Abstract
Binge drinking of alcoholic beverages among adolescents is a common practice that can have serious health consequences. Alcohol is a potent immunomodulator that alters a wide range of immune responses. However, it is unclear whether there is a differential immune response to alcoholic beverages with a high versus low concentration of ethanol. In this study, we used a PCR array containing 46 primer pairs of selected genes to compare mRNA expression in the spleen, an immune system organ, of adolescent rats following binge drinking of alcohol solutions containing either 20% or 52% ethanol (v/v, 4.8 g/kg daily dosage), or water (control) for 3 d. We found that, expression of IL-1β, IL-6, CCL2, and GABAA receptor α2 subunit in the spleen were decreased, and mGluR5 and 5-HT3A receptor expression were increased after administration of an ethanol solution containing 52% ethanol, but not one with 20% ethanol. Our data suggest that alcohol-mediated immunomodulatory effects are, in part, dependent on the ethanol by volume concentration. This is the first study to show that exposure to a high ethanol percentage beverage can have more profound effects on immune responses than one with a low percentage of ethanol.
Keywords: Binge drinking, ethanol, adolescent rat spleen, PCR array
Introduction
Binge drinking is the pattern of alcohol consumption that is of greatest concern from a public health perspective [1], particularly because binge drinking appears to be common among adolescents [2]. Binge drinking often has a harmful societal impact, including violence and vehicle accidents. The National Survey on Drug Use and Health shows that 1.6% of 12-13 year olds, 7.0% of 14-15 year olds, 17.0% of 16-17 year olds, and 34.7% of 18-20 year old individuals engaged in binge alcohol drinking in 2009 [3].
Ethanol, has been shown to be a potent immunomodulator [4]. Heavy alcohol consumption can suppress a wide range of immune responses, including the inflammatory response to pathogen-derived signals [5]. The effects of alcohol on innate immunity are complicated. There is ample evidence that the ethanolmediated immunomodulatory effects depend on many factors, including the pattern of drinking, the amount of ethanol consumed, and the age of the drinker [6]. Binge drinking, in particular, has been reported to suppress cytokine responses mediated through toll-like receptors (TLRs), which play a key role in innate immunity [7].
The hypothalamic supraoptic nucleus (SON) is involved in regulating the hypothalamic-pituitary -adrenal (HPA) axis. The SON is responsible for maintaining plasma osmolality by producing vasopressin [8], and it partially mediates the responses of the HPA axis to ethanol [8-10]. Using the immunocytochemical staining of the FOS proto-oncogene protein as a marker of neuronal activation, we previously reported that FOS immunoreactivity (FOSir) is induced in the SON by alcohol solutions containing 48%, 64%, and 80% ethanol (w/v), but not by solutions containing 16% or 32% ethanol [11].
The HPA axis has been shown to regulate the bidirectional communication between the nervous and immune systems [12]. Thus, we hypothesized that exposure to alcohol solutions with a high versus low ethanol concentration will cause differential immune responses. To test our hypothesis, we created a PCR array containing 46 primer pairs of selected genes to evaluate the differential effects of binge drinking of alcohol solutions containing a high concentration of ethanol (52%, v/v) versus a low ethanol concentration (20%, v/v) on gene expression in the spleen of adolescent rats. We examined the spleen because it is one of the largest lymphoid organs in mammals, and contains important cells involved in immune responses [13]. We found that, in the spleen, binge drinking of alcohol solutions with a high ethanol content, but not a low ethanol content, modulates the expression of genes involved in innate immunity.
Materials and methods
Animals
Adolescent (post-natal day 25) male Fischer 344 rats (Harlan, Indianapolis, IN, USA) were group-housed immediately upon arrival, and stayed in group cages during the experiment. The animals were maintained in a controlled temperature (21-22 °C) environment and a 12-h light/12-h dark illumination cycle, with lights on at 7:00 AM. Food and tap water were provided ad libitum. This research was approved by the Institutional Animal Care and Use Committee (IACUC) at Seton Hall University, South Orange, NJ, USA.
