstein S., Lammert M., Baumuller A., Bolt H.M. Renal cell cancer risk and occupational exposure to trichloroethylene: Results of a consecutive case-control study in Arnsberg, Germany. Am. J. Ind. Med. 2003;43:274–285. doi: 10.1002/ajim.10185. [DOI] [PubMed] [Google Scholar]
- 50.Charbotel B., Fevotte J., Hours M., Martin J.L., Bergeret A. Case-control study on renal cell cancer and occupational exposure to trichloroethylene. Part II: Epidemiological aspects. Ann. Occup. Hyg. 2006;50:777–787. doi: 10.1093/annhyg/mel039. [DOI] [PubMed] [Google Scholar]
- 51.Dosemeci M., Cocco P., Chow W.H. Gender differences in risk of renal cell carcinoma and occupational exposures to chlorinated aliphatic hydrocarbons. Am. J. Ind. Med. 1999;36:54–59. doi: 10.1002/(sici)1097-0274(199907)36:1<54::aid-ajim8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 52.Pesch B., Haerting J., Ranft U., Klimpel A., Oelschlagel B., Schill W. Occupational risk factors for renal cell carcinoma: Agent-specific results from a case-control study in Germany. MURC study group. Multicenter urothelial and renal cancer study. Int. J. Epidemiol. 2000;29:1014–1024. doi: 10.1093/ije/29.6.1014. [DOI] [PubMed] [Google Scholar]
- 53.Siemiatycki J. Risk Factors for Cancer in the Workplace. CRC Press; Boca Raton, FL, USA: 1991. [Google Scholar]
- 54.Ikeda M., Otsuji H., Imamura T., Komoike Y. Urinary excretion of total trichloro-compounds, trichloroethanol, and trichloroacetic acid as a measure of exposure to trichloroethylene and tetrachloroethylene. Br. J. Ind. Med. 1972;29:328–333. doi: 10.1136/oem.29.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pannett B., Coggon D., Acheson E.D. A job-exposure matrix for use in population based studies in England and Wales. Br. J. Ind. Med. 1985;42:777–783. doi: 10.1136/oem.42.11.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomez M.R., Cocco P., Dosemeci M., Stewart P.A. Occupational exposure to chlorinated aliphatic hydrocarbons: Job exposure matrix. Am. J. Ind. Med. 1994;26:171–183. doi: 10.1002/ajim.4700260204. [DOI] [PubMed] [Google Scholar]
- 57.Morgan R.W., Kelsh M.A., Zhao K., Heringer S. Letter from Paul A. Cammer, Ph.D., Trichloroethylene Issues Group, to Cheryl Siegel Scott, US EPA, ID 645806. US EPA, Health and Environmental Reserach Online (HERO); Durham, NC, USA: 1997. [accessed on 30 August 2011]. Final Report “Cohort Mortality Study of Aerospace Workers with Exposure to Trichloroethylene.”. Available online: http://hero.epa.gov/index.cfm?heroid=645806&action=search.do&submit=Search&portal=true&sort=year&all=&exact=&any=&author=&journal=&singleyear=&startyear=&endyear= [Google Scholar]
- 58.Bruning T., Weirich G., Hornauer M.A., Hofler H., Brauch H. Renal cell carcinomas in trichloroethene (TRI) exposed persons are associated with somatic mutations in the von Hippel-Lindau (VHL) tumour suppressor gene. Arch. Toxicol. 1997;71:332–335. doi: 10.1007/s002040050394. [DOI] [PubMed] [Google Scholar]
- 59.Pesch B. Correspondence from Beate Pesch, Ph.D., BGFA—Research Institute of Occupational Medicine to Cheryl Siegel Scott, US EPA, ID 783486. US EPA, Health and Environmental Reserach Online (HERO); Durham, NC, USA: 2008. [accessed on 30 August 2011]. Available online: http://hero.epa.gov/index.cfm?heroid=783486&action=search.do&submit=Search&portal=true&sort=year&all=&exact=&any=&author=&journal=&singleyear=&startyear=&endyear= [Google Scholar]
- 60.Environmental Protection Agency (US EPA) Toxicological Review of Trichloroethylene (CAS No. 79-01-6) in Support of Summary Information on the Integrated Risk Information System (IRIS), EPA/635/R-09/011F. EPA; Washington, DC, USA: 2011. [Google Scholar]
- 61.Vlaanderen J., Portengen L., Rothman N., Lan Q., Kromhout H., Vermeulen R. Flexible meta-regression to assess the shape of the benzene-leukemia exposure-response curve. Environ. Health Perspect. 2010;118:526–532. doi: 10.1289/ehp.0901127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henschler D., Vamvakas S., Lammert M., Dekant W., Kraus B., Thomas B., Ulm K. Increased incidence of renal cell tumors in a cohort of cardboard workers exposed to trichloroethene. Arch. Toxicol. 1995;69:291–299. doi: 10.1007/s002040050173. [DOI] [PubMed] [Google Scholar]
