Abstract
Inflammatory breast cancer (IBC) represents the most aggressive form of breast cancer, characterized by rapid progression, involvement of dermal lymphatic emboli and extensive metastatic lymph nodes. Matrix metalloproteinases (MMPs) are proteolytic enzymes that play an important role in cancer invasion and metastasis. Although the role of MMPs in non-IBC is well studied, little is known about its role in IBC. Thus the goal of the present study was to 1) investigate the expression and activity levels of membrane type matrix metalloproteinase-1 (MT1-MMP) and matrix metalloproteinase-2 and-9 (MMP-2 and MMP-9) in IBC versus non-IBC tissue samples and; 2) test correlation between expression of MT1-MMP and pro- and active forms of MMP-2 and MMP-9. We enrolled 51 breast cancer patients, 21 were diagnosed as IBC and 30 as non-IBC. Level of expression of MT1-MMP in carcinoma tissue was assessed by immunoblot and immunohistochemistry techniques. The expression and activation of MMP-2 and MMP-9 was measured by gelatin zymography. Our results revealed that MT1-MMP, pro-MMP-2, pro-MMP-9 and active MMP-2 were more expressed in IBC tissue versus non-IBC. Furthermore, we found that MT1-MMP expression correlates with expression of pro-MMP-2, pro-MMP-9 and active MMP-2 in IBC tissue samples and with MMP-9 in non-IBC tissue sample. In conclusion, our study suggests a role of MT1-MMP in inflammatory breast cancer disease progression.
Keywords: Inflammatory breast cancer, matrix metalloproteinases, MT1-MMP, MMP-2, MMP-9
Introduction
Inflammatory breast cancer (IBC) is the most lethal subtype of breast cancer with unique clinical and pathological characteristics. Although relatively rare, it is more prominent among young ages [1] and its incidence rates appear to be increasing over the last 20 years [2]. Clinically, IBC is defined by distinct features, including rapid onset, erythema, edema of the breast, and a “peaud'orange” appearance of the skin. High metastatic potential characterized by formation of dermal and lymphatic emboli and extensive axillary lymph node involvement are the major symptoms at time of diagnosis [3-4]. Proteases are known to be involved in cancer progression through enhancement of cell motility, invasion and lymph node metastasis [5]. Among large family of proteases are the matrix metalloproteinases (MMPs) consists of 23 members including 17 secreted as soluble enzymes and 6 of them are membrane type-metalloproteinases [6].Depend on substrate specificity and domain structure, MMPs are divided into five subgroups: collagenases, gelatinases, matrilysins, stromelysins, and membrane -bound MMPs (MT-MMP) [7]. Within the tumor microenvironment MMPs expressed and secreted by carcinoma and stromal cells. MMPs playan essential role in cancer invasion and metastasis by degrading components of the basement membrane, thereby facilitating carcinoma cell intravasation and dissemination [8-9]. Furthermore, MMPs also may modulate carcinoma cells physiology in vivo as a consequence of their ability to cleave growth factors, cell surface receptors, cell adhesion molecules, or chemokines/cytokines. For instance, by cleaving pro-apoptotic factors, MMPs may be able to generate a more aggressive phenotype via production of apoptosis-resistant cells [10]. MMPs are produced as inactive forms (pro-MMPs), which are activated by other proteases. Their activity is inhibited by tissue inhibitors of matrix metalloproteinases (TIMPs) that can form complexes either with pro- or activated enzymes [8, 11].
MT1-MMP, also known as MMP-14, was the first identified membranous enzyme of the MT-MMP family [12] that appears to be important in the degradation of the extracellular matrix proteins and activation of other MMPs such as pro-MMP-2, pro-MMP-13 and pro-MMP-8 [13-15]. It also participates in cell migration, invasion and angiogenesis [16]. Inactive form of MT1-MMP is cleaved intracellulary by furin and migrates to cell surface as active form [17]. Within the tumor microenvironment MT1-MMP enhance invasion and motility of stromal cells such as fibroblasts [18] and carcinoma cells [14]. Secretary MMP-2 and -9 contribute to tumor invasion and metastasis by degradation of collagen type IV that is considered as the basic component of the basement membrane [19]. Activation of MMP-2 is regulated through the formation of a molecular complex with MT1-MMP and TIMP-2, which serves as a receptor for pro-MMP-2. The pro-MMP-2 in the trimeric complex is bound to complex and activated by TIMP-2-free MT1-MMP to generate the active form of MMP-2, then the active MMPs usually activates pro-MMP-9 [5, 15]. Co-expression of MT1-MMP and MMP-2 found to be correlated with melanoma invasion and metastasis in human xenograft models [20]. Expression of MT1-MMP, MMP-2 and MMP-9 stimulate lymph node and tongue metastasis in orthotopic murine model of head and neck cancer [21] and invasive potential of prostate cancer [22]. Similarly breast cancer progression from early stages to highly invasive and metastatic carcinomas found to be accompanied by increased in the expression of MT1-MMP, MMP-2 and MMP-9 [23]. Therefore, MT1-MMP, MMP-2 and MMP-9 may be considered as a predictive biomarker for tumor aggressiveness, invasiveness and poor prognosis [23-26]. The aim of the present research was to test the expression levels of MT1-MMP, MMP-2 and -9 in the aggressive breast cancer phenotype IBC versus non-IBC and to test whether MT1-MMP correlates with expression and activation of MMP-2 and MMP-9. We found that MT1-MMP was overexpressed in IBC versus non-IBC carcinoma tissues. Furthermore, MT1-MMP in IBC carcinoma tissues correlates with expression of pro-MMP-2 and pro-MMP-9 and the activity of MMP-2, while in non-IBC, expression of MT1-MMP correlates with expression ofpro-MMP-9. Our results indicated that MT1-MMP may play important role in IBC progression via inducing the expression of pro-MMP-2 and pro-MMP-9 and activation of MMP-2.