Ethanol administration
The rats were randomly assigned to one of three groups (n = 4 per group): 0% ethanol, 20% ethanol, or 52% ethanol. Prior to the initiation of the ethanol treatment, the animals were conditioned to the gavage (i.g.) procedure for 3 d to minimize the potential stress due to the procedure. An ethanol dose of 2.4 g/kg body weight was administered by i.g. twice a day (8:00 AM and 10:00 AM) for 3 d (total daily = 4.8 g/kg). The i.g. procedure and ethanol doses were modified from Ward et al. [14]. The volume/ body weight was different for the 0%, 20%, and 52% ethanol solutions. For 20% ethanol, 10.53 mL of 190 proof alcohol (Pharmco, Brookfield, CT) was diluted to 50 mL in distilled water; for 52% ethanol, 27.37 mL of 190 proof alcohol was diluted to 50 mL in distilled water. The control (0%) animal received water in a volume that was equal to a 36% ethanol solution as per a previous study [15]. By the end of alcohol treatment, the animals were at post-natal day 35. Two hours after the last i.g. administration of alcohol, the rats were sacrificed by rapid decapitation, the trunk blood was collected for serum preparation, and the spleens were collected, frozen on dry ice, and stored at −80°C.
Corticosterone radioimmunoassay
The rats were conditioned for 3 d prior to the study to eliminate the possible effects of corticosterone, produced as a stress response to the i.g. procedure, on gene expression in the spleen. Serum was prepared from trunk blood 30 min following blood collection and stored at -80°C until analyzed by the corticosterone RIA assay as per the manufacturer's protocol (MP Biomedicals, Orangeburg, NY). A one-way ANOVA, followed by a Newman-Kuels post hoc test, was used to determine if the serum corticosterone levels (mean ± SEM) between the animals given ethanol concentrations of 0%, 20%, or 52% were statistically different.
RNA isolation and purification
A 100 mg aliquot of each spleen was used to isolate total RNA using the TRIzol® protocol (Invitrogen, Carlsbad, CA, USA). The total RNA was further purified using an RNeasy® mini kit (Qiagen, Valencia, CA, USA).
PCR array and data analysis
Based on the existing literature, 46 genes reportedly regulated by ethanol were chosen and custom made by SABioscience Corporation (Frederick, MD, USA). The PCR array was conducted using an RT2 Custom Profiler™ PCR Array (Catalog #CAPR10026, SABiosciences, Frederick, MD, USA) according to the manufacturer's instructions. The threshold and baseline were set manually according to the manufacturer's instructions. The resulting threshold cycle values (CT) were transferred into the data analysis template and uploaded onto the manufacturer's website (http://www.sabiosciences.com/per/arrayanalysis.php). The mRNA expression level of each gene was normalized using the expression of two housekeeping genes (beta tubulin and beta actin) as controls. The relative expression of each gene was compared with the expression in the control group and calculated on the website using the ΔΔCT method. Each reported value represented the mean increase (or decrease) of mRNA expression relative to the control levels for four biological replicates. A P-value of ≤0.05 and a fold change in gene expression of ≥2 were taken as significant.
Results
Serum corticosterone levels
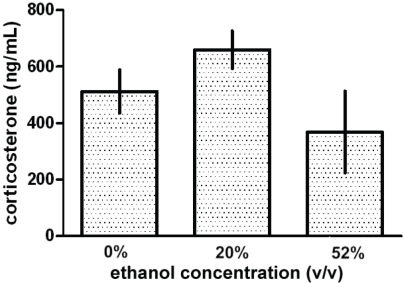
Serum corticosterone levels in the trunk blood of the experimental animals given ethanol concentrations of 0%, 20%, or 52% were examined. The serum corticosterone levels (mean ± SEM) in the animals given 0% (512 ± 76.78 ng/mL), 20% (659.7 ± 66.29 ng/mL), or 52% (368.5 ± 144.8 ng/mL) ethanol were found not to be statistically different (Figure 1).
Figure 1.
Effects of gavage alcohol administration on serum corticosterone levels in adolescent rats. Adolescent rats were administered water (0%), or alcohol solutions containing 20% or 52% ethanol (v/v) by a gavage (i.g.) procedure for 3 d. Serum corticosterone levels (ng/mL) were then determined by radioimmunoassay. The serum levels of corticosterone from the 20% and 52% ethanol groups were not statistically different from the control (0%) group. Data are represented as the mean ± SEM (n = 4).