- 63.Vamvakas S., Bruning T., Thomasson B., Lammert M., Baumuller A., Bolt H.M., Dekant W., Birner G., Henschler D., Ulm K. Renal cell cancer correlated with occupational exposure to trichloroethene. J. Cancer Res. Clin. Oncol. 1998;124:374–382. doi: 10.1007/s004320050186. [DOI] [PubMed] [Google Scholar]
- 64.Steinmaus C., Smith A.H., Jones R.M., Smith M.T. Meta-analysis of benzene exposure and non-Hodgkin lymphoma: Biases could mask an important association. Occup. Environ. Med. 2008;65:371–378. doi: 10.1136/oem.2007.036913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vlaanderen J., Lan Q., Kromhout H., Rothman N., Vermeulen R. Occupational benzene exposure and the risk of lymphoma subtypes: A meta-analysis of cohort studies incorporating three study quality dimensions. Environ. Health Perspect. 2011;119:159–167. doi: 10.1289/ehp.1002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang L., Steinmaus C., Eastmond D.A., Xin X.K., Smith M.T. Formaldehyde exposure and leukemia: A new meta-analysis and potential mechanisms. Mutat. Res. 2009;681:150–168. doi: 10.1016/j.mrrev.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 67.Vardiman J.W. The World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues: An overview with emphasis on the myeloid neoplasms. Chem. Biol. Interact. 2010;184:16–20. doi: 10.1016/j.cbi.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 68.Chow W.H., Dong L.M., Devesa S.S. Epidemiology and risk factors for kidney cancer. Nat. Rev. Urol. 2010;7:245–257. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.IARC. Tobacco Smoke and Involuntary Smoking. Vol. 83 International Agency for Research on Cancer; Lyon, France: 2004. [Google Scholar]
- 70.Charbotel B., Fevotte J., Martin J.L., Bergeret A. Renal cell carcinoma and exposure to trichloroethylene: Are the French limits of occupational exposure relevant? Rev. Epidemiol. Sante Publique. 2009;57:41–47. doi: 10.1016/j.respe.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 71.Karami S., Boffetta P., Brennan P., Stewart P.A., Zaridze D., Matveev V., Janout V., Kollarova H., Bencko V., Navratilova M., et al. Renal cancer risk and occupational exposure to polycyclic aromatic hydrocarbons and plastics. J. Occup. Environ. Med. 2011;53:218–223. doi: 10.1097/JOM.0b013e31820a40a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Calvert G.M., Ward E., Schnorr T.M., Fine L.J. Cancer risks among workers exposed to metalworking fluids: A systematic review. Am. J. Ind. Med. 1998;33:282–292. doi: 10.1002/(sici)1097-0274(199803)33:3<282::aid-ajim10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 73.Mirer F.E. New evidence on the health hazards and control of metalworking fluids since completion of the OSHA advisory committee report. Am. J. Ind. Med. 2010;53:792–801. doi: 10.1002/ajim.20853. [DOI] [PubMed] [Google Scholar]
- 74.Venook A.P., Papandreou C., Furuse J., de Guevara L.L. The incidence and epidemiology of hepatocellular carcinoma: A global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 75.El-Serag H.B. Epidemiology of hepatocellular carcinoma in USA. Hepatol. Res. 2007;37(Suppl 2):S88–S94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 76.Alexander D.D., Mink P.J., Adami H.O., Chang E.T., Cole P., Mandel J.S., Trichopoulos D. The non-Hodgkin lymphomas: A review of the epidemiologic literature. Int. J. Cancer. 2007;120(Suppl 12):1–39. doi: 10.1002/ijc.22719. [DOI] [PubMed] [Google Scholar]
- 77.Morton L.M., Hartge P., Holford T.R., Holly E.A., Chiu B.C., Vineis P., Stagnaro E., Willett E.V., Franceschi S., La Vecchia C., et al. Cigarette smoking and risk of non-Hodgkin lymphoma: A pooled analysis from the International Lymphoma Epidemiology Consortium (INTERLYMPH) Cancer Epidemiol. Biomarkers Prev. 2005;14:925–933. doi: 10.1158/1055-9965.EPI-04-0693. [DOI] [PubMed] [Google Scholar]
- 78.Lan Q., Zhang L., Tang X., Shen M., Smith M.T., Qiu C., Ge Y., Ji Z., Xiong J., He J., et al. Occupational exposure to trichloroethylene is associated with a decline in lymphocyte subsets and soluble CD27 and CD30 markers. Carcinogenesis. 2010;31:1592–1596. doi: 10.1093/carcin/bgq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.