Materials and methods
Patient selection and tissue samples
For the purpose of patients’ enrolment, we obtained Institutional Review Board (IRB) approval from ethics committee of Ain Shams University. Fifty one breast cancer patients were enrolled from breast clinic of Ain Shams University Hospitals. According to the American Joint Committee on Cancer (AJCC), breast cancer patients were subdivided into 2 groups IBC and non-IBC. IBC patients (n=21) were clinically identified by a diffuse erythema, peaud’ orange and edema of the breast. Diagnostic examination for both core and skin biopsies were reviewed at the time of diagnosis by pathologist. The pathological evidence of IBC was confirmed by the observation of dermal tumor emboli or lymphovascular invasion [1]. Non-IBC group consisted of thirty (n=30) invasive ductal carcinoma patients that were defined as stage II and III.
Tissue specimens were obtained from pretreated diagnostic biopsy and modified radical mastectomy (MRM). Each tissue specimen was divided into 2 halves; one fixed in 10% neutral formalin buffered for histopathological diagnosis and immunohistochemistry and second half snap frozen in liquid nitrogen for further biochemical and molecular studies. For routine diagnosis, the expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (Her-2) were assessed by immunohistochemical techniques using mouse anti-ER clone 1D5, anti-PR clone PR 636 (both from Dako, Glostrup, Denmark) and mouse monoclonal antibody HER-2 (clone SPM 495) from Lab vision (Thermo Fisher Scientific Inc, USA), respectively.
Soduim dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting
Cancer tissue samples obtained from MRM were homogenized in RIPA buffer (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS). Protein content was determined using Bradford assay (Fermentas, Burlington, ON, Canada). SDS-PAGE and immunoblotting were performed as we described before [27]. Briefly, equal amounts of protein (50 μg/well) from each tissue homogenate were electrophoresed on 12% SDS- polyacrlamide gels under reducing conditions then transferred onto Immobilon-PPolyvinylidene Difluoride (PVDF) membrane (Millipore, USA). The membrane was blocked overnight with 5% nonfat dried milk in TBS- 0.05% Tween 20. Then the membrane was incubated with 1:1500 dilution of anti-MT1-MMP antibody (Millipore, USA) and followed by washing and incubation with 1:10,000 diluted peroxidase-labeled goat antirabbit secondary antibody (Kirkegaard and Perry Laboratories (KPL), Inc. Gaithersburg, MD, USA). Following washing, bands were visualized using 3, 3', 5, 5'-tetramethylbenzidine (TMB) membrane peroxidase substrate (KPL, Inc. Gaithersburg, MD, USA). Once the color appeared the reaction was stopped by immersing membrane in water for 20-30 seconds.
Immunohistochemistry (IHC)
Immunohistochemical staining was performed on 4 μm-thick paraffin tissue sections. Tissue sections were first deparaffinized followed by antigen retrieval in Tris -EDTA buffer (10mM Tris Base, 1mM EDTA Solution, 0.05% Tween 20, pH 9.0) in the microwave. After cooling to room temperature, endogenous peroxidase activity of the tissue was blocked with 3% hydrogen peroxide for 10 min (BioGenex, San Ramon, CA, USA) then slides were washed in TBS with 0.1% Tween 20. Non specific protein binding was blocked with serum blocking for 5 min (BioGenex, San Ramon, CA) followed by incubating the slides with polyclonal rabbit-MMP-14 antibody from Chemicon (Temecula, CA, USA). MT1-MMP antibody dilution used was 1:100 for 2 h. Followed by washing twice inTris-buffered saline TBS (0.05 mol/L Tris-HCl, pH 7.6, 0.15 mol/L NaCl and 0.05% Tween 20) for 5min. After washing tissue sections were incubated 100 μl of horse radish peroxidase (HRP) labeled rabbit or mouse secondary antibody [EnVision+ Dual Link System-HRP (DAB+)] for 45 min, washed, stained by 100 μl of DAB+ diluted 1:50 in substrate buffer [EnVision+ Dual Link System-HRP (DAB+)] for 15 min. Staining was achieved by adding 100 μl of DAB+ diluted 1:50 in substrate buffer. As positive control, samples from normal human placenta were used. For negative control the primary antibody was replaced by TBS on slides with specimens. Finally, specimens were rinsed in TBS, nuclei were counterstained with hematoxylin and mounted using Permount® for microscopic examination.
The immunostaining of MT1-MMP was evaluated using light microscope (Olympus, CX41, Japan), which mainly detected in the cytoplasm of malignant cells. The expression of MT1-MMP was scored according staining intensity and the number of stained cells as follows: 0, no staining or weak staining in < 10% of positive cells; score 1, weak to moderate staining detected in 11-20% of carcinoma cells, score 2, moderate to strong staining in 21-50% of cells and score 3, strong staining in >50% of cells [28].
Gelatin zymography
Gelatin zymograpy was performed as we described before [27]. Briefly, samples were denatured without reducing or heating conditions and electrophoresed on 10% containing 1% gelatin (w/v) at 4°C until the dye reached the bottom of the gel. Gels were subsequently washed twice for 15 min in renaturation buffer containing 2.5% Triton X-100 at room temperature, and then incubated overnight at 37°C in developing buffer [5 mM CaCl2, 0.05% Brij 35, and 50 mM Tris (pH 7.8)]. After incubation time, gels were stained with 0.5% Coomassie brilliant blue R-250 in 50% methanol and 10% acetic acid for 1 h and then de-stained in a 50% methanol and 10% acetic acid solution until clear bands appeared. Clear bands represent areas of gelatinolytic activity of MMP-2 and MMP-9. Human recombinant MMP-2 and MMP-9 were loaded separately as positive controls. Gelatinolytic bands of MMP-2 and MMP-9 were assessed using an arbitrary graded scale as described before [29-30]. Scale categories were divided into the following: +/- no or faint band (< 1.0 mm in width); 1+, clear or intense band detected (1-3 mm in width); and 2+, very intense band detected (> 3 mm in width).