Ethanol modulation of gene expression in the rat spleen
Treatment with a 20% ethanol solution did not significantly change the expression of the 46 genes examined when compared to the 0% ethanol control treatment (Table 1). Treatment with a 52% ethanol solution significantly decreased the expression of IL-1β, IL-6, chemokine (C-C motif) ligand 2 (CCL2, MCP-1), and gamma-aminobutyric acid A receptor alpha 2 (GABAA receptor α2 subunit), but increased the expression of metabotropic glutamate receptor 5 (mGluR5) and 5-hydroxytryptamine (serotonin) receptor 3a (5-HT3A) when compared to the 0% ethanol control treatment. The fold change and p-value of these genes are noted in bold in Table 1.
Table 1.
Effect of high ethanol (52%, v/v) versus low ethanol (20%, v/v) concentration binge drinking on gene expression in the spleen of adolescent rats.
| Gene | NCBI Reference Sequence | 20% vs 0% Ethanol | 52% vs 0% Ethanol | ||
|---|---|---|---|---|---|
| Fold Change | p-value | Fold Change | p-value | ||
| IL-4 | NM_201270 | −1.296 | 0.515 | 1.141 | 0.893 |
| IL-10 | NM_012854 | 1.180 | 0.230 | 1.291 | 0.072 |
| TNF-α | NM_012675 | 1.010 | 0.948 | 1.113 | 0.603 |
| IL-1β | NM_031512 | −1.169 | 0.400 | −2.125 | 0.002 |
| IL-2 | NM_053836 | 1.980 | 0.055 | 1.099 | 0.941 |
| IL-6 | NM_012589 | −1.083 | 0.683 | −2.629 | 0.007 |
| CCR5 | NM_053960 | −1.497 | 0.013 | −1.915 | 0.003 |
| TLR2 | NM_198769 | −1.069 | 0.652 | −1.444 | 0.040 |
| TLR4 | NM_019178 | −1.154 | 0.182 | −1.098 | 0.240 |
| NF-κB p65 | NM_199267 | 1.040 | 0.817 | −1.115 | 0.438 |
| Dopamine receptor D1A | NM_012546 | −2.713 | 0.261 | 1.283 | 0.813 |
| Dopamine receptor D2 | NM_012547 | −2.000 | 0.158 | −1.200 | 0.373 |
| Dopamine receptor D3 | NM_017140 | −1.001 | 0.904 | 1.333 | 0.188 |
| Dopamine receptor D4 | NM_012944 | −1.088 | 0.412 | 1.087 | 0.979 |
| Dopamine receptor D5 | NM_012768 | −1.025 | 0.972 | 1.302 | 0.210 |
| PDGF-B | XM_343293 | −1.221 | 0.266 | −1.151 | 0.234 |
| CCL2 | NM_031530 | −1.969 | 0.036 | −3.180 | 0.006 |
| mGluR1 | NM_017011 | −1.079 | 0.838 | 1.500 | 0.072 |
| mGluR2 | XM_343470 | 1.077 | 0.644 | 1.333 | 0.188 |
| mGluR5 | NM_017012 | 1.443 | 0.115 | 2.096 | 0.013 |
| 5-HT3A receptor | NM_024394 | 1.454 | 0.195 | 2.275 | 0.033 |
| nAChR β2 | NM_019297 | 1.042 | 0.670 | 1.568 | 0.559 |
| GABA A receptor, α 1 | NM_183326 | −1.155 | 0.956 | −1.243 | 0.437 |
| GABA A receptor, α 2 | XM_223378 | −1.354 | 0.630 | −2.148 | 0.031 |
| GABA A receptor, α 3 | NM_017069 | 1.939 | 0.033 | −1.167 | 0.613 |
| GABA A receptor, α 4 | NM_080587 | −1.423 | 0.190 | −1.148 | 0.352 |
| GABA A receptor, α 5 | NM_017295 | −1.120 | 0.673 | 1.333 | 0.194 |
| GABA A receptor, α 6 | NM_021841 | −1.001 | 0.904 | 1.333 | 0.188 |
| Dopamine transporter | NM_012694 | −1.094 | 0.615 | 1.370 | 0.498 |
| Tyrosine hydroxylase | NM_012740 | −1.001 | 0.904 | 1.904 | 0.049 |
| GAD67 | NM_O17OO7 | −1.278 | 0.794 | −1.763 | 0.133 |
| GAD65 | NM_012563 | −1.342 | 0.378 | 1.217 | 0.372 |
| Choline transporter | NM_053521 | −1.283 | 0.319 | 1.151 | 0.820 |
| Alcohol dehydrogenase 1 | NM_019286 | 1.078 | 0.682 | −1.010 | 0.671 |
| Alcohol dehydrogenase 4 | NM_017270 | 1.373 | 0.394 | −1.249 | 0.663 |
| Aldehyde dehydrogenase 2 | NM_032416 | 1.070 | 0.573 | −1.160 | 0.359 |
| Cytochrome P450 2E | NM_031543 | 1.759 | 0.788 | −1.196 | 0.432 |
| Adenylate cyclase 5 | NM_022600 | 1.045 | 0.694 | 1.085 | 0.456 |
| CREB1 | NM_031017 | 1.097 | 0.572 | 1.068 | 0.696 |
| CREB binding protein | NM_133381 | −1.238 | 0.085 | −1.305 | 0.012 |
| MAPK8 | XM_341399 | 1.030 | 0.760 | −1.072 | 0.619 |
| PKCα | XM_343975 | −1.001 | 0.955 | −1.032 | 0.894 |
| Caspase 3 | NM_012922 | −1.021 | 0.748 | −1.190 | 0.180 |
| CXCL1 | NM_030845 | 1.013 | 0.741 | 1.335 | 0.153 |
| BDNF | NM_012513 | 1.238 | 0.349 | 1.131 | 0.494 |
| SNAP25 | NM_030991 | 1.119 | 0.482 | 1.236 | 0.305 |
Discussion
Alcohol is a potent immunomodulator, and alcohol consumption alters a wide range of immune responses. However, it is unclear whether alcohol-mediated changes in immune responses correlate with the relative concentration of ethanol in the alcohol consumed. To address this question, we designed a PCR array containing the primer pairs of 46 genes, the expression of which is regulated by alcohol, in order to examine gene expression in the spleen of adolescent rats following binge drinking with alcohol solutions containing 20% or 52% ethanol (v/v) for 3 d compared to water (0%) consumption. The protein products of these 46 genes include cyto-kines, chemokines, and other signaling molecules involved in immune responses. Because the nervous and immune systems share many signaling and messenger molecules, we included several neurotransmitters and related molecules in our PCR array.
We had some concerns that the i.g. administration of alcohol would induce stress in the animals. The conditioning procedure conducted for 3 d prior to the study was designed to limit the stress related to the i.g. procedure. Our RIA data showed that the serum levels of corticosterone for the three treatment groups were close to the previously reported baseline corticosterone levels (50 to 400 ng/mL) in rats [16]. Further, the serum levels of corticosterone were not different in the animals given 20% ethanol or 52% ethanol. Thus, the possible effects of corticosterone were not a determining factor on gene expression in the spleen.
Our data showed that gene expression in the spleen of adolescent rats administered with a 20% ethanol solution was not significantly different from that in animals receiving water. However, IL-1β, IL-6, and CCL2 (MCP-1) expression were significantly decreased in the animals administered an alcohol solution with 52% ethanol. Using blood monocytes as an in vitro cell model, Szabo et al. [17,18,19] showed that treatment with alcohol inhibits induction of proinflammatory mediators, including IL-1β, IL-6, and the chemokine, MCP-1 [5, 17-19]. Our data indicate that down-regulation of proinflammatory cytokines and chemokines following binge drinking of alcohol with 52% ethanol could be due to ethanol's direct effects on immune cells.
The amount of ethanol in the alcohol solutions given to the animals was 4.8 g/kg for both the 20% or 52% ethanol concentrations. Thus, a direct mechanism alone cannot explain why a similar decrease in IL-1β, IL-6, and CCL2 (MCP-1) mRNA levels was not seen in the spleen of animals given binge alcohol with 20% ethanol. One possible explanation is that there may be a difference in the absorption rate of alcohol at various ethanol concentrations, resulting in a difference in distribution, elimination, and effects of alcohol in the body. In fact, we found that 52% ethanol can lead to a higher blood ethanol concentration at 90 min after the last ethanol administration than an equal dose of 20% ethanol (data not shown). Another explanation is that intake of alcohol with different ethanol concentrations could lead to stimulation and activation of different cell types and tissues.