Statistical analysis
Statistical analyses were performed using SPSS 16.0 software (SPSS). Qualitative data were expressed as frequencies and percentages while quantitative data were expressed as mean ±SD. Difference or comparison between two groups was done by Student T- test, Fisher's exact test and Mann-Whitney U-test. Association between different variables was computed using Fisher's exact test. Differences were considered statistically significant at P- value < 0.05.
Results
Patient clinical and pathological characteristic
Clinical and pathological characterization of IBC and non-IBC patients is shown in Table 1. Mean age of IBC patients was 46.7 ± 10.2 years with age range from 29 to 65 years old while mean age of non-IBC patients was 53.6 ± 11.2 years with age range from 30 to 76 years. Therefore, IBC patients were significantly (P= 0.041) younger than non-IBC patients. In IBC patients, tumor sizes were varied from 1-11 cm with mean size of 6.25 ± 3 cm, with approximately 15.0% of them having tumor sizes less than 2 cm and 85.0% having a tumor mass greater than 2 cm. Whereas, non- IBC patients showed tumor masses ranging from 1.4-11 cm with mean size of 4.48 ± 2.4 cm in which 17.9% had size less than 2cm and 82.1% patients exhibited tumor size more than 2cm. The mean tumor size of IBC patients was significantly higher (P= 0.04) than in non-IBC patients.
Table 1.
Clinical and pathological characteristics of patients with IBC and non-IBC
| Characteristics | IBC n =21(%) | Non-IBC n =30(%) | P-value |
|---|---|---|---|
| Age | |||
| Mean± SD | 46.7± 10.29 | 53.6±11.27 | 0.041*a |
| Range | (29-65) | (30-76) | |
| Tumor size | |||
| Mean± SD | 6.25±3.0 | 4.48±2.4 | 0.04*a |
| <2cm | 3(15.0%) | 5(17.9%) | |
| >2cm | 17(85.0%) | 23(82.1%) | 1.000b |
| NA | 1 | 2 | |
| Tumor grade | |||
| G2 | 17(81.0%) | 23(76.7%) | 1.000b |
| G3 | 4(19.0%) | 7(23.3%) | |
| Number of lymph nodes † | |||
| 0 | 0 | 5(18.5%) | |
| 1-4 | 6(31.6%) | 15(55.6) | 0.007*b |
| >4 | 13(68.4%) | 7(25.9%) | |
| Not examined | 2 | 3 | |
| Lymphovascular invasion | |||
| Positive | 17(81.0%) | 3(10.0%) | 0.000*b |
| Negative | 4(19.0%) | 27(90.0%) | |
| ER status | |||
| Positive | 7(36.8%) | 9(30.0%) | |
| Negative | 12(63.2%) | 21(70.0 %) | 0.757b |
| NA | 2 | - | |
| PR status | |||
| Positive | 9(47.4%) | 10(33.3%) | |
| Negative | 10(52.6%) | 20(66.7%) | 0.377b |
| NA | 2 | - | |
| HER-2 status | |||
| Positive | 3(15.8%) | 7(23.3%) | |
| Negative | 16(84.2%) | 23(76.7%) | 0.720b |
| NA | 2 | - | |
Data are reported as means± SD. NA= not available
Significant p value calculated by
Student-T test or
Fisher's exact test.
P value was calculated between < 4 involved 0 and >4
The tumor grade among IBC patients revealed that 81.0% were diagnosed as grade 2 (G2) and 19.0% were grade 3 (G3). In non-IBC patients, 76.7% were classified as grade 2 (G2) and 23.3% were tumor grade 3 (G3).
Lymph nodes metastasis was subdivided according to number of positive metastatic lymph nodes into 0, 1-4 and > 4. All IBC patients who underwent surgery had positive lymph nodes metastasis, 31.6% had 1-4 positive lymph nodes and 68.4% had greater than 4 positive metastatic lymph nodes. In non-IBC patients, 18.5% were node negative and 55.6% had 1-4 positive lymph nodes whereas 25.9% had more than 4 positive lymph nodes. Thus, women with IBC were more likely to present with 4 or more positive lymph nodes compared with non-IBC women (P= 0.007).Pathological examination of IBC and non-IBC tissue sections revealed that lymphovascular invasion characterized by migration of carcinoma cells into lymphatic vessels is significantly (P= 0.000) more than in IBC 81.0% versus non-IBC 10.0% patients.
ER, PR and HER-2 status were assessed as negative and positive for all patients. In IBC patients, positive staining for ER, PR and HER-2 was detected in 36.8%, 47.4% and 15.8%, respectively. While in non-IBC patients positive staining for ER, PR and HER-2 was 30.0%, 33.3% and 23.3% in non-IBC patients, respectively. There was no statistically significant difference among ER, PR and Her-2 in IBC versus non-IBC patients.
Overexpression of MT1-MMP in IBC versus non-IBC patients
Immunoblot analysis was used for assessment of MT1-MMP in tissue lysates of IBC and non-IBC patients. Results of immunoblot analysis showed MT1-MMP (Mwt 60 kDa) was highly expressed in IBC tissues as compared to non-IBC tissues. In which, 66.7% of IBC patients’ breast tissues expressed high level of MT1-MMP characterized by heavy band at 60 kDa (Figure 1A) while, 40.0% of non-IBC patients’ breast tissues expressed light band of MT1-MMP (Figure 1B).Immunohistochemical analysis confirmed immunoblot results where IBC tissues highly expressing (score 3) MT1-MMP (Figure 2A and B) compared to moderate expression (score 2) of MT1-MMP by non-IBC carcinoma cells (Figure 2C and D).