Adolescence is the period during which a number of crucial neuroendocrine changes occur, and alcohol can interfere with many of these processes, including the functions of the HPA axis [20]. We previously reported that neuronal activation in the SON by alcohol only takes place with alcohol solutions containing a high ethanol concentration [11]. The SON is involved in regulating the responsiveness of the HPA axis. In particular, the SON is responsible for maintaining plasma osmolality by producing vasopressin, which partially mediates the responses of the HPA axis to ethanol [8-10]. Our present study showed that binge drinking with alcohol with 52% ethanol, but not 20% ethanol, down-regulated GABAA receptor α2 subunit gene expression, but up-regulated mGluR5 and 5-HT3A receptor expression. The regulation of gene expression of neurotransmitter receptors in the spleen further indicates that there is a close inter-relationship between the nervous and immune systems. Taken together, our data suggest that binge drinking with alcohol containing a high ethanol concentration can indirectly affect immune responses by stimulating neuronal activation, leading to alterations in the brain-immune axis.
The HPA axis has been proposed to play a role in changes in immune cell function [21]. We know that cross-talk does exist between the immune system and the central nervous system [22]. It has been suggested that the activation of the HPA axis elicits powerful antiinflammatory actions, inhibiting inflammatory mediators, such as cytokines, via glucocorticoid production. Although our RIA determination of corticosterone levels, the final product of the HPA axis, after alcohol treatment indicated that any possible effect of corticosterone is limited, the effects of other hormones that originate in the HPA axis, including CRH, ACTH, growth hormone, prolactin, FSH, LH, and TSH, on gene expression in the spleen cannot be excluded [12].
In summary, our results indicate that binge drinking of alcohol with a high ethanol concentration can have more profound effects on immune responses than alcohol with a lower ethanol concentration. The molecular and cellular mechanisms underlying this differential alcohol regulation of gene expression in the spleen are complex, and may be due to ethanol's direct effects on the spleen as well as indirect effects on neuronal activation in the brain. Considering that hard liquor, which has a high ethanol concentration, is the preferred (43.8%) alcoholic beverage among high school youth [23], this finding is of practical significance and can have a high impact on the way researchers view and study the effects of exposure to alcohol.
Acknowledgments
This work was supported, in part, by K02 DA016149 from the National Institute on Drug Abuse and by RC2 AA019415 from the National Institute on Alcohol Abuse and Alcoholism to SL Chang. The authors also thank Drs. Chuang Liu and Erik Langsdorf for animal treatment and tissue collection, and corticosterone radioimmunoassay, respectively.
References
- 1.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Bethesda, MD: National Institute on Drug Abuse; 2010. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2009 (NIH Publication No. 10-7583) [Google Scholar]
- 2.Nelson DE, Naimi TS, Brewer RD, Nelson HA. State alcohol-use estimates among youth and adults, 1993-2005. Am J Prev Med. 2009;36:218–224. doi: 10.1016/j.amepre.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 3.SAMHSA . Rockville, MD: 2010. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings (Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10-4586Findings) [Google Scholar]
- 4.Romeo J, Wärnberg J, Marcos A. Drinking pattern and socio-cultural aspects on immune response: an overview. Proc Nutr Soc. 2010;69:341–346. doi: 10.1017/S0029665110001904. [DOI] [PubMed] [Google Scholar]