Figure 1.

Immunoblot analysis showing expression of MT1-MMP (MMP-14) in tissue lysates of IBC (A) and non-IBC patients (B). A. Lanes from 1-6 represent tissue lysates of different IBC patients and shows increased expression of MT1-MMP (60 kDa). B. Lanes from 1-6 represent tissue lysates of different non-IBC patients and shows weak expression of MT1 -MMP(60kDa).
Figure 2.
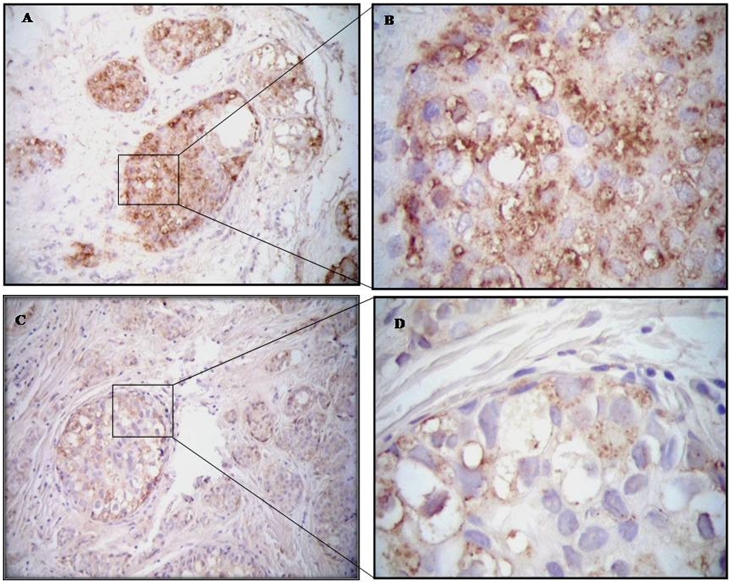
Photomicrographs represent immunohistochemistry staining of MT1-MMP expression. [A & B] IBC patient shows overexpression (score 3) of MT1-MMP. [C & D] Non-IBC invasive intraductal carcinoma showing moderate expression (score 2) of MT1-MMP; magnification 100X and 400X, respectively.
Increase activity of MMP-2 and MMP-9 in the IBC versus non-IBC tissue samples
To determine the activity of MMP-2 and MMP-9 in tissues of IBC and non-IBC patients, we used the gelatin zymography assay. For MMP-2, both pro-form at Mwt 72 kDa and active form at Mwt 62 kDa were detected in tissue homogenate of IBC (Figure 3A) and non-IBC (Figure 3B). While for MMP-9, the pro-form was detected at Mwt 92 kDa and active form at Mwt 82kDa in the tissue lysates of IBC (Figure 3A) and non-IBC (Figure 3B).
Figure 3.

Representative zymograms of tissue lysates from IBC (A) and non-IBC patients (B) depicting gelatinolytic activity of proMMP-2 (72 kDa), MMP-2 (62 kDa), proMMP-9 (92 kDa) and MMP-9 (82 kDa). Samples were equally loaded (50|jg protein/well), separated by 10% SDS-PAGE containing 1% gelatin undernon-reducing conditions without heating. Regions of enzymatic activity were appeared as clear zones over the black background.
Using an arbitrary graded scale as described before [29-30], we assessed gelatinolytic activity of MMP-2 and MMP-9. As described in Table 2, MMP-2 and MMP-9 activities were variable among tumors of IBC and non-IBC. Using Semi-quantitative analysis, we found that intense bands (1+) of pro- MMP-9 in 66.7% of IBC tissues versus 93.3% of non-IBC whereas very intense bands (2+) in was detected only in 33.3% of IBC not in non-IBC tissue. IBC patients were significantly higher (P= 0.001) for pro-MMP-9 than non-IBC patients.
Table 2.
Semi-quantitative analysis of MMP-2 and MMP-9 activity in IBC versus non-IBC patients
| Patients | +/− | 1+ | 2+ | P-value | |
|---|---|---|---|---|---|
| Pro-MMP-9 | IBC | 0 | 14(66.7%) | 7(33.3%) | P = 0.001* |
| Non-IBC | 2(6.7%) | 28(93.3%) | 0 | ||
| Active MMP-9 | IBC | 9(42.9%) | 8(38.1%) | 4(19%) | P = 0.093 |
| Non-IBC | 18(60.0%) | 12(40.0%) | 0 | ||
| Pro-MMP-2 | IBC | 1(4.8%) | 19(90.4%) | 1(4.8%) | P = 0.009* |
| Non-IBC | 10(33.3%) | 20(66.6%) | 0 | ||
| Active MMP-2 | IBC | 1(4.8%) | 9(42.8%) | 11(52.4%) | P = 0.000* |
| Non-IBC | 12(40.0%) | 15(50.0%) | 3(10.0%) |
Significant p value calculated by Mann-Whitney U-test. Gelatinolytic bands were assessed and divided into the following: +/-, no or faint band detected (< 1mm in width); 1+, clear or intense band detected (1-3mm in width) and 2+, very intense band detected (> 3mm in width).
Regarding active form of MMP-9, 38.1% showed intense bands of 1+ and 19% showed very intense bands of 2+ in IBC patients. In non-IBC patients, intense bands (1+) in 40.0% and no detectable bands of 2+ were showed. However, this trend was not statistically significant (P = 0.093).
In IBC patients, pro- and active MMP-2 of intense bands (1+) were detected in 90.4% and 42.8%, respectively. Very intense bands (2+) of pro- and active MMP-2 were observed in 4.8% and 52.4%, respectively.
In non-IBC, detection of pro- and active MMP-2 were almost negative/weak (+/-) in 33.3% and 40.0%, respectively in the examined tissues. Intense bands (1+) of pro- and active MMP-2 were detected in 66.6% and 50.0%, respectively. While very intense band (+2) of active MMP-2 was detected in 10% of the examined non-IBC tissue homogenates. There were statistically significant in MMP-2 expression (P= 0.009) and activity (P= 0.000) between IBC versus non-IBC carcinoma tissue.
Correlation between MT1-MMP and both forms of MMP-9 and MMP-2 in tissue lysates of IBC and non-IBC patient
To test whether the MT1-MMP expression correlates with MMP-2 and MMP-9 activities in IBC versus non-IBC patient tissues, we used Fisher's exact test (Table 3). MT1-MMP showed statistically significant association (P= 0.0086, P= 0.0448 and P= 0.0448) with pro-MMP-9, pro-MMP-2 and active MMP-2 in the IBC patients, respectively. While in non-IBC tissues, a significant correlation of MT1-MMP (P< 0.0001) was detected only with pro-MMP-9 and correlation between pro-MMP-2 and MT1-MMP was observed within the limits of significance (P= 0.06).
Table 3.
Correlation of MT1-MMP with MMP-2 and MMP-9 in IBC versus non-IBC tissues
| IBC, n (%) | P-value | Non-IBC, n (%) | P-value | ||||
|---|---|---|---|---|---|---|---|
| +ve | −ve | +ve | −ve | ||||
| MT1-MMP | 14(66.7%) | 7(33.3%) | 12(40.0%) | 18(60.0%) | |||
| Pro-MMP-9 | 21(100%) | 0(0%) | 0.0086* | 28(93.3%) | 2(6.7%) | <0.0001* | |
| Active MMP-9 | 12(57.1%) | 9(42.9%) | 0.7513 | 18(60.0%) | 12(40.0%) | 1.000 | |
| Pro-MMP-2 | 20(95.2%) | 1(4.8%) | 0.0448* | 10(33.3%) | 20(66.7%) | 0.06 | |
| Active MMP-2 | 20(95.2%) | 1(4.8%) | 0.0448* | 12(40.0%) | 18(60.0%) | 0.1964 | |
Significant p value analyzed by Fisher's exact test. n: number of patient
Discussion
Tumor invasion and metastasis are associated with a complex cascade of proteolytic events and a crucial role played by MMPs in the degradation of extracellular matrix (ECM) proteins and basement membrane [8]. MT1-MMP is considered as one of the critical MMPs involved in the tumor invasion by functioning as a pericellular collagenase and an activator of the latent form of MMP-2 [15]. MT1-MMP found to be directly associated with metastasis and poor prognosis [31] either directly or through activation of gelatinases MMP-2 [32] and MMP-9 [33].
In the present study, we compared the expression of MMPs (MT1-MMP, MMP2 and MMP-9) in IBC versus non-IBC patients in an attempt to provide a more validated data on biological behavior of IBC phenotype. We assessed the level of expression of MT1-MMP in breast cancer tissues of the study groups using immunoblotting and immunohistochemistry. MT1-MMP was strongly detected in cytoplasm of IBC cells, with slightly staining in the adjacent stromal cells [unpublished data]. Our results agree with other studies which detected positive staining of MT1-MMP in the cytoplasm of breast tumor cells with slight staining of stromal cells [25].
There are many studies investigated the relationship between expression of MMPs and clinicopathological characteristics of breast cancer patients. Highest expression of MT1-MMP [34-35] and MMP-2 [36] was found in cases with larger tumor size. However, others [28, 37-38] found non-significant association of MT1-MMP expression either from breast tumor cells or stromal cells with tumor size. In regard to lymph node metastasis, studies showed that high expression of MMPs especially MT1-MMP, MMP-2 and MMP-9 [34-35, 38-40] was significantly associated with positive lymph node metastasis. Metastatic cancer cells in the metastatic axillary lymph nodes (MALNs) can induce MT1-MMP and MMP-7 production by fibroblast-like cells and mononuclear inflammatory cells; this may prove role of MMPs expressed by the stromal cells in tumor progression [41]. Additionally, MMP-2 and MMP-9 can induce releasing ECM-bound proangiogenic factors such as vascular endothelial growth factor (VEGF), thus accelerating angiogenesis process [42]. Increased expression of MMP-2, MMP-9 and VEGF was correlated with increased number of lymphatic vessels; this may enhance lymphangiogenesis and metastatic lymph node of breast cancer [43].
However, in early reports, MT1-MMP exhibit to have a single function as an activator for MMPs such as MMP-2. Recent data have provided evidence that MT1-MMP plays essential role in the degradation of the ECM, cleavage of cell adhesion molecules and cell surface receptors [44]. Due to participation of active MMP-2, MMP -8 and MMP-13 in the degradation of the interstitial collagen, MT1-MMP mediated the activation of pro- form of these enzymes [15] indicating that MT1-MMP can contribute in tumor progression and metastasis either by cleavage extracellular components or by activating other MMPs.
For that increase in the activity of MMP-2 and MMP-9 in breast cancer patients tissues and sera postulated to be strong marker for predicting development and severity of breast cancer invasion [36, 45]. For instance, high activity of MMP-2 and MMP-9 was detected in carcinoma tissues [40] and sera of breast cancer patients [45-46] suggesting important role of both enzymes as biomarkers in breast cancer progression and aggressiveness.
In the present study, we detected increased expression of MT1-MMP in aggressive phenotype IBC compared to non-IBC. In addition, there was also significant increase in the expression of pro-MMP-9 (P= 0.001), pro- MMP-2 (P= 0.009) and active MMP-2 (P= 0.000) in IBC relative to non-IBC carcinoma tissues.
IBC is known as an aggressive form of breast cancer characterized by high invasiveness, poor prognosis and low survival rate [47]. Although MMPs are probably important mediators for the invasiveness, motility and metastatic potential of non-IBC [19, 48], they are not well studied in IBC. Since high levels and activity of MMPs (MT1-MMP, MMP-2 and MMP-9) is closely linked to breast cancer disease aggressiveness, increase in the number of positive metastatic lymph node, and poor prognosis, all previous properties coincided with IBC characteristic nature.