- 5.Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 2009;33:220–232. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goral J, Karavitis J, Kovacs EJ. Exposuredependent effects of ethanol on the innate immune system. Alcohol. 2008;42:237–247. doi: 10.1016/j.alcohol.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pruett SB, Zheng Q, Fan R, Matthews K, Schwab C. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol. 2004;33:147–155. doi: 10.1016/j.alcohol.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Itoi K, Jiang YQ, Iwasaki Y, Watson SJ. Regulatory mechanisms of corticotropinreleasing hormone and vasopressin gene expression in the hypothalamus. J Neuroendocrinol. 2004;16:348–355. doi: 10.1111/j.0953-8194.2004.01172.x. [DOI] [PubMed] [Google Scholar]
- 9.Slattery DA, Neumann ID. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology. 2010;58:56–61. doi: 10.1016/j.neuropharm.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 10.Ogilvie KM, Lee S, Rivier C. Role of arginine vasopressin and corticotropin-releasing factor in mediating alcohol-induced adrenocorticotropin and vasopressin secretion in male rats bearing lesions of the paraventricular nuclei. Brain Res. 1997;744:83–95. doi: 10.1016/s0006-8993(96)01082-7. [DOI] [PubMed] [Google Scholar]
- 11.Chang SL, Patel NA, Romero AA. Activation and desensitization of Fos immunoreactivity in the rat brain following ethanol administration. Brain Res. 1995;679:89–98. doi: 10.1016/0006-8993(95)00210-h. [DOI] [PubMed] [Google Scholar]
- 12.Petrovsky N. Towards a unified model of neuroendocrine-immune interaction. Immunol Cell Biol. 2OO1;79:350–357. doi: 10.1046/j.1440-1711.2001.01029.x. [DOI] [PubMed] [Google Scholar]
- 13.Thomsen RW, Schoonen WM, Farkas DK, Riis A, Jacobsen J, Fryzek JP, Sorensen HT. Risk for hospital contact with infection in patients with splenectomy: a population-based cohort study. Ann Intern Med. 2009;151:546–555. doi: 10.7326/0003-4819-151-8-200910200-00008. [DOI] [PubMed] [Google Scholar]
- 14.Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, de Witte P, Ballini C, Corte LD, Dexter D. Neuro-inflammation induced in the hippocampus of ‘binge drinking’ rats may be mediated by elevated extracellular glutamate content. J Neurochem. 2009;111:1119–1128. doi: 10.1111/j.1471-4159.2009.06389.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Showalter J, Grigson PS. Ethanolinduced conditioned taste avoidance: reward or aversion? Alcohol Clin Exp Res. 2009;33:522–530. doi: 10.1111/j.1530-0277.2008.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu K, Amagaya S, Ogihara Y. Analysis of corticosterone in the serum of mice and rats using high-performance liquid chromatography. J Chromatogr. 1983;272:170–175. doi: 10.1016/s0378-4347(00)86114-9. [DOI] [PubMed] [Google Scholar]
- 17.Szabo G, Mandrekar P, Girouard L, Catalano D. Regulation of human monocyte functions by acute ethanol treatment: decreased tumor necrosis factor-alpha, interleukin-1 beta and elevated interleukin-10, and transforming growth factor-beta production. Alcohol Clin Exp Res. 1996;20:900–907. doi: 10.1111/j.1530-0277.1996.tb05269.x. [DOI] [PubMed] [Google Scholar]
- 18.Szabo G, Mandrekar P, Catalano D. Inhibition of superantigen-induced T cell proliferation and monocyte IL-1 beta, TNF-alpha, and IL-6 production by acute ethanol treatment. J Leukoc Biol. 1995;58:342–350. doi: 10.1002/jlb.58.3.342. [DOI] [PubMed] [Google Scholar]
- 19.Szabo G, Chavan S, Mandrekar P, Catalano D. Acute alcohol consumption attenuates interleukin-8 (IL-8) and monocyte chemoattractant peptide-1 (MCP-1) induction in response to ex vivo stimulation. J Clin Immunol. 1999;19:67–76. doi: 10.1023/a:1020518703050. [DOI] [PubMed] [Google Scholar]
- 20.Prendergast MA, Little HJ. Adolescence, glucocorticoids and alcohol. Pharmacol Biochem Behav. 2007;86:234–245. doi: 10.1016/j.pbb.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Padgett EL, Sibley DA, Jerrells TR. Effect of adrenalectomy on ethanol-associated changes in lymphocyte cell numbers and subpopulations in thymus, spleen, and gut-associated lymphoid tissues. Int J Immunopharmacol. 2000;22:285–298. doi: 10.1016/s0192-0561(99)00083-1. [DOI] [PubMed] [Google Scholar]
- 22.Gaillard RC. Interactions between the immune and neuroendocrine systems: clinical implications. J Soc Biol. 2003;197:89–95. [PubMed] [Google Scholar]
- 23.Siegel MB, Naimi TS, Cremeens JL, Nelson DE. Alcoholic beverage preferences and associated drinking patterns and risk behaviors among high school youth. Am J Prev Med. 2011;40:419–426. doi: 10.1016/j.amepre.2010.12.011. [DOI] [PubMed] [Google Scholar]