Since the activation of MMP-2 is associated with increase in the probability of cancer metastasis [49-50], a mechanism may be mediated by MT1-MMP activity at cell surface through TIMP/MT1-MMP complex [15]. We also correlated between expression of MT1-MMP and MMP-2 in cancer tissues of IBC versus non-IBC. We detected a statistically significant correlation between MT1-MMP and both forms (pro-and active) of MMP-2 among IBC patients (P= 0.0086, P= 0.0448). The present results agree with studies which have shown MT1-MMP to play significant role in the activation of pro-MMP -2 in the presence low amount of TIMP-2. It is know that pro-MMP-2 binds to MT1-MMP and TIMP-2, forming ternary complex and then to be activated by an adjacent TIMP-2 free MT1-MMPon the cell surface [12, 32, 34, 51]. Furthermore, MT1-MMP and TIMP-2 were induced by H-Ras in MCF10A human breast epithelial cells, leading to activation of MMP-2 [52]. Interestingly, co-localization of MT1-MMP and MMP-2 is implicated in the tumor progression from early steps of initial tumor development, growth and angiogenesis to invasion and metastasis [53].
Moreover, our results showed that the pro-form of MMP-9 was significantly (P= 0.0086) associated with MT1-MMP expression in IBC and not non-IBC. This is in accordance with other pervious findings which detected association between the expression of cytoplasmic MMP-9 and MT1-MMP in breast cancer patients [28]. However, the activation of MMP-9 is still debated, some studies found MT1-MMP participates in MMP-9 activation [33] and others found pro-MMP-9 is activated by other MMPs such as MMP-2 [54], MMP-3 [55-56], MMP-7 [57], MMP-13 [58] and MMP-26 [59].
In conclusion, overexpression of MT1-MMP by IBC carcinoma tissues may be associated with disease aggressiveness either directly through inducing cell motility, or indirectly by activation of MMP-2 which participate in extracellular matrix degradation subsequently enhance IBC invasiveness and metastasis. Further studies are warranted to confirm our present findings and elucidate role of MT1-MMP in IBC progression.
Acknowledgments
Authors acknowledge with special gratitude the collaboration of Prof. Bonnie F. Sloane Wayne State University, Detroit, MI, USA for providing MT1-MMP antibody. We thank Prof. Akram N. Nouh (Department of Pathology, National Cancer Institute, Cairo University, Giza, Egypt) for his assistance in reviewing and scoring of pathology slides. We also thank Mr. Ayman Maher (Department of Zoology, Cairo University, Giza, Egypt) for his assistance in IHC stain. The authors are supported by Avon Foundation USA (M.M.M) and Science and Technology Development Funds (Grant # 408), Egypt (M.M.M.).
References
- 1.Nouh MA, Mohamed MM, El-Shinawi M, Shaalan MA, Cavallo-Medved D, Khaled HM, Sloane BF. Cathepsin B: a potential prognostic marker for inflammatory breast cancer. J Transl Med. 2011;9:1. doi: 10.1186/1479-5876-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97:966–975. doi: 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giordano SH, Hortobagyi GN. Inflammatory breast cancer: clinical progress and the main problems that must be addressed. Breast Cancer Res. 2003;5:284–288. doi: 10.1186/bcr608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Laere SJ, Van den Eynden GG, Van der Auwera I, Vandenberghe M, van Dam P, Van Marck EA, van Golen KL, Vermeulen PB, Dirix LY. Identification of cell-of-origin breast tumor subtypes in inflammatory breast cancer by gene expression profiling. Breast Cancer Res Treat. 2006;95:243–255. doi: 10.1007/s10549-005-9015-9. [DOI] [PubMed] [Google Scholar]
- 5.Lee M, Fridman R, Mobashery S. Extracellular proteases as targets for treatment of cancer metastases. Chem Soc Rev. 2004;33:401–409. doi: 10.1039/b209224g. [DOI] [PubMed] [Google Scholar]
- 6.Radisky ES, Radisky DC. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:201–212. doi: 10.1007/s10911-010-9177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 9.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez LO, Junquera S, del Casar JM, Gonzalez L, Marin L, Gonzalez-Reyes S, Andicoechea A, Gonzalez-Fernandez R, Gonzalez JM, Perez-Fernandez R, Vizoso FJ. Immunohistochemical study of matrix metalloproteinases and their inhibitors in pure and mixed invasive and in situ ductal carcinomas of the breast. Hum Pathol. 2010;41:980–989. doi: 10.1016/j.humpath.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6:480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 12.Cao J, Sato H, Takino T, Seiki M. The C-terminal region of membrane type matrix metalloproteinase is a functional transmembrane domain required for pro-gelatinase A activation. J Biol Chem. 1995;270:801–805. doi: 10.1074/jbc.270.2.801. [DOI] [PubMed] [Google Scholar]
- 13.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 14.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbolina MV, Stack MS. Membrane type 1-matrix metalloproteinase: substrate diversity in pericellular proteolysis. Semin Cell Dev Biol. 2008;19:24–33. doi: 10.1016/j.semcdb.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seiki M. Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion. Cancer Lett. 2003;194:1–11. doi: 10.1016/s0304-3835(02)00699-7. [DOI] [PubMed] [Google Scholar]
- 17.Yana I, Weiss SJ. Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol Biol Cell. 2000;11:2387–2401. doi: 10.1091/mbc.11.7.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H, Sodek KL, Hwang Q, Brown TJ, Ringuette M, Sodek J. Phagocytosis of collagen by fibroblasts and invasive cancer cells is mediated by MT1-MMP. Biochem Soc Trans. 2007;35:704–706. doi: 10.1042/BST0350704. [DOI] [PubMed] [Google Scholar]
- 19.Duffy MJ, Maguire TM, Hill A, McDermott E, O'Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2:252–257. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann UB, Westphal JR, Zendman AJ, Becker JC, Ruiter DJ, van Muijen GN. Expression and activation of matrix metalloproteinase-2 (MMP-2) and its co-localization with membrane-type 1 matrix metalloproteinase (MT1-MMP) correlate with melanoma progression. J Pathol. 2000;191:245–256. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH632>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Dasgupta S, Bhattacharya Chatterjee M, O'Malley BW, Jr, Chatterjee SK. Tumor me tastasis in an orthotopic murine model of head and neck cancer: possible role of TGF-beta 1 secreted by the tumor cells. J Cell Biochem. 2006;97:1036–1051. doi: 10.1002/jcb.20647. [DOI] [PubMed] [Google Scholar]
- 22.Wilson SR, Gallagher S, Warpeha K, Hawthorne SJ. Amplification of MMP-2 and MMP-9 production by prostate cancer cell lines via activation of protease-activated receptors. Prostate. 2004;60:168–174. doi: 10.1002/pros.20047. [DOI] [PubMed] [Google Scholar]
- 23.Figueira RC, Gomes LR, Neto JS, Silva FC, Silva ID, Sogayar MC. Correlation between MMPs and their inhibitors in breast cancer tumor tissue specimens and in cell lines with different metastatic potential. BMC Cancer. 2009;9:20. doi: 10.1186/1471-2407-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang WG, Davies G, Martin TA, Parr C, Watkins G, Mason MD, Mansel RE. Expression of membrane type-1 matrix metalloproteinase, MT1-MMP in human breast cancer and its impact on invasiveness of breast cancer cells. Int J Mol Med. 2006;17:583–590. [PubMed] [Google Scholar]
- 25.Kohrmann A, Kammerer U, Kapp M, Dietl J, Anacker J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC Cancer. 2009;9:188. doi: 10.1186/1471-2407-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez LO, Pidal I, Junquera S, Corte MD, Vazquez J, Rodriguez JC, Lamelas ML, Merino AM, Garcia-Muniz JL, Vizoso FJ. Overexpression of matrix metalloproteinases and their inhibitors in mononuclear inflammatory cells in breast cancer correlates with metastasis-relapse. Br J Cancer. 2007;97:957–963. doi: 10.1038/sj.bjc.6603963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohamed MM, Cavallo-Medved D, Rudy D, Anbalagan A, Moin K, Sloane BF. Interleukin-6 increases expression and secretion of cathepsin B by breast tumor-associated monocytes. Cell Physiol Biochem. 2010;25:315–324. doi: 10.1159/000276564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mylona E, Nomikos A, Magkou C, Kamberou M, Papassideri I, Keramopoulos A, Nakopoulou L. The clinicopathological and prognostic significance of membrane type 1 matrix metalloproteinase (MT1-MMP) and MMP-9 according to their localization in invasive breast carcinoma. Histopathology. 2007;50:338–347. doi: 10.1111/j.1365-2559.2007.02615.x. [DOI] [PubMed] [Google Scholar]
- 29.Young TN, Rodriguez GC, Rinehart AR, Bast RC, Jr, Pizzo SV, Stack MS. Characterization of gelatinases linked to extracellular matrix invasion in ovarian adenocarcinoma: purification of matrix metalloproteinase 2. Gynecol Oncol. 1996;62:89–99. doi: 10.1006/gyno.1996.0195. [DOI] [PubMed] [Google Scholar]
- 30.Sun XM, Dong WG, Yu BP, Luo HS, Yu JP. Detection of type IV collagenase activity in malignant ascites. World J Gastroenterol. 2003;9:2592–2595. doi: 10.3748/wjg.v9.i11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozanov DV, Savinov AY, Williams R, Liu K, Golubkov VS, Krajewski S, Strongin AY. Molecular signature of MT1-MMP: transactivation of the downstream universal gene network in cancer. Cancer Res. 2008;68:4086–4096. doi: 10.1158/0008-5472.CAN-07-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 33.Toth M, Chvyrkova I, Bernardo MM, Hernandez-Barrantes S, Fridman R. Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP -3: role of TIMP-2 and plasma membranes. Biochem Biophys Res Commun. 2003;308:386–395. doi: 10.1016/s0006-291x(03)01405-0. [DOI] [PubMed] [Google Scholar]
- 34.Ueno H, Nakamura H, Inoue M, Imai K, Noguchi M, Sato H, Seiki M, Okada Y. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in human invasive breast carcinomas. Cancer Res. 1997;57:2055–2060. [PubMed] [Google Scholar]
- 35.Gonzalez LO, Gonzalez-Reyes S, Marin L, Gonzalez L, Gonzalez JM, Lamelas ML, Merino AM, Rodriguez E, Pidal I, del Casar JM, Andicoechea A, Vizoso F. Comparative analysis and clinical value of the expression of metalloproteases and their inhibitors by intratumour stromal mononuclear inflammatory cells and those at the invasive front of breast carcinomas. Histopathology. 2010;57:862–876. doi: 10.1111/j.1365-2559.2010.03723.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu SC, Yang SF, Yeh KT, Yeh CM, Chiou HL, Lee CY, Chou MC, Hsieh YS. Relationships between the level of matrix metalloproteinase-2 and tumor size of breast cancer. Clin Chim Acta. 2006;371:92–96. doi: 10.1016/j.cca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 37.Ishigaki S, Toi M, Ueno T, Matsumoto H, Muta M, Koike M, Seiki M. Significance of membrane type 1 matrix metalloproteinase expression in breast cancer. Jpn J Cancer Res. 1999;90:516–522. doi: 10.1111/j.1349-7006.1999.tb00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tetu B, Brisson J, Wang CS, Lapointe H, Beaudry G, Blanchette C, Trudel D. The influence of MMP-14, TIMP-2 and MMP-2 expression on breast cancer prognosis. Breast Cancer Res. 2006;8:R28. doi: 10.1186/bcr1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HJ, Park CI, Park BW, Lee HD, Jung WH. Expression of MT-1 MMP, MMP2, MMP9 and TIMP2 mRNAs in ductal carcinoma in situ and invasive ductal carcinoma of the breast. Yonsei Med J. 2006;47:333–342. doi: 10.3349/ymj.2006.47.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah FD, Shukla SN, Shah PM, Shukla HK, Patel PS. Clinical significance of matrix metalloproteinase 2 and 9 in breast cancer. Indian J Cancer. 2009;46:194–202. doi: 10.4103/0019-509X.52953. [DOI] [PubMed] [Google Scholar]
- 41.Garcia MF, Gonzalez Reyes S, Gonzalez LO, Junquera S, Berdize N, Del Casar JM, Medina M, Vizoso FJ. Comparative study of the expression of metalloproteases and their inhibitors in different localizations within primary tumours and in metastatic lymph nodes of breast cancer. Int J Exp Pathol. 2010;91:324–334. doi: 10.1111/j.1365-2613.2010.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duffy MJ, McGowan PM, Gallagher WM. Cancer invasion and metastasis: changing views. J Pathol. 2008;214:283–293. doi: 10.1002/path.2282. [DOI] [PubMed] [Google Scholar]
- 43.Zheng SQ, Huang RQ, Zhang YJ. Role of matrix metalloproteinase (MMP)-2 and -9 and vascular endothelial growth factor C in lymph node metastasis of breast cancer. Zhonghua Bing Li Xue Za Zhi. 2010;39:240–244. [PubMed] [Google Scholar]
- 44.Strongin AY. Proteolytic and non-proteolytic roles of membrane type-1 matrix metalloproteinase in malignancy. Biochim Biophys Acta. 2010;1803:133–141. doi: 10.1016/j.bbamcr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel S, Sumitra G, Koner BC, Saxena A. Role of serum matrix metalloproteinase-2 and -9 to predict breast cancer progression. Clin Biochem. 2011;44:869–872. doi: 10.1016/j.clinbiochem.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 46.Stankovic S, Konjevic G, Gopcevic K, Jovic V, Inic M, Jurisic V. Activity of MMP-2 and MMP-9 in sera of breast cancer patients. Pathol Res Pract. 2010;206:241–247. doi: 10.1016/j.prp.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Kleer CG, van Golen KL, Merajver SD. Molecular biology of breast cancer metastasis. Inflammatory breast cancer: clinical syndrome and molecular determinants. Breast Cancer Res. 2000;2:423–429. doi: 10.1186/bcr89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kousidou OC, Roussidis AE, Theocharis AD, Karamanos NK. Expression of MMPs and TIMPs genes in human breast cancer epithelial cells depends on cell culture conditions and is associated with their invasive potential. Anticancer Res. 2004;24:4025–4030. [PubMed] [Google Scholar]
- 49.Banerji A, Chakraborti J, Mitra A, Chatterjee A. Cell membrane-associated MT1-MMP-dependent activation of pro-MMP-2 in A375 melanoma cells. J Environ Pathol Toxicol Oncol. 2005;24:3–17. doi: 10.1615/jenvpathtoxoncol.v24.i1.20. [DOI] [PubMed] [Google Scholar]
- 50.Di Nezza LA, Misajon A, Zhang J, Jobling T, Quinn MA, Ostor AG, Nie G, Lopata A, Salamonsen LA. Presence of active gelatinases in endometrial carcinoma and correlation of matrix metalloproteinase expression with increasing tumor grade and invasion. Cancer. 2002;94:1466–1475. doi: 10.1002/cncr.10355. [DOI] [PubMed] [Google Scholar]
- 51.Itoh Y, Takamura A, Ito N, Maru Y, Sato H, Suenaga N, Aoki T, Seiki M. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 2001;20:4782–4793. doi: 10.1093/emboj/20.17.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim IY, Jeong SJ, Kim ES, Kim SH, Moon A. Type I collagen-induced pro-MMP-2 activation is differentially regulated by H-Ras and N-Ras in human breast epithelial cells. J Biochem Mol Biol. 2007;40:825–831. doi: 10.5483/bmbrep.2007.40.5.825. [DOI] [PubMed] [Google Scholar]
- 53.Sounni NE, Noel A. Membrane type-matrix metalloproteinases and tumor progression. Biochimie. 2005;87:329–342. doi: 10.1016/j.biochi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Fridman R, Toth M, Pena D, Mobashery S. Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2) Cancer Res. 1995;55:2548–2555. [PubMed] [Google Scholar]
- 55.Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267:3581–3584. [PubMed] [Google Scholar]
- 56.Ramos DeSimone N, Hahn Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999;274:13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- 57.Sang QX, Birkedal Hansen H, Van Wart HE. Proteolytic and non-proteolytic activation of human neutrophil progelatinase B. Biochim Biophys Acta. 1995;1251:99–108. doi: 10.1016/0167-4838(95)00086-a. [DOI] [PubMed] [Google Scholar]
- 58.Knauper V, Smith B, Lopez Otin C, Murphy G. Activation of progelatinase B (proMMP-9) by active collagenase-3 (MMP-13) Eur J Biochem. 1997;248:369–373. doi: 10.1111/j.1432-1033.1997.00369.x. [DOI] [PubMed] [Google Scholar]
- 59.Uria JA, Lopez Otin C. Matrilysin-2, a new matrix metalloproteinase expressed in human tumors and showing the minimal domain organization required for secretion, latency, and activity. Cancer Res. 2000;60:4745–4751. [PubMed] [Google Scholar